Wearable Tendon Kinetics
Abstract
1. Introduction
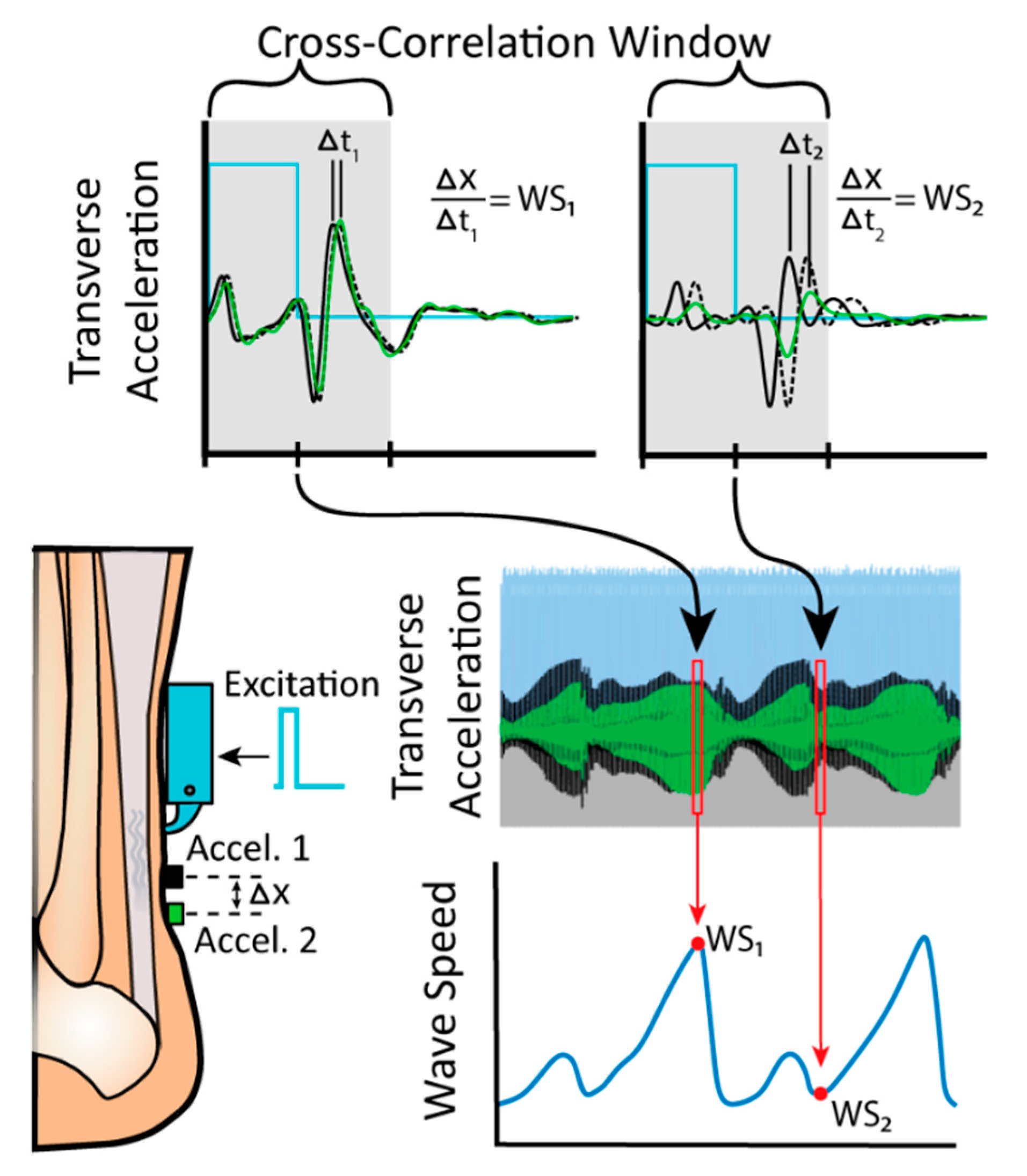
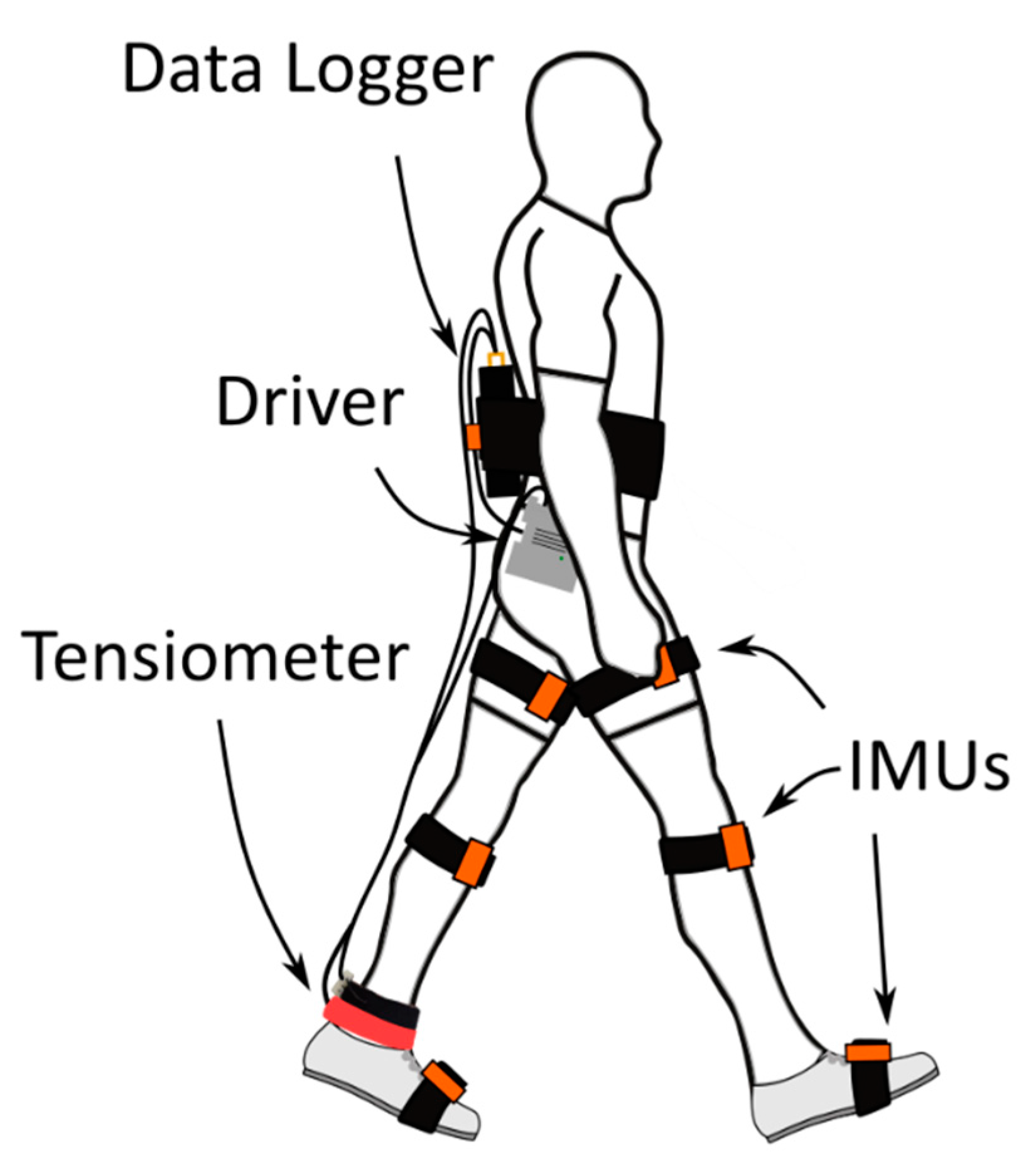
2. Materials and Methods
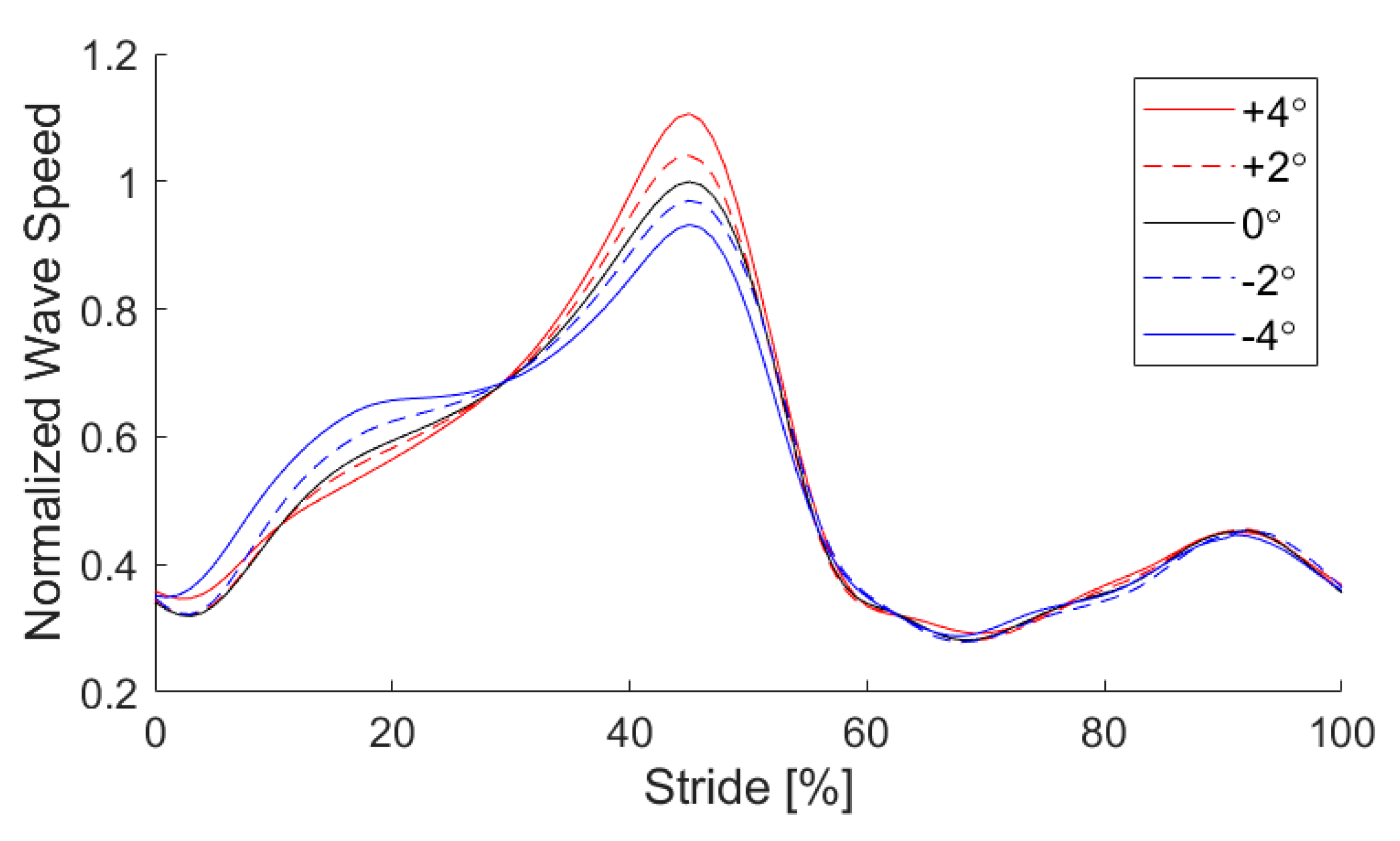
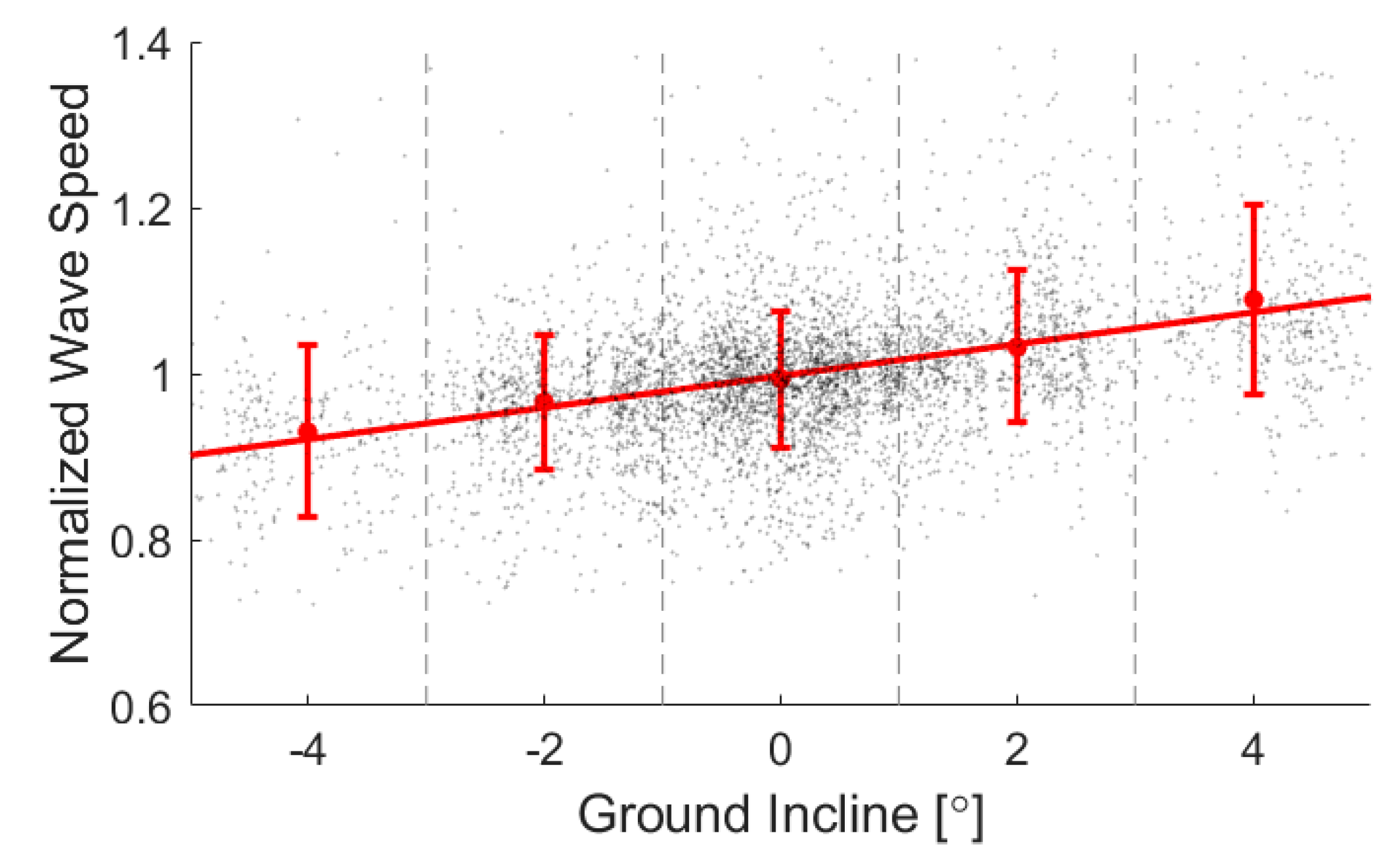
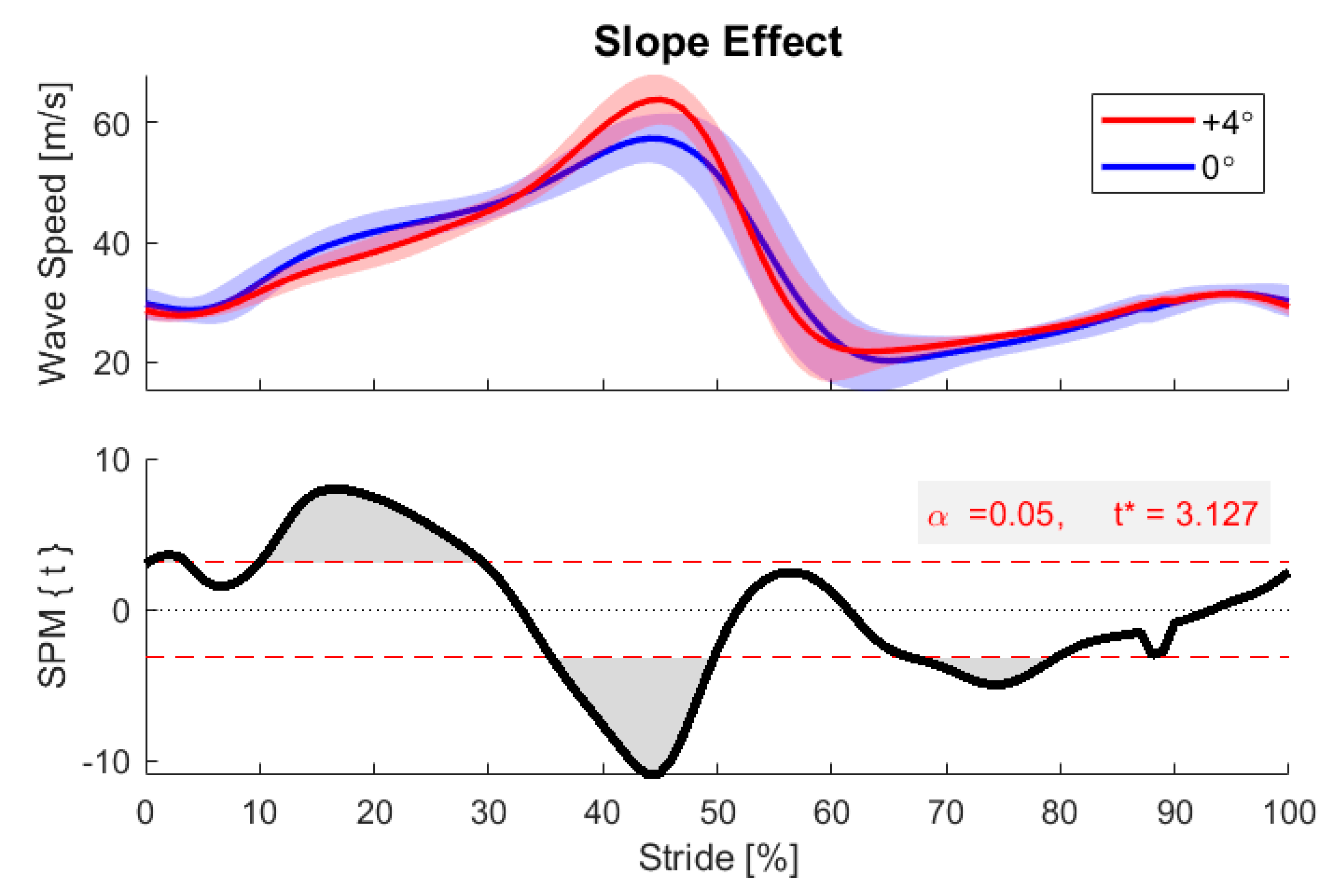
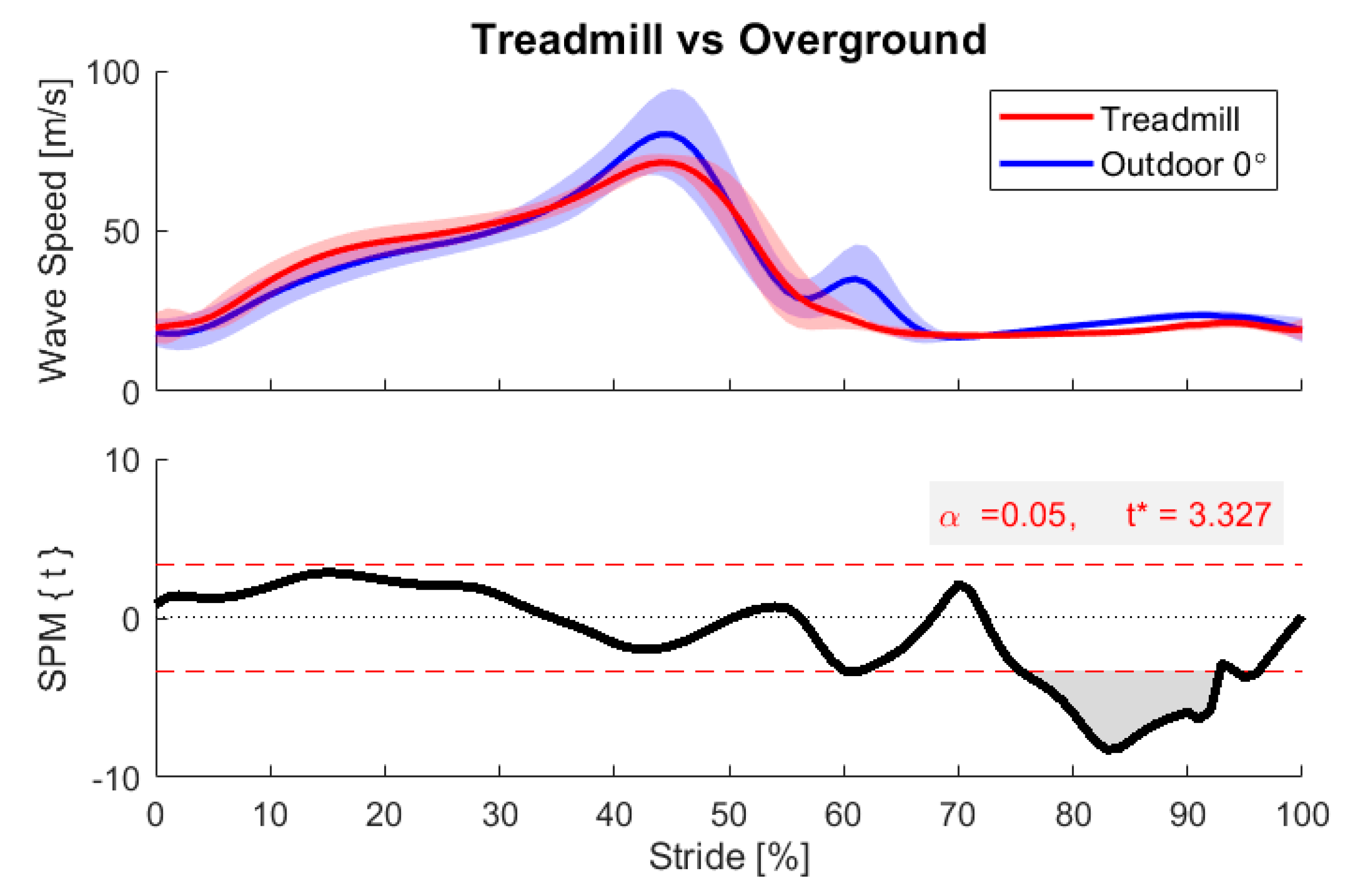
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Participant | Gender | Age (years) | Height (m) | Weight (kg) | Strides Analyzed |
|---|---|---|---|---|---|
| 1 | M | 24 | 1.90 | 85.0 | 580 |
| 2 | M | 24 | 1.96 | 83.9 | 588 |
| 3 | F | 26 | 1.63 | 56.7 | 624 |
| 4 | M | 25 | 1.83 | 88.5 | 505 |
| 5 | F | 19 | 1.83 | 68.0 | 545 |
| 6 | M | 23 | 1.88 | 74.8 | 505 |
| 7 | F | 23 | 1.65 | 70.3 | 534 |
| 8 | M | 24 | 1.70 | 70.0 | 614 |
| 9 | M | 23 | 1.85 | 90.7 | 613 |
| Mean | 3F/6M | 23.44 ± 1.94 | 1.80 ± 0.12 | 76.4 ± 11.3 | 567.6 ± 46.7 |
| Participant | Stride Length (m) | Ground Incline 1 (degrees) | Walking Speed (m/s) | Minimum Wave Speed 2 (m/s) | Peak Wave Speed 2 (m/s) |
|---|---|---|---|---|---|
| 1 | 1.60 ± 0.079 | 0.11 ± 1.93 | 1.46 ± 0.10 | 15.13 ± 0.75 | 59.08 ± 6.82 |
| 2 | 1.68 ± 0.085 | −0.08 ± 1.96 | 1.51 ± 0.17 | 14.78 ± 0.95 | 59.39 ± 1.90 |
| 3 | 1.46 ± 0.139 | 0.17 ± 1.96 | 1.42 ± 0.15 | 17.82 ± 1.92 | 78.68 ± 5.63 |
| 4 | 1.54 ± 0.059 | 0.22 ± 1.95 | 1.34 ± 0.19 | 15.31 ± 0.78 | 82.36 ± 11.91 |
| 5 | 1.52 ± 0.114 | −0.02 ± 1.96 | 1.53 ± 0.20 | 13.35 ± 0.97 | 62.16 ± 2.43 |
| 6 | 1.69 ± 0.087 | 0.15 ± 1.97 | 1.57 ± 0.13 | 15.82 ± 0.65 | 58.06 ± 1.85 |
| 7 | 1.53 ± 0.136 | 0.23 ± 1.92 | 1.56 ± 0.10 | 13.33 ± 0.56 | 53.16 ± 2.08 |
| 8 | 1.45 ± 0.115 | 0.21 ± 1.94 | 1.42 ± 0.12 | 17.98 ± 3.70 | 59.11 ± 3.08 |
| 9 | 1.53 ± 0.102 | 0.24 ± 1.84 | 1.37 ± 0.10 | 15.92 ± 0.43 | 79.99 ± 9.41 |
| Mean | 1.55 ± 0.134 | 0.14 ± 1.94 | 1.46 ± 0.16 | 15.58 ± 2.23 | 66.13 ± 12.23 |
Appendix B
| Participant | Coefficient | Intercept | p-Value | R2 |
|---|---|---|---|---|
| 1 | 0.0443 | 0.9948 | 1.17 × 10−54 | 0.3428 |
| 2 | 0.0053 | 1.0004 | 1.58 × 10−13 | 0.0888 |
| 3 | 0.0276 | 0.9954 | 8.70 × 10−58 | 0.3384 |
| 4 | 0.0184 | 0.9959 | 7.17 × 10−8 | 0.0561 |
| 5 | 0.0176 | 1.0003 | 4.18 × 10−82 | 0.4929 |
| 6 | 0.0137 | 0.9979 | 1.30 × 10−60 | 0.4154 |
| 7 | 0.0149 | 0.9966 | 9.00 × 10−46 | 0.3158 |
| 8 | 0.0117 | 0.9975 | 7.11 × 10−21 | 0.1338 |
| 9 | 0.0182 | 0.9956 | 1.06 × 10−10 | 0.0660 |
| Overall | 0.0191 | 0.9974 | 2.96 × 10−184 | 0.1514 |
References
- Chambers, R.; Gabbett, T.J.; Cole, M.H.; Beard, A. The Use of Wearable Microsensors to Quantify Sport-Specific Movements. Sports Med. 2015, 45, 1065–1081. [Google Scholar] [CrossRef] [PubMed]
- Li, R.T.; Kling, S.R.; Salata, M.J.; Cupp, S.A.; Sheehan, J.; Voos, J.E. Wearable Performance Devices in Sports Medicine. Sports Health 2016, 8, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Peppoloni, L.; Filippeschi, A.; Ruffaldi, E.; Avizzano, C.A. (WMSDs Issue) A Novel Wearable System for the Online Assessment of Risk for Biomechanical Load in Repetitive Efforts. Int. J. Ind. Ergon. 2014, 52, 1. [Google Scholar] [CrossRef]
- Alberto, R.; Draicchio, F.; Varrecchia, T.; Silvetti, A.; Iavicoli, S. Wearable Monitoring Devices for Biomechanical Risk Assessment at Work: Current Status and Future Challenges—A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2001. [Google Scholar] [CrossRef]
- Conforti, I.; Mileti, I.; Del Prete, Z.; Palermo, E. Assessing Ergonomics and Biomechanical Risk in Manual Handling of Loads through a Wearable System. In Proceedings of the 2019 IEEE International Workshop on Metrology for Industry 4.0 and IoT, MetroInd 4.0 and IoT, Naples, Italy, 4–6 June 2019. [Google Scholar] [CrossRef]
- Kwon, J.; Park, J.H.; Ku, S.; Jeong, Y.H.; Paik, N.J.; Park, Y.L. A Soft Wearable Robotic Ankle-Foot-Orthosis for Post-Stroke Patients. IEEE Robot. Autom. Lett. 2019, 4, 2547–2552. [Google Scholar] [CrossRef]
- Iosa, M.; De Sanctis, M.; Summa, A.; Bergamini, E.; Morelli, D.; Vannozzi, G. Usefulness of Magnetoinertial Wearable Devices in Neurorehabilitation of Children with Cerebral Palsy. Appl. Bionics Biomech. 2018, 2018. [Google Scholar] [CrossRef]
- Havens, K.L.; Cohen, S.C.; Pratt, K.A.; Sigward, S.M. Accelerations from Wearable Accelerometers Reflect Knee Loading during Running after Anterior Cruciate Ligament Reconstruction. Clin. Biomech. 2018, 58, 57–61. [Google Scholar] [CrossRef]
- Hullfish, T.J.; Qu, F.; Stoeckl, B.D.; Gebhard, P.M.; Mauck, R.L.; Baxter, J.R. Measuring Clinically Relevant Knee Motion with a Self-Calibrated Wearable Sensor. J. Biomech. 2019, 89, 105–109. [Google Scholar] [CrossRef]
- Camomilla, V.; Bergamini, E.; Fantozzi, S.; Vannozzi, G. Trends Supporting the In-Field Use of Wearable Inertial Sensors for Sport Performance Evaluation: A Systematic Review. Sensors 2018, 18, 873. [Google Scholar] [CrossRef]
- Adesida, Y.; Papi, E.; McGregor, A.H. Exploring the Role of Wearable Technology in Sport Kinematics and Kinetics: A Systematic Review. Sensors 2019, 19, 1597. [Google Scholar] [CrossRef]
- Johansson, D.; Malmgren, K.; Alt Murphy, M. Wearable Sensors for Clinical Applications in Epilepsy, Parkinson’s Disease, and Stroke: A Mixed-Methods Systematic Review. J. Neurol. 2018, 265, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Thorp, J.E.; Adamczyk, P.G.; Ploeg, H.-L.; Pickett, K.A. Monitoring Motor Symptoms During Activities of Daily Living in Individuals With Parkinson’s Disease. Front. Neurol. 2018, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Dadashi, F.; Mariani, B.; Rochat, S.; Büla, C.J.; Santos-Eggimann, B.; Aminian, K. Gait and Foot Clearance Parameters Obtained Using Shoe-Worn Inertial Sensors in a Large-Population Sample of Older Adults. Sensors 2013, 14, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Tunca, C.; Pehlivan, N.; Ak, N.; Arnrich, B.; Salur, G.; Ersoy, C. Inertial Sensor-Based Robust Gait Analysis in Non-Hospital Settings for Neurological Disorders. Sensors 2017, 17, 825. [Google Scholar] [CrossRef] [PubMed]
- Rebula, J.R.; Ojeda, L.V.; Adamczyk, P.G.; Kuo, A.D. Measurement of Foot Placement and Its Variability with Inertial Sensors. Gait Posture 2013, 38, 974–980. [Google Scholar] [CrossRef]
- Ojeda, L.V.; Adamczyk, P.G.; Rebula, J.R.; Nyquist, L.V.; Strasburg, D.M.; Alexander, N.B. Reconstruction of Body Motion during Self-Reported Losses of Balance in Community-Dwelling Older Adults. Med. Eng. Phys. 2019, 64, 86–92. [Google Scholar] [CrossRef]
- Ueberschär, O.; Fleckenstein, D.; Warschun, F.; Kränzler, S.; Walter, N.; Hoppe, M.W. Measuring Biomechanical Loads and Asymmetries in Junior Elite Long-Distance Runners through Triaxial Inertial Sensors. Sport. Orthop. Traumatol. 2019, 35, 296–308. [Google Scholar] [CrossRef]
- Sheerin, K.R.; Reid, D.; Besier, T.F. The Measurement of Tibial Acceleration in Runners—A Review of the Factors That Can Affect Tibial Acceleration during Running and Evidence-Based Guidelines for Its Use. Gait Posture 2019, 67, 12–24. [Google Scholar] [CrossRef]
- Howell, A.M.; Kobayashi, T.; Hayes, H.A.; Foreman, K.B.; Bamberg, S.J.M. Kinetic Gait Analysis Using a Low-Cost Insole. IEEE Trans. Biomed. Eng. 2013, 60, 3284–3290. [Google Scholar] [CrossRef]
- Bonnet, V.; Mazza, C.; Fraisse, P.; Cappozzo, A. Real-time Estimate of Body Kinematics During a Planar Squat Task Using a Single Inertial Measurement Unit. IEEE Trans. Biomed. Eng. 2013, 60, 1920–1926. [Google Scholar] [CrossRef]
- Latella, C.; Kuppuswamy, N.; Romano, F.; Traversaro, S.; Nori, F. Whole-Body Human Inverse Dynamics with Distributed Micro-Accelerometers, Gyros and Force Sensing. Sensors 2016, 16, 727. [Google Scholar] [CrossRef] [PubMed]
- Disselhorst-Klug, C.; Schmitz-Rode, T.; Rau, G. Surface electromyography and muscle force: Limits in sEMG–force relationship and new approaches for applications. Clin. Biomech. 2009, 24, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ginn, K.; Eastburn, G.; Lee, M. Evaluation of a buckle force transducer for measuring tissue tension. Aust. J. Physiother. 1993, 39, 31–38. [Google Scholar] [CrossRef]
- Erdemir, A.; Hamel, A.J.; Piazza, S.J.; Sharkey, N.A. Fiberoptic measurement of tendon forces is influenced by skin movement artifact. J. Biomech. 2003, 36, 449–455. [Google Scholar] [CrossRef]
- Komi, P.V. Relevance of in vivo force measurements to human biomechanics. J. Biomech. 1990, 23, 23–34. [Google Scholar] [CrossRef]
- Fukashiro, S.; Komi, P.; Järvinen, M.; Miyashita, M. Comparison between the directly measured achilles tendon force and the tendon force calculated from the ankle joint moment during vertical jumps. Clin. Biomech. 1993, 8, 25–30. [Google Scholar] [CrossRef]
- Fukashiro, S.; Komi, P.V.; Järvinen, M.; Miyashita, M. In vivo achilles tendon loading’ during jumping in humans. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995, 71, 453–458. [Google Scholar] [CrossRef]
- Finni, T.; Komi, P.V.; Lukkariniemi, J. Achilles tendon loading during walking: Application of a novel optic fiber technique. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 77, 289–291. [Google Scholar] [CrossRef]
- Ancillao, A.; Tedesco, S.; Barton, J.; O’Flynn, B. Indirect Measurement of Ground Reaction Forces and Moments by Means of Wearable Inertial Sensors: A Systematic Review. Sensors 2018, 18, 2564. [Google Scholar] [CrossRef]
- Karatsidis, A.; Bellusci, G.; Schepers, H.M.; De Zee, M.; Andersen, M.S.; Veltink, P. Estimation of Ground Reaction Forces and Moments During Gait Using Only Inertial Motion Capture. Sensors 2016, 17, 75. [Google Scholar] [CrossRef]
- Schepers, H.M.; Koopman, H.F.J.M.; Veltink, P. Ambulatory Assessment of Ankle and Foot Dynamics. IEEE Trans. Biomed. Eng. 2007, 54, 895–902. [Google Scholar] [CrossRef]
- Hurkmans, H.L.; Bussmann, J.; Benda, E.; Verhaar, J.A.N.; Stam, H. Accuracy and repeatability of the Pedar Mobile system in long-term vertical force measurements. Gait Posture 2006, 23, 118–125. [Google Scholar] [CrossRef]
- Saggin, B.; Scaccabarozzi, D.; Tarabini, M. Metrological Performances of a Plantar Pressure Measurement System. IEEE Trans. Instrum. Meas. 2013, 62, 766–776. [Google Scholar] [CrossRef]
- Fong, D.T.; Chan, Y.-Y.; Hong, Y.; Yung, P.S.; Fung, K.-Y.; Chan, K.-M. Estimating the complete ground reaction forces with pressure insoles in walking. J. Biomech. 2008, 41, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Matijevich, E.S.; Branscombe, L.M.; Scott, L.R.; Zelik, K.E. Ground reaction force metrics are not strongly correlated with tibial bone load when running across speeds and slopes: Implications for science, sport and wearable tech. PLoS ONE 2019, 14, e0210000. [Google Scholar] [CrossRef]
- Meardon, S.A.; Willson, J.D.; Gries, S.R.; Kernozek, T.W.; Derrick, T.R. Bone stress in runners with tibial stress fracture. Clin. Biomech. 2015, 30, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Vergari, C.; Ravary-Plumioën, B.; Evrard, D.; Laugier, P.; Mitton, D.; Pourcelot, P.; Crevier-Denoix, N. Axial speed of sound is related to tendon’s nonlinear elasticity. J. Biomech. 2012, 45, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Hug, F.; Tucker, K.; Gennisson, J.-L.; Tanter, M.; Nordez, A. Elastography for Muscle Biomechanics. Exerc. Sport Sci. Rev. 2015, 43, 125–133. [Google Scholar] [CrossRef] [PubMed]
- DeWall, R.J.; Slane, L.C.; Lee, K.S.; Thelen, D.G. Spatial variations in Achilles tendon shear wave speed. J. Biomech. 2014, 47, 2685–2692. [Google Scholar] [CrossRef]
- Martin, J.A.; Brandon, S.C.E.; Keuler, E.M.; Hermus, J.R.; Ehlers, A.C.; Segalman, D.J.; Allen, M.S.; Thelen, D.G. Gauging force by tapping tendons. Nat. Commun. 2018, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Biedrzycki, A.H.; Lee, K.S.; DeWall, R.J.; Brounts, S.H.; Murphy, W.L.; Markel, M.D.; Thelen, D.G. In Vivo Measures of Shear Wave Speed as a Predictor of Tendon Elasticity and Strength. Ultrasound Med. Boil. 2015, 41, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Bolus, N.; Jeong, H.-K.; Blaho, B.M.; Safaei, M.; Young, A.; Inan, O. Fit to Burst: Toward Noninvasive Estimation of Achilles Tendon Load Using Burst Vibrations. IEEE Trans. Biomed. Eng. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, I.; Huang, Y.; Ophir, J.; Spratt, S. Methods for Estimation of Subsample Time Delays of Digitized Echo Signals. Ultrason. Imaging 1995, 17, 142–171. [Google Scholar] [CrossRef]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Region-of-interest analyses of one-dimensional biomechanical trajectories: Bridging 0D and 1D theory, augmenting statistical power. PeerJ 2016, 4, e2652. [Google Scholar] [CrossRef]
- Pataky, T.C. One-dimensional statistical parametric mapping in Python. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 295–301. [Google Scholar] [CrossRef]
- Pataky, T.C. One-Dimensional Statistical Parametric Mapping in MATLAB; Free Software Foundation: Boston, MA, USA, 2019. [Google Scholar]
- Keuler, E.M.; Loegering, I.F.; Martin, J.A.; Roth, J.D.; Thelen, D.G. Shear Wave Predictions of Achilles Tendon Loading during Human Walking. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Lichtwark, G.A.; Wilson, A.M. Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. J. Exp. Boil. 2006, 209, 4379–4388. [Google Scholar] [CrossRef]
- Lay, A.N.; Hass, C.J.; Gregor, R.J. The effects of sloped surfaces on locomotion: A kinematic and kinetic analysis. J. Biomech. 2006, 39, 1621–1628. [Google Scholar] [CrossRef]
- Silder, A.; Besier, T.F.; Delp, S.L. Predicting the metabolic cost of incline walking from muscle activity and walking mechanics. J. Biomech. 2012, 45, 1842–1849. [Google Scholar] [CrossRef]
- Stoquart, G.; Detrembleur, C.; Lejeune, T. Effect of speed on kinematic, kinetic, electromyographic and energetic reference values during treadmill walking. Neurophysiol. Clin. Neurophysiol. 2008, 38, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R.; Stanhope, S.J. Sensitivity of joint moments to changes in walking speed and body-weight-support are interdependent and vary across joints. J. Biomech. 2013, 46, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Adamczyk, P.G. Analyzing Gait in the Real World Using Wearable Movement Sensors and Frequently Repeated Movement Paths. Sensors 2019, 19, 1925. [Google Scholar] [CrossRef] [PubMed]
- Hollman, J.H.; Watkins, M.K.; Imhoff, A.C.; Braun, C.E.; Akervik, K.A.; Ness, D.K. A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait Posture 2016, 43, 204–209. [Google Scholar] [CrossRef]
- Tamburini, P.; Storm, F.A.; Buckley, C.; Bisi, M.C.; Stagni, R.; Mazza, C. Moving from laboratory to real life conditions: Influence on the assessment of variability and stability of gait. Gait Posture 2018, 59, 248–252. [Google Scholar] [CrossRef]
- Komisar, V.; Novak, A.C.; Haycock, B. A novel method for synchronizing motion capture with other data sources for millisecond-level precision. Gait Posture 2017, 51, 125–131. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Martin, J.A.; Schmitz, D.G.; Thelen, D.G. Shear Wave Tensiometry Reveals an Age-Related Deficit in Triceps Surae Work at Slow and Fast Walking Speeds. Front. Sports Act. Living 2020, 2. [Google Scholar] [CrossRef]
- Schmitt, L.C.; Paterno, M.V.; Ford, K.R.; Myer, G.D.; Hewett, T.E. Strength Asymmetry and Landing Mechanics at Return to Sport after Anterior Cruciate Ligament Reconstruction. Med. Sci. Sports Exerc. 2015, 47, 1426–1434. [Google Scholar] [CrossRef]
- Firminger, C.R.; Asmussen, M.J.; Cigoja, S.; Fletcher, J.R.; Nigg, B.M.; Edwards, W.B. Cumulative Metrics of Tendon Load and Damage Vary Discordantly with Running Speed. Med. Sci. Sports Exerc. 2020. [Google Scholar] [CrossRef]
- Roy, S.; Edan, Y. Investigating Joint-Action in Short-Cycle Repetitive Handover Tasks: The Role of Giver Versus Receiver and its Implications for Human-Robot Collaborative System Design. Int. J. Soc. Robot. 2018, 1–16. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.; Peternel, L.; Tsagarakis, N.; Ajoudani, A. Anticipatory Robot Assistance for the Prevention of Human Static Joint Overloading in Human–Robot Collaboration. IEEE Robot. Autom. Lett. 2017, 3, 68–75. [Google Scholar] [CrossRef]
- Someshwar, R.; Meyer, J.; Edan, Y. A Timing Control Model for H-R Synchronization. IFAC Proc. Vol. 2012, 45, 698–703. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harper, S.E.; Roembke, R.A.; Zunker, J.D.; Thelen, D.G.; Adamczyk, P.G. Wearable Tendon Kinetics. Sensors 2020, 20, 4805. https://doi.org/10.3390/s20174805
Harper SE, Roembke RA, Zunker JD, Thelen DG, Adamczyk PG. Wearable Tendon Kinetics. Sensors. 2020; 20(17):4805. https://doi.org/10.3390/s20174805
Chicago/Turabian StyleHarper, Sara E., Rebecca A. Roembke, John D. Zunker, Darryl G. Thelen, and Peter G. Adamczyk. 2020. "Wearable Tendon Kinetics" Sensors 20, no. 17: 4805. https://doi.org/10.3390/s20174805
APA StyleHarper, S. E., Roembke, R. A., Zunker, J. D., Thelen, D. G., & Adamczyk, P. G. (2020). Wearable Tendon Kinetics. Sensors, 20(17), 4805. https://doi.org/10.3390/s20174805






