Label-Free Electrochemical Biosensors for the Determination of Flaviviruses: Dengue, Zika, and Japanese Encephalitis
Abstract
1. Introduction
2. Electrochemical Detection Methods
2.1. Electrochemical Impedance Spectroscopy and Conductometry
2.2. Voltammetry, Amperometry
3. Electrochemical Biosensors for DENV Diagnostic
3.1. DNA and RNA Biosensors for DENV Diagnostic
3.2. Immunosensors for DENV Diagnostic
3.3. Peptide-Based Biosensors for DENV Diagnostic
4. Electrochemical Biosensors for ZIKV Diagnostic
5. Electrochemical Biosensors for JEV Diagnostic
6. Conclusions and Future Perspectives of Biosensors
Author Contributions
Funding
Conflicts of Interest
References
- Aleyas, A.G.; George, J.A.; Han, Y.W.; Kim, H.K.; Kim, S.J.; Yoon, H.A.; Eo, S.K. Flaviviruses Induce Pro-inflammatory and Anti-inflammatory Cytokines from Murine Dendritic Cells through MyD88-dependent Pathway. Immune Netw. 2007, 7, 66. [Google Scholar] [CrossRef]
- Chong, H.Y.; Leow, C.Y.; Abdul Majeed, A.B.; Leow, C.H. Flavivirus infection—A review of immunopathogenesis, immunological response, and immunodiagnosis. Virus Res. 2019, 274, 197770. [Google Scholar] [CrossRef]
- Nazmi, A.; Dutta, K.; Hazra, B.; Basu, A. Role of pattern recognition receptors in flavivirus infections. Virus Res. 2014, 185, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R.N. Diagnosis of arboviral infections-A quagmire of cross reactions and complexities. Travel Med. Infect. Dis. 2016, 14, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Daep, C.A.; Muñoz-Jordán, J.L.; Eugenin, E.A. Flaviviruses, an expanding threat in public health: Focus on dengue, West Nile, and Japanese encephalitis virus. J. Neurovirol. 2014, 20, 539–560. [Google Scholar] [CrossRef]
- Li, X.F.; Dong, H.L.; Wang, H.J.; Huang, X.Y.; Qiu, Y.F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.Q.; et al. Development of a chimeric Zika vaccine using a licensed live-attenuated flavivirus vaccine as backbone. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2015, 14, 45–54. [Google Scholar] [CrossRef]
- Chokephaibulkit, K.; Houillon, G.; Feroldi, E.; Bouckenooghe, A. Safety and immunogenicity of a live attenuated Japanese encephalitis chimeric virus vaccine (IMOJEV®) in children. Expert Rev. Vaccines 2016, 15, 153–166. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Y.; Kang, X.; Zhang, X.; Hao, H.; Chen, W.; Liu, L.; Li, X.; Li, L.; Yuan, Q.; et al. A multiplex ELISA-based protein array for screening diagnostic antigens and diagnosis of Flaviviridae infection. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J. Climate Change and the Arboviruses: Lessons from the Evolution of the Dengue and Yellow Fever Viruses. Annu. Rev. Virol. 2016, 3, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Gugliemini, O.; Harber, S.; Harrison, A.; Houle, L.; Ivory, J.; Kersten, S.; Khan, R.; Kim, J.; LeBoa, C.; et al. Environmental and Social Change Drive the Explosive Emergence of Zika Virus in the Americas. PLoS Negl. Trop. Dis. 2017, 11, e0005135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Du, S.; Shan, C.; Nie, K.; Zhang, R.; Li, X.F.; Zhang, R.; Wang, T.; Qin, C.F.; et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature 2017, 545, 482–486. [Google Scholar] [CrossRef]
- Baronti, C.; Sire, J.; de Lamballerie, X.; Quérat, G. Nonstructural NS1 proteins of several mosquito-borne Flavivirus do not inhibit TLR3 signaling. Virology 2010, 404, 319–330. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar]
- Young, P.R.; Hilditch, P.A.; Bletchly, C.; Halloran, W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000, 38, 1053–1057. [Google Scholar] [CrossRef]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef]
- Herrada, C.A.; Kabir, M.A.; Altamirano, R.; Asghar, W. Advances in Diagnostic Methods for Zika Virus Infection. J. Med. Dev. 2018, 12, 0408021–04080211. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Abele-Horn, M.; Donoso Mantke, O.; Enders, M.; Fingerle, V.; Gärtner, B.; Hagedorn, J.; Rabenau, H.F.; Reiter-Owona, I.; Tintelnot, K.; et al. Immunological Methods for the Detection of Infectious Diseases; Dustri-Verlag Dr. Karl Feistle: Oberhaching, Germany, 2017. [Google Scholar]
- Parkash, O.; Shueb, R.H. Diagnosis of dengue infection using conventional and biosensor based techniques. Viruses 2015, 7, 5410–5427. [Google Scholar] [CrossRef]
- Zainuddin, A.A.; Nordin, A.N.; Rahim, R.A. Recent trends in dengue detection methods using biosensors. IIUM Eng. J. 2018, 19, 134–153. [Google Scholar] [CrossRef]
- Alzate, D.; Cajigas, S.; Robledo, S.; Muskus, C.; Orozco, J. Genosensors for differential detection of Zika virus. Talanta 2020, 210, 120648. [Google Scholar] [CrossRef] [PubMed]
- Ohan, N.W.; Heikkila, J.J. Reverse transcription-polymerase chain reaction: An overview of the technique and its applications. Biotechnol. Adv. 1993, 11, 13–29. [Google Scholar] [CrossRef]
- Sinawang, P.D.; Rai, V.; Ionescu, R.E.; Marks, R.S. Electrochemical lateral flow immunosensor for detection and quantification of dengue NS1 protein. Biosens Bioelectron. 2016, 77, 400–408. [Google Scholar] [CrossRef]
- Moço, A.C.R.; Guedes, P.H.; Flauzino, J.M.R.; da Silva, H.S.; Vieira, J.G.; Castro, A.C.H.; Gomes, É.V.R.; Tolentino, F.M.; Soares, M.M.C.N.; Madurro, J.M.; et al. Electrochemical Detection of Zika Virus in Biological Samples: A Step for Diagnosis Point-of-care. Electroanalysis 2019, 31, 1580–1587. [Google Scholar] [CrossRef]
- Hien, H.T.; Giang, H.T.; Trung, T.; Van Tuan, C. Enhancement of biosensing performance using a polyaniline/multiwalled carbon nanotubes nanocomposite. J. Mater. Sci. 2017, 52, 1694–1703. [Google Scholar] [CrossRef]
- Singhal, C.; Pundir, C.S.; Narang, J. A genosensor for detection of consensus DNA sequence of Dengue virus using ZnO/Pt-Pd nanocomposites. Biosens. Bioelectron. 2017, 97, 75–82. [Google Scholar] [CrossRef]
- Khristunova, Y.; Korotkova, E.; Kratochvil, B.; Barek, J.; Dorozhko, E.; Vyskocil, V.; Plotnikov, E.; Voronova, O.; Sidelnikov, V. Preparation and Investigation of Silver Nanoparticle–Antibody Bioconjugates for. Sensors 2019, 19, 2103. [Google Scholar] [CrossRef]
- Darwish, N.T.; Alias, Y.B.; Khor, S.M. An introduction to dengue-disease diagnostics. TrAC-Trends Anal. Chem. 2015, 67, 45–55. [Google Scholar] [CrossRef]
- Cecchetto, J.; Fernandes, F.C.B.; Lopes, R.; Bueno, P.R. The capacitive sensing of NS1 Flavivirus biomarker. Biosens. Bioelectron. 2017, 87, 949–956. [Google Scholar] [CrossRef]
- Silva, M.M.S.; Dias, A.C.M.S.; Cordeiro, M.T.; Marques, E.; Goulart, M.O.F.; Dutra, R.F. A thiophene-modified screen printed electrode for detection of dengue virus NS1 protein. Talanta 2014, 128, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Tan, O.K.; Tse, M.S.; Ooi, E.E. A label-free immunosensor for diagnosis of dengue infection with simple electrical measurements. Biosens. Bioelectron. 2010, 25, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Tiwari, S.; Jayant, R.D.; Vashist, A.; Nikkhah-Moshaie, R.; El-Hage, N.; Nair, M. Electrochemical Biosensors for Early Stage Zika Diagnostics. Trends Biotechnol. 2017, 35, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, V.; Yu, Y.; Clayton, N.; Chuang, Y.C.; Wang, Y.; Mueller, S.; Levon, K.; Simon, M.; Rafailovich, M. A chip-based potentiometric sensor for a Zika virus diagnostic using 3D surface molecular imprinting. Analyst 2019, 144, 4266–4280. [Google Scholar] [CrossRef] [PubMed]
- Ozer, T.; Geiss, B.J.; Henry, C.S. Review—Chemical and Biological Sensors for Viral Detection. J. Electrochem. Soc. 2020, 167, 037523. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, J.; Carvalho, F.C.; Santos, A.; Fernandes, F.C.B.; Bueno, P.R. An impedimetric biosensor to test neat serum for dengue diagnosis. Sens. Actuators B Chem. 2015, 213, 150–154. [Google Scholar] [CrossRef]
- van Tuan, C.; Huy, T.Q.; Van Hieu, N.; Tuan, M.A.; Trung, T. Polyaniline Nanowires-Based Electrochemical Immunosensor for Label Free Detection of Japanese Encephalitis Virus. Anal. Lett. 2013, 46, 1229–1240. [Google Scholar] [CrossRef]
- Channon, R.B.; Yang, Y.; Feibelman, K.M.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Development of an Electrochemical Paper-Based Analytical Device for Trace Detection of Virus Particles. Anal. Chem. 2018, 90, 7777–7783. [Google Scholar] [CrossRef]
- Syahir, A.; Usui, K.; Tomizaki, K.; Kajikawa, K.; Mihara, H. Label and Label-Free Detection Techniques for Protein Microarrays. Microarrays 2015, 4, 228–244. [Google Scholar] [CrossRef]
- Sang, S.; Wang, Y.; Feng, Q.; Wei, Y.; Ji, J.; Zhang, W. Progress of new label-free techniques for biosensors: A review. Crit. Rev. Biotechnol. 2015, 36, 1–17. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, R.; Chai, Y.; Chen, S.; Wang, N.; Zhu, Q. Layer-by-layer self-assembly of films of nano-Au and Co(bpy)33+ for the determination of Japanese B encephalitis vaccine. Biochem. Eng. J. 2006, 28, 231–236. [Google Scholar] [CrossRef]
- Figueiredo, A.; Vieira, N.C.S.; Dos Santos, J.F.; Janegitz, B.C.; Aoki, S.M.; Junior, P.P.; Lovato, R.L.; Nogueira, M.L.; Zucolotto, V.; Guimarães, F.E.G. Electrical detection of dengue biomarker using egg yolk immunoglobulin as the biological recognition element. Sci. Rep. 2015, 5, 7865. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Slouka, Z.; Shah, S.S.; Behura, S.K.; Shi, Z.; Stack, M.S.; Severson, D.W.; Chang, H.C. An ion-exchange nanomembrane sensor for detection of nucleic acids using a surface charge inversion phenomenon. Biosens. Bioelectron. 2014, 60, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Zhang, L.; Li, Q.; Chai, Y.; Cao, S. A label-free amperometric immunosenor based on multi-layer assembly of polymerized o-phenylenediamine and gold nanoparticles for determination of Japanese B encephalitis vaccine. Anal. Chim. Acta 2005, 531, 1–5. [Google Scholar] [CrossRef]
- Silva, M.M.S.; Dias, A.C.M.S.; Silva, B.V.M.; Gomes-Filho, S.L.R.; Kubota, L.T.; Goulart, M.O.F.; Dutra, R.F. Electrochemical detection of dengue virus NS1 protein with a poly(allylamine)/carbon nanotube layered immunoelectrode. J. Chem. Technol. Biotechnol. 2015, 90, 194–200. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. The utilization of SiNWs/AuNPs-modified indium tin oxide (ITO) in fabrication of electrochemical DNA sensor. Mater. Sci. Eng. C 2014, 45, 270–276. [Google Scholar] [CrossRef]
- Faria, H.A.M.; Zucolotto, V. Label-free electrochemical DNA biosensor for zika virus identification. Biosens. Bioelectron. 2019, 131, 149–155. [Google Scholar] [CrossRef]
- Kaushik, A.; Yndart, A.; Kumar, S.; Jayant, R.D.; Vashist, A.; Brown, A.N.; Li, C.Z.; Nair, M. A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein. Sci. Rep. 2018, 8, 9700. [Google Scholar] [CrossRef]
- Santos, A.; Bueno, P.R.; Davis, J.J. A dual marker label free electrochemical assay for Flavivirus dengue diagnosis. Biosens. Bioelectron. 2018, 100, 519–525. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Rocha-Santos, T.A.; Duarte, A.C. Review of analytical figures of merit of sensors and biosensors in clinical applications. TrAC-Trends Anal. Chem. 2010, 29, 1172–1183. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29, S49–S52. [Google Scholar] [PubMed]
- Long, G.L.; Winefordner, J.D. Limit of Detection. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Lim, J.M.; Kim, J.H.; Ryu, M.Y.; Cho, C.H.; Park, T.J.; Park, J.P. An electrochemical peptide sensor for detection of dengue fever biomarker NS1. Anal. Chim. Acta 2018, 1026, 109–116. [Google Scholar] [CrossRef]
- Alves, F.; Leoni, D.; Tenório, M.; Marques, E.; Júnior, D.O.; Amalia, R.; Dutra, F.; Del, M.; Taboada, P. Novel electrochemical genosensor for Zika virus based on a poly- (3-amino- 4-hydroxybenzoic acid) -modified pencil carbon graphite electrode. Sens. Actuators B. Chem. 2019, 296, 126681. [Google Scholar] [CrossRef]
- Peh, A.E.K.; Li, S.F.Y. Dengue virus detection using impedance measured across nanoporous alumina membrane. Biosens. Bioelectron. 2013, 42, 391–396. [Google Scholar] [CrossRef]
- Lisdat, F.; Schäfer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef]
- Kafka, J.; Pänke, O.; Abendroth, B.; Lisdat, F. A label-free DNA sensor based on impedance spectroscopy. Electrochim. Acta 2008, 53, 7467–7474. [Google Scholar] [CrossRef]
- Gan, T.; Shi, Z.; Sun, J.; Liu, Y. Simple and novel electrochemical sensor for the determination of tetracycline based on iron/zinc cations-exchanged montmorillonite catalyst. Talanta 2014, 121, 187–193. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- George, A.; Amrutha, M.S.; Srinivasan, R.; Srivastava, P.; Sai, V.V.R.; Sunil, S. Label-Free Detection of Chikungunya Non-Structural Protein 3 Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2019, 166, 1356–1363. [Google Scholar] [CrossRef]
- Garrote, B.L.; Santos, A.; Bueno, P.R. Perspectives on and Precautions for the Uses of Electric Spectroscopic Methods in Label-free Biosensing Applications. ACS Sens. 2019, 4, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Forzani, E.S.; Li, X.; Tao, N. Hybrid amperometric and conductometric chemical sensor based on conducting polymer nanojunctions. Anal. Chem. 2007, 79, 5217–5224. [Google Scholar] [CrossRef]
- Poghossian, A.; Schöning, M.J. Label-Free Sensing of Biomolecules with Field-Effect Devices for Clinical Applications. Electroanalysis 2014, 26, 1197–1213. [Google Scholar] [CrossRef]
- Cui, J.; Gao, L.; Chen, S.; Huang, Z.; Wang, X. Electrochemical voltammetric behaviors of synthetic dengue virus RNAs at ITO sensing electrode. J. Electroanal. Chem. 2019, 851, 113463. [Google Scholar] [CrossRef]
- Brainina, K.; Kozitsina, A.; Beikin, J. Electrochemical immunosensor for Forest-Spring encephalitis based on protein a labeled with colloidal gold. Anal. Bioanal. Chem. 2003, 376, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, C.H.; Ryu, M.Y.; Kim, J.G.; Lee, S.J.; Park, T.J.; Park, J.P. Development of peptide biosensor for the detection of dengue fever biomarker, nonstructural 1. PLoS ONE 2019, 14, e0222144. [Google Scholar] [CrossRef]
- Abdul Rashid, J.I.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. Surface modifications to boost sensitivities of electrochemical biosensors using gold nanoparticles/silicon nanowires and response surface methodology approach. J. Mater. Sci. 2016, 51, 1083–1097. [Google Scholar] [CrossRef]
- Lai, H.C.; Chin, S.F.; Pang, S.C.; Henry Sum, M.S.; Perera, D. Carbon Nanoparticles Based Electrochemical Biosensor Strip for Detection of Japanese Encephalitis Virus. J. Nanomater. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Khristunova, E.; Barek, J.; Kratochvíl, B.; Korotkova, E.; Dorozhko, E.; Vyskočil, V. Comparison of Two Immunoanalytical Methods for Determination of Antibodies to Tick-Borne Encephalitis Virus. Chem. Listy 2020, 114. in press. [Google Scholar]
- Lu, L.; Liu, B.; Liu, C.; Xie, G. Amperometric immunosensor for myeloperoxidase in human serum based on a multi-wall carbon nanotubes-ionic liquid-cerium dioxide film-modified electrode. Bull. Korean Chem. Soc. 2010, 31, 3259–3264. [Google Scholar] [CrossRef]
- Oliveira, M.D.L.; Nogueira, M.L.; Correia, M.T.S.; Coelho, L.C.B.B.; Andrade, C.A.S. Detection of dengue virus serotypes on the surface of gold electrode based on Cratylia mollis lectin affinity. Sens. Actuators B Chem. 2011, 155, 789–795. [Google Scholar] [CrossRef]
- Balmaseda, A.; Hammond, S.N.; Pérez, L.; Tellez, Y.; Saborío, I.; Mercado, J.C.; Cuadra, R.; Rocha, J.; Pérez, M.A.; Silva, S.; et al. Serotype-specific differences in clinical manifestations of dengue. Am. J. Trop. Med. Hyg. 2006, 74, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Anusha, J.R.; Kim, B.C.; Yu, K.H.; Raj, C.J. Electrochemical biosensing of mosquito-borne viral disease, dengue: A review. Biosens. Bioelectron. 2019, 142, 111511. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinsky, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective in Vitro and in Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef]
- Nawaz, M.H.; Hayat, A.; Catanante, G.; Latif, U.; Marty, J.L. Development of a portable and disposable NS1 based electrochemical immunosensor for early diagnosis of dengue virus. Anal. Chim. Acta 2018, 1026, 1–7. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Tripathy, S.; Joseph, J.; Pothuneedi, S.; Das, D.; Vanjari, S.R.K.; Rao, A.V.S.S.N.; Singh, S.G. A miniaturized electrochemical platform with an integrated PDMS reservoir for label-free DNA hybridization detection using nanostructured Au electrodes. Analyst 2019, 144, 6953–6961. [Google Scholar] [CrossRef]
- Tripathy, S.; Krishna Vanjari, S.R.; Singh, V.; Swaminathan, S.; Singh, S.G. Electrospun manganese (III) oxide nanofiber based electrochemical DNA-nanobiosensor for zeptomolar detection of dengue consensus primer. Biosens. Bioelectron. 2017, 90, 378–387. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Hapuarachchi, H.C.; Ng, L.C.; Soh, S.H.; Leo, Y.S.; Toh, C.S. Ultrasensitive cDNA detection of dengue virus RNA using electrochemical nanoporous membrane-based biosensor. PLoS ONE 2012, 7, e42346. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Cooper, E.B.; Gaudet, S.; Sorger, P.K.; Manalis, S.R. Electronic detection of DNA by its intrinsic molecular charge. Proc. Natl. Acad. Sci. USA 2002, 99, 14142–14146. [Google Scholar] [CrossRef]
- Zhang, G.J.; Zhang, L.; Huang, M.J.; Luo, Z.H.H.; Tay, G.K.I.; Lim, E.J.A.; Kang, T.G.; Chen, Y. Silicon nanowire biosensor for highly sensitive and rapid detection of Dengue virus. Sens. Actuators B Chem. 2010, 146, 138–144. [Google Scholar] [CrossRef]
- Cheng, M.S.; Ho, J.S.; Tan, C.H.; Wong, J.P.S.; Ng, L.C.; Toh, C.S. Development of an electrochemical membrane-based nanobiosensor for ultrasensitive detection of dengue virus. Anal. Chim. Acta 2012, 725, 74–80. [Google Scholar] [CrossRef]
- Darwish, N.T.; Alrawi, A.H.; Sekaran, S.D.; Alias, Y.; Khor, S.M. Electrochemical Immunosensor Based on Antibody-Nanoparticle Hybrid for Specific Detection of the Dengue Virus NS1 Biomarker. J. Electrochem. Soc. 2016, 163, B19–B25. [Google Scholar] [CrossRef]
- Wasik, D.; Mulchandani, A.; Yates, M. Salivary Detection of Dengue Virus NS1 Protein with a Label-Free Immunosensor for Early Dengue Diagnosis. Sensors 2018, 18, 2641. [Google Scholar] [CrossRef]
- Cecchetto, J.; Santos, A.; Mondini, A.; Cilli, E.M.; Bueno, P.R. Serological point-of-care and label-free capacitive diagnosis of dengue virus infection. Biosens. Bioelectron. 2020, 151, 111972. [Google Scholar] [CrossRef]
- da Cruz Santos, C.; Santos, P.C.M.; Rocha, K.L.S.; Thomasini, R.L.; de Oliveira, D.B.; Franco, D.L.; Ferreira, L.F. A new tool for dengue virus diagnosis: Optimization and detection of anti-NS1 antibodies in serum samples by impedimetric transducers. Microchem. J. 2020, 154, 104544. [Google Scholar] [CrossRef]
- Fischer, M.J.E. Amine coupling through EDC/NHS: A practical approach. Methods Mol. Biol. 2010, 627, 55–73. [Google Scholar] [PubMed]
- Nguyen, B.T.T.; Peh, A.E.K.; Chee, C.Y.L.; Fink, K.; Chow, V.T.K.; Ng, M.M.L.; Toh, C.S. Electrochemical impedance spectroscopy characterization of nanoporous alumina dengue virus biosensor. Bioelectrochemistry 2012, 88, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Darwish, N.T.; Alias, Y.; Khor, S.M. Indium tin oxide with zwitterionic interfacial design for biosensing applications in complex matrices. Appl. Surf. Sci. 2015, 325, 91–99. [Google Scholar] [CrossRef]
- Arshad, R.; Rhouati, A.; Hayat, A.; Nawaz, M.H.; Yameen, M.A.; Mujahid, A.; Latif, U. MIP-Based Impedimetric Sensor for Detecting Dengue Fever Biomarker. Appl. Biochem. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realising their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef]
- Cieplak, M.; Kutner, W. Artificial Biosensors: How Can Molecular Imprinting Mimic Biorecognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef]
- Vieira, N.C.S.; Figueiredo, A.; Dos Santos, J.F.; Aoki, S.M.; Guimarães, F.E.G.; Zucolotto, V. Label-free electrical recognition of a dengue virus protein using the SEGFET simplified measurement system. Anal. Methods 2014, 6, 8882–8885. [Google Scholar] [CrossRef]
- Luna, D.M.N.; Avelino, K.Y.P.S.; Cordeiro, M.T.; Andrade, C.A.S.; Oliveira, M.D.L. Electrochemical immunosensor for dengue virus serotypes based on 4-mercaptobenzoic acid modified gold nanoparticles on self-assembled cysteine monolayers. Sens. Actuators B Chem. 2015, 220, 565–572. [Google Scholar] [CrossRef]
- Navakul, K.; Warakulwit, C.; Yenchitsomanus, P.T.; Panya, A.; Lieberzeit, P.A.; Sangma, C. A novel method for dengue virus detection and antibody screening using a graphene-polymer based electrochemical biosensor. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 549–557. [Google Scholar] [CrossRef]
- Wang, S.M.; Sekaran, S.D. Early diagnosis of dengue infection using a commercial dengue duo rapid test kit for the detection of NS1, IGM, and IGG. Am. J. Trop. Med. Hyg. 2010, 83, 690–695. [Google Scholar] [CrossRef]
- Alere SD Product Catalogo. 2016. Available online: https://www.globalpointofcare.abbott/en/product-details/sd-bioline-dengue-duo-ns1-ag---ab-combo.html (accessed on 3 May 2018).
- Andries, A.-C.; Duong, V.; Ong, S.; Ros, S.; Sakuntabhai, A.; Horwood, P.; Dussart, P.; Buchy, P. Evaluation of the performances of six commercial kits designed for dengue NS1 and anti-dengue IgM, IgG and IgA detection in urine and saliva clinical specimens. BMC Infect. Dis. 2016, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Human Membrane Attack Complex(MAC) ELISA Kit. Cat No. MBS268481. pp. 1–8. Available online: https://www.mybiosource.com/human-elisa-kits/membrane-attack-complex-mac/268481 (accessed on 24 April 2016).
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Bosch, I.; De Puig, H.; Hiley, M.; Carré-Camps, M.; Perdomo-Celis, F.; Narváez, C.F.; Salgado, D.M.; Senthoor, D.; Grady, M.O.; Phillips, E.; et al. Rapid antigen tests for dengue virus serotypes and zika virus in patient serum. Sci. Transl. Med. 2017, 9, eaan1589. [Google Scholar] [CrossRef] [PubMed]
- Sikka, V.; Chattu, V.K.; Popli, R.K.; Galwankar, S.C.; Kelkar, D.; Sawicki, S.G.; Stawicki, S.P.; Papadimos, T.J. The emergence of zika virus as a global health security threat: A review and a consensus statement of the INDUSEM Joint working Group (JWG). J. Glob. Infect. Dis. 2016, 8, 3–15. [Google Scholar] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Faria, A.M.; Mazon, T. Early diagnosis of Zika infection using a ZnO nanostructures-based rapid electrochemical biosensor. Talanta 2019, 203, 153–160. [Google Scholar] [CrossRef]
- Cabral-miranda, G.; Cardoso, A.R.; Ferreira, C.S.; Sales, M.G.F.; Martin, F. Biosensor-based selective detection of Zika virus specific antibodies in infected individuals. Biosens. Bioelectron. 2018, 113, 101–107. [Google Scholar] [CrossRef]
- Song, H.; Qi, J.; Haywood, J.; Shi, Y.; Gao, G.F. Zika virus NS1 structure reveals diversity of electrostatic surfaces among flaviviruses. Nat. Struct. Mol. Biol. 2016, 23, 456–458. [Google Scholar] [CrossRef]
- Xu, X.; Song, H.; Qi, J.; Liu, Y.; Wang, H.; Su, C.; Shi, Y.; Gao, G.F. Contribution of intertwined loop to membrane association revealed by Zika virus full-length NS 1 structure. EMBO J. 2016, 35, 2170–2178. [Google Scholar] [CrossRef]
- Afsahi, S.; Lerner, M.B.; Goldstein, J.M.; Lee, J.; Tang, X.; Bagarozzi, D.A.; Pan, D.; Locascio, L.; Walker, A.; Barron, F.; et al. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron. 2018, 100, 85–88. [Google Scholar] [CrossRef]
- da Fonseca Alves, R.; da Silva, A.G.; Ferreira, L.F.; Franco, D.L. Synthesis and characterization of a material derived from 4-mercaptobenzoic acid: A novel platform for oligonucleotide immobilization. Talanta 2017, 165, 69–75. [Google Scholar] [CrossRef]
- Tancharoen, C.; Sukjee, W.; Thepparit, C.; Jaimipuk, T.; Auewarakul, P.; Thitithanyanont, A.; Sangma, C. Electrochemical Biosensor Based on Surface Imprinting for Zika Virus Detection in Serum. ACS Sens. 2019, 4, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Limitations, P.U.S.E. Rx ONLY ZIKV DetectTM IgM Capture ELISA Instructions for Use For Use Under an Emergency Use Authorization Only. 2018; pp. 1–18. Available online: https://www.fda.gov/media/99521/download (accessed on 21 May 2018).
- Granger, D.; Hilgart, H.; Misner, L.; Christensen, J.; Bistodeau, S.; Palm, J.; Strain, A.K.; Konstantinovski, M.; Liu, D.; Tran, A.; et al. Serologic testing for zika virus: Comparison of three zika virus IgM screening enzyme-linked immunosorbent assays and initial laboratory experiences. J. Clin. Microbiol. 2017, 55, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Anti-Zika Virus ELISA (IgG) First Specifi c Serological Test Worldwide for the Detection of Antibodies Against Zika Virus. 2015, pp. 1–2. Available online: https://www.euroimmun.com/documents/Indications/Infections/Zika-virus/EI_2668_D_UK_B.pdf (accessed on 9 July 2017).
- Zhang, L.; Yuan, R.; Huang, X.; Chai, Y.; Cao, S. Potentiometric immunosensor based on antiserum of Japanese B encephalitis immobilized in nano-Au/polymerized o-phenylenediamine film. Electrochem. Commun. 2004, 6, 1222–1226. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Tung, T.T.; Losic, D. Carbon Nanomaterial Based Biosensors for Non-Invasive Detection of Cancer and Disease Biomarkers for Clinical Diagnosis. Sensors 2017, 17, 1919. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.F.; Lim, L.S.; Pang, S.C.; Sia, M.; Sum, H. Carbon nanoparticle modified screen printed carbon electrode as a disposable electrochemical immunosensor strip for the detection of Japanese encephalitis virus. Microchim. Acta 2016, 491–497. [Google Scholar] [CrossRef]
- Huy, T.Q.; Hanh, N.T.H.; Thuy, N.T.; Van Chung, P.; Nga, P.T.; Tuan, M.A. A novel biosensor based on serum antibody immobilization for rapid detection of viral antigens. Talanta 2011, 86, 271–277. [Google Scholar] [CrossRef]
- JE DetectTM IgM ANTIBODY CAPTURE ELISA (MAC-ELISA). Available online: https://inbios.com/je-detect-igm-antibody-capture-elisa-for-japanese-encephalitis-intl-2/ (accessed on 8 July 2018).
- Robinson, J.S.; Featherstone, D.; Vasanthapuram, R.; Biggerstaff, B.J.; Desai, A.; Ramamurty, N.; Chowdhury, A.H.; Sandhu, H.S.; Cavallaro, K.F.; Johnson, B.W. Evaluation of three commercially available Japanese encephalitis virus IgM enzyme-linked immunosorbent assays. Am. J. Trop. Med. Hyg. 2010, 83, 1146–1155. [Google Scholar] [CrossRef]

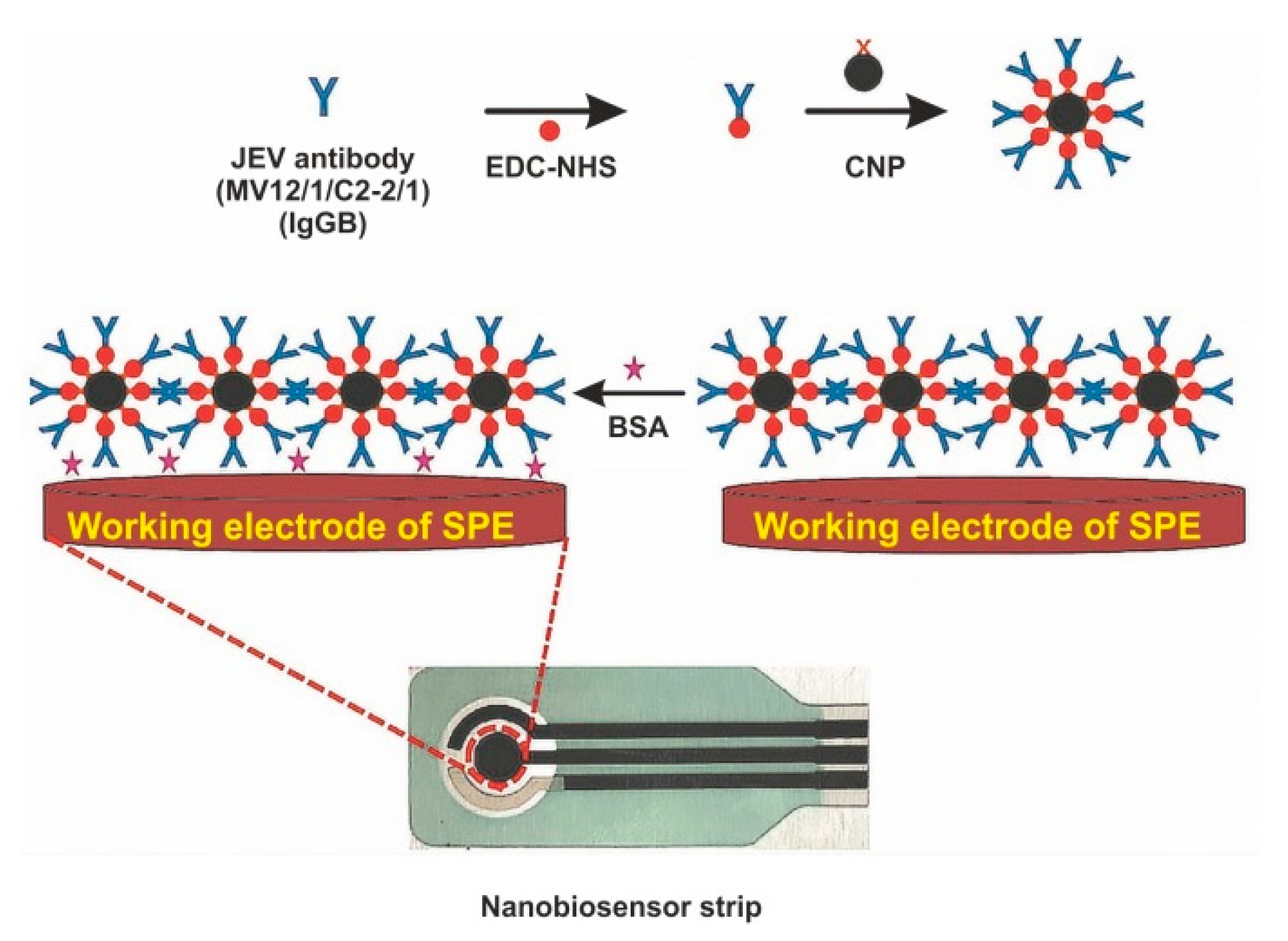
| Electrochemical Label-Free Biosensors | ||||||||
| Electrode/Platform Material | Method | Bio-Recognition Layer | Target | Limit of Detection | Possible Cross-Reactivity | Adv. | Dis. | Ref. |
| A nanoporous alumina membrane/Pt | DPV, CV | Mouse anti-DENV2 monoclonal antibody | DENV2 | 1 pfu/mL | Chikungunya virus, West Nile virus, DENV3 | a, f | b, d, e | [87] |
| A nanoporous alumina membrane/Pt | DPV, CV | DENV probe ssDNA | DENV1 | 9.55 × 10−12 mol/L | DENV3 | e, f | b, d, e | [81] |
| Mn2O3/GCE | DPV | DENV probe ssDNA | DENV comple- mentary DNA | 1.2 × 10−19 mol/L | DENV non-complementary DNA | d, e, f | c, e | [81] |
| Nafion/ITO | SWV | DENV probe ssDNA | DENV2 RNA | 2 × 10−18 mol/L | RNAs (DENV1, −3, −4) | a, e | b, e, f | [66] |
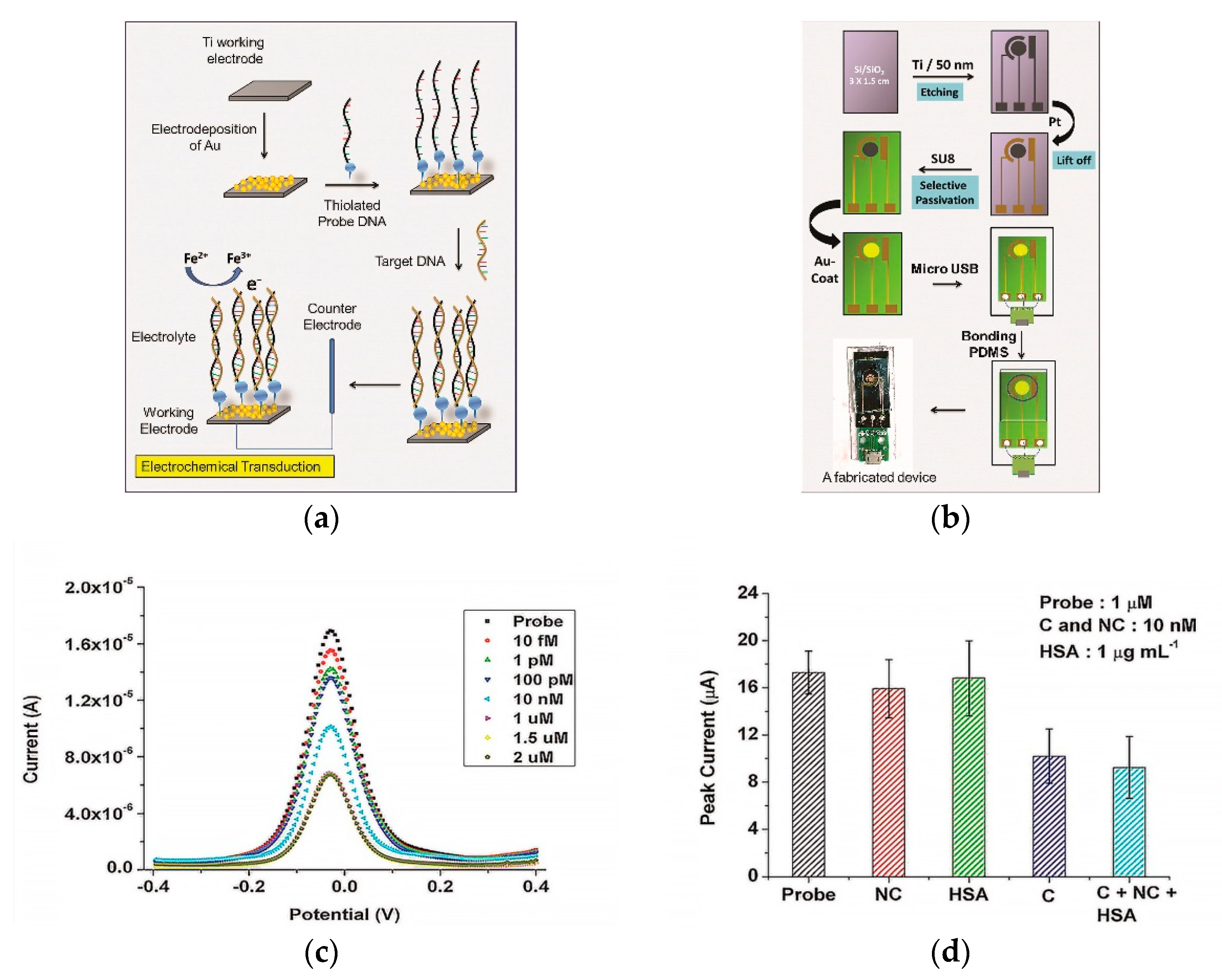
| Au nanostructures/Ti | DPV, CV | DENV thiolated probe ssDNA | DENV comple- mentary DNA | 9.7 × 10−16 mol/L | DENV non-comple- mentary DNA, human serum albumin | b, c, e | e | [80] |
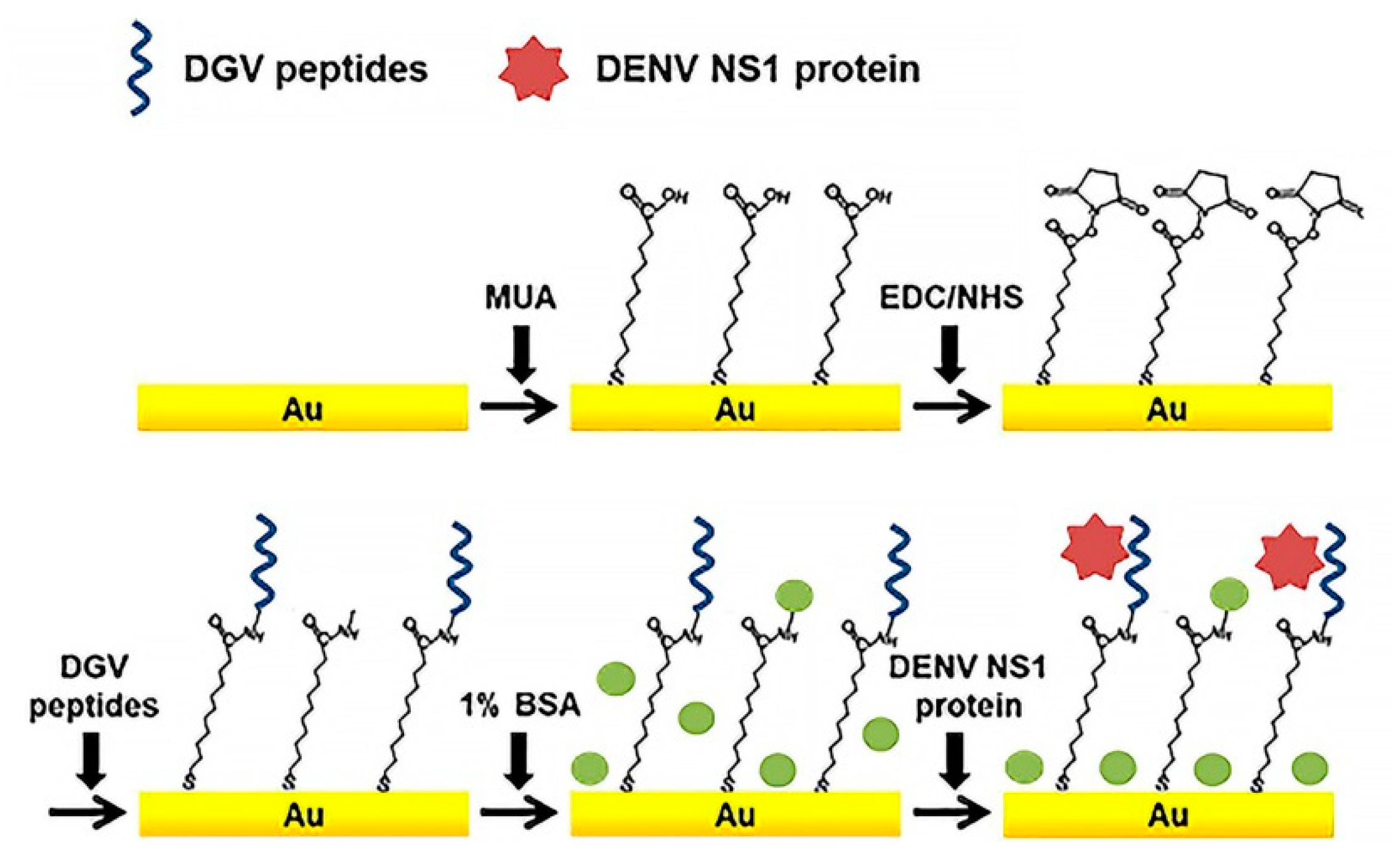
| AuE | SWV, EIS | Synthetic peptides (DGV BP1–BP5) | DENV NS1antigen | 1.49 μg/mL | Bovine serum albumin | a, f | b, e | [68] |
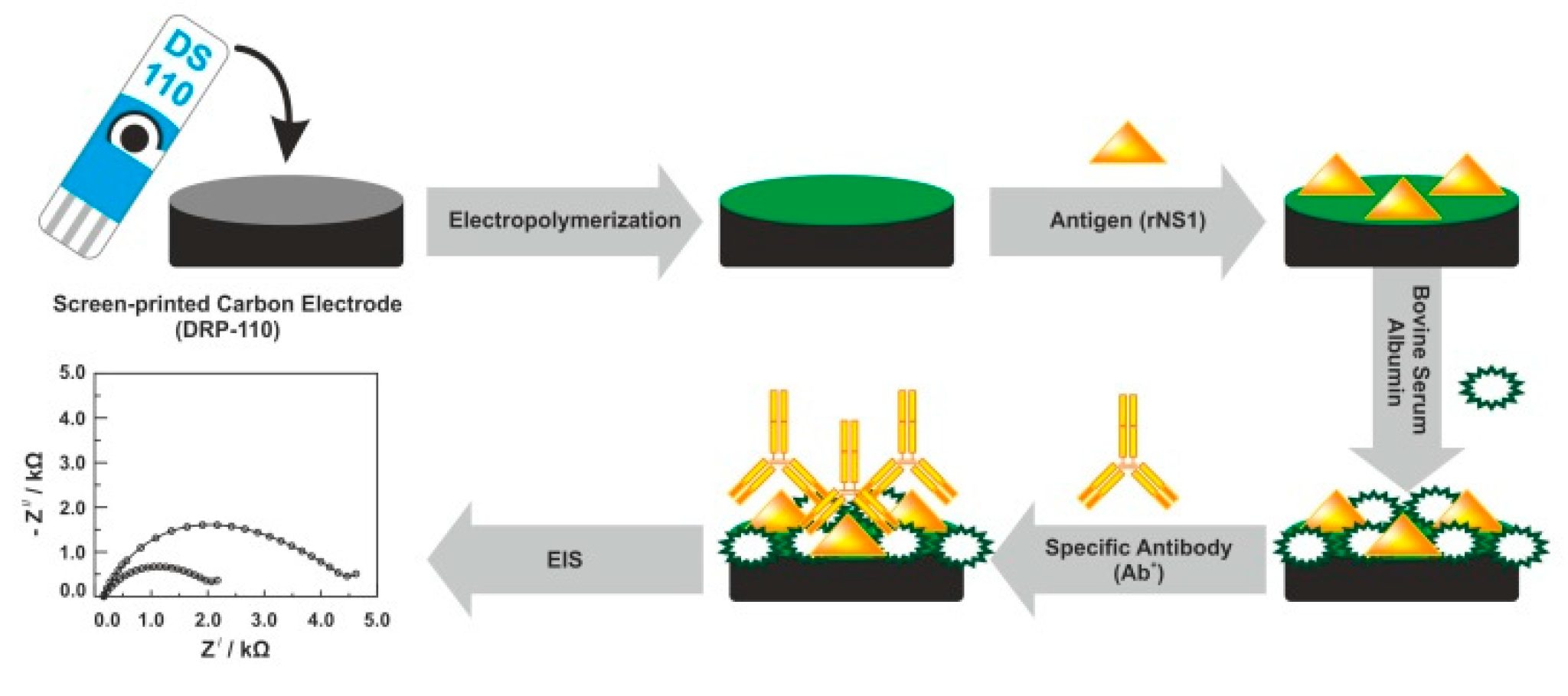
| MUA/6COH/AuE | EIS | Anti-DENV NS1 antibody | DENV NS1 antigen | 30 ng/mL | – | b, e, f, | c, e | [37] |
| A nanoporous alumina electrode | EIS | Anti-DENV2 antibody | DENV2 | 1 pfu/mL | Chikungunya virus, West Nile virus | a, f | b, d, e | [93] |
| Pt film/alumina membrane | EIS | Anti-DENV2 antibody | DENV2 DENV3 | 0.23 and0.71 pfu/mL | Chikungunya virus | a, b, e | c, d, e, f | [57] |
| 4-mercaptobenzoic acid/AuNPs/AuE | EIS | Anti-DENV antibody | DENV1–4 | – | – | a, b, | c, e | [99] |
| AuNPs/1,4-phenylenediamine/ITO | EIS | Anti-DENV NS1 antibody | DENV NS1antigen | 5 ng/mL | Malaria-infected sera | b, d, e, f | a, c, f | [88] |
| 1-pyrenebutyric acid/SWNT/Au microelectrode | EIS | Anti-DENV NS1 antibody | DENV NS1 antigen | 1 ng/mL | Artificial human saliva | a, b, e | b, c, d, e | [89] |
| 11-(ferrocenyl)undecanethiol/PEG (poly(ethylene glycol)- thiol/AuE | EIS | Anti-DENV NS1 antibody, DENV NS1 antigen | DENV NS1 antigen, anti-DENV NS1 antibody | 1.2 ng/mL, 6.1 ng/mL | – | b, d, e | a, c, d, e, f | [50] |
| Ferrocene-tagged peptide/AuE | EIS | Anti-DENV NS1 antibody | DENV NS1 antigen | – | – | b, d | c, d, e, f | [90] |
| Poly(4-aminobenzoic acid)/screen-printed electrode | EIS | DENV NS1 antigen | Anti-DENV NS1 antibody | – | Uric acid, glucose, water, HBS-EP buffer | b, c, d, f | c | [91] |
| Copolymers + graphene oxide/AuE | EIS | DENV antigen | DENV2 antibody | 0.12 pfu/mL | Influenza A virus | b, e | c, d, e, f | [100] |
| Dopamine/polysulfone nanofibers/SPCE | EIS | Imprinted NS1 protein | DENV NS1 antigen | 0.3 ng/mL | Fetal bovine serum, lysozyme | b, c, d, e, f | c, d, e | [95] |
| PVB (polyvinyl formal chloroform solution)—Fe3O4/AuE | EIS, CV | CramoLL (lectin and fetuin isolated from Cratylia mollis seeds) | Glycoproteins of DENV2, DENV3 | – | – | f | b, c, d, e | [73] |
| PNA/SiNW (silicon nanowire) | Electronic conductivity | DENV comple- mentary fragment | DENV2 | 1.0 × 10−14 mol/L | – | b, c, f | b, c, d, e | [86] |
| AuE | Electronic conductivity | Anti-DENV NS1 antibody | DENV NS1antigen | 0.25 μg/mL | – | [98] | ||
| Anion exchange nanoporous membrane | Conducto-metry | Negatively charged DNA oligoprobes | DENV2, DENV3 RNA | 1.0 × 10−12 mol/L | – | b, d, f | e | [44] |
| Commercially Available Assays | ||||||||
| Platform/Com-pany | Method | Bio-Recognition Molecules | Target | Detection Rates | Possible Cross-Reactivity | Adv. | Dis. | Ref. |
| Test Strips/Abbott SD BIOLINE Dengue Duo | In-vitro immunochromatogra-phic | DENV envelope proteins—Au colloidal | DENV NS1 antigen, DENV Ig M, Ig G Antibodies | 92.4% | RNAs (DENV1, −3, −4) and other flaviviruses | b, d, e, f | a, c | [101,102] |
| Microtiter plate/Panbio Dengue Ig M | Membrane attack complex—ELISA | Anti-DENV human-IgM antibody, DENV NS1 antigen, antibody-HRP conjugates | DENV Ig M antibodies | 81% | RNAs (DENV1, −3, −4) and other flaviviruses | d, f | a, c | [103,104] |
| Microtiter plate/Abbott SD ELISA Dengue | Indirect ELISA | Anti-DENV human-IgM antibody, DENV NS1antigen, antibody-HRP conjugates | DENV Ig M antibodies | 69.2% | RNAs (DENV1, −3, −4) and other flaviviruses | d, f | a, c | [103,105] |
| Electrode Material | Method | Bio-Recognition Layer | Target | Limit of Detection | Possible Cross-Reactivity | Adv. | Dis. | Ref. |
| DTSP (dithiobis(succi-nimidyl propionate))/IDE (interdigitated micro-Au electrode) | EIS | ZIKV envelope protein antibody (Zev-Abs) | ZIKV antigen | 1 × 10−11 mol/L | Chikungunya virus, West Nile virus, DENV | c, e, f | b, c | [49] |
| p-Phenylenediamine/SPCE | EIS, CV, SWV | ZIKV EDIII and NS1 | ZIKV antibodies | 17 fg/mL | DENV | d, e | a, c | [110] |
| PEG/Ti-Pt leads on SiO2/graphene chip | Capacitan-ce measure-ment | Mouse anti- ZIKV monoclonal antibody | ZIKV NS1 antigen | 4.5 × 10−10 mol/L | JEV | b, c | a, c, e | [113] |
| SIPs-GO composites/AuE | DPV | ZIKV imprinted to the polymer | ZIKV antigen | 2 × 10−4 pfu/mL | DENV2 | d | a, c, d | [115] |
| 3-4-AHBA/ PCGE | SWV, EIS | ZIKV aminated ssDNA | ZIKV antigen | 2.54 × 10−11 mol/L | DENV2, −3 | f | b, c, e | [56] |
| ZnO nanostructures/PCB | CV | Anti-ZIKV NS1 antibody | ZIKV NS1 antigen | 1 pg/mL | DENV NS1 antigen | e, f | a, c, e | [109] |
| Disposable AuE/PET | EIS, DPV, CV | ZIKV thiolated probe ssDNA | ZIKV NS5 antigen | 2.5 × 10−8 mol/L | DENV NS5 protein | a, c | b, e | [48] |
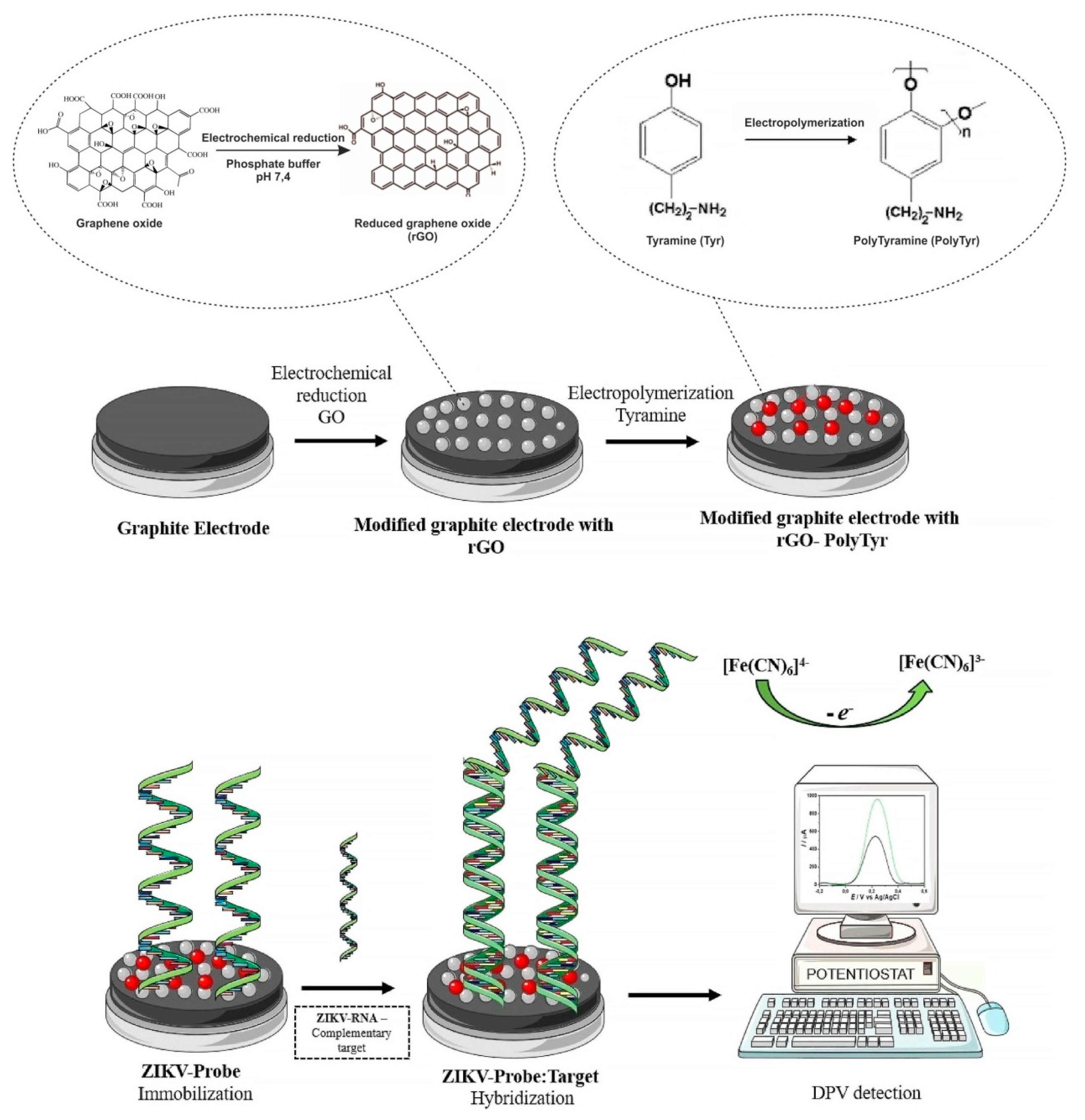
| Poly-tyramine/ rGO/graphite electrode | DPV | ZIKV oligonucleotide | ZIKV genomic RNA | 0.1 fg/mL | – | d, f | f | [26] |
| Commercially Available Assays | ||||||||
| Platform/Company | Method | Bio-Recognition Molecules | Target | Detection Rates | Possible Cross-Reactivity | Adv. | Dis. | Ref. |
| Test Strips/Abbott SD BIOLINE Zika Ig M | In-vitro immunochromatographic | ZIKV envelope proteins—Aucolloidal | ZIKV NS1 antigen, DENV Ig M, Ig G antibodies | 95.6% | RNAs (DENV1, −3, −4) and other flaviviruses | b, d, e, f | a, c | [101] |
| Microtiter plate/InBios ZIKV Detect™ | Membrane attack complex—ELISA | Anti-DENV human-IgM antibody, DENV NS1antigen, antibody-HRP conjugates | DENV Ig M antibodies | 96.5% | Yellow Fever virus, Chikungunya virus | d, e, f | a, c | [116,117] |
| Microtiter plate precoated with ZIKV NS1/ Euroimmun anti-ZIKV IgM | Indirect ELISA | Antibody-HRP conjugates | DENV Ig M antibodies | 56% | West Nile virus | d, f | a, c | [118,117] |
| Electrode Material | Method | Bio-Recognition Layer | Target | Limit of Detection | Adv. | Dis. | Ref. |
| Nano-Au/o-PDA polymer film/PtE | Amperometry | Antiserum of JEV | JEV antigen | 6 × 10−9 pfu/mL | b, f | c, b | [119] |
| Nano-Au/o-PDA polymer film with deposited Prussian blue/PtE | Amperometry | Antiserum of JEV | JEV antigen | 6 × 10−9 pfu/mL | b, f | a, b, c | [45] |
| l-cysteine + nano-Au and [Co(bpy)3]3+/AuE | Potentiometry | Antiserum of JEV | JEV antigen | 3.5 × 10−8 pfu/mL | b | a, b, c | [42] |
| Silanized surface with protein A/screen-printed electrode | EIS | Serum containing antibodies to JEV | JEV antigen | 0.75 µg/mL | a, b c, d | d, e, f | [122] |
| PANI nanowires/PtE | EIS | Anti-JEV antibodies | JEV antigen | 10 ng/mL | e | c, d, e, f, | [38] |
| CNPs/3-aminopropyl triethoxysilane/SPCE | EIS, CV | JEV antibody | JEV antigen | 2 ng/mL | b, d | c, d, e, f, | [121] |
| PANI/multiwalled carbon nanotubes/PtE | EIS | Anti-JEV antibodies | JEV antigen | – | a, b | d, e, f | [27] |
| CNPs/chitosan/SPCE | EIS, CV | JEV antibody | JEV antigen | 0.36 ng/mL | b, d, c | a, c, d, e, f | [70] |
| Commercially Available Assays | |||||||
| Platform/Company | Method | Bio-Recognition Molecules | Target | Detection Rates | Adv. | Adv. | Ref. |
| Microtiter plate/InBios JEV Detect™ | Membrane attack complex—ELISA | Anti-JEV human-IgM antibody, JEV NS1 antigen, antibody-HRP conjugates | JEV Ig M antibodies | 56% | d, f | a, c | [123,124] |
| Microtiter plate/XCyton JEV Chex | Membrane attack complex—ELISA | Anti-JEV human-IgM antibody, JEV NS1 antigen, antibody-HRP conjugates | JEV Ig M antibodies | 19% | d, f | a, c | [124] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khristunova, E.; Dorozhko, E.; Korotkova, E.; Kratochvil, B.; Vyskocil, V.; Barek, J. Label-Free Electrochemical Biosensors for the Determination of Flaviviruses: Dengue, Zika, and Japanese Encephalitis. Sensors 2020, 20, 4600. https://doi.org/10.3390/s20164600
Khristunova E, Dorozhko E, Korotkova E, Kratochvil B, Vyskocil V, Barek J. Label-Free Electrochemical Biosensors for the Determination of Flaviviruses: Dengue, Zika, and Japanese Encephalitis. Sensors. 2020; 20(16):4600. https://doi.org/10.3390/s20164600
Chicago/Turabian StyleKhristunova, Ekaterina, Elena Dorozhko, Elena Korotkova, Bohumil Kratochvil, Vlastimil Vyskocil, and Jiri Barek. 2020. "Label-Free Electrochemical Biosensors for the Determination of Flaviviruses: Dengue, Zika, and Japanese Encephalitis" Sensors 20, no. 16: 4600. https://doi.org/10.3390/s20164600
APA StyleKhristunova, E., Dorozhko, E., Korotkova, E., Kratochvil, B., Vyskocil, V., & Barek, J. (2020). Label-Free Electrochemical Biosensors for the Determination of Flaviviruses: Dengue, Zika, and Japanese Encephalitis. Sensors, 20(16), 4600. https://doi.org/10.3390/s20164600







