Artificial Neural Networks in Motion Analysis—Applications of Unsupervised and Heuristic Feature Selection Techniques
Abstract
1. Introduction
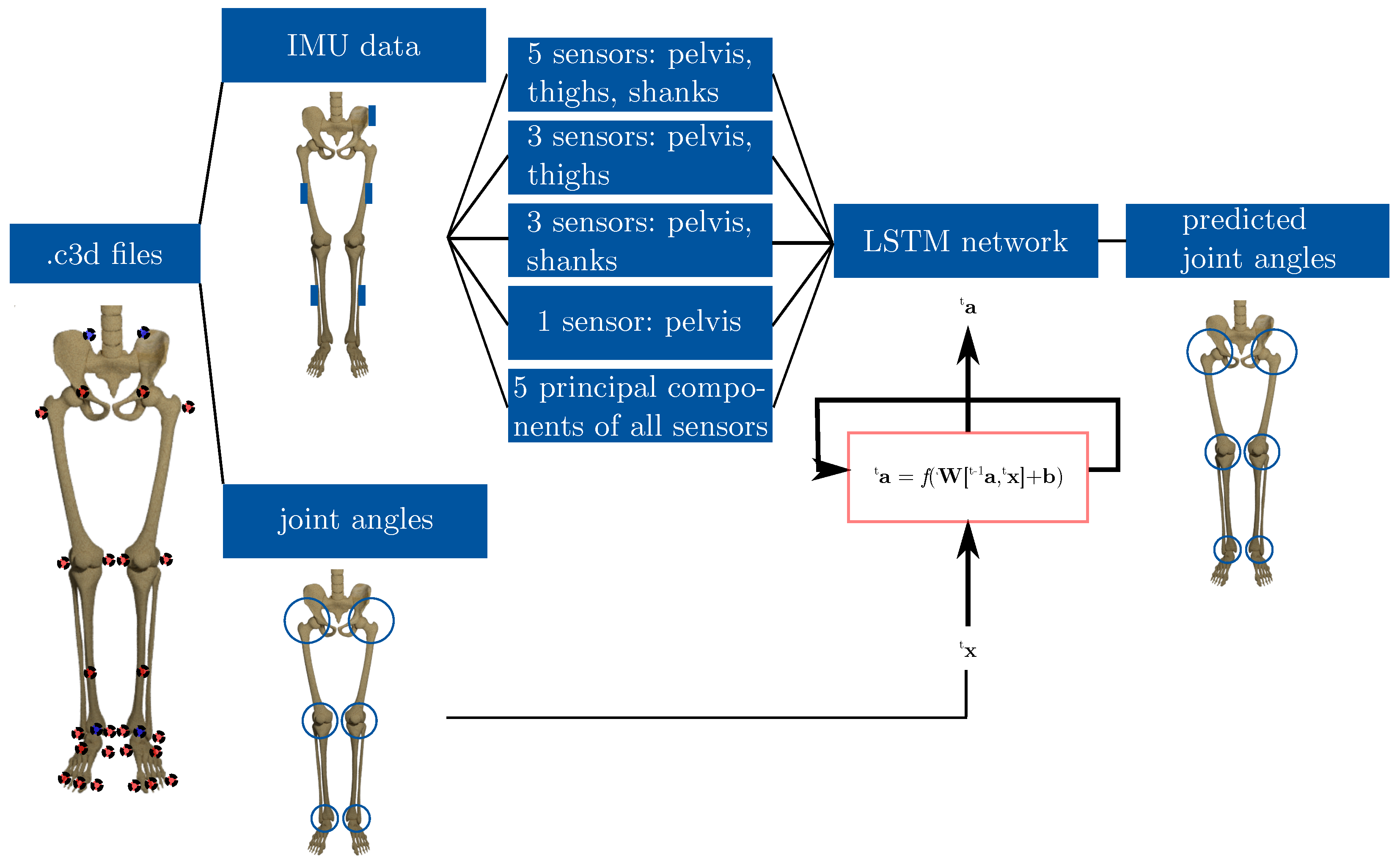
2. Materials and Methods
2.1. Data Set
2.2. Feature Selection
- 5 sensors: pelvis, right thigh, left thigh, right shank, left shank (PTS-net)
- 3 sensors: pelvis, right thigh, left thigh (PT-net)
- 3 sensors: pelvis, right shank, left shank (PS-net)
- 1 sensor: pelvis (P-net).
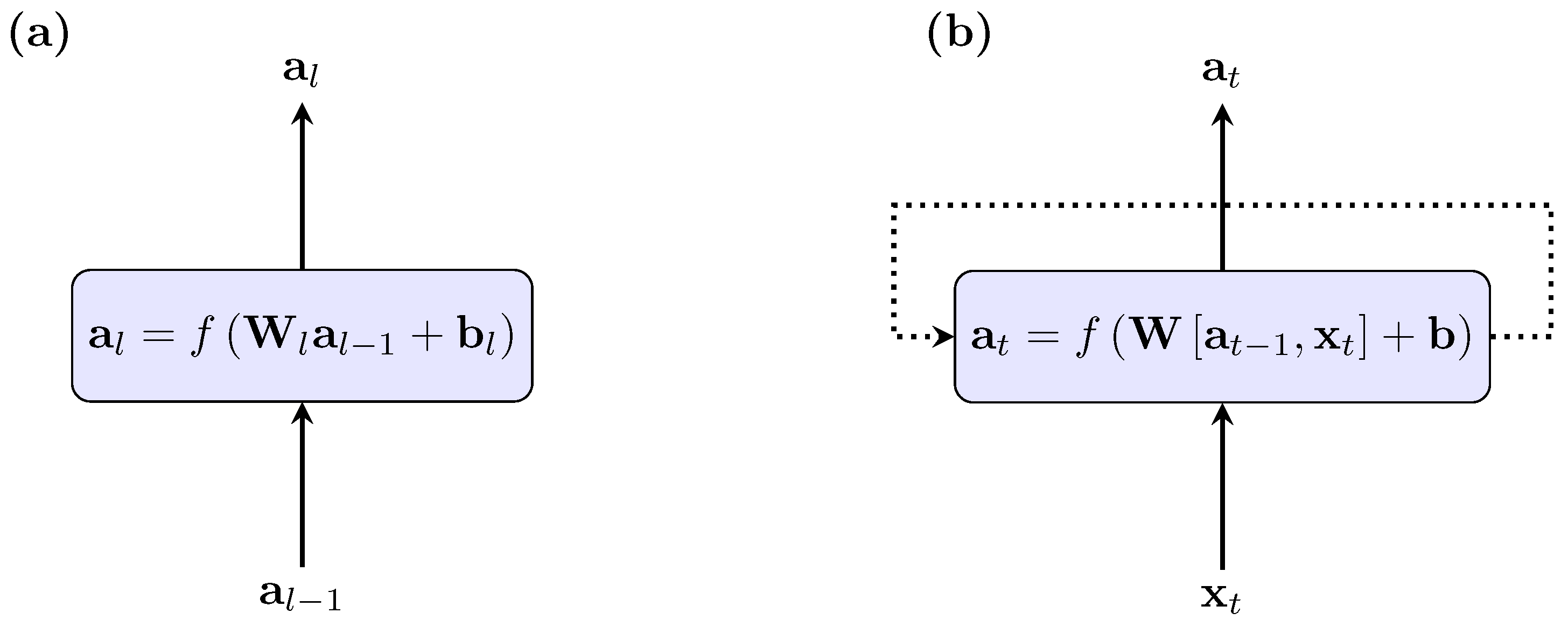
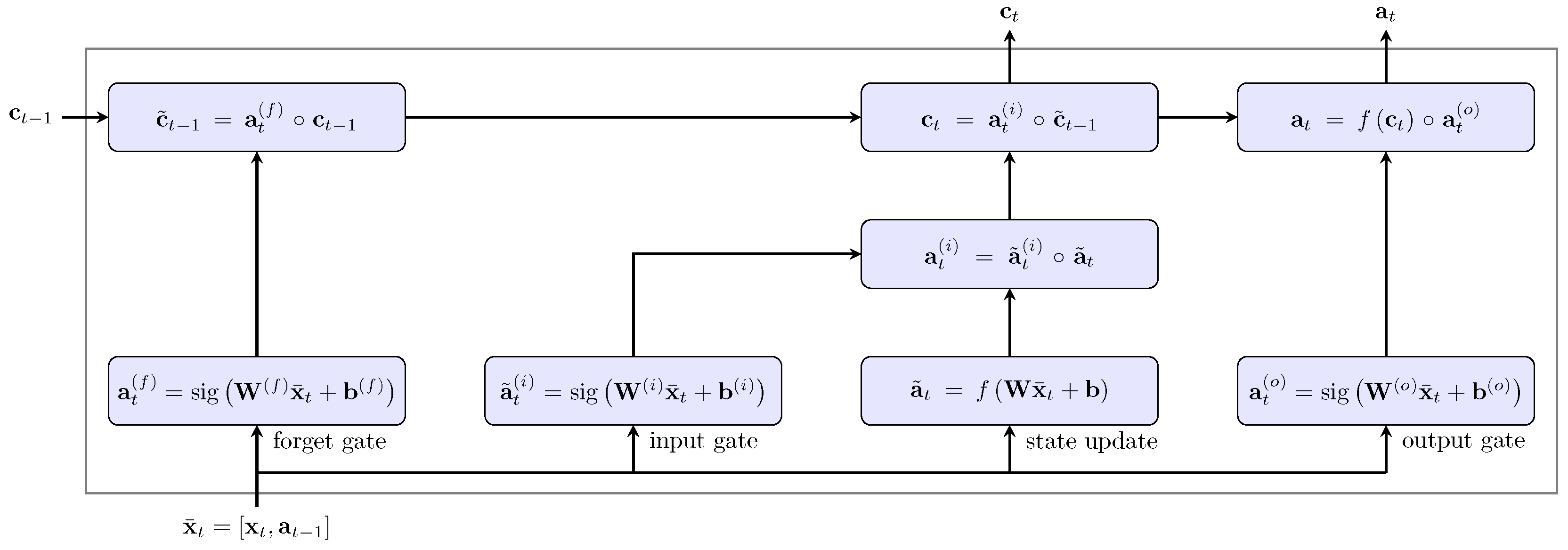
2.3. Long Short-Term Memory Neural Network
2.4. Data Analysis
3. Results
3.1. Principle Component Analysis
3.2. Prediction Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adesida, Y.; Papi, E.; McGregor, A.H. Exploring the role of wearable technology in sport kinematics and kinetics: A systematic review. Sensors 2019, 19, 1597. [Google Scholar] [CrossRef] [PubMed]
- Kobsar, D.; Ferber, R. Wearable sensor data to track subject-specific movement patterns related to clinical outcomes using a machine learning approach. Sensors 2018, 18, 2828. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Trojaniello, D.; Cereatti, A.; Sabatini, A.M. A machine learning framework for gait classification using inertial sensors: Application to elderly, post-stroke and huntington’s disease patients. Sensors 2016, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Aroganam, G.; Manivannan, N.; Harrison, D. Review on Wearable Technology Sensors Used in Consumer Sport Applications. Sensors 2019, 19, 1983. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, U.; Zijlstra, W.; Aminian, K.; Chastin, S.F.; de Bruin, E.D.; Helbostad, J.L.; Bussmann, J.B. Recommendations for standardizing validation procedures assessing physical activity of older persons by monitoring body postures and movements. Sensors 2014, 14, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- van den Noort, J.C.; Steenbrink, F.; Roeles, S.; Harlaar, J. Real-time visual feedback for gait retraining: Toward application in knee osteoarthritis. Med. Biol. Eng. Comput. 2015, 53, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Bessone, V.; Höschele, N.; Schwirtz, A.; Seiberl, W.; Bessone, V. Validation of a new inertial measurement unit system based on different dynamic movements for future in-field applications applications. Sport. Biomech. 2019, 1–16. [Google Scholar] [CrossRef]
- Lebleu, J.; Gosseye, T.; Detrembleur, C.; Mahaudens, P.; Cartiaux, O.; Penta, M. Lower Limb Kinematics Using Inertial Sensors during Locomotion: Accuracy and Reproducibility of Joint Angle Calculations with Different. Sensors 2020, 20, 715. [Google Scholar] [CrossRef]
- Poitras, I.; Dupuis, F.; Bielmann, M.; Campeau-Lecours, A.; Mercier, C.; Bouyer, L.J.; Roy, J.S. Validity and reliability of wearable sensors for joint angle estimation: A systematic review. Sensors 2019, 19, 1555. [Google Scholar] [CrossRef]
- Mundt, M.; Thomsen, W.; David, S.; Dupré, T.; Bamer, F.; Potthast, W.; Markert, B. Assessment of the measurement accuracy of inertial sensors during different tasks of daily living. J. Biomech. 2019, 84, 81–86. [Google Scholar] [CrossRef]
- Seel, T.; Ruppin, S. Eliminating the Effect of Magnetic Disturbances on the Inclination Estimates of Inertial Sensors. IFAC-PapersOnLine 2017, 50, 8798–8803. [Google Scholar] [CrossRef]
- Mundt, M.; Koeppe, A.; David, S.; Witter, T.; Bamer, F.; Potthast, W.; Markert, B. Estimation of Gait Mechanics Based on Simulated and Measured IMU Data Using an Artificial Neural Network. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Laidig, D.; Lehmann, D.; Begin, M.A.; Seel, T. Magnetometer-free Realtime Inertial Motion Tracking by Exploitation of Kinematic Constraints in 2-DoF Joints. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1233–1238. [Google Scholar] [CrossRef]
- Nowka, D.; Kok, M.; Seel, T. On motions that allow for identification of hinge joint axes from kinematic constraints and 6D IMU data. In Proceedings of the 2019 18th European Control Conference, ECC 2019, Naples, Italy, 25–28 June 2019; pp. 4325–4331. [Google Scholar] [CrossRef]
- Gurchiek, R.D.; Cheney, N.; Mcginnis, R.S. Estimating Biomechanical Time-Series with Wearable Sensors: A Systematic Review of Machine Learning Techniques. Sensors 2019, 19, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T. Adaptive Forward-Backward Greedy Algorithm for Learning Sparse Represenations. IEEE Trans. Inf. Theory 2011, 57, 4689–4708. [Google Scholar] [CrossRef]
- Choi, A.; Jung, H.; Lee, K.Y.; Lee, S.; Mun, J.H. Machine learning approach to predict center of pressure trajectories in a complete gait cycle: A feedforward neural network vs. LSTM network. Med. Biol. Eng. Comput. 2019. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.B.; Oh, S.E.; Mun, J.H. Improving the ground reaction force prediction accuracy using one-axis plantar pressure: Expansion of input variable for neural network. J. Biomech. 2016, 49, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Aljaaf, A.J.; Hussain, A.J.; Fergus, P.; Przybyla, A.; Barton, G.J. Evaluation of machine learning methods to predict knee loading from the movement of body segments. In Proceedings of the International Joint Conference on Neural Networks, Vancouver, BC, Canada, 24–29 July 2016. [Google Scholar] [CrossRef]
- Ardestani, M.M.; Chen, Z.; Wang, L.; Lian, Q.; Liu, Y.; He, J.; Li, D.; Jin, Z. Feed forward artificial neural network to predict contact force at medial knee joint: Application to gait modification. Neurocomputing 2014, 139, 114–129. [Google Scholar] [CrossRef]
- Kapelner, T.; Vujaklija, I.; Jiang, N.; Negro, F.; Aszmann, O.C.; Principe, J.; Farina, D. Predicting wrist kinematics from motor unit discharge timings for the control of active prostheses. J. Neuroeng. Rehabil. 2019, 16, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, R.; Chen, W.; Xiong, C. Simultaneous and continuous estimation of shoulder and elbow kinematics from surface EMG signals. Front. Neurosci. 2017, 11, 1–12. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Cao, S.; Zhang, X.; Chen, X. Feasibility study of advanced neural networks applied to sEMG-based force estimation. Sensors 2018, 18, 3226. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Cheng, Y.; Xi, N. Surface EMG based continuous estimation of human lower limb joint angles by using deep belief networks. Biomed. Signal Process. Control 2018, 40, 335–342. [Google Scholar] [CrossRef]
- Oh, S.E.; Choi, A.; Mun, J.H. Prediction of ground reaction forces during gait based on kinematics and a neural network model. J. Biomech. 2013, 46, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Rezaei, A.; Cuthbert, T.J.; Napier, C.; Menon, C. Lower body kinematics monitoring in running using fabric-based wearable sensors and deep convolutional neural networks. Sensors 2019, 19, 5325. [Google Scholar] [CrossRef] [PubMed]
- Ziai, A.; Menon, C. Comparison of regression models for estimation of isometric wrist joint torques using surface electromyography. J. Neuroeng. Rehabil. 2011, 8, 1–12. [Google Scholar] [CrossRef]
- Wouda, F.J.; Giuberti, M.; Rudigkeit, N.; van Beijnum, B.J.F.; Poel, M.; Veltink, P. Time Coherent Full-Body Poses Estimated Using Only Five Inertial Sensors: Deep versus Shallow Learning. Sensors 2019, 19, 3716. [Google Scholar] [CrossRef]
- Mundt, M.; Thomsen, W.; Witter, T.; Koeppe, A.; David, S.; Bamer, F.; Potthast, W.; Markert, B. Prediction of lower limb joint angles and moments during gait using artificial neural networks. Med Biol. Eng. Comput. 2020, 58, 211–225. [Google Scholar] [CrossRef]
- Findlow, A.H.; Goulermas, J.Y.; Nester, C.J.; Howard, D.; Kenney, L.P. Predicting lower limb joint kinematics using wearable motion sensors. Gait Posture 2008, 28, 120–126. [Google Scholar] [CrossRef]
- Stetter, B.J.; Ringhof, S.; Krafft, F.C.; Sell, S.; Stein, T. Estimation of Knee Joint Forces in Sport Movements Using Wearable Sensors and Machine Learning. Sensors 2019, 19, 3690. [Google Scholar] [CrossRef]
- Zago, M.; Sforza, C.; Dolci, C.; Tarabini, M.; Galli, M. Use of Machine Learning and Wearable Sensors to Predict Energetics and Kinematics of Cutting Maneuvers. Sensors 2019, 19, 3094. [Google Scholar] [CrossRef]
- Jie-han Ngoh, K.; Gouwanda, D.; Gopalai, A.A.; Yu, C. Estimation of vertical ground reaction force during running using neural network model and uniaxial accelerometer. J. Biomech. 2018, 76, 269–273. [Google Scholar] [CrossRef]
- Lim, H.; Kim, B.; Park, S. Prediction of Lower Limb Kinetics and Kinematics during Walking by a Single IMU on the Lower Back Using Machine Learning. Sensors 2020, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Shahabpoor, E.; Pavic, A.; Brownjohn, J.M.W.; Billings, S.A.; Guo, L.Z.; Bocian, M. Real-life measurement of tri-axial walking ground reaction forces using optimal network of wearable inertial measurement units. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Chiasson, D.P.; Hu, H.; Shull, P.B. Influence of IMU position and orientation placement errors on ground reaction force estimation. J. Biomech. 2019, 97. [Google Scholar] [CrossRef] [PubMed]
- Komnik, I.; Peters, M.; Funken, J.; David, S.; Weiss, S.; Potthast, W. Non-sagittal knee joint kinematics and kinetics during gait on level and sloped grounds with unicompartmental and total knee arthroplasty patients. PLoS ONE 2016, 11, e0168566. [Google Scholar] [CrossRef]
- Dupré, T.; Dietzsch, M.; Komnik, I.; Potthast, W.; David, S. Agreement of measured and calculated muscle activity during highly dynamic movements modelled with a spherical knee joint. J. Biomech. 2019, 84, 73–80. [Google Scholar] [CrossRef]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, L.; Witte, H.; et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion—Part I: Ankle, hip, and spine. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef]
- Bamer, F.; Kazemi, A.A.; Bucher, C. A new model order reduction strategy adapted to nonlinear problems in earthquake engineering. Earthq. Eng. Struct. Dyn. 2017, 46, 537–559. [Google Scholar] [CrossRef]
- Bamer, F.; Markert, B. An efficient response identification strategy for nonlinear structures subject to nonstationary generated seismic excitations. Mech. Based Des. Struct. Mach. 2017, 45, 313–330. [Google Scholar] [CrossRef]
- Shirafkan, N.; Bamer, F.; Stoffel, M.; Markert, B. Quasistatic analysis of elastoplastic structures by the proper generalized decomposition in a space-time approach. Mech. Res. Commun. 2020, 104, 103500. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016; Available online: http://www.deeplearningbook.org (accessed on 15 August 2020).
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Gers, F.A.; Schmidhuber, J.; Cummins, F. Learning to Forget: Continual Prediction with {LSTM}. In Proceedings of the 1999 Ninth International Conference on Artificial Neural Networks, ICANN’99, Edinburgh, UK, 7–10 September 1999; Volume 2471, pp. 850–855. [Google Scholar] [CrossRef]
- Li, L.; Jamieson, K.; DeSalvo, G.; Rostamizadeh, A.; Talwalkar, A. Hyperband: A novel bandit-based approach to hyperparameter optimization. J. Mach. Learn. Res. 2018, 18, 1–52. [Google Scholar]
- Seel, T.; Raisch, J.; Schauer, T. IMU-Based Joint Angle Measurement for Gait Analysis. Sensors 2014, 14, 6891–6909. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, A.M. Quaternion-Based Extended Kalman Filter for Determining Orientation by Inertial and Magnetic Sensing. IEEE Trans. Biomed. Eng. 2006, 53, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Teufl, W.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Validity, test-retest reliability and long-term stability of magnetometer free inertial sensor based 3D joint kinematics. Sensors 2018, 18, 1980. [Google Scholar] [CrossRef]
- Zihajehzadeh, S.; Park, E.J. A Novel Biomechanical Model-Aided IMU/UWB Fusion for Magnetometer-Free Lower Body Motion Capture. IEEE Trans. Syst. Man Cybern. Syst. 2017, 47, 927–938. [Google Scholar] [CrossRef]
- Zimmermann, T.; Taetz, B.; Bleser, G. IMU-to-segment assignment and orientation alignment for the lower body using deep learning. Sensors 2018, 18, 302. [Google Scholar] [CrossRef]
- Brunner, T.; Lauffenburger, J.P.; Changey, S.; Basset, M. Magnetometer-augmented IMU simulator: In-depth elaboration. Sensors 2015, 15, 5293–5310. [Google Scholar] [CrossRef]
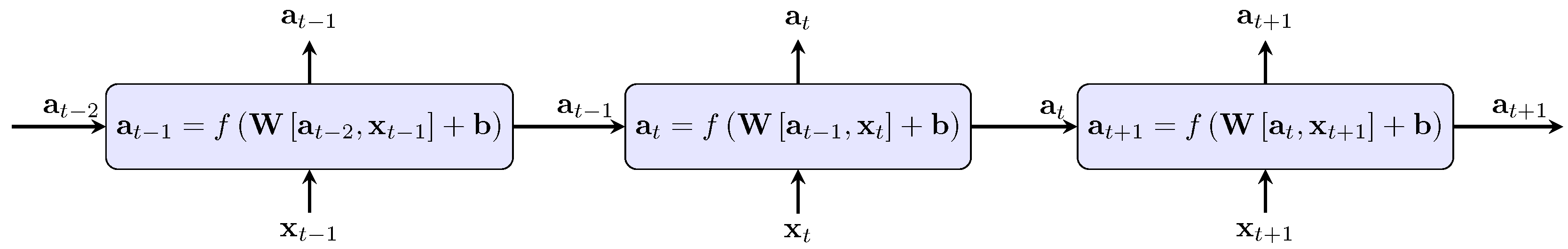
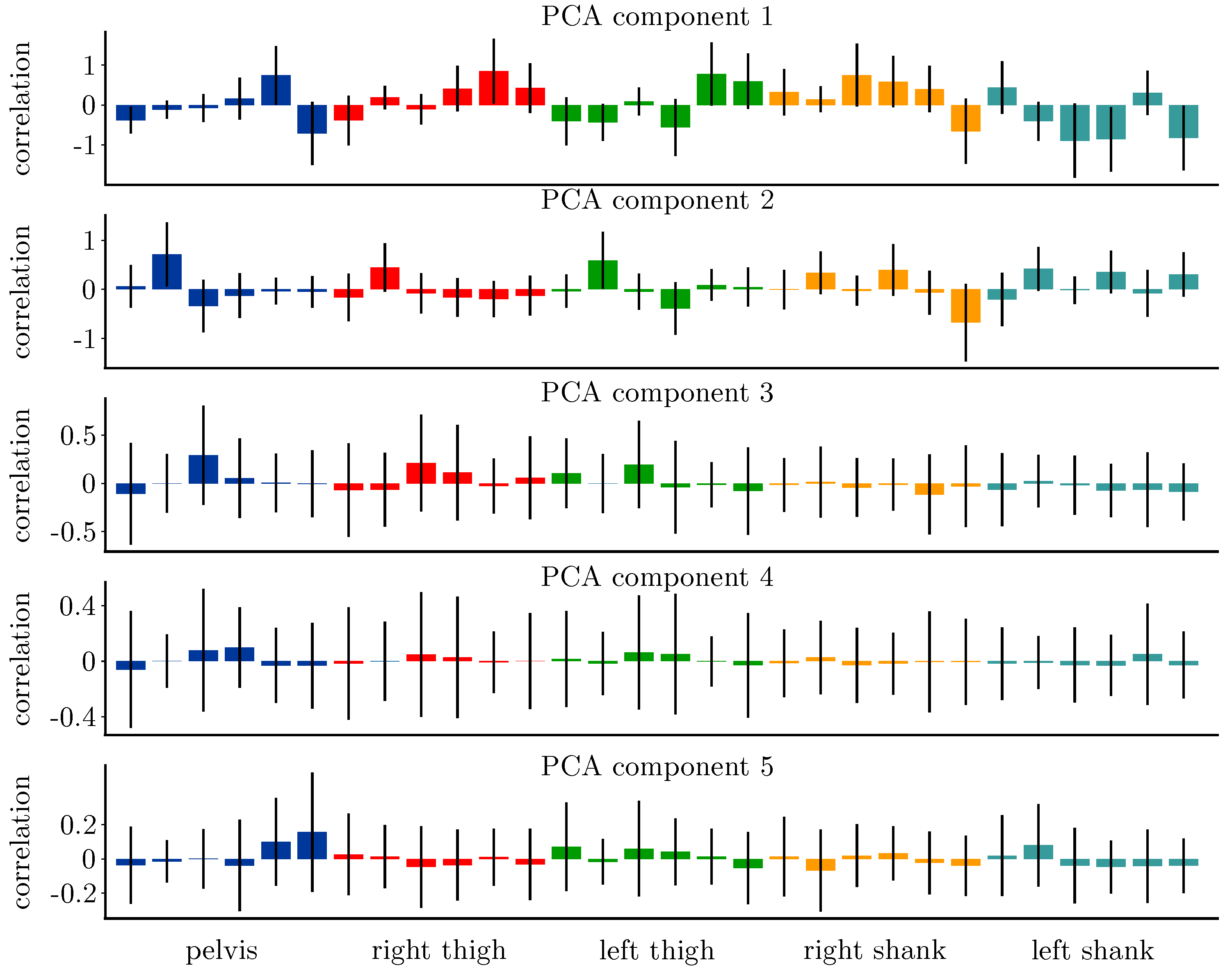
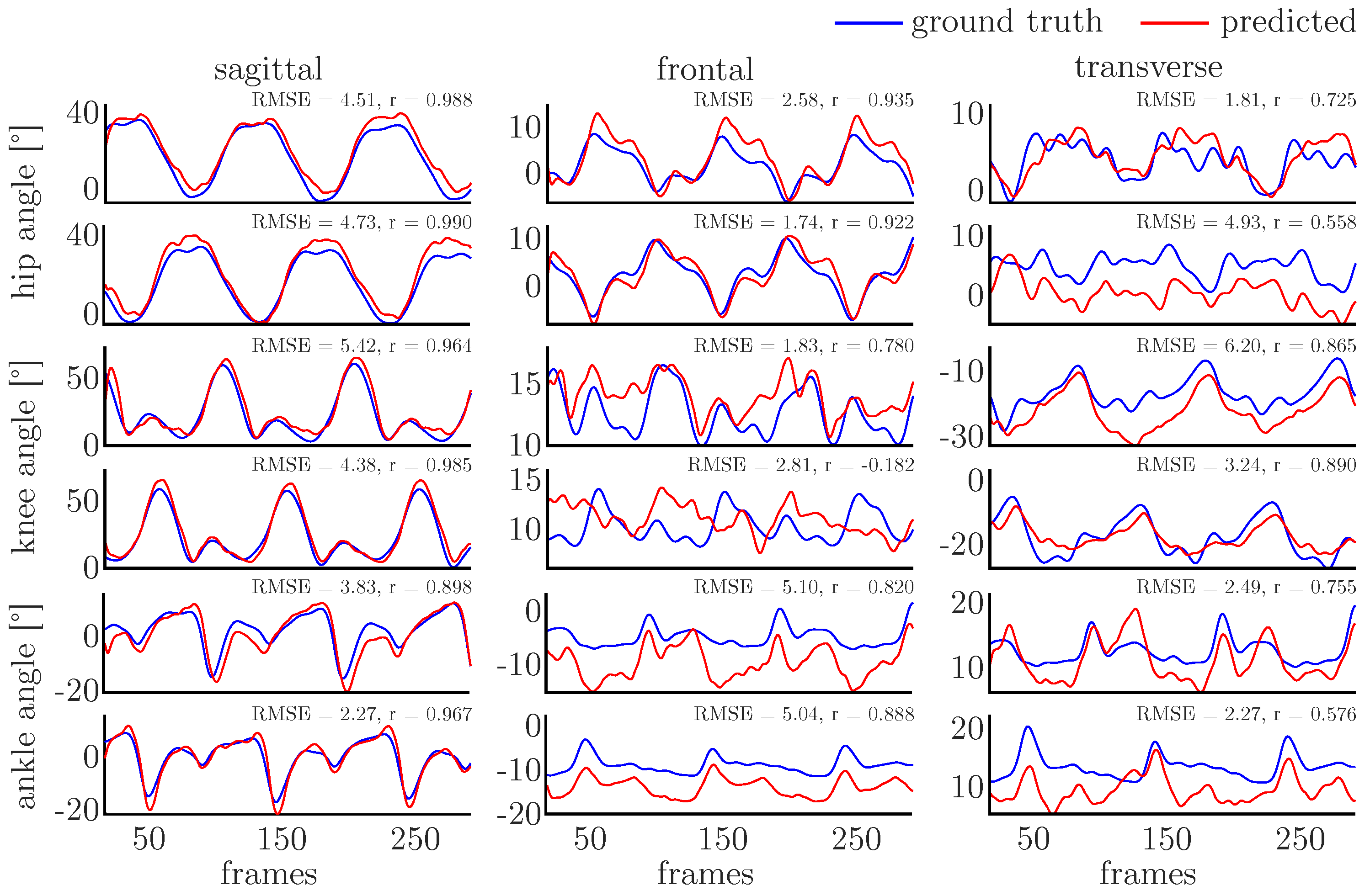


| CV | No. of Training Subjects (Samples) | No. of Validation Subjects (Samples) | No. of Test Subjects (Samples) |
|---|---|---|---|
| 1 | 73 (56,764) | 15 (14,398) | 27 (16,905) |
| 2 | 70 (60,145) | 18 (11,017) | 27 (16,905) |
| 3 | 71 (59,823) | 17 (11,339) | 27 (16,905) |
| 4 | 73 (57,270) | 15 (13,892) | 27 (16,905) |
| 5 | 73 (58,190) | 15 (12,972) | 27 (16,905) |
| PCA-Net | PTS-Net | PT-Net | PS-Net | P-Net | ||
|---|---|---|---|---|---|---|
| hip | sagittal | 0.891 (0.047) | 0.985 (0.006) | 0.988 (0.007) | 0.989 (0.006) | 0.979 (0.010) |
| frontal | 0.747 (0.104) | 0.935 (0.034) | 0.938 (0.031) | 0.942 (0.032) | 0.908 (0.037) | |
| transverse | 0.408 (0.229) | 0.677 (0.207) | 0.653 (0.190) | 0.646 (0.188) | 0.459 (0.238) | |
| knee | sagittal | 0.925 (0.032) | 0.992 (0.003) | 0.990 (0.004) | 0.993 (0.003) | 0.978 (0.007) |
| frontal | 0.934 (0.043) | 0.947 (0.036) | 0.940 (0.039) | 0.950 (0.032) | 0.928 (0.052) | |
| transverse | 0.894 (0.072) | 0.929 (0.059) | 0.926 (0.057) | 0.936 (0.054) | 0.913 (0.063) | |
| ankle | sagittal | 0.760 (0.088) | 0.927 (0.035) | 0.920 (0.040) | 0.937 (0.033) | 0.873 (0.050) |
| frontal | 0.941 (0.023) | 0.952 (0.041) | 0.956 (0.022) | 0.965 (0.018) | 0.938 (0.036) | |
| transverse | 0.934 (0.036) | 0.947 (0.041) | 0.951 (0.029) | 0.958 (0.028) | 0.939 (0.033) |
| PCA-Net | PTS-Net | PT-Net | PS-Net | P-Net | ||
|---|---|---|---|---|---|---|
| hip | sagittal | 4.11 (0.96) | 1.74 (0.54) | 1.70 (0.58) | 1.62 (0.55) | 2.31 (0.62) |
| frontal | 1.67 (0.38) | 0.95 (0.28) | 0.91 (0.28) | 0.87 (0.30) | 1.16 (0.26) | |
| transverse | 2.77 (0.87) | 2.13 (0.86) | 2.13 (0.91) | 2.12 (0.92) | 2.72 (0.97) | |
| knee | sagittal | 4.60 (1.06) | 1.77 (0.38) | 1.98 (0.51) | 1.69 (0.44) | 2.97 (0.55) |
| frontal | 2.16 (0.71) | 1.58 (0.66) | 1.77 (0.82) | 1.54 (0.72) | 2.18 (0.85) | |
| transverse | 3.49 (1.10) | 2.62 (1.06) | 2.85 (1.25) | 2.48 (1.09) | 3.36 (1.27) | |
| ankle | sagittal | 2.49 (0.31) | 1.50 (0.36) | 1.58 (0.43) | 1.35 (0.37) | 2.01 (0.35) |
| frontal | 2.17 (0.56) | 1.71 (0.72) | 1.76 (0.72) | 1.51 (0.62) | 2.21 (0.72) | |
| transverse | 1.79 (0.38) | 1.39 (0.47) | 1.39 (0.48) | 1.21 (0.41) | 1.68 (0.47) |
| FFNN | LSTM | PS-Net | |||||
|---|---|---|---|---|---|---|---|
| RMSE | r | RMSE | r | RMSE | r | ||
| sagittal | 1.31 | 0.999 | 1.74 | 0.997 | 1.62 | 0.989 | |
| hip | frontal | 1.25 | 0.980 | 1.30 | 0.965 | 0.87 | 0.942 |
| transverse | 2.48 | 0.864 | 2.70 | 0.889 | 2.12 | 0.646 | |
| sagittal | 1.37 | 0.997 | 1.92 | 0.997 | 1.69 | 0.993 | |
| knee | frontal | 1.55 | 0.793 | 1.92 | 0.681 | 1.54 | 0.950 |
| transverse | 1.74 | 0.957 | 3.73 | 0.945 | 2.48 | 0.936 | |
| sagittal | 1.56 | 0.983 | 1.80 | 0.983 | 1.35 | 0.937 | |
| ankle | frontal | 1.31 | 0.892 | 1.35 | 0.912 | 1.51 | 0.965 |
| transverse | 1.76 | 0.891 | 2.14 | 0.920 | 1.21 | 0.958 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundt, M.; Koeppe, A.; Bamer, F.; David, S.; Markert, B. Artificial Neural Networks in Motion Analysis—Applications of Unsupervised and Heuristic Feature Selection Techniques. Sensors 2020, 20, 4581. https://doi.org/10.3390/s20164581
Mundt M, Koeppe A, Bamer F, David S, Markert B. Artificial Neural Networks in Motion Analysis—Applications of Unsupervised and Heuristic Feature Selection Techniques. Sensors. 2020; 20(16):4581. https://doi.org/10.3390/s20164581
Chicago/Turabian StyleMundt, Marion, Arnd Koeppe, Franz Bamer, Sina David, and Bernd Markert. 2020. "Artificial Neural Networks in Motion Analysis—Applications of Unsupervised and Heuristic Feature Selection Techniques" Sensors 20, no. 16: 4581. https://doi.org/10.3390/s20164581
APA StyleMundt, M., Koeppe, A., Bamer, F., David, S., & Markert, B. (2020). Artificial Neural Networks in Motion Analysis—Applications of Unsupervised and Heuristic Feature Selection Techniques. Sensors, 20(16), 4581. https://doi.org/10.3390/s20164581







