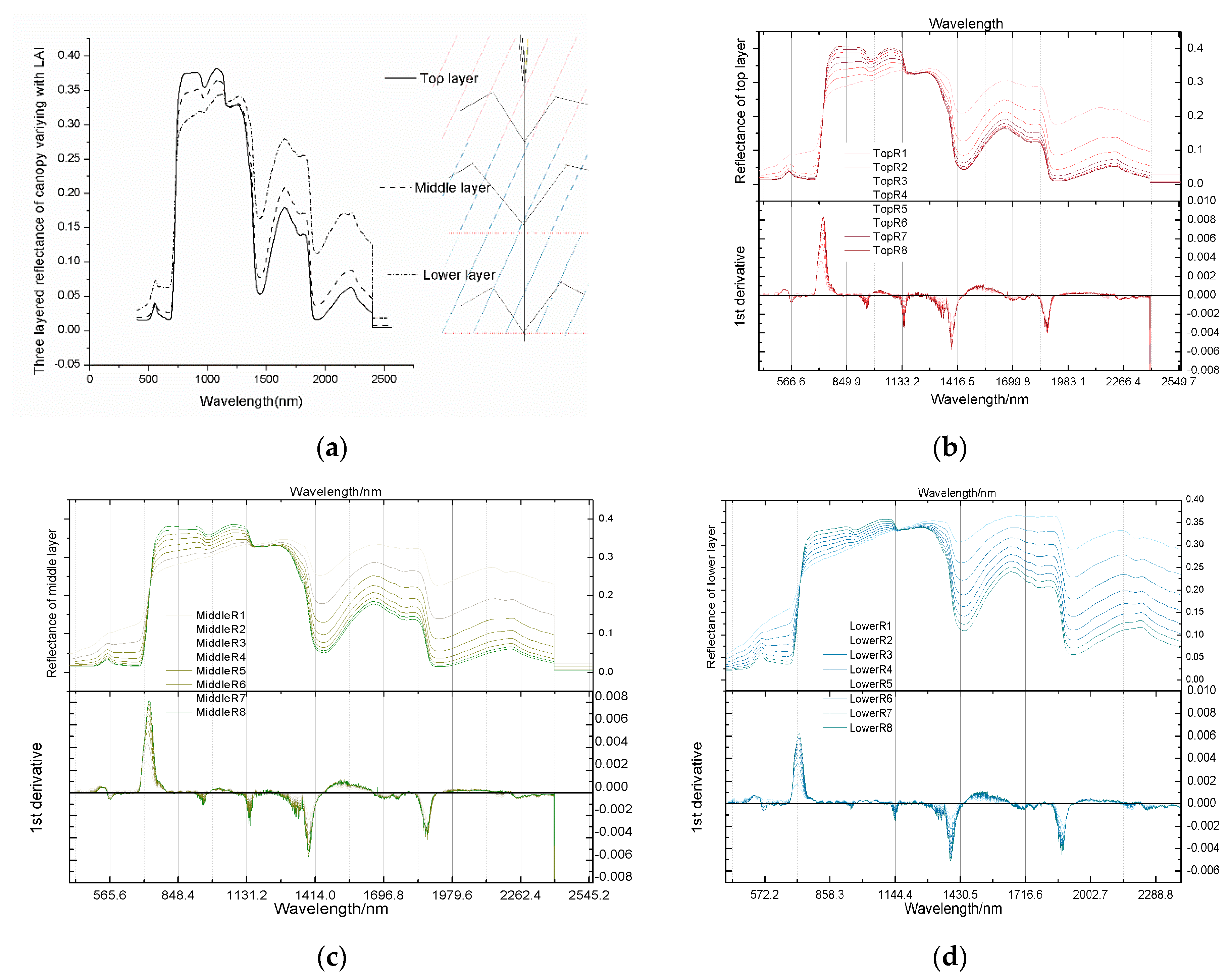
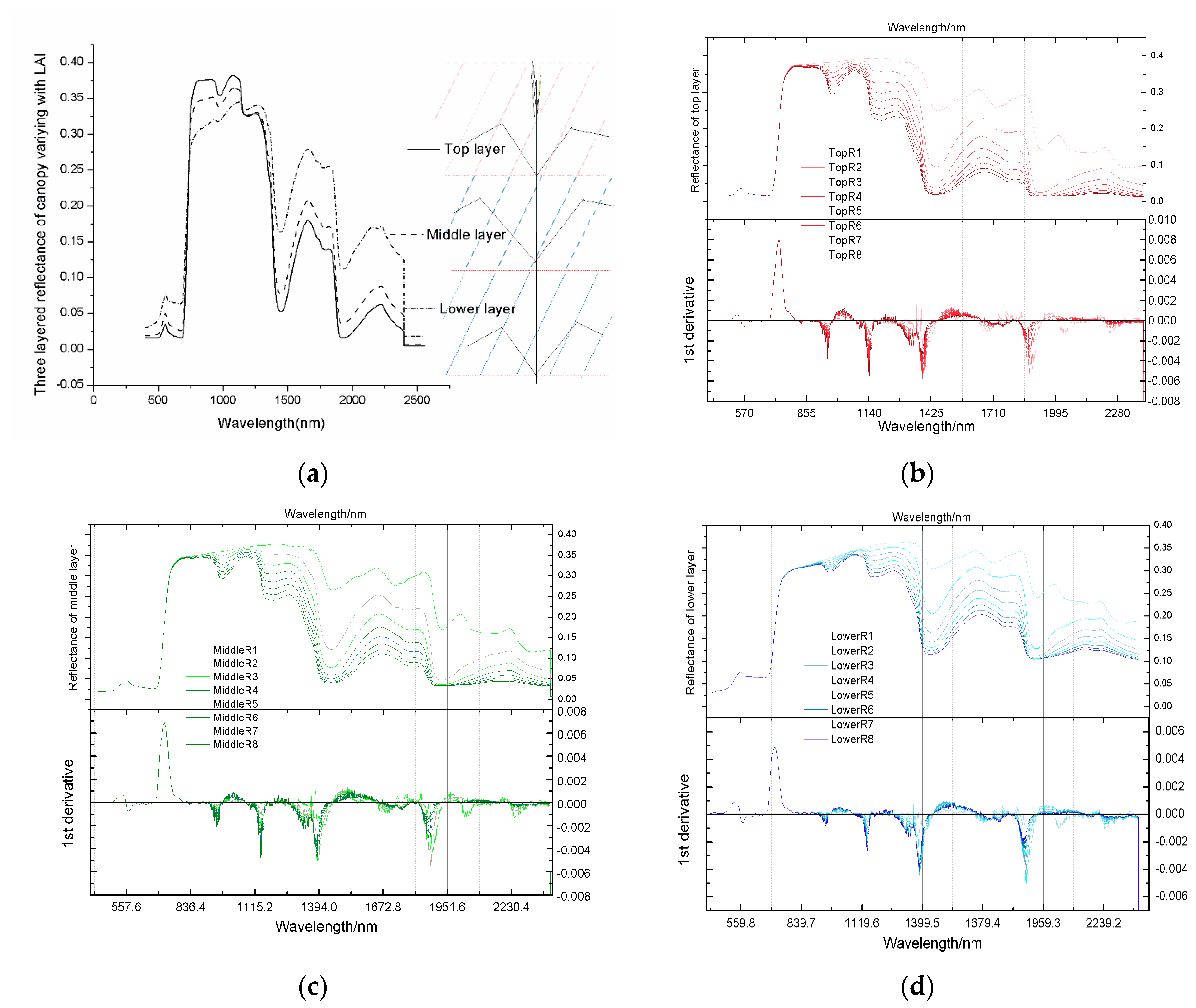
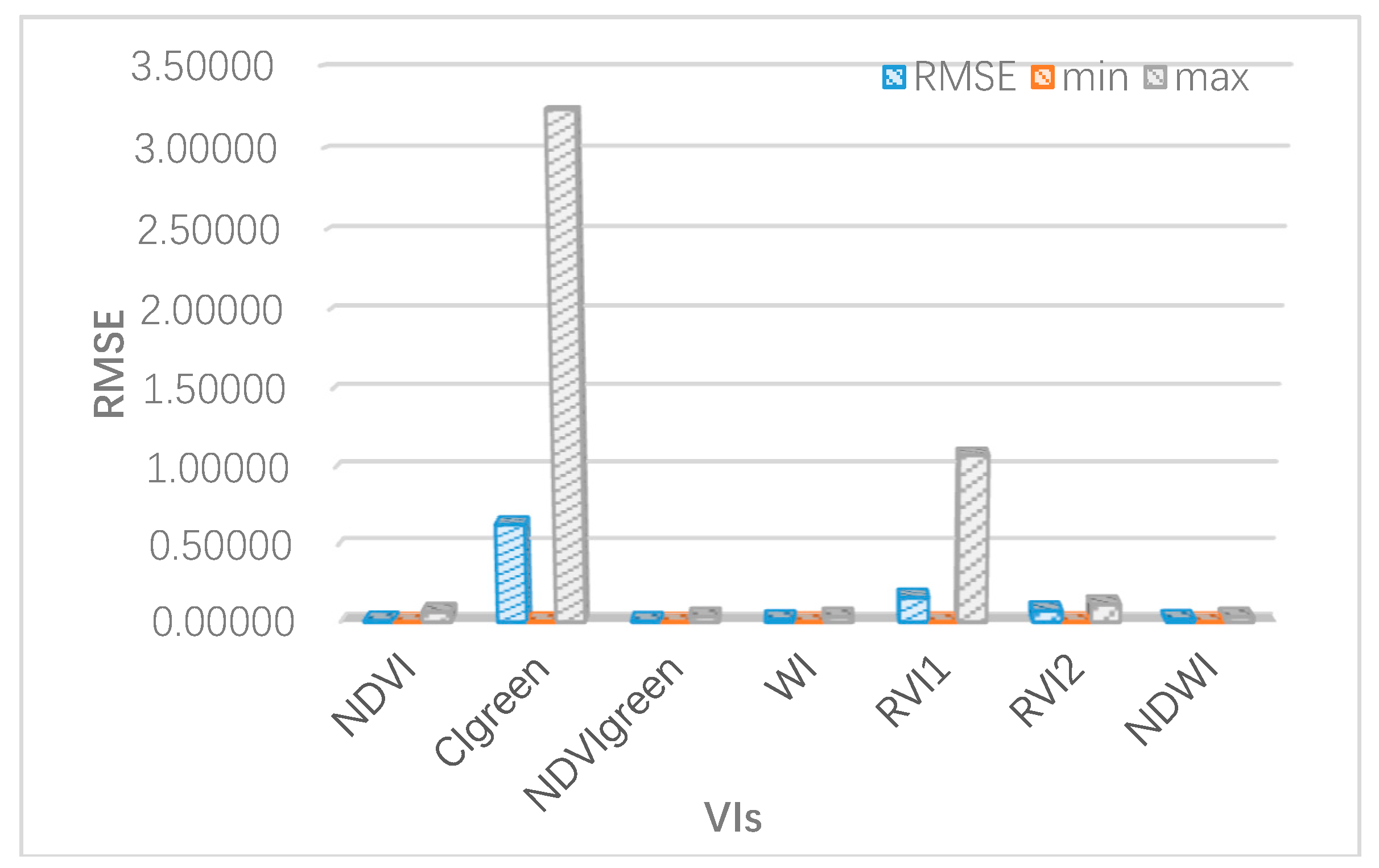
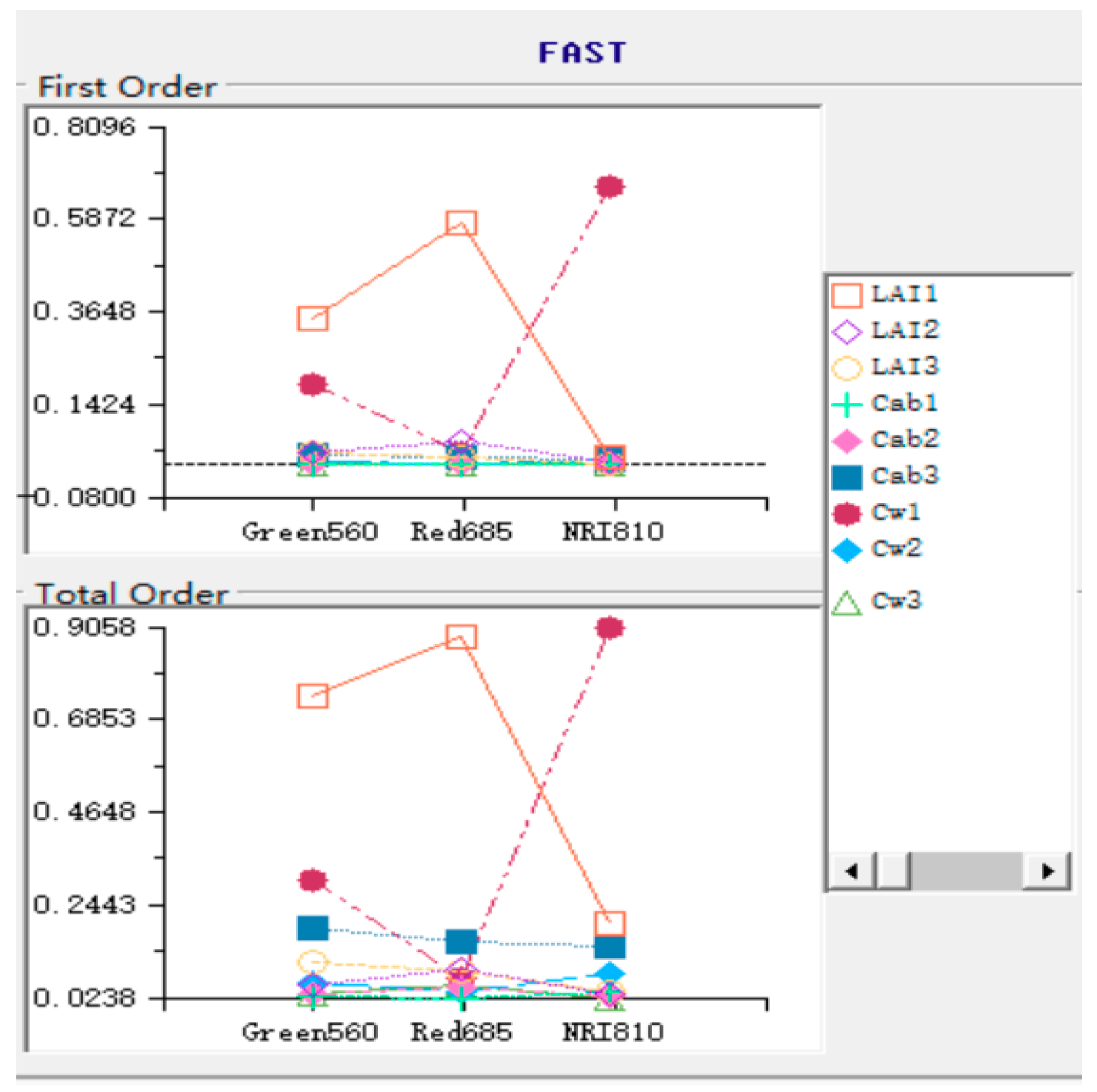
Vegetation modelling is a nondestructive technique for quantifying vegetation properties and analysing the physiological conditions of crops, such as growth potential and nutrition [
1]. The soil canopy observation, photochemistry, and energy fluxes (SCOPE) model simulates the transmission law of light in uniform canopy [
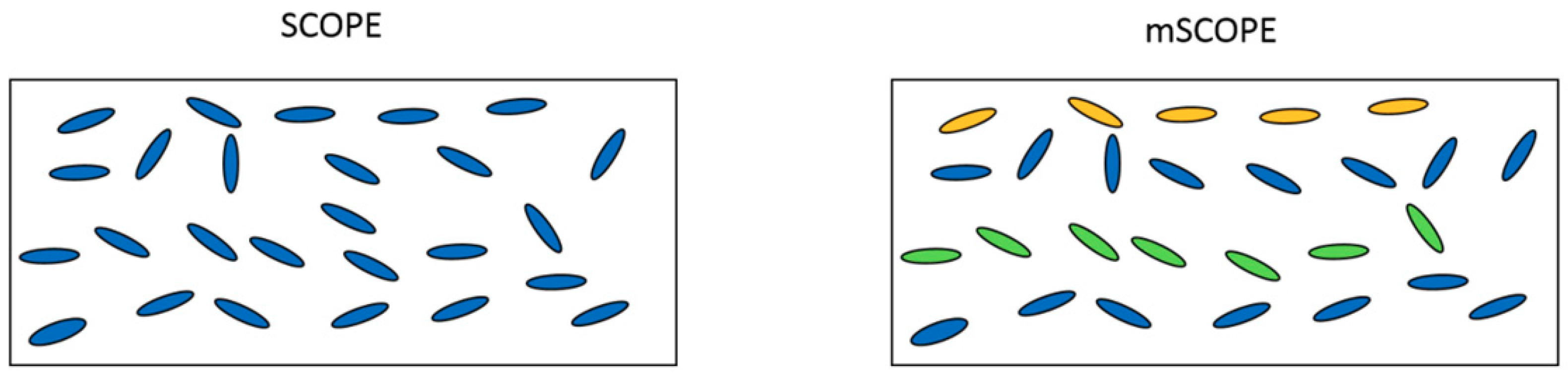
2]. However, the SCOPE model is a vertically integrated radiative transfer and energy balance model based on the classical 1-D SAIL model [
3,
4]. Disregarding vertical heterogeneity in vegetation canopy may negatively affect fluorescence and reflectivity spectra, a condition that leads to erroneous interpretation of the physiological characteristics of natural plants [
4]. In reality, the biochemical components of the canopy with 3D structure are not evenly distributed and exhibit vertical heterogeneity. Therefore, the influence of vertical heterogeneity on spectral response is not negligible [
5,
6,
7]. Furthermore, vertical heterogeneity may greatly influence the accuracy of actual growth detection and remote estimation of nutrient characteristics of crops [
8]. Zhao et al. [
8] also found that the chlorophyll, leaf moisture, and leaf area index (LAI) of winter wheat have vertical heterogeneity. Moreover, the physiological and biochemistry indices of crops and canopy are correlated with the spectra at visible light, near-infrared, and middle-infrared bands [
8,
9].
Remote sensing techniques are rapidly developing. These techniques allow time-effective and noninvasive data collection over large scales [
10,
11,
12,
13,
14]. Remote sensing techniques have two directions in estimating vegetation characteristics: one is an empirical statistical method for analysing the physiological variables and spectral vegetation indexes (VIs) of vegetation; the other involves the establishment of a physical canopy reflection model [
15]. Harnessing the advantages of spectral technology will allow the accurate and efficient monitoring and evaluation of the growth and yield of large-scale crops [
16]. However, spectral technology can obtain canopy spectra only. Combining the spectral data obtained by satellite and the vegetation model mentioned herein can invert the biochemical information of different layers in vegetation. On the basis of the SCOPE model, Yang et al. [
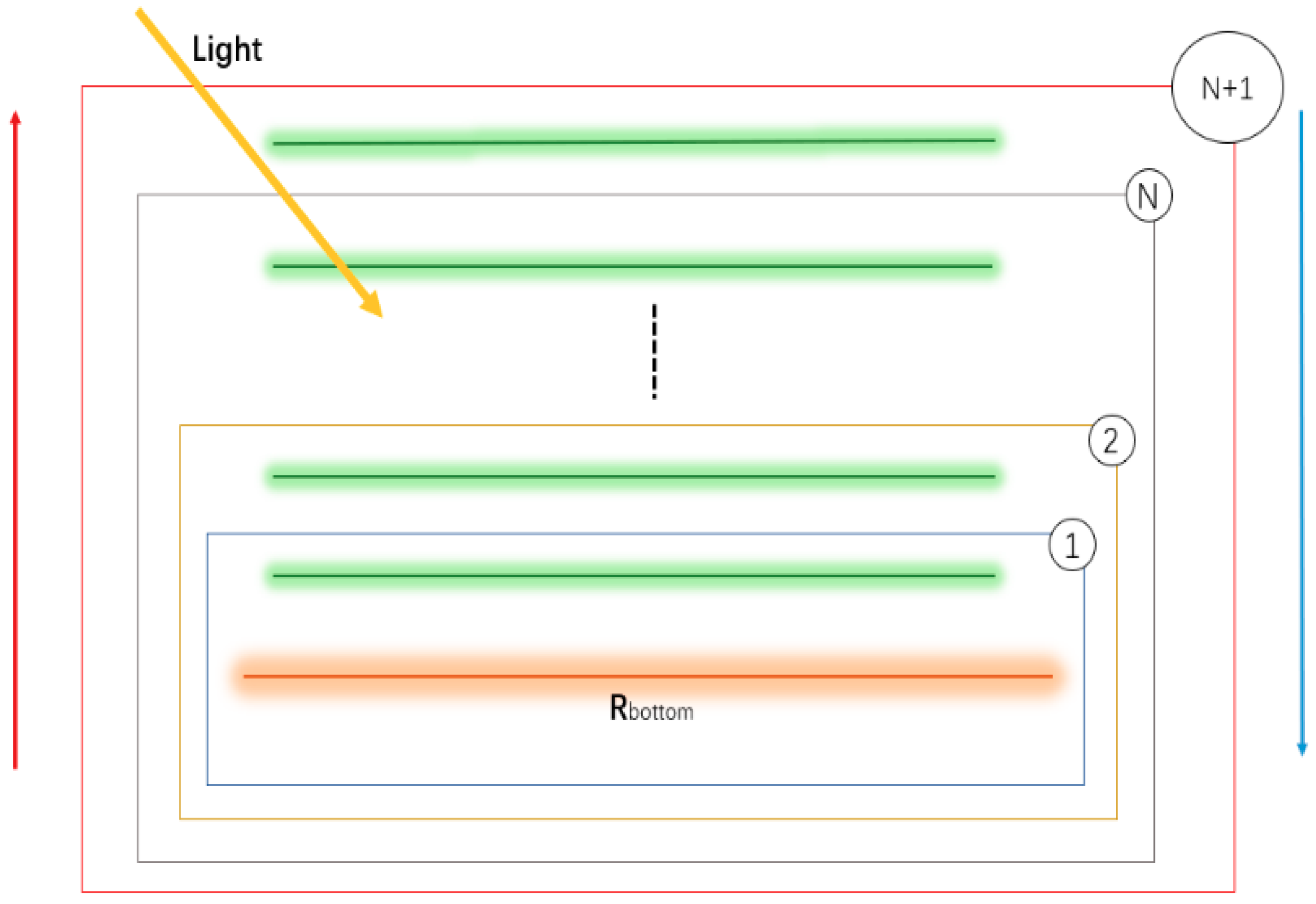
3] developed the mSCOPE model. This model adds vertical stratification of crops to explain better the correlations between remote sensing observations and plant functional traits. However, few studies using this multilayer model have been conducted on the spectral effects of crops on vertical biochemical contents. The interdependence between vegetation biophysical parameters and spectral data must be understood to infer crop status from spectral data that represent the shape of crops and the colour of their leaves [
17]. The laws of radiative transfer within vegetation can be obtained through empirical and theoretical studies [
17]. In the field, the statistical distribution of reflectivity denotes the comprehensive effects of canopy variables, soil environment, growth stage, and other factors. Numerous studies have approximated this reflectivity as a Gaussian distribution. However, in practice, this assumption is too harsh because the physiological distribution of the canopy is always not uniform. Leaf area index (LAI,
) [
16], chlorophyll (
,
) [
18], and vegetation water content (
,
) [
19] are important parameters used in characterizing photosynthesis, biomass, and evapotranspiration. Previous studies have demonstrated that many remote sensing spectral vegetation indexes have good correlation with LAI. Shibayama et al. [
20] confirmed the correlation between LAI and spectra. The normalized vegetation index (NDVI) is closely related to the biochemical parameters of crop canopy scale [
21]. Tan et al. [
16] found that the ratio of near-infrared to green light (reflectance at 810 and 560 nm) has a remarkable relationship with LAI. In consideration of the penetration characteristics of chlorophyll absorption feature bands in inner canopy, a combination of sensitive spectral bands (near-infrared band at 810 nm and green band at 560 nm) was selected to evaluate vertically layered chlorophyll [
22]. Previous studies that adopted hyperspectral technique in measuring the water content in the upper, middle, and lower layers of potato have revealed different spectral characteristic reflectance and water content distributions in different leaf positions [
19]. Previous works have observed good correlations between vegetation indexes and water content. Wang et al. [
23] found that the reflectivity ratios of 1450 and 1940 nm have a good effect on water condition estimation. Ceccato et al. [
24,
25] highlighted that the reflectance ratios of 1600 and 820 nm have a strong correlation with the equivalent water thickness of canopy leaves. Furthermore, sun-induced chlorophyll fluorescence (SIF) can track the photosynthetic activity of vegetation, and this information is important in determining plant stress and yield [
26]. The measurement of chlorophyll fluorescence is nondestructive and noninvasive [
27]. The effects of SIF on plant physiology can be monitored by exploring the relationship between reflectance and fluorescence spectrum and chlorophyll. The effects of chlorophyll concentration on chlorophyll fluorescence ratio (
) in intact leaves mainly depends on the reabsorption of fluorescence in the 685 nm band, which increases with the increase in chlorophyll concentration [
18]. Owing to the complexity of internal and external factors in the canopy [
28], the relationship between spectral characteristics of a single band and canopy variables is usually not universal. Converting vegetation index from spectral data, which always contain at least two or three spectral bands to minimize interference from external factors [
28], is simple with high computational efficiency, but it lacks universality. Thus, combining empirical methods and models is necessary. Klem et al. [
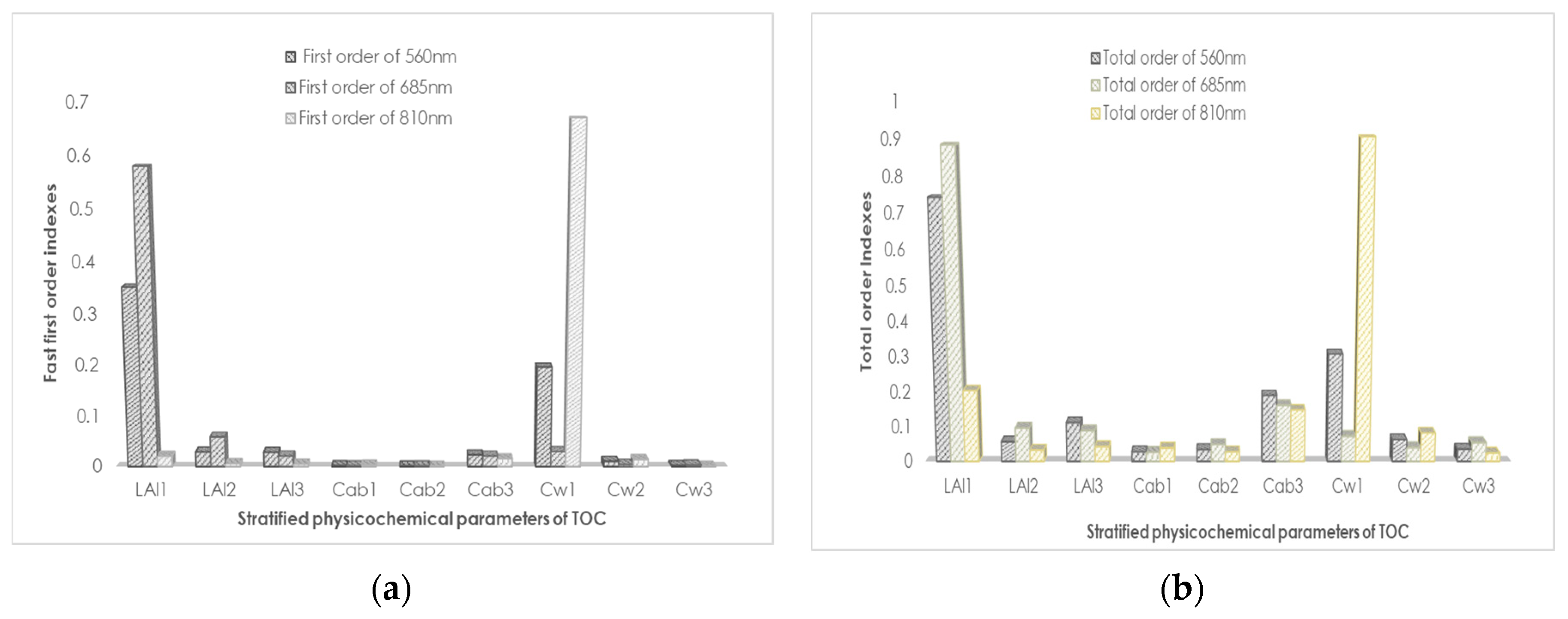
29] emphasized the influence of canopy vertical heterogeneity on spectral reflectivity by calculating the spectral index of spring wheat in different vertical parts. The mSCOPE model proposed by Yang et al. can be used as an effective tool to study the influence of vertical heterogeneity on canopy reflectance. On the basis of the understanding of the law of radiation transmission in the canopy, the authors extended the 1D model SCOPE to vertical stratification and simply verified the performance of the mSCOPE model in simulating canopy reflectivity. However, the model requires further verification to analyse the influence of various physiological parameters. The reliability and generalization of a model must be verified via sensitivity analysis before it is employed. On the basis of a global sensitivity analysis of selected stratified crop parameters via the Fourier amplitude sensitivity test (FAST), we discussed the sensitivity of these selected parameters to the spectrum of winter wheat [
30]. FAST is a sensitivity test method that converts all multidimensional integrals of the input of an uncertain model into one-dimensional integrals [
31,
32]. FAST mainly calculates the variances and means of results by combining the distribution ranges of input factors. The transformation from multidimensional to one-dimensional is essentially the sensitivity analysis of means and variances. First-order and global sensitivity analyses of the selected parameters were conducted to explore the executable ability in this model. Winter wheat, an important crop worldwide, was selected to test this model. The reflectivity and fluorescence spectra of winter wheat at different vertical strata were measured using the mSCOPE model. Furthermore, vegetation index was calculated from simulation data to analyse further the physiological state of vegetation. Sensitivity analysis (SA) helps in selecting the parameter factors that contribute most to the instability of model output. Furthermore, SA simplifies the difficulty of analysis and provides the necessary foundation and prerequisite for the subsequent simplification of model running time.
The purpose of this study is to explore the method of simulating the winter wheat spectrum with the mSCOPE model and verifying its rationality from multiple perspectives. Firstly, the appropriate number of layers was selected. Based on the spectral data generated by mSCOPE simulation, the correlation between vegetation index and spectrum was analysed. Then, the accuracy of vegetation index (VIs) inversion was evaluated using statistical indicators. Finally, SA was carried out to further verify the rationality of model operation and parameter selection. The rest of this study was organized as follows.
Section 2.1 introduced the parameterization of mSCOPE model;
Section 2.2 introduced the selection of vegetation index; in
Section 2.3, two scenarios were designed, which were used for selecting the optimal number of layers and for accuracy assessment using statistical indicators, respectively; in
Section 2.4, SA was applied to verify the accuracy.
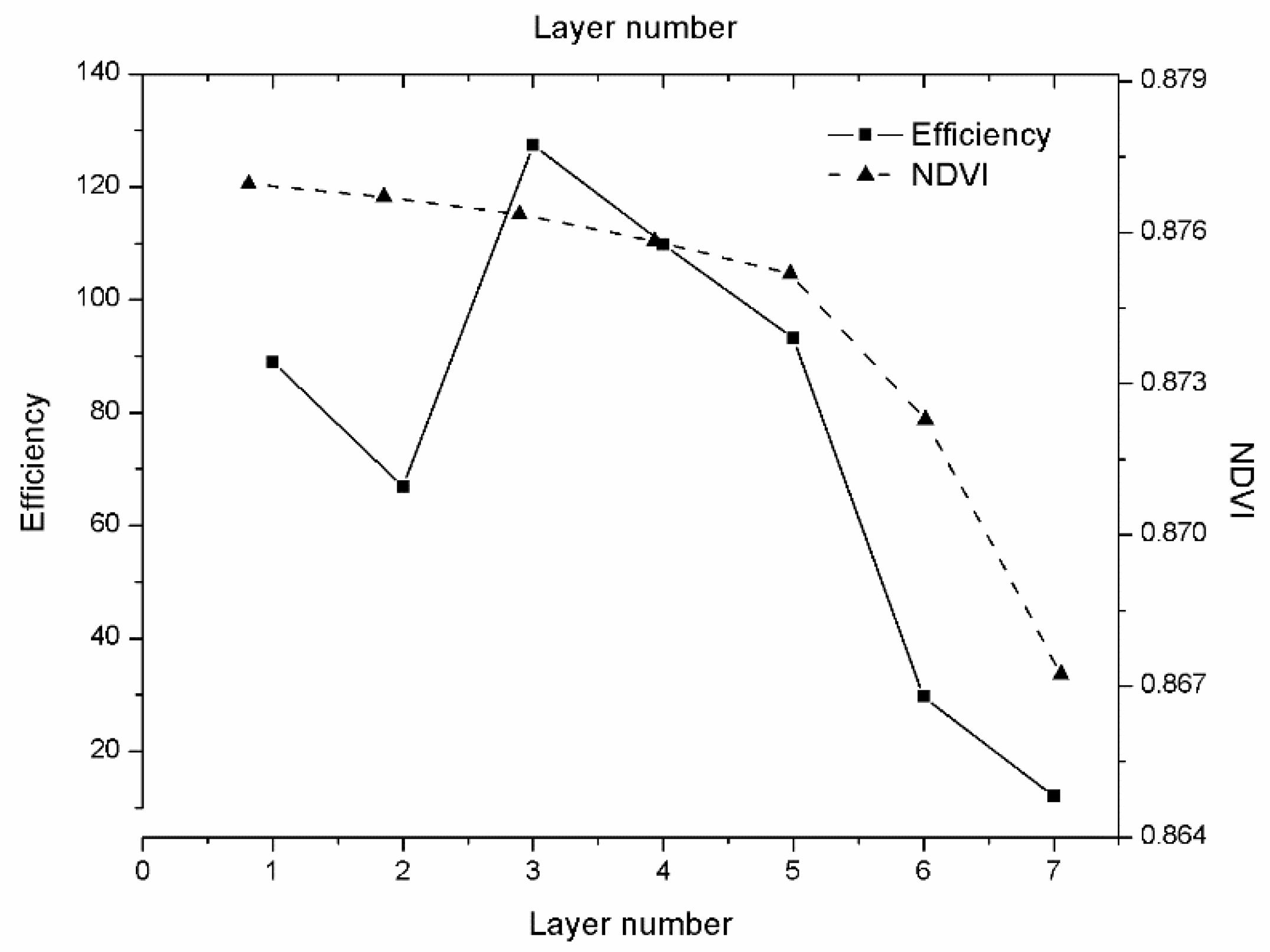
Section 3.1 gave the analysis results between simulated spectral and VIs; in
Section 3.2, the inversion accuracy was verified by field measured data;
Section 3.3 gave the SA results. In
Section 4, the strengths and weaknesses of the developed approach were analysed in detail.
Section 5 summarized the results of this study.