Indication of Electromagnetic Field Exposure via RBF-SVM Using Time-Series Features of Zebrafish Locomotion
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Acquisition
2.2. Behavior Test, Video-Tracking, and Time-Series Acquisition
2.3. Feature Calculation and Extraction
2.3.1. Feature Calculation and Normalization
2.3.2. Feature Extraction
2.4. Correlation between Zebrafish Behaviors and the AEPs
2.4.1. Experimental Procedure
2.4.2. AEPs Collection
2.4.3. Time-Series Establishment for Feature Extraction
2.4.4. Regression
2.5. Zebrafish Behavior under MF Exposure
2.5.1. Experimental Procedure
2.5.2. EMF Exposure
2.5.3. Time-Series Establishment, Feature Calculation, and Extraction for Classification
Time-Series Establishment
Feature Calculation
Feature Extraction for Classification
2.5.4. RBF-SVM Classification
3. Results
3.1. Verification Result of the AEPs Impact on Zebrafish Locomotion
3.2. Classification Results of the Features of Zebrafish Locomotion
4. Discussion
4.1. Reliability of the RBF-SVM Models
4.1.1. Classification Accuracy
4.1.2. Robustness
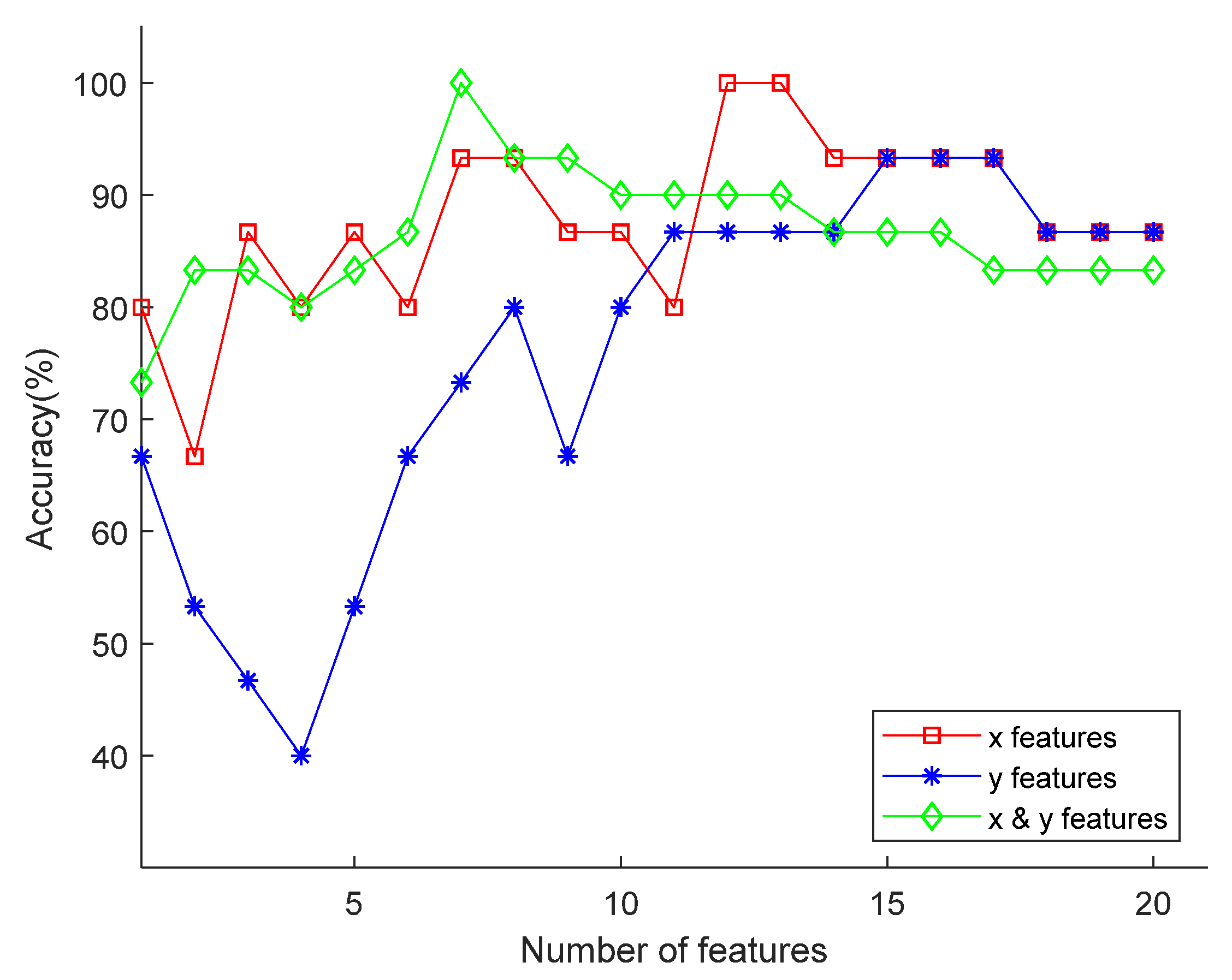
4.1.3. Dimension Selections of the Feature Sets
4.2. Possible Reason behind the Achievement
4.3. Further Improvement
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Series | Names | Descriptions |
|---|---|---|
| 15 | rms | Root-mean-square of the time series |
| 18 | standard_deviation | Measure of spread of the input time series |
| 32 | DN_Moments_raw_4 | A moment of the distribution of the input time series |
| 34 | DN_Moments_raw_6 | A moment of the distribution of the input time series |
| 36 | DN_Moments_raw_8 | A moment of the distribution of the input time series |
| 399 | ’IN_AutoMutualInfoStats_diff_20_kraskov1_4_ami19’ | Statistics on automutual information function for a time series |
| 431 | ’CO_CompareMinAMI_quantiles_2_80_nlocmax’ | Variability in first minimum of automutual information |
| 1093 | ’CO_AddNoise_1_std1_10_firstUnder75’ | Changes in the automutual information with the addition of noise |
| 1712 | ’CO_Embed2_tau_stdb1’ | Statistics of the time-series in a 2-dimensional embedding space |
| 1741 | ’CO_Embed2_Dist_tau_d_ac1’ | Analyzes distances in a 2-d embedding space of a time series |
| 1830 | ’NW_VisibilityGraph_norm_olu90’ | Visibility graph analysis of a time series |
| 1902 | ’SY_SpreadRandomLocal_100_100_meanac2’ | Bootstrap-based stationarity measure |
| 1925 | ’SY_SpreadRandomLocal_200_100_stdac1’ | Bootstrap-based stationarity measure |
| 2096 | ’FC_Surprise_dist_50_3_udq_500_uq’ | How surprised you would be of the next data point given recent memory |
| 2131 | ’FC_Surprise_T1_100_4_udq_500_median’ | How surprised you would be of the next data point given recent memory |
| 2164 | ’FC_Surprise_T2_100_4_udq_500_tstat’ | How surprised you would be of the next data point given recent memory |
| 2900 | ’DN_OutlierInclude_n_001_nfexprmse’ | How statistics depend on distributional outliers |
| 3416 | ’SC_FluctAnal_2_nothing_50_logi_linfitint’ | Implements fluctuation analysis by a variety of methods |
| 3774 | ’NL_crptool_fnn_10_2_ac_fnn7’ | Analyzes the false-nearest neighbors statistic |
| 5209 | ’MF_GARCHfit_ar_P1_Q2_engle_mean_diff_p’ | Generalized autoregressive conditional heteroscedasticity (GARCH) time-series modeling |
| 5366 | ’ST_MomentCorr_002_02_median_iqr_none_R’ | Correlations between simple statistics in local windows of a time series. |
| 5400 | ’MD_rawHRVmeas_SD1’ | Heart rate variability (HRV) measures of a time series. |
| 5401 | ’MD_rawHRVmeas_SD2’ | Heart rate variability (HRV) measures of a time series. |
References
- International Commission on Non-Ionizing Radiation Protection. ICNIRP Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields. Health Phys. 1998, 74, 494–522. [Google Scholar]
- Reilly, J.P.; Hirata, A. Low-frequency electrical dosimetry: Research agenda of the IEEE International Committee on Electromagnetic Safety. Phys. Med. Biol. 2016, 61, R138. [Google Scholar] [CrossRef] [PubMed]
- IEEE. Approved Draft Standard for Safety Levels with Respect to Human Exposure to Electric, Magnetic and Electromagnetic Fields, 0 Hz to 300 GHz; IEEE C95.1-2019; IEEE Standards Association: Piscataway, NJ, USA, 2019. [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection. ICNIRP Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz–100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar]
- Van der Schalie, W.H.; Shedd, T.R.; Knechtges, P.L.; Widder, M.W. Using higher organisms in biological early warning systems for real-time toxicity detection. Biosens. Bioelectron. 2001, 16, 457–465. [Google Scholar] [CrossRef]
- Netto, I. Assessing the Usefulness of the Automated Monitoring Systems Ecotox and Daphniatox in an Integrated Early-Warning System for Drinking Water. Ph.D. Thesis, Ryerson University, Toronto, ON, Canada, 2010. [Google Scholar]
- Amorim, J.; Fernandes, M.; Vasconcelos, V.; Teles, L.O. Evaluation of the sensitivity spectrum of a video tracking system with zebrafish (Danio rerio) exposed to five different toxicants. Environ. Sci. Pollut. Res. 2017, 24, 16086–16096. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.; Fernandes, M.; Vasconcelos, V.; Teles, L.O. Stress test of a biological early warning system with zebrafish (Danio rerio). Ecotoxicology 2017, 26, 13–21. [Google Scholar] [CrossRef]
- Fernandes, M.; Amorim, J.; Vasconcelos, V.; Teles, L.O. Resilience assessment of a biological early warning system based on the locomotor behavior of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2016, 23, 18858–18868. [Google Scholar] [CrossRef]
- Bae, M.J.; Park, Y.S. Biological early warning system based on the responses of aquatic organisms to disturbances: A review. Sci. Total Environ. 2014, 466–467, 635–649. [Google Scholar] [CrossRef]
- Nüßer, L.K.; Skulovich, O.; Hartmann, S.; Seiller, T.B.; Cofalla, C.; Schuettrumpf, H.; Hollert, H.; Salomons, E.; Ostfeld, A. A sensitive biomarker for the detection of aquatic contamination based on behavioral assays using zebrafish larvae. Ecotoxicol. Environ. Saf. 2016, 133, 271–280. [Google Scholar] [CrossRef]
- Teles, L.O.; Fernandes, M.; Amorim, J.; Vasconcelos, V. Video-tracking of zebrafish (Danio rerio) as a biological early warning system using two distinct artificial neural networks: Probabilistic neural network (PNN) and self-organizing map (SOM). Aquat. Toxicol. 2015, 165, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V. The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish; Springer International Publishing: Cham, Geermany, 2017. [Google Scholar]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyazar, E.; Wu, N.; Kalueff, A.V. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef]
- Guo, S. Using zebrafish to assess the impact of drugs on neural development and function. Exp. Opin. Drug Discov. 2009, 4, 715–726. [Google Scholar] [CrossRef]
- Best, J. Zebrafish: An in vivo model for the study of neurological diseases. Neuropsychiatr. Dis. Treat. 2008, 4, 567. [Google Scholar] [CrossRef] [PubMed]
- Miklósi, Á.; Andrew, R.J. The zebrafish as a model for behavioral studies. Zebrafish 2006, 3, 227–234. [Google Scholar] [CrossRef]
- Fonseka, T.M.; Wen, X.Y.; Foster, J.A.; Kennedy, S.H. Zebrafish models of major depressive disorders. J. Neurosci. Res. 2016, 94, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Takagi, T.; Hiraishi, T. Video analysis of fish schooling behavior in finite space using a mathematical model. Fish. Res. 2003, 60, 3–10. [Google Scholar] [CrossRef]
- Blaser, R.; Gerlai, R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav. Res. Methods 2006, 38, 456–469. [Google Scholar] [CrossRef]
- Panula, P.; Chen, Y.-C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.; Bartels, B. Video-aided analysis of zebrafish locomotion and anxiety-related behavioral responses. Neuromethods 2011, 51, 1–14. [Google Scholar]
- Sun, W.; He, Y.; Leung, S.-W.; Kong, Y.-C. In Vivo analysis of embryo development and behavioral response of medaka fish under static magnetic field exposures. Int. J. Environ. Res. Public Health 2019, 16, 844. [Google Scholar] [CrossRef]
- Valente, D.; Golani, I.; Mitra, P.P. Analysis of the trajectory of Drosophila melanogaster in a circular open field arena. PLoS ONE 2007, 2, e1083. [Google Scholar] [CrossRef]
- VKrylov, V.; Izyumov, Y.G.; EIzvekov, I.; Nepomnyashchikh, V.A. Magnetic fields and fish behavior. Biol. Bull. Rev. 2014, 4, 222–231. [Google Scholar] [CrossRef]
- Osipova, E.A.; Pavlova, V.V.; Nepomnyashchikh, V.A.; Krylov, V.V. Influence of magnetic field on zebrafish activity and orientation in a plus maze. Behav. Process. 2016, 122, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Myklatun, A.; Lauri, A.; Eder, S.H.K.; Cappetta, M.; Shcherbakov, D.; Wurst, W.; Winklhofer, M.; Westmeyer, G.G. Zebrafish and medaka offer insights into the neurobehavioral correlates of vertebrate magnetoreception. Nat. Commun. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Sedigh, E.; Heidari, B.; Roozati, A.; Valipour, A. The effect of different intensities of static magnetic field on stress and selected reproductive indices of the zebrafish (Danio rerio) during acute and subacute exposure. Bull. Environ. Contam. Toxicol. 2019, 102, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.N.; Cummins, R.A. The open-field test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Wong, K.; Elegante, M.; Bartels, B.; Elkhayat, S.; Tien, D.; Roy, S.; Goodspeed, J.; Suciu, C.; Tan, J.; Grimes, C.; et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 450–457. [Google Scholar] [CrossRef]
- Rosemberg, D.B.; Rico, E.P.; Mussulini, B.H.M.; Piato, A.L.; Calcagnotto, M.E.; Bonan, C.D.; Dias, R.D.; Blaser, R.E.; Souza, D.O.; de Oliveira, D.L. Differences in spatio-temporal behavior of zebrafish in the open tank paradigm after a short-period confinement into dark and bright environments. PLoS ONE 2011, 6, e19397. [Google Scholar] [CrossRef]
- Fulcher, B.D.; Njones, S. Hctsa: A computational framework for automated time-series phenotyping using massive feature extraction. Cell Syst. 2017, 5, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, B.D.; Little, M.A.; Jones, N.S. Highly comparative time-series analysis: The empirical structure of time series and their methods. J. R. Soc. Interface 2013, 10, 20130048. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Long, F.; Ding, C. Feature selection based on mutual information: Criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans. Pattern Anal. Mach. Intell. 2005, 27, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Peng, H. Minimum redundancy feature selection from microarray gene expression data. J. Bioinform. Comput. Biol. 2005, 3, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Vapnik, V.N. The Nature of Statistical Learning Theory Second Edition With 50 IlIustrations; Springer: Berlin/Heidelber, Germany, 1999. [Google Scholar]
- Vapnik, V.; Golowich, S.E. Support vector method for function approximation, regression estimation, and signal processing. In Advances in Neural Information Proccessing Systems; Mozer, M., Jordan, M., Eds.; MIT Press: Cambridge, UK, 2007; pp. 281–287. [Google Scholar]
- Wang, W.; Xu, Z.; Lu, W.; Zhang, X. Determination of the spread parameter in the Gaussian kernel for classification and regression. Neurocomputing 2003, 55, 643–663. [Google Scholar] [CrossRef]
- He, Y.; Leung, P.S.W.; Chow, Y.-T.; Diao, Y. Dosimetry Analysis of a 3-D Open-Structured Wireless Power Transfer System. In Proceedings of the 2019 Joint International Symposium on Electromagnetic Compatibility, Sapporo and Asia-Pacific International Symposium on Electromagnetic Compatibility (EMC Sapporo/APEMC), Sapporo, Japan, 3–7 June 2019; pp. 750–753. [Google Scholar]
- Shimamoto, T.; Iwahashi, M.; Sugiyama, Y.; Laakso, I.; Hirata, A.; and Onishi, T. SAR evaluation in models of an adult and a child for magnetic field from wireless power transfer systems at 6.78 MHz. Biomed. Phys. Eng. Express 2016, 2, 027001. [Google Scholar] [CrossRef]
- Bululukova, D.; Kramer, M. Application of existing wireless power transfer standards in automotive applications. In Proceedings of the 2014 Int. Conf. Connect. Veh. Expo, (ICCVE 2014), Wienna, Austria, 3–7 November 2014; pp. 863–864. [Google Scholar]
- Chow, J.P.W.; Chung, S.H.; Chan, L.L.H.; Shen, R.; Tang, S.C. Optimal design and experimental assessment of a wireless power transfer system for home-cage monitoring. IEEE Trans. Power Electron. 2019, 1, 1. [Google Scholar] [CrossRef]
- Campbell, W.M.; Sturim, D.E.; Reynolds, D.A. Support vector machines using GMM supervectors for speaker verification. IEEE Sign. Process. Lett. 2006, 13, 308–311. [Google Scholar] [CrossRef]
- Platt, J.C. Sequential Minimal Pptimization: A Fast Algorithm for Training Support Vector Machines; Technical Report; Springer: Zurich, Switzerland, 1998. [Google Scholar]
- Amari, S.; Wu, S. Improving support vector machine classifiers by modifying kernel functions. Neural Networks 1999, 12, 783–789. [Google Scholar] [CrossRef]
- Hsu, C.W.; Lin, C.J. A comparison of methods for multiclass support vector machines. IEEE Trans. Neural Networks 2002, 13, 415–425. [Google Scholar]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the International Joint Conference of Artificial Intelligence, Montreal, QB, Canada, 20–25 August 1995. [Google Scholar]
- Ward, D.M. Evaluation: From precision, recall and F-measure to ROC, informedness, markedness & correlation. J. Mach. Learn. Technol. 2011, 2, 37–63. [Google Scholar]
- IEEE. IEEE Standard for Safety Levels with Respect to Human Exposure to Radio Frequency Electromagnetic Fields, 3 kHz to 300 GHz; Report 0_1-238; IEEE Standards Association: Piscataway, NJ, USA, 2005. [Google Scholar]
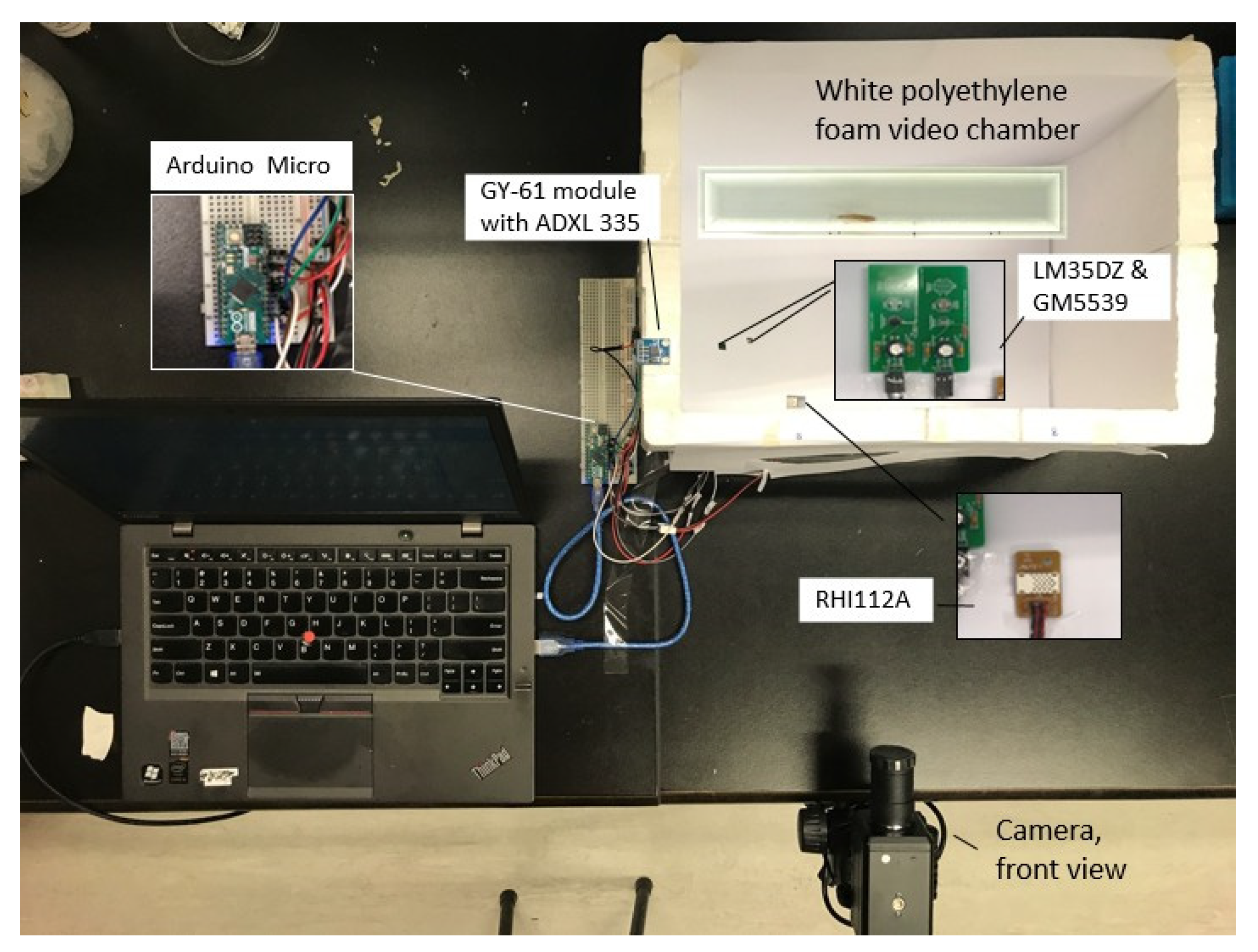
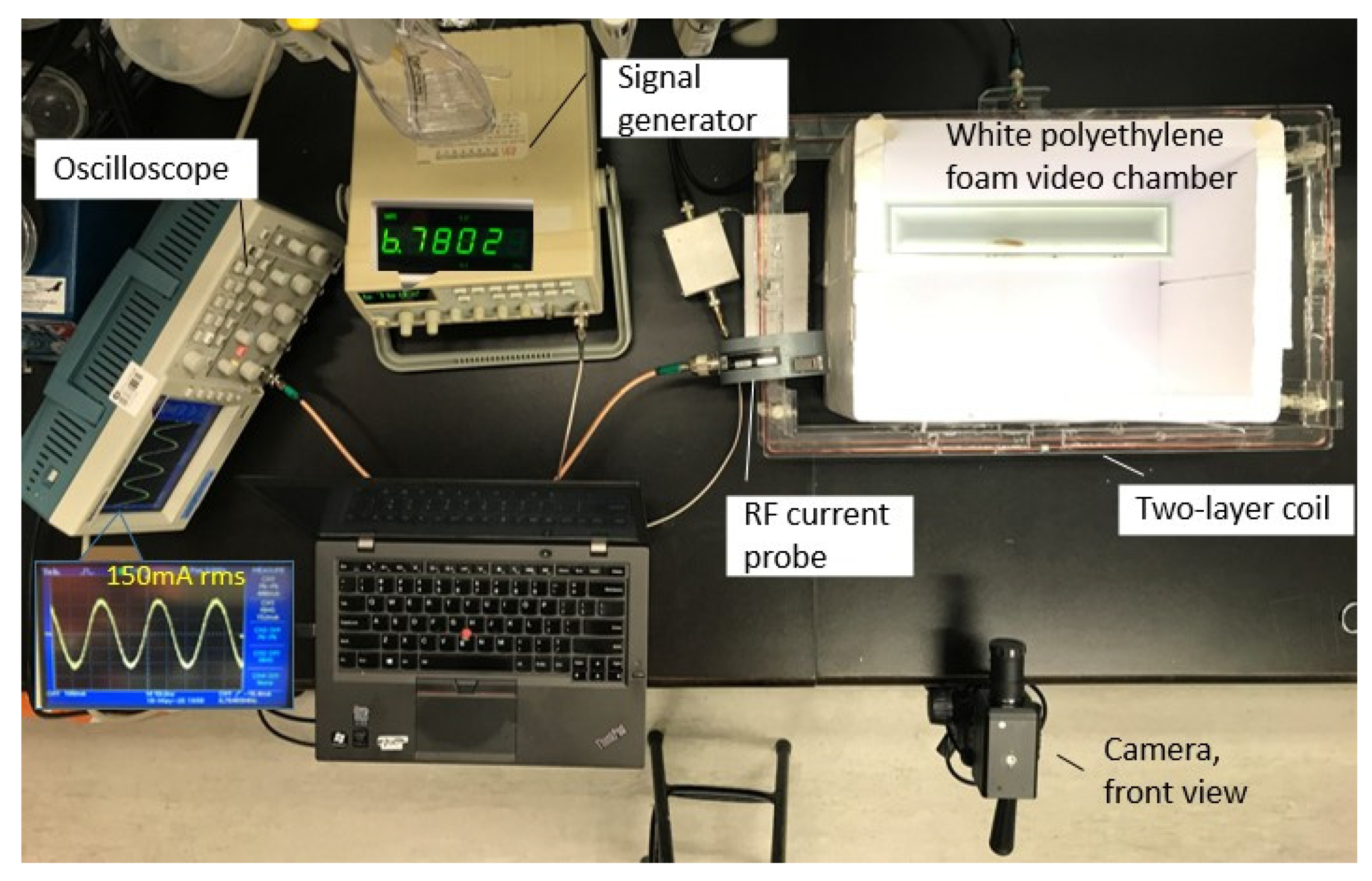


| Class | MF Exposure Conditions |
|---|---|
| −1 | Sham MF exposure |
| 1 | Real MF exposure |
| τ (minutes) | M | Feature Sets | In Total of 14 Zebrafish |
|---|---|---|---|
| 1 | 15 | 30 | 420 |
| 3 | 5 | 10 | 140 |
| 5 | 3 | 6 | 84 |
| τ | Serial Numbers of Selected Features | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
| 1 | 34 | 1741 | 399 | 15 | 5209 | 32 | 1925 | 431 | 1830 | 3416 |
| 3 | 32 | 2131 | 15 | 36 | 1902 | 5366 | 1712 | 34 | 18 | 2096 |
| 5 | 18 | 2900 | 1093 | 2164 | 32 | 36 | 5400 | 3774 | 5401 | 34 |
| τ (mins) | Feature Sets | Feature Sets for Training | Feature Sets for Testing | Numbers of the Features with Following FDs for Each Class | |
|---|---|---|---|---|---|
| 5 | 10 | ||||
| 1 | 420 | 300 | 120 | 1050 | 10500 |
| 3 | 140 | 100 | 40 | 350 | 3500 |
| 5 | 84 | 60 | 24 | 210 | 2100 |
| Exposure Condition | Averaged X Coordinate (mm) | Averaged Y Coordinate (mm) | Averaged Velocity (mm/s) | Averaged Acceleration (mm/s2) | Averaged Distance Moved (mm) |
|---|---|---|---|---|---|
| Sham | −233.25 ± 1.38 | −65.41 ± 14.32 | 50.30 ± 25.15 | 104.21 ± 52.10 | 1.68 ± 0.84 |
| Real | 52.43 ± 60.95 | −36.38 ± 16.65 | 9.51 ± 14.82 | 24.42 ± 1.51 | 0.32 ± 0.49 |
| Coordinate Time-Series | Feature Dimension | Temperature | Humidity | Acceleration | Illumination | ||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | ||
| x | 1 | −0.08 | 1.0299 | −0.02 | 1.0910 | −0.05 | 1.0854 | −0.32 | 1.1677 |
| 2 | −0.09 | 1.0319 | −0.01 | 1.0843 | −0.05 | 1.0884 | −0.24 | 1.1308 | |
| 3 | −0.16 | 1.0660 | −0.02 | 1.0939 | −0.09 | 1.1055 | −0.27 | 1.1478 | |
| 4 | −0.29 | 1.1257 | −0.06 | 1.1145 | −0.14 | 1.1301 | −0.25 | 1.1379 | |
| 5 | −0.27 | 1.1179 | −0.07 | 1.1209 | −0.14 | 1.1302 | −0.32 | 1.1689 | |
| 6 | −0.2 | 1.0825 | −0.06 | 1.1132 | −0.06 | 1.0936 | −0.19 | 1.1102 | |
| 7 | −0.12 | 1.0457 | −0.03 | 1.0985 | −0.04 | 1.0801 | −0.08 | 1.0589 | |
| 8 | −0.07 | 1.0253 | −0.01 | 1.0850 | 0 | 1.0612 | −0.03 | 1.0351 | |
| 9 | −0.03 | 1.0033 | 0.01 | 1.0777 | 0.02 | 1.0498 | −0.01 | 1.0213 | |
| 10 | −0.01 | 0.9930 | 0.01 | 1.0734 | 0.03 | 1.0413 | 0.01 | 1.0104 | |
| 11 | 0.01 | 0.9858 | 0.02 | 1.0702 | 0.03 | 1.0436 | 0.03 | 1.0045 | |
| 12 | 0.02 | 0.9816 | 0.02 | 1.0703 | 0.04 | 1.0399 | 0.02 | 1.0054 | |
| 13 | 0.02 | 0.9811 | 0.03 | 1.0676 | 0.04 | 1.0401 | 0.03 | 1.0037 | |
| 14 | 0.02 | 0.9807 | 0.03 | 1.0673 | 0.04 | 1.0403 | 0.03 | 1.0035 | |
| 15 | 0.02 | 0.9808 | 0.03 | 1.0673 | 0.04 | 1.0405 | 0.03 | 1.0032 | |
| 16 | 0.02 | 0.9809 | 0.03 | 1.0677 | 0.04 | 1.0404 | 0.03 | 1.0029 | |
| 17 | 0.02 | 0.9809 | 0.03 | 1.0678 | 0.04 | 1.0404 | 0.03 | 1.0029 | |
| 18 | 0.02 | 0.9809 | 0.03 | 1.0678 | 0.04 | 1.0404 | 0.03 | 1.0029 | |
| 19 | 0.02 | 0.9810 | 0.03 | 1.0678 | 0.04 | 1.0404 | 0.03 | 1.0029 | |
| 20 | 0.02 | 0.9810 | 0.03 | 1.0678 | 0.04 | 1.0404 | 0.03 | 1.0029 | |
| y | 1 | −0.08 | 1.0314 | −0.02 | 1.0986 | −0.05 | 1.1027 | −0.29 | 1.1456 |
| 2 | −0.11 | 1.0423 | −0.03 | 1.1038 | −0.07 | 1.1098 | −0.2 | 1.1049 | |
| 3 | −0.14 | 1.0579 | −0.06 | 1.1179 | −0.06 | 1.1086 | −0.22 | 1.1118 | |
| 4 | −0.2 | 1.0881 | −0.05 | 1.1135 | −0.04 | 1.0945 | −0.25 | 1.1271 | |
| 5 | −0.18 | 1.0766 | −0.07 | 1.1226 | −0.06 | 1.1044 | −0.2 | 1.1021 | |
| 6 | −0.13 | 1.0542 | −0.04 | 1.1049 | −0.04 | 1.0956 | −0.08 | 1.0486 | |
| 7 | −0.09 | 1.0359 | −0.02 | 1.0948 | −0.04 | 1.0951 | −0.04 | 1.0266 | |
| 8 | −0.06 | 1.0216 | −0.01 | 1.0904 | 0.01 | 1.0700 | 0 | 1.0051 | |
| 9 | 0.01 | 0.9861 | 0.01 | 1.0818 | 0.02 | 1.0621 | 0.02 | 0.9983 | |
| 10 | 0.02 | 0.9810 | 0.02 | 1.0766 | 0.03 | 1.0591 | 0.02 | 0.9984 | |
| 11 | 0.02 | 0.9805 | 0.02 | 1.0727 | 0.04 | 1.0554 | 0.02 | 0.9963 | |
| 12 | 0.02 | 0.9796 | 0.02 | 1.0729 | 0.04 | 1.0541 | 0.02 | 0.9957 | |
| 13 | 0.03 | 0.9785 | 0.02 | 1.0723 | 0.04 | 1.0523 | 0.03 | 0.9936 | |
| 14 | 0.03 | 0.9781 | 0.02 | 1.0722 | 0.04 | 1.0530 | 0.03 | 0.9938 | |
| 15 | 0.03 | 0.9776 | 0.02 | 1.0723 | 0.04 | 1.0527 | 0.03 | 0.9934 | |
| 16 | 0.03 | 0.9771 | 0.03 | 1.0718 | 0.04 | 1.0528 | 0.03 | 0.9933 | |
| 17 | 0.03 | 0.9770 | 0.03 | 1.0718 | 0.04 | 1.0529 | 0.03 | 0.9935 | |
| 18 | 0.03 | 0.9771 | 0.03 | 1.0718 | 0.04 | 1.0529 | 0.03 | 0.9935 | |
| 19 | 0.03 | 0.9772 | 0.03 | 1.0718 | 0.04 | 1.0529 | 0.03 | 0.9935 | |
| 20 | 0.03 | 0.9772 | 0.03 | 1.0718 | 0.04 | 1.0529 | 0.03 | 0.9935 | |
| (a)Cmin = 2e−10 | |||||||
| τ (mins) | Feature Size | Parameters | Accuracy | Precision | Recall | F1-Score | |
| C | Gamma | ||||||
| 1 | 5 | 2e−10 | 0.0039063 | 100% | 54.7619 | 76.67 | 0.6389 |
| 10 | 0.03125 | 0.0625 | 100% | 100% | 100% | 1 | |
| 3 | 5 | 2e−10 | 0.03125 | 100% | 75.5102% | 74% | 0.7475 |
| 10 | 2e−10 | 0.0039063 | 100% | 70% | 56% | 0.6222 | |
| 5 | 5 | 1 | 9.53674e−7 | 100% | 76.6667% | 76.6667% | 0.7667 |
| 10 | 2e−10 | 9.5367e−07 | 100% | 76.6667% | 76.6667% | 0.7667 | |
| (b) Cmin = 2e−5 | |||||||
| τ (mins) | Feature Size | Parameters | Accuracy | Precision | Recall | F1-Score | |
| C | Gamma | ||||||
| 1 | 5 | 2e−5 | 0. 0039063 | 100% | 54% | 54% | 0.54 |
| 10 | 0.0625 | 100% | 100% | 100% | 1 | ||
| 3 | 5 | 0.03125 | 100% | 52% | 52% | 0.52 | |
| 10 | 0. 0039063 | 100% | 48% | 48% | 0.48 | ||
| 5 | 5 | 9.5367e−07 | 100% | 62.5% | 50% | 0.5556 | |
| 10 | 9.5367e−07 | 100% | 53.33% | 53.33% | 53.33 | ||
| (c) Cmin = 2e−4 | |||||||
| τ (mins) | Feature Size | Parameters | Accuracy | Precision | Recall | F1-Score | |
| C | Gamma | ||||||
| 1 | 5 | 2e−4 | 0.0039603 | 100% | 74.5902% | 60.6667% | 0.6691 |
| 10 | 0.03125 | 100% | 100% | 99.3333% | 0.9967 | ||
| 3 | 5 | 0.03125 | 100% | 96% | 96% | 0.96 | |
| 10 | 0.0039063 | 100% | 54% | 54% | 0.54 | ||
| 5 | 5 | 9.5367e−7 | 100% | 73.33% | 73.33% | 0.7333 | |
| 10 | 9.5367e−7 | 100% | 53.33% | 53.33% | 0.5333 | ||
| (d) Cmin = 2e−3 | |||||||
| τ (mins) | Feature Size | Parameters | Accuracy | Precision | Recall | F1-Score | |
| C | Gamma | ||||||
| 1 | 5 | 2e−3 | 0.0078125 | 100% | 100% | 98.6667% | 0.9933 |
| 10 | 0.03125 | 100% | 100% | 100% | 1 | ||
| 3 | 5 | 0.015625 | 100% | 100% | 100% | 1 | |
| 10 | 0.0039063 | 100% | 100% | 92.5% | 0.9583 | ||
| 5 | 5 | 9.5367e−7 | 100% | 73.3333% | 73.3333% | 0.7333 | |
| 10 | 9.5367e−7 | 100% | 62.5% | 50% | 0.5556 | ||
| (e) Cmin = 1 | |||||||
| τ (mins) | Feature Size | Parameters | Accuracy | Precision | Recall | F1-Score | |
| C | Gamma | ||||||
| 1 | 5 | 1 | 0.00097656 | 100% | 100% | 99.3333% | 0.9967 |
| 10 | 0.0039063 | 100% | 100% | 100% | 1 | ||
| 3 | 5 | 0.0019531 | 100% | 100% | 96% | 0.9796 | |
| 10 | 0.00097656 | 100% | 100% | 100% | 1 | ||
| 5 | 5 | 9.5367e−7 | 100% | 66.67% | 53.33% | 0.5925 | |
| 10 | 9.5367e−7 | 100% | 47.619% | 66.67% | 0.5556 | ||
| τ | Total Numbers by HCTSA | Number of Features Getting Cumulative Contribution Rate Threshold | |
|---|---|---|---|
| 85% | 95% | ||
| 1 | 5210 | 130 | 259 |
| 3 | 5367 | 61 | 103 |
| 5 | 5401 | 41 | 65 |
| Standard/ Guidelines | Schemes | General Public (A/m) | Occupational (A/m) | |||
|---|---|---|---|---|---|---|
| Specification | Value | Specification | Value | |||
| ICNIRP | 1998 | Averaged over 6 min. | 0.73/fM | 0.1077 | 1.6/fM | 0.2360 |
| 2020 | Averaged over 30 min whole body | 2.2/fM | 0.3560 | 4.9/fM | 0.7929 | |
| Averaged over 6 min local | 4.9/fM | 0.7929 | 10.8/fM | 1.5929 | ||
| IEEE C95.1 | 2005 | 6 min whole body | 16.3/fM | 2.4041 | 16.3/fM | 2.4041 |
| 2019 | Averaged 30 min RMS for whole body | 16.3/fM | 2.4041 | 16.3/fM | 2.4041 | |
| Averaged 30 min local | 36.4/fM | 5.3687 | 36.4/fM | 5.3687 | ||
| τ | Serial Numbers of Selected Features | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 *** | 1741 *** | 399 *** | 15 *** | 5209 *** | 32 *** | 1925 *** | 431 *** | 1830 *** | 3416 *** |
| 3 | 32 *** | 2131 *** | 15 *** | 36 *** | 1902 *** | 5366 *** | 1712 *** | 34 *** | 18 *** | 2096 *** |
| 5 | 18 *** | 2900 *** | 1093 *** | 2164 *** | 32 *** | 36 *** | 5400 *** | 3774 *** | 5401 *** | 34 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Tsang, K.F.; Kong, R.Y.-C.; Chow, Y.-T. Indication of Electromagnetic Field Exposure via RBF-SVM Using Time-Series Features of Zebrafish Locomotion. Sensors 2020, 20, 4818. https://doi.org/10.3390/s20174818
He Y, Tsang KF, Kong RY-C, Chow Y-T. Indication of Electromagnetic Field Exposure via RBF-SVM Using Time-Series Features of Zebrafish Locomotion. Sensors. 2020; 20(17):4818. https://doi.org/10.3390/s20174818
Chicago/Turabian StyleHe, Yaqing, Kim Fung Tsang, Richard Yuen-Chong Kong, and Yuk-Tak Chow. 2020. "Indication of Electromagnetic Field Exposure via RBF-SVM Using Time-Series Features of Zebrafish Locomotion" Sensors 20, no. 17: 4818. https://doi.org/10.3390/s20174818
APA StyleHe, Y., Tsang, K. F., Kong, R. Y.-C., & Chow, Y.-T. (2020). Indication of Electromagnetic Field Exposure via RBF-SVM Using Time-Series Features of Zebrafish Locomotion. Sensors, 20(17), 4818. https://doi.org/10.3390/s20174818






