Development of an Anthropomorphic Phantom of the Axillary Region for Microwave Imaging Assessment
Abstract
1. Introduction
2. Dielectric Properties Measurement of Lymph Nodes
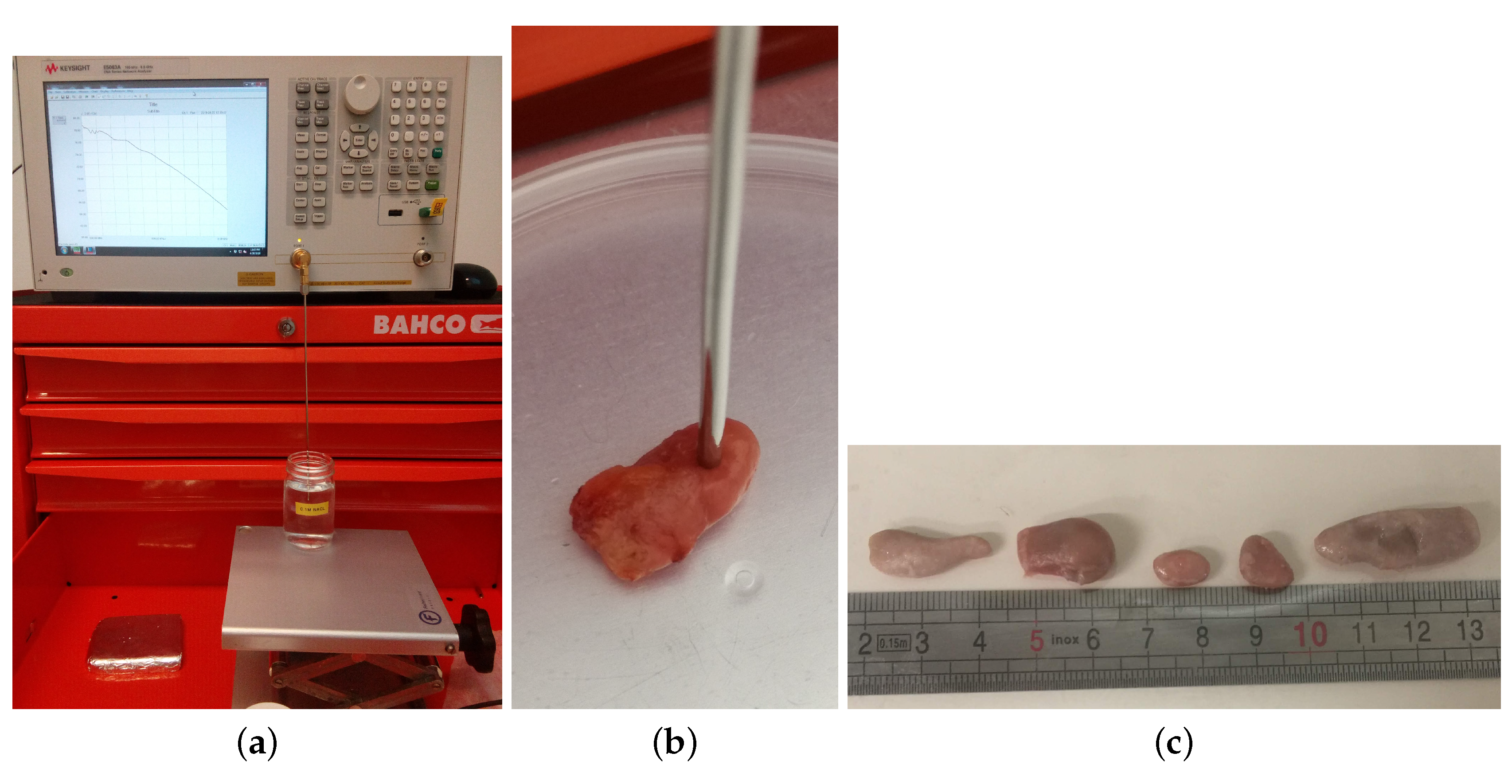
2.1. Measurement Procedure
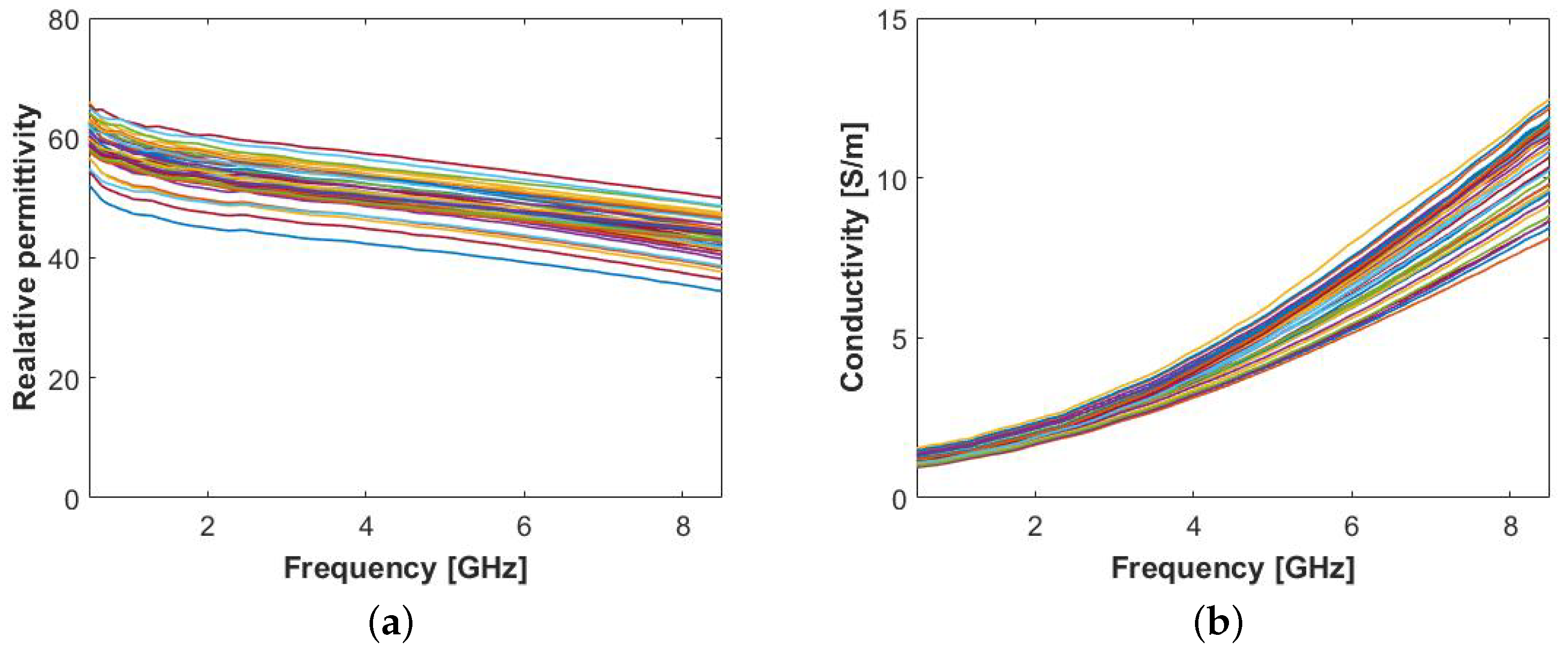
2.1.1. Characterization of Human Axillary Lymph Nodes
- included measurement where ;
- included measurement where ;
- included measurement where .
2.1.2. Characterization of Animal Lymph Nodes
3. Tissue Mimicking Materials
4. Axillary Phantom Design and Development
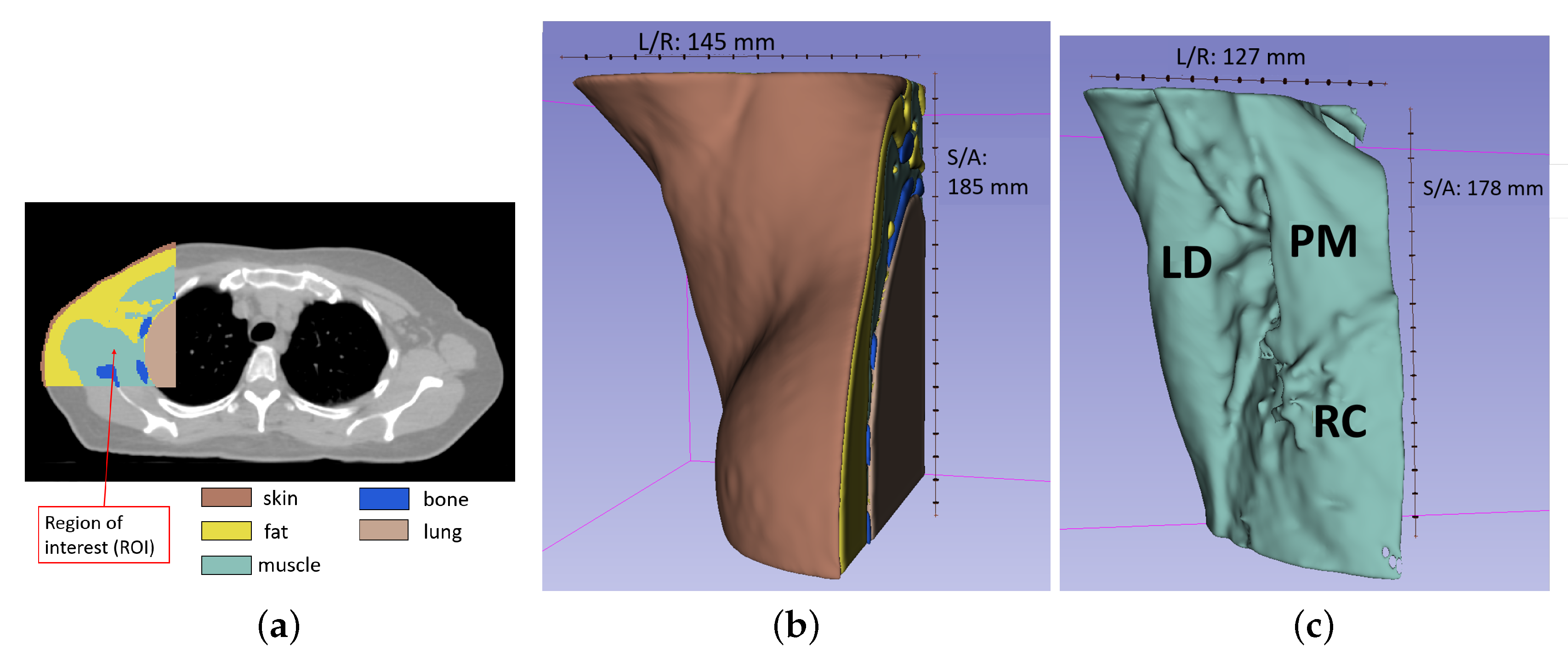
4.1. Patient Position and Intended MWI Setup Description
4.2. Axillary Phantom Development and Fabrication
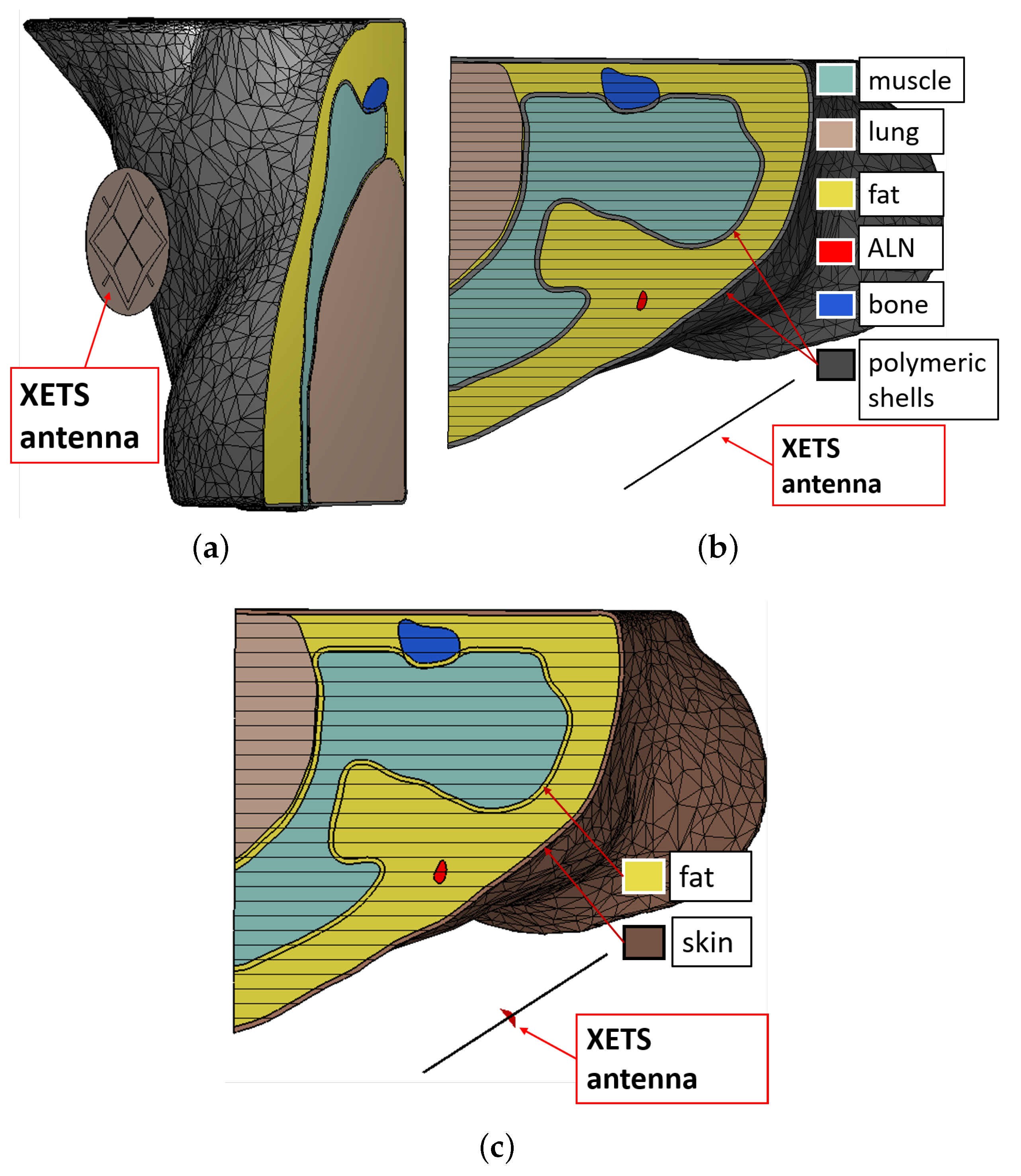
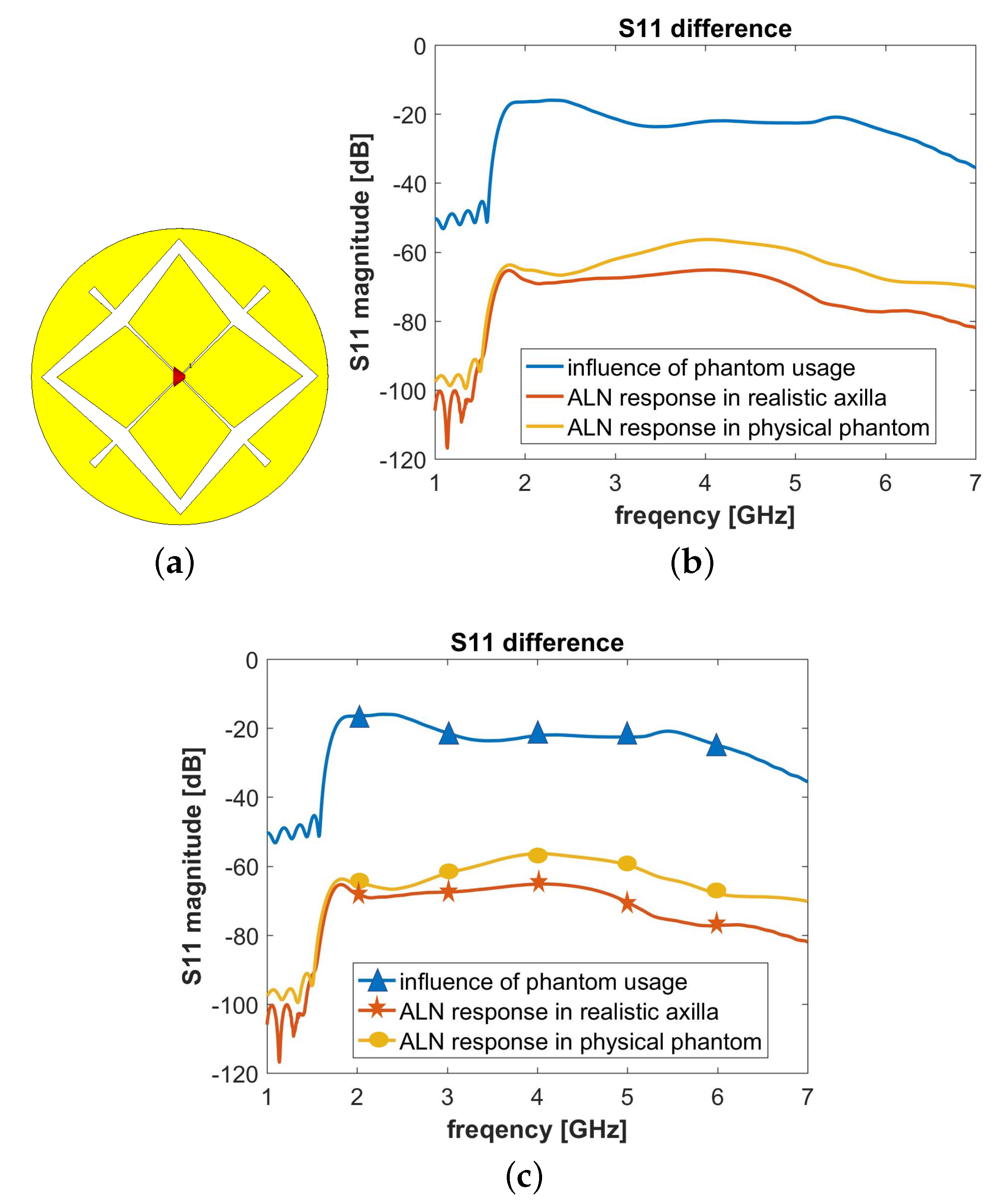
5. Numerical Assessment
6. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Giuliano, A.E.; Somerfield, M.R.; Benson, A.B., III; Bodurka, D.C.; Burstein, H.J.; Cochran, A.J.; Cody, H.S., III; Edge, S.B.; Galper, S.; et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J. Clin. Oncol. 2005, 23, 7703–7720. [Google Scholar] [CrossRef] [PubMed]
- Facts for Life: Axillary Lymph Node. 2009. Available online: https://ww5.komen.org/uploadedFiles/_Komen/Content/About_Breast_Cancer/Tools_and_Resources/Fact_Sheets_and_Breast_Self_Awareness_Cards/AxillaryLymphNodes.pdf (accessed on 1 September 2020).
- Rahbar, H.; Partridge, S.C.; Javid, S.H.; Lehman, C.D. Imaging axillary lymph nodes in patients with newly diagnosed breast cancer. Curr. Probl. Diagn. Radiol. 2012, 41, 149–158. [Google Scholar] [CrossRef]
- Hagness, S.C.; Taflove, A.; Bridges, J.E. Two-dimensional FDTD analysis of a pulsed microwave confocal system for breast cancer detection: Fixed-focus and antenna-array sensors. IEEE Trans. Biomed. Eng. 1998, 45, 1470–1479. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, B.; Xu, L.; Li, J.; Stoica, P. Multistatic adaptive microwave imaging for early breast cancer detection. IEEE Trans. Biomed. Eng. 2006, 53, 1647–1657. [Google Scholar] [CrossRef]
- Conceição, R.C.; Mohr, J.J.; O’Halloran, M. An Introduction to Microwave Imaging for Breast Cancer Detection; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Eleutério, R.; Medina, A.; Conceição, R.C. Initial study with microwave imaging of the axilla to aid breast cancer diagnosis. In Proceedings of the 2014 USNC-URSI Radio Science Meeting (Joint with AP-S Symposium), Memphis, TN, USA, 6–11 July 2014; IEEE: Piscataway, NJ, USA, 2014; p. 306. [Google Scholar]
- Eleutério, R.; Conceição, R.C. Initial study for detection of multiple lymph nodes in the axillary region using Microwave Imaging. In Proceedings of the 2015 9th European Conference on Antennas and Propagation (EuCAP), Lisbon, Portugal, 13–17 April 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–3. [Google Scholar]
- Godinho, D.M.; Felício, J.M.; Castela, T.; Silva, N.M.; Orvalho, L.M.; Fernandes, C.A.; Conceição, R.C. Extracting Dielectric Properties for MRI-based Phantoms for Axillary Microwave Imaging Device. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020; IEEE: Piscataway, NJ, USA, 2020. [Google Scholar]
- Savazzi, M.; Porter, E.; O’Halloran, M.; Costa, J.R.; Fernandes, C.A.; Felício, J.M.; Conceição, R.C. Development of a Transmission-Based Open-Ended Coaxial-Probe Suitable for Axillary Lymph Node Dielectric Measurements. In Proceedings of the 2020 14th European Conference on Antennas and Propagation (EuCAP), Copenhagen, Denmark, 15–20 March 2020; IEEE: Piscataway, NJ, USA, 2020. [Google Scholar]
- Liu, J.; Hay, S.G. Prospects for microwave imaging of the lymphatic system in the axillary. In Proceedings of the 2016 IEEE-APS Topical Conference on Antennas and Propagation in Wireless Communications (APWC), Cairns, QLD, Australia, 19–23 September 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 183–186. [Google Scholar]
- Lazebnik, M.; Madsen, E.L.; Frank, G.R.; Hagness, S.C. Tissue-mimicking phantom materials for narrowband and ultrawideband microwave applications. Phys. Med. Biol. 2005, 50, 4245. [Google Scholar] [CrossRef] [PubMed]
- Mashal, A.; Gao, F.; Hagness, S.C. Heterogeneous anthropomorphic phantoms with realistic dielectric properties for microwave breast imaging experiments. Microw. Opt. Technol. Lett. 2011, 53, 1896–1902. [Google Scholar] [CrossRef]
- Bakar, A.A.; Abbosh, A.; Bialkowski, M. Fabrication and characterization of a heterogeneous breast phantom for testing an ultrawideband microwave imaging system. In Proceedings of the Asia-Pacific Microwave Conference 2011, Melbourne, Australia, 5–8 December 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1414–1417. [Google Scholar]
- Hahn, C.; Noghanian, S. Heterogeneous breast phantom development for microwave imaging using regression models. Int. J. Biomed. Imaging 2012, 2012, 607. [Google Scholar] [CrossRef]
- Klemm, M.; Leendertz, J.; Gibbins, D.; Craddock, I.; Preece, A.; Benjamin, R. Microwave radar-based breast cancer detection: Imaging in inhomogeneous breast phantoms. IEEE Antennas Wirel. Propag. Lett. 2009, 8, 1349–1352. [Google Scholar] [CrossRef]
- Burfeindt, M.J.; Colgan, T.J.; Mays, R.O.; Shea, J.D.; Behdad, N.; Van Veen, B.D.; Hagness, S.C. MRI-derived 3-D-printed breast phantom for microwave breast imaging validation. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 1610–1613. [Google Scholar] [CrossRef]
- Garrett, J.; Fear, E. Stable and flexible materials to mimic the dielectric properties of human soft tissues. IEEE Antennas Wirel. Propag. Lett. 2014, 13, 599–602. [Google Scholar] [CrossRef]
- Garrett, J.; Fear, E. A new breast phantom with a durable skin layer for microwave breast imaging. IEEE Trans. Antennas Propag. 2015, 63, 1693–1700. [Google Scholar] [CrossRef]
- Santorelli, A.; Laforest, O.; Porter, E.; Popović, M. Image classification for a time-domain microwave radar system: Experiments with stable modular breast phantoms. In Proceedings of the 2015 9th European Conference on Antennas and Propagation (EuCAP), Lisbon, Portugal, 13–17 April 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–5. [Google Scholar]
- Oliveira, B.L.; O’Loughlin, D.; O’Halloran, M.; Porter, E.; Glavin, M.; Jones, E. Microwave Breast Imaging: Experimental tumor phantoms for the evaluation of new breast cancer diagnosis systems. Biomed. Phys. Eng. Express 2018, 4, 025036. [Google Scholar] [CrossRef]
- Joachimowicz, N.; Conessa, C.; Henriksson, T.; Duchêne, B. Breast phantoms for microwave imaging. IEEE Antennas Wirel. Propag. Lett. 2014, 13, 1333–1336. [Google Scholar] [CrossRef]
- Joachimowicz, N.; Duchêne, B.; Conessa, C.; Meyer, O. Anthropomorphic breast and head phantoms for microwave imaging. Diagnostics 2018, 8, 85. [Google Scholar] [CrossRef]
- Mohammed, B.J.; Abbosh, A.M. Realistic head phantom to test microwave systems for brain imaging. Microw. Opt. Technol. Lett. 2014, 56, 979–982. [Google Scholar] [CrossRef]
- McDermott, B.; Porter, E.; Santorelli, A.; Divilly, B.; Morris, L.; Jones, M.; McGinley, B.; O’Halloran, M. Anatomically and dielectrically realistic microwave head phantom with circulation and reconfigurable lesions. Prog. Electromagn. Res. B 2017, 8, 47–60. [Google Scholar] [CrossRef]
- Choi, J.W.; Cho, J.; Lee, Y.; Yim, J.; Kang, B.; Oh, K.K.; Jung, W.H.; Kim, H.J.; Cheon, C.; Lee, H.D.; et al. Microwave Detection of Metastasized Breast Cancer Cells in the Lymph Node; Potential Application for Sentinel Lymphadenectomy. Breast Cancer Res. Treat. 2004, 86, 107–115. [Google Scholar] [CrossRef]
- Cameron, T.; Okoniewski, M.; Fear, E.; Mew, D.; Banks, B.; Ogilvie, T. A Preliminary Study of the Electrical Properties of Healthy and Diseased Lymph Nodes. In Proceedings of the 2010 14th International Symposium on Antenna Technology and Applied Electromagnetics & the American Electromagnetics Conference, Ottawa, ON, Canada, 5–8 July 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–3. [Google Scholar]
- Gabriel, S.; Lau, R.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251. [Google Scholar] [CrossRef]
- Salahuddin, S.; McDermott, B.; Porter, E.; O’Halloran, M.; Elahi, M.A.; Shahzad, A. An Empirical Dielectric Mixing Model for Biological Tissues. In Proceedings of the 2019 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–5. [Google Scholar]
- Gabriel, C.; Peyman, A. Dielectric measurement: Error analysis and assessment of uncertainty. Phys. Med. Biol. 2006, 51, 6033. [Google Scholar] [CrossRef]
- Keysight 85070E, Dielectric Probe Kit 200 MHz to 50 GHz. Available online: https://www.keysight.com/us/en/assets/7018-01196/technical-overviews/5989-0222.pdf (accessed on 1 September 2020).
- La Gioia, A.; Porter, E.; Merunka, I.; Shahzad, A.; Salahuddin, S.; Jones, M.; O’Halloran, M. Open-ended coaxial probe technique for dielectric measurement of biological tissues: Challenges and common practices. Diagnostics 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A.; Gabriel, C.; Grant, E. Complex permittivity of sodium chloride solutions at microwave frequencies. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2007, 28, 264–274. [Google Scholar] [CrossRef]
- Maenhout, G.; Markovic, T.; Ocket, I.; Nauwelaers, B. Effect of open-ended coaxial probe-to-tissue contact pressure on dielectric measurements. Sensors 2020, 20, 2060. [Google Scholar] [CrossRef]
- Porter, E.; La Gioia, A.; Salahuddin, S.; Decker, S.; Shahzad, A.; Elahi, M.A.; O’Halloran, M.; Beyan, O. Minimum information for dielectric measurements of biological tissues (MINDER): A framework for repeatable and reusable data. Int. J. Microw.-Comput.-Aided Eng. 2018, 28, e21201. [Google Scholar] [CrossRef]
- Campbell, A.; Land, D. Dielectric properties of female human breast tissue measured in vitro at 3.2 GHz. Phys. Med. Biol. 1992, 37, 193. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093. [Google Scholar] [CrossRef] [PubMed]
- Bio-MINDER: Dielectric Database for Biological Tissues. 2017. Available online: https://www.bio-minder.com/about-minder (accessed on 1 September 2020).
- University Hospital Galway (Galway, Ireland). Available online: https://saolta.ie/hospital/university-hospital-galway (accessed on 1 September 2020).
- La Gioia, A.; O’Halloran, M.; Elahi, A.; Porter, E. Investigation of histology radius for dielectric characterisation of heterogeneous materials. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1064–1079. [Google Scholar] [CrossRef]
- Meaney, P.M.; Gregory, A.P.; Seppälä, J.; Lahtinen, T. Open-ended coaxial dielectric probe effective penetration depth determination. IEEE Trans. Microw. Theory Tech. 2016, 64, 915–923. [Google Scholar] [CrossRef]
- Hurt, W.D. Multiterm Debye dispersion relations for permittivity of muscle. IEEE Trans. Biomed. Eng. 1985, BME-32, 60–64. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.; Gabriel, C. The dielectric properties of biological tissues: III. Parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef]
- Pawlina, W.; Ross, M.H. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology; Lippincott Williams & Wilkins: London, UK, 2018. [Google Scholar]
- Tissue Properties Database V4.0. Available online: https://itis.swiss/virtual-population/tissue-properties/downloads/database-v4-0/ (accessed on 1 September 2020).
- Proto-Pasta Conductive PLA. Available online: https://www.proto-pasta.com/pages/conductive-pla (accessed on 1 September 2020).
- Faenger, B.; Ley, S.; Helbig, M.; Sachs, J.; Hilger, I. Breast phantom with a conductive skin layer and conductive 3D-printed anatomical structures for microwave imaging. In Proceedings of the 2017 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1065–1068. [Google Scholar]
- Triton X-100. Available online: https://www.sigmaaldrich.com/catalog/product/sial/x100?lang=it®ion=IT (accessed on 1 September 2020).
- Ruvio, G.; Solimene, R.; Cuccaro, A.; Gaetano, D.; Browne, J.E.; Ammann, M.J. Breast cancer detection using interferometric MUSIC: Experimental and numerical assessment. Med Phys. 2014, 41, 103101. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.; Sarafianou, M.; Craddock, I.J. Compound radar approach for breast imaging. IEEE Trans. Biomed. Eng. 2016, 64, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Solis-Nepote, M.; Reimer, T.; Pistorius, S. An Air-Operated Bistatic System for Breast Microwave Radar Imaging: Pre-Clinical Validation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1859–1862. [Google Scholar]
- Felicio, J.M.; Bioucas-Dias, J.M.; Costa, J.R.; Fernandes, C.A. Microwave Breast Imaging using a Dry Setup. IEEE Trans. Comput. Imaging 2019, 6, 167–180. [Google Scholar] [CrossRef]
- Fundação Champalimaud (Lisbon, Portugal). Available online: https://fchampalimaud.org/ (accessed on 2 September 2020).
- FormLabs. Available online: https://formlabs.com/ (accessed on 1 September 2020).
- 3D Slicer. Available online: https://www.slicer.org/ (accessed on 6 February 2020).
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Van Eijnatten, M.; van Dijk, R.; Dobbe, J.; Streekstra, G.; Koivisto, J.; Wolff, J. CT image segmentation methods for bone used in medical additive manufacturing. Med Eng. Phys. 2018, 51, 6–16. [Google Scholar] [CrossRef]
- Van Eijnatten, M.; Koivisto, J.; Karhu, K.; Forouzanfar, T.; Wolff, J. The impact of manual threshold selection in medical additive manufacturing. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 607–615. [Google Scholar] [CrossRef]
- Rydholm, T.; Fhager, A.; Persson, M.; Geimer, S.D.; Meaney, P.M. Effects of the plastic of the realistic GeePS-L2S-Breast phantom. Diagnostics 2018, 8, 61. [Google Scholar] [CrossRef]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An Open-Source Mesh Processing Tool. In Eurographics Italian Chapter Conference; Scarano, V., Chiara, R.D., Erra, U., Eds.; The Eurographics Association: Munich, Germany, 2008. [Google Scholar] [CrossRef]
- Herrmann, L.R. Laplacian-isoparametric grid generation scheme. J. Eng. Mech. Div. 1976, 102, 749–907. [Google Scholar]
- Blender. Available online: https://www.blender.org/ (accessed on 1 September 2020).
- Ultimaker 3. Available online: https://ultimaker.com/ (accessed on 1 September 2020).
- STRATASYS F170. Available online: https://www.stratasys.com/3d-printers/f123 (accessed on 1 September 2020).
- CST Studio Suite. Available online: https://www.3ds.com/products-services/simulia/products/cst-studio-suite/ (accessed on 26 February 2020).
- Felício, J.M.; Fernandes, C.A.; Costa, J.R. Complex permittivity and anisotropy measurement of 3D-printed PLA at microwaves and millimeter-waves. In Proceedings of the 2016 22nd International Conference on Applied Electromagnetics and Communications (ICECOM), Croatia, Balkans, 19–21 September 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–6. [Google Scholar]
- Costa, J.R.; Medeiros, C.R.; Fernandes, C.A. Performance of a crossed exponentially tapered slot antenna for UWB systems. IEEE Trans. Antennas Propag. 2009, 57, 1345–1352. [Google Scholar] [CrossRef]
- Felício, J.M.; Bioucas-Dias, J.M.; Costa, J.R.; Fernandes, C.A. Antenna Design and Near-Field Characterization for Medical Microwave Imaging Applications. IEEE Trans. Antennas Propag. 2019, 67, 4811–4824. [Google Scholar] [CrossRef]







| Human ALNs | Animal LNs | |||
|---|---|---|---|---|
| [%] | [%] | [%] | [%] | |
| V1 | 1.06 | 2.09 | 0.93 | 2.93 |
| V2 | 2.26 | 2.92 | 5.50 | 2.13 |
| [S/m] | [s] | [s] | ||||
|---|---|---|---|---|---|---|
| ALN | 1.00 | 0.96 | 34.17 | 7.05× 10 | 16.22 | 1.36× 10 |
| ALN | 1.00 | 1.23 | 119.39 | 1.0× 10 | 38.25 | 1.0× 10 |
| Animal LN (inner content) | 0.97 | 1.44 | 211.99 | n1.65× 10 | 45.18 | 1.12× 10 |
| Tissue | Mixture Composition | Average (±st.dev) Absolute Error in TMM Dielectric Properties | ||
|---|---|---|---|---|
| TX-100 | NaCl | Relative Permittivity | Conductivity | |
| [vol%] | [g/l] | [S/m] | ||
| muscle | 26.5 | 7.4 | 0.4 (±0.3) | 0.21 (±0.13) |
| lung | 55.0 | 4.51 | 4.2 (±3.7) | 0.89 (±0.43) |
| fat | 100 | 0 | 0.3 (±0.2) | 0.11 (±0.11) |
| ALN | 25.0 | 8.59 | 1.6 (±0.9) | 0.33 (±0.21) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savazzi, M.; Abedi, S.; Ištuk, N.; Joachimowicz, N.; Roussel, H.; Porter, E.; O’Halloran, M.; Costa, J.R.; Fernandes, C.A.; Felício, J.M.; et al. Development of an Anthropomorphic Phantom of the Axillary Region for Microwave Imaging Assessment. Sensors 2020, 20, 4968. https://doi.org/10.3390/s20174968
Savazzi M, Abedi S, Ištuk N, Joachimowicz N, Roussel H, Porter E, O’Halloran M, Costa JR, Fernandes CA, Felício JM, et al. Development of an Anthropomorphic Phantom of the Axillary Region for Microwave Imaging Assessment. Sensors. 2020; 20(17):4968. https://doi.org/10.3390/s20174968
Chicago/Turabian StyleSavazzi, Matteo, Soroush Abedi, Niko Ištuk, Nadine Joachimowicz, Hélène Roussel, Emily Porter, Martin O’Halloran, Jorge R. Costa, Carlos A. Fernandes, João M. Felício, and et al. 2020. "Development of an Anthropomorphic Phantom of the Axillary Region for Microwave Imaging Assessment" Sensors 20, no. 17: 4968. https://doi.org/10.3390/s20174968
APA StyleSavazzi, M., Abedi, S., Ištuk, N., Joachimowicz, N., Roussel, H., Porter, E., O’Halloran, M., Costa, J. R., Fernandes, C. A., Felício, J. M., & Conceição, R. C. (2020). Development of an Anthropomorphic Phantom of the Axillary Region for Microwave Imaging Assessment. Sensors, 20(17), 4968. https://doi.org/10.3390/s20174968











