Blood Pressure Sensors: Materials, Fabrication Methods, Performance Evaluations and Future Perspectives
Abstract
1. Introduction
2. Blood Pressure Measurement
2.1. Invasive and Minimally Invasive Blood Pressure Measurement and Materials
2.2. Non-Invasive Blood Pressure Measurement and Materials
2.2.1. Full Occlusion
2.2.2. Semi Occlusion
2.2.3. No Occlusion
3. Transducing Modalities and Materials for Non-Invasive Blood Pressure Measurement
4. Sensing Principles
4.1. Piezoresistive
- The deformation in the composite geometry that may lead to changes in its length and cross-section area.
- The change in resistivity of the composite by changing the resistivity and/or volume of the conductive filler.
4.2. Pizocapacitive
4.3. Optical
4.4. Field Effect Transistor
4.5. Triboelectric
4.6. Sensor Performance
5. Sensor Building Blocks
5.1. Substrate
5.2. Active Materials
5.2.1. Carbon Compound
5.2.2. Graphene and Graphene Derivatives
5.2.3. Conducting Polymers
5.2.4. Emerging Low Dimensional Materials
5.2.5. Metal-Organic Frameworks and MXenes
5.3. Electrodes
5.4. Operational Lifecycle
5.4.1. Self-Cleaning
5.4.2. Self-Protection
5.4.3. Self-Diagnosis and Reporting
5.4.4. Self-Healing
5.4.5. Self-Degradation
6. Outlook
Funding
Conflicts of Interest
Acronyms and Abbreviations
| CB | Carbon Black. |
| CCE | Capacitive Coupled electrode, which is a non-contact electrode that works on the principle of capacitive charges between the user’s skin and the electrode. |
| CMC | Carboxymethyl Chitosan. |
| C-MOF | Carbonized Metal Organic Framework. |
| CNT | Carbon Nanotube. |
| CuTCNQ | Copper 7,7,8,8-tetracyano-p-quinodimethane. |
| CPH | Conducting polymer hydrogel. |
| CVD | Chemical Vapor Deposition. |
| DETA | Diethylenetriamine. |
| DGEBA | Diglycidyl ether of bisphenol A (Epon™ 8132). |
| DMSO | Dimethyl sulfoxide. |
| DN Hydrogel | Double Network Hydrogel. |
| DPP-DTT:PCBM | N-alkyl diketopyrrolo-pyrrole dithienylthienothiophene, and a fullerene derivative, phenyl-C61-butyric acid Methylester. |
| EmFi | Electromechanical Film. |
| EPDM | ethylene propylene diene monomer. |
| EToH | Ethanol. |
| F | Force. |
| FFR | Fractional Flow Reserve is a physiological index that invasively measures the ratio between distal and proximal pressure of stenosis at maximum hyperaemia. Also, it is considered a gold standard. |
| FFRCT | Fractional Flow Reserve based on Computed Tomography. |
| GF | Graphene Foam. |
| GO | Graphene Oxide. |
| GNS | Graphene Nanosheet. |
| GPN | Graphene Porous Network. |
| GS | Graphene Sponge. |
| LED | Light Emitting Diode. |
| LZT | Lead Zirconate Titanate. |
| MEMS | Micro-electromechanical System. |
| MOF-5 | Zn4O(BDC)3, where BDC =1,4-benzodicarboxylate. |
| MP | Microparticles. |
| m-PCL | poly(ɛ-caprolactone). |
| MWCNT | Multi-Walled Carbon Nanotube. |
| NITEC | Nitrile imine-mediated tetrazole-ene cycloaddition. |
| NIR | Near-Infrared. |
| NP | Nanoparticles. |
| NS | Nanosheet. |
| NW | Nanowire. |
| OLED | Organic Light Emitting Diode. |
| P | Pressure. |
| PA | Polyamide. |
| PAA | Polyacrylic acid. |
| PANI | Polyaniline. |
| PANIF | Polyaniline Nanofiber. |
| PANIPAm | Poly(N-isopropylacrylamide). |
| PBS | Polyborosiloxane |
| PCL | Polycaprolactone. |
| PDMS | Polydimethylsiloxane. |
| PdOx | Palladium Oxides. |
| PE | Poly(ethelene). |
| PEDOT: PSS | Poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate). |
| PEG | Poly(ethylene glycol). |
| PEN | Polyethylene Naphthalene. |
| PES | Polyether Sulfone. |
| PET | Polyethylene Terephthalate. |
| PETMP | Pentaerythritol tetrakis(3-mercaptopropionate). |
| PFDTS | 1H,1H,2H,2H-Perfluorodecyltrichlorosilane |
| PGS | Poly(glycerol sebacate). |
| PHB/PHV | Polyhydroxybutyrate/Polyhydroxyvalerate. |
| PI | Polyimide. |
| PLA | Polylactic Acid. |
| PLGA | Poly(lactic-co-glycolic acid). |
| PMMA | Polymethylmethacrylate. |
| POE | Polyolefin Elastomer. |
| POMaC | Poly(octamethyle nemaleate (anhydride) citrate). |
| PP | Polypropylene. |
| PPy | Polypyrrole. |
| PRF | Passive Radio Frequency. |
| PSR | Pressure Sensitive Rubber. |
| PTFE | Polytetrafluoroethylene. |
| PU | Polyurethane. |
| PUD | Polyurethane Dispersion. |
| PVA | Polyvinyl Alcohol. |
| PVDF | Polyvinylidene fluoride. |
| PVDF-HFP | Poly(vinylidene fluoride)-co-Hexafluoropropylene. |
| P(VDF-TrFe) | Poly(vinylidene fluoride-co-trifluoroethylene). |
| rGO | Reduced Graphene Oxide. |
| SEM | Scanning Electron Microscope. |
| sh-crl-PU | Disulfide-cross-linked polyurethane. |
| SP | Spiropyran |
| S-R | Self -Resonant. |
| SWCNT | Single-Walled Carbon Nanotube. |
| TAA | Terephthalaldehyde. |
| T-CVD | Thermal -Chemical Vapor Deposition. |
| TEMPO | 2, 2, 6, 6-tetrametylpiperidine-1-oxyl. |
| TETA | triethylenetetramine. |
| TOCNF | TEMPO-oxidized cellulose nanofiber. |
| TPU | Thermoplastic polyurethane. |
| Triton X-100 | Polyethylene glycol p-(1,1,3,3-tetramethylbutyl)-phenyl ether. |
| TREN | Tris(2-aminoethyl)amine. |
| TTT | 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione. |
| VHB | Very High Bond. |
References
- World Health Organization. World Health Organization Cardiovascular Diseases (CVDs) Report; World Health Organization (WHO): Geneva, Switzerland, 2019. [Google Scholar]
- COVID-19 Surveillance Group Characteristics of COVID-19 Patients dying in Italy Report Based on Available Data on 24 March 2020; The Italian National Health Service: Rome, Italy, 27 March 2020.
- World Health Organization (WHO)-China Joint Mission. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19); World Health Organization (WHO): Geneva, Switzerland, 28 February 2020. [Google Scholar]
- Kovacs, R.J.; Moyer, D.V. Statement on the Need for Increased Access to Telehealth to Combat Community Spread of COVID-19; American College of Cardiology and American College of Physicians: Washington, DC, USA, 2020; p. 1. [Google Scholar]
- Perl, T.M.; Price, C.S. Managing emerging infectious diseases: Should travel be the fifth vital sign? Ann. Intern. Med. 2020, 45, 235. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- RenJi, H. Early Detection of Cardiac Impairment and Prediction of RV Hypertrophy in Patients With CTD. Available online: https://ClinicalTrials.gov/show/NCT04297371 (accessed on 4 August 2020).
- Sierra, C.; de la Sierra, A. Early detection and management of the high-risk patient with elevated blood pressure. Vasc. Health Risk Manag. 2008, 4, 289–296. [Google Scholar] [CrossRef]
- Handler, J. The importance of accurate blood pressure measurement. Perm. J. 2009, 13, 51–54. [Google Scholar] [CrossRef]
- Poncette, A.S.; Spies, C.; Mosch, L.; Schieler, M.; Weber-Carstens, S.; Krampe, H.; Balzer, F. Clinical Requirements of Future Patient Monitoring in the Intensive Care Unit: Qualitative Study. JMIR Med. Inform. 2019, 7, e13064. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Zhou, Z.; Padgett, S.; Cai, Z.; Conta, G.; Wu, Y.; He, Q.; Zhang, S.; Sun, C.; Liu, J.; Fan, E.; et al. Single-layered ultra-soft washable smart textiles for all-around ballistocardiograph, respiration, and posture monitoring during sleep. Biosens. Bioelectron. 2020, 155, 112064. [Google Scholar] [CrossRef]
- Lisi, F.; Peterson, J.R.; Gooding, J.J. The application of personal glucose meters as universal point-of-care diagnostic tools. Biosens. Bioelectron. 2020, 148, 111835. [Google Scholar] [CrossRef]
- Shandilya, R.; Bhargava, A.; Bunkar, N.; Tiwari, R.; Goryacheva, I.Y.; Mishra, P.K. Nanobiosensors: Point-of-care approaches for cancer diagnostics. Biosens. Bioelectron. 2019, 130, 147–165. [Google Scholar] [CrossRef]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, P.; Erenas, M.M.; Martinez-Olmos, A.; Carvajal, M.A.; Gonzalez-Chocano, S.; Capitan-Vallvey, L.F.; Palma, A.J. General-purpose passive wireless point-of-care platform based on smartphone. Biosens. Bioelectron. 2019, 141, 111360. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Mei, Q.; Tao, Z.; Wu, H.; Zhao, M.; Wang, S.; Liu, Y. A smartphone-integrated ratiometric fluorescence sensing platform for visual and quantitative point-of-care testing of tetracycline. Biosens. Bioelectron. 2020, 148, 111791. [Google Scholar] [CrossRef] [PubMed]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoci, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89 Pt 2, 886–898. [Google Scholar] [CrossRef]
- Song, Y.; Min, J.; Gao, W. Wearable and Implantable Electronics: Moving toward Precision Therapy. ACS Nano 2019, 13, 12280–12286. [Google Scholar] [CrossRef]
- Iftikhar, Z.; Lahdenoja, O.; Jafari Tadi, M.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Multiclass classifier based cardiovascular condition detection using smartphone mechanocardiography. Sci. Rep. 2018, 8, 9344. [Google Scholar] [CrossRef]
- Pfeiffer, S. The Vision of Industrie 4.0 in the making-a case of future told, tamed, and traded. Nanoethics 2017, 11, 107–121. [Google Scholar] [CrossRef]
- Schwab, K. The Fourth Industrial Revolution; World Economic Forum: Cologny, Switzerland, 2016. [Google Scholar]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Chrysant, S.G. A new paradigm in the treatment of the cardiovascular disease continuum: Focus on prevention. Hippokratia 2011, 15, 7–11. [Google Scholar]
- Engel, J.; van der Wulp, I.; Poldervaart, J.M.; Reitsma, J.B.; de Bruijne, M.C.; Wagner, C. Clinical decision-making of cardiologists regarding admission and treatment of patients with suspected unstable angina or non-ST-elevation myocardial infarction: Protocol of a clinical vignette study. BMJ Open 2015, 5, e006441. [Google Scholar] [CrossRef]
- Karunathilake, S.P.; Ganegoda, G.U. Secondary prevention of cardiovascular diseases and application of technology for early diagnosis. Biomed. Res. Int. 2018, 2018, 5767864. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C., Jr.; McMahan, C.A.; Gidding, S.S. Preventing heart disease in the 21st century: Implications of the pathobiological determinants of Atherosclerosis in youth (PDAY) study. Circulation 2008, 117, 1216–1627. [Google Scholar] [CrossRef] [PubMed]
- D’Addona, D.M.; Rongo, R.; Teti, R.; Martina, R. Bio-compatible cyber-physical system for cloud-based customizable sensor monitoring of pressure conditions. Proc. CIRP 2018, 67, 150–155. [Google Scholar] [CrossRef]
- Seminara, L.; Pinna, L.; Ibrahim, A.; Noli, L.; Capurro, M.; Caviglia, S.; Gastaldo, P.; Valle, M. Electronic Skin: Achievements, Issues and Trends. Proc. Technol. 2014, 15, 549–558. [Google Scholar] [CrossRef]
- Shimonomura, K. tactile image sensors employing camera: A review. Sensors 2019, 19, 3933. [Google Scholar] [CrossRef]
- Xu, S.; Jayaraman, A.; Rogers, J.A. Skin sensors are the future of health care. Nature 2019, 571, 319–321. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef]
- Chung, H.U.; Kim, B.H.; Lee, J.Y.; Lee, J.; Xie, Z.; Ibler, E.M.; Lee, K.; Banks, A.; Jeong, J.Y.; Kim, J.; et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019, 363, eaau0780. [Google Scholar] [CrossRef]
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-enabled wearable sensors for healthcare. Adv. Healthcare Mater. 2018, 7, 1700889. [Google Scholar] [CrossRef]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-Integrated wearable systems: A comprehensive review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Unal, B.; Khademhosseini, A.; Butt, H. Wearables in medicine. Adv. Mater. 2018, 30, e1706910. [Google Scholar] [CrossRef] [PubMed]
- Dooley, E.E.; Golaszewski, N.M.; Bartholomew, J.B. Estimating Accuracy at exercise intensities: A comparative study of self-monitoring heart rate and physical activity wearable devices. JMIR M.Health UHealth 2017, 5, e34. [Google Scholar] [CrossRef] [PubMed]
- Izmailova, E.S.; Wagner, J.A.; Perakslis, E.D. Wearable devices in clinical trials: Hype and hypothesis. Clin. Pharmacol. Ther. 2018, 104, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef]
- Kaisti, M.; Panula, T.; Leppanen, J.; Punkkinen, R.; Jafari Tadi, M.; Vasankari, T.; Jaakkola, S.; Kiviniemi, T.; Airaksinen, J.; Kostiainen, P.; et al. Clinical assessment of a non-invasive wearable MEMS pressure sensor array for monitoring of arterial pulse waveform, heart rate and detection of atrial fibrillation. NPJ Digit. Med. 2019, 2, 39. [Google Scholar] [CrossRef]
- Duking, P.; Fuss, F.K.; Holmberg, H.C.; Sperlich, B. Recommendations for assessment of the reliability, sensitivity, and validity of data provided by wearable sensors designed for monitoring physical activity. JMIR MHealth UHealth 2018, 6, e102. [Google Scholar] [CrossRef]
- Magder, S. The meaning of blood pressure. Crit. Care 2018, 22, 257. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; O’Rourke, M.; Nichols, W.W. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kleinstreuer, C. Biofluid Dynamics: Principles And Selected Applications; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83. [Google Scholar] [CrossRef]
- Ogedegbe, G.; Pickering, T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef]
- Kim, C.S.; Ober, S.L.; McMurtry, M.S.; Finegan, B.A.; Inan, O.T.; Mukkamala, R.; Hahn, J.O. Ballistocardiogram: Mechanism and potential for unobtrusive cardiovascular health monitoring. Sci. Rep. 2016, 6, 31297. [Google Scholar] [CrossRef]
- Varghees, V.N.; Ramachandran, K.I. A novel heart sound activity detection framework for automated heart sound analysis. Biomed. Sign. Process. Control 2014, 13, 174–188. [Google Scholar] [CrossRef]
- Chowdhury, M.H.; Cheung, R.C.C. Reconfigurable architecture for multi-lead ecg signal compression with high-frequency noise reduction. Sci. Rep. 2019, 9, 17233. [Google Scholar] [CrossRef] [PubMed]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central blood pressure: Current evidence and clinical importance. Eur. Heart J. 2014, 35, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, S.; Ricci, Z.; Quattrone, D.; Tofani, L.; Tujjar, O.; Villa, G.; Romano, S.M.; De Gaudio, A.R. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: An observational study. Crit. Care 2014, 18, 644. [Google Scholar] [CrossRef] [PubMed]
- Crystal, G.J.; Assaad, S.I.; Heerdt, P.M. Cardiovascular Physiology. In Pharmacology and Physiology for Anesthesia; Hemmings, H.C., Egan, T.D., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 473–519. [Google Scholar]
- Gaukroger, P.B.; Roberts, J.G.; Manners, T.A. Infusion thrombophlebitis: A prospective comparison of 645 Vialon and Teflon cannulae in anaesthetic and postoperative use. Anaesth. Intens. Care 1988, 16, 265–271. [Google Scholar] [CrossRef]
- Pizzoferrato, A.; Arciola, C.R.; Cenni, E.; Ciapetti, G.; Sassi, S. In vitro biocompatibility of a polyurethane catheter after deposition of fluorinated film. Biomaterials 1995, 16, 361–367. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, S.; Marsh, N.; Ray-Barruel, G.; Flynn, J.; Larsen, E.; Rickard, C.M. Infection risks associated with peripheral vascular catheters. J. Infect. Prev. 2016, 17, 207–213. [Google Scholar] [CrossRef]
- Lambert, J.M.; Lee, M.-S.; Taller, R.A.; Solomon, D.D. Medical grade tubing: Criteria for catheter applications. J. Vinyl Addit. Technol. 1991, 13, 204–207. [Google Scholar] [CrossRef]
- Cohen, A.B.; Dagli, M.; Stavropoulos, S.W., Jr.; Mondschein, J.I.; Soulen, M.C.; Shlansky-Goldberg, R.D.; Solomon, J.A.; Chittams, J.L.; Trerotola, S.O. Silicone and polyurethane tunneled infusion catheters: A comparison of durability and breakage rates. J. Vasc. Interv. Radiol. 2011, 22, 638–641. [Google Scholar] [CrossRef]
- Wall, C.; Moore, J.; Thachil, J. Catheter-related thrombosis: A practical approach. J. Intens. Care Soc. 2016, 17, 160–167. [Google Scholar] [CrossRef]
- Auffan, M.; Santaella, C.; Thiéry, A.; Paillès, C.; Rose, J.; Achouak, W.; Thill, A.; Masion, A.; Wiesner, M.; Bottero, J.-Y. Electrostatic MEMS microphones. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 775–783. [Google Scholar]
- Meena, K.V.; Mathew, R.; Sankar, A.R. Design and optimization of a three-terminal piezoresistive pressure sensor for catheter based in vivo biomedical applications. Biomed. Phys. Eng. Express 2017, 3, 045003. [Google Scholar] [CrossRef]
- Allen, H.; Ramzan, K.; Knutti, J.; Withers, S. A novel ultra-miniature catheter tip pressure sensor fabricated using silicon and glass thinning techniques. MRS Proc. 2011, 681, I7.4. [Google Scholar] [CrossRef]
- Hasenkamp, W.; Forchelet, D.; Pataky, K.; Villard, J.; Van Lintel, H.; Bertsch, A.; Wang, Q.; Renaud, P. Polyimide/SU-8 catheter-tip MEMS gauge pressure sensor. Biomed. Microdev. 2012, 14, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Pandya, H.J.; Sheng, J.; Desai, J.P. MEMS-based flexible force sensor for tri-axial catheter contact force measurement. J. Microelectromech. Syst. 2017, 26, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Dario, P.; De Rossi, D.; Bedini, R.; Francesconi, R.; Trivella, M.G. PVF2catheter-tip transducers for pressure, sound and flow measurements. Ferroelectrics 2011, 60, 149–162. [Google Scholar] [CrossRef]
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57. [Google Scholar] [CrossRef]
- Qingsong, X.; Guoxing, W.; Zhengchun, P. Machine Learning Methods for Real-Time Blood Pressure Measurement Based on Photoplethysmography. In Proceedings of the 2018 IEEE 23rd International Conference on Digital Signal Processing (DSP) Digital Signal Processing (DSP), Shanghai, China, 19–21 November 2018. [Google Scholar]
- Versi, E. Gold standard is an appropriate term. BMJ 1992, 305, 187. [Google Scholar] [CrossRef]
- Pour-Ghaz, I.; Manolukas, T.; Foray, N.; Raja, J.; Rawal, A.; Ibebuogu, U.N.; Khouzam, R.N. Accuracy of non-invasive and minimally invasive hemodynamic monitoring: Where do we stand? Ann. Transl. Med. 2019, 7, 421. [Google Scholar] [CrossRef]
- Murphy, O.H.; Bahmanyar, M.R.; Borghi, A.; McLeod, C.N.; Navaratnarajah, M.; Yacoub, M.H.; Toumazou, C. Continuous in vivo blood pressure measurements using a fully implantable wireless SAW sensor. Biomed. Microdev. 2013, 15, 737–749. [Google Scholar] [CrossRef]
- Melki, S.; Todani, A.; Cherfan, G. An implantable intraocular pressure transducer: Initial safety outcomes. JAMA Ophthalmol. 2014, 132, 1221–1225. [Google Scholar] [CrossRef]
- Kawoos, U.; McCarron, R.M.; Auker, C.R.; Chavko, M. Advances in intracranial pressure monitoring and its significance in managing traumatic brain injury. Int. J. Mol. Sci. 2015, 16, 28979–28997. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Brox, D.; Assadsangabi, B.; Mohamed Ali, M.S.; Takahata, K. A stainless-steel-based implantable pressure sensor chip and its integration by microwelding. Sens. Actuators A Phys. 2017, 257, 134–144. [Google Scholar] [CrossRef]
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; MacEwan, M.R.; Huang, Y.; et al. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci. Adv. 2019, 5, eaaw1899. [Google Scholar] [CrossRef]
- Potkay, J.A. Long term, implantable blood pressure monitoring systems. Biomed. Microdev. 2008, 10, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Benmira, A.; Perez-Martin, A.; Schuster, I.; Aichoun, I.; Coudray, S.; Bereksi-Reguig, F.; Dauzat, M. From Korotkoff and Marey to automatic non-invasive oscillometric blood pressure measurement: Does easiness come with reliability? Exp. Rev. Med. Dev. 2016, 13, 179–189. [Google Scholar] [CrossRef]
- Raamat, R.; Talts, K.J.J.; Kivastik, J. A model-based retrospective analysis of the fixed-ratio oscillometric blood pressure measurement. In Proceedings of the 13th IEEE International Conference on BioInformatics and BioEngineering (IEEE BIBE 2013), Chania, Greece, 10–13 November 2013. [Google Scholar]
- Dinesh, S.; M, B.; Charles, F.; Upendra, K.; Subramaniam, N.; Jamshed, D. Palpatory method of measuring diastolic blood pressure. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 528. [Google Scholar]
- Odagiri, T.; Morita, T.; Yamauchi, T.; Imai, K.; Tei, Y.; Inoue, S. Convenient measurement of systolic pressure: The reliability and validity of manual radial pulse pressure measurement. J. Palliat. Med. 2014, 17, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Non-invasive Sphygmomanometers-Part 2: Clinical Investigation of Intermittent Automated Measurement Type; ISO 81060-2:2018; International Organization for Standardization: Geneva, Switzerland, 2018; p. 36. [Google Scholar]
- Ringrose, J.S.; McLean, D.; Ao, P.; Yousefi, F.; Sankaralingam, S.; Millay, J.; Padwal, R. Effect of cuff design on auscultatory and oscillometric blood pressure measurements. Am. J. Hypertens. 2016, 29, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Bilo, G.; Sala, O.; Perego, C.; Faini, A.; Gao, L.; Gluszewska, A.; Ochoa, J.E.; Pellegrini, D.; Lonati, L.M.; Parati, G. Impact of cuff positioning on blood pressure measurement accuracy: May a specially designed cuff make a difference? Hypertens. Res. 2017, 40, 573–580. [Google Scholar] [CrossRef]
- O’Brien, E. Review: A century of confusion; which bladder for accurate blood pressure measurement? J. Hum. Hypertens. 1996, 10, 565–572. [Google Scholar]
- Tochikubo, O.; Watanabe, J.; Hanada, K.; Miyajima, E.; Kimura, K. A new double cuff sphygmotonometer for accurate blood pressure measurement. Hypertens. Res. 2001, 24, 353–357. [Google Scholar] [CrossRef][Green Version]
- Brown, M.A.; Buddle, M.L.; Whitworth, J.A. Measurement of blood pressure during pregnancy: Evaluation of the ‘TriCUFF’. Aust. N. Z. J. Obstet. Gynaecol. 1993, 33, 48–50. [Google Scholar] [CrossRef]
- Bonso, E.; Saladini, F.; Zanier, A.; Benetti, E.; Dorigatti, F.; Palatini, P. Accuracy of a single rigid conical cuff with standard-size bladder coupled to an automatic oscillometric device over a wide range of arm circumferences. Hypertens. Res. 2010, 33, 1186–1191. [Google Scholar] [CrossRef]
- Duffy, M.K.; Williams, M. Blood Pressure Cuff and to a Method of Making the Same. U.S. Patent 1,999,900,625,804, 11 December 1992. [Google Scholar]
- Ledford, J.; Drake, R.; Ellenburg, L.; Jarvis, G.; Edward, L.P. Bladderless Blood Pressure Cuff. U.S. Patent 6,036,718A, 2 July 1998. [Google Scholar]
- Garrett, R.J. Disposable Medical Pressure Cuffs and Method of Production. U.S. Patent 5,392,782A, 7 February 1994. [Google Scholar]
- Vivenzio Ian, R.L.; Edwards, I.K.; Lia, R.A.; Perkins, J.; Karla, S.R. Recyclable or Biodegradable Blood Pressure Cuff. U.S. Patent 8,652,057B2, 19 May 2014. [Google Scholar]
- Li, H.; Bao, H.; Bok, K.X.; Lee, C.Y.; Li, B.; Zin, M.T.; Kang, L. High durability and low toxicity antimicrobial coatings fabricated by quaternary ammonium silane copolymers. Biomater. Sci. 2016, 4, 299–309. [Google Scholar] [CrossRef]
- McCaughey, E.; Higgins, T.; Shlisky, T. Antimicrobial Blood Pressure Cuff Liner. U.S. Patent 201,000,894,081A, 7 February 2010. [Google Scholar]
- Deselle, C.T.; Durgag, K.; Paul, B.; Gunn, V.; Pendleton, B.; Provonchee, R. Blood Pressure Cuff Shield Incorporating Antimicrobial Technology. U.S. Patent 20,150,351,851A1, 22 March 2014. [Google Scholar]
- De Smedt, S. Noninvasive intraocular pressure monitoring: Current insights. Clin. Ophthalmol. 2015, 9, 1385–1392. [Google Scholar] [CrossRef][Green Version]
- Drzewiecki, G.; Krishna, G.; Katta, H. Method of deflection corrected tonometry with phantom vessel experiments. Comput. Biol. Med. 2019, 104, 329–334. [Google Scholar] [CrossRef]
- Garcia-Ortiz, L.; Recio-Rodriguez, J.I.; Agudo-Conde, C.; Maderuelo-Fernandez, J.A.; Patino-Alonso, M.C.; de Cabo-Laso, A.; Rodriguez-Martin, C.; Gonzalez-Sanchez, J.; Rodriguez-Sanchez, E.; Gomez-Marcos, M.A.; et al. Noninvasive validation of central and peripheral augmentation index estimated by a novel wrist-worn tonometer. J. Hypertens. 2018, 36, 2204–2214. [Google Scholar] [CrossRef]
- Hirano, H.; Fukuchi, T.; Kurita, Y.; Kandori, A.; Sano, Y.; Nakamura, R.; Saeki, N.; Kawamoto, M.; Yoshizumi, M.; Tsuji, T. Development of a palpable carotid pulse pressure sensor using electromagnetic induction. IEEJ Trans. Electron. Inform. Syst. 2012, 132, 1934–1942. [Google Scholar]
- Okafor, K.C.; Brandt, J.D. Measuring intraocular pressure. Curr. Opin. Ophthalmol. 2015, 26, 103–109. [Google Scholar] [CrossRef]
- Ozcura, F.; Yildirim, N.; Sahin, A.; Colak, E. Comparison of Goldmann applanation tonometry, rebound tonometry and dynamic contour tonometry in normal and glaucomatous eyes. Int. J. Ophthalmol. 2015, 8, 299–304. [Google Scholar]
- Leonardi, M.; Leuenberger, P.; Bertrand, D.; Bertsch, A.; Renaud, P. Digest of Technical Papers (Cat. No.03TH8664). In A Soft Contact Lens with a MEMS Strain Gage Embedded For Intraocular Pressure Monitoring, Proceedings of the Transducers 03, 12th International Conference on Solid-State Sensors, Actuators and Microsystems, Boston, MA, USA, 8–12 June 2003; IEEE: Piscataway, NJ, USA, 2003; Volume 2, pp. 1043–1046. [Google Scholar]
- Chen, G.Z.; Chan, I.S.; Leung, L.K.; Lam, D.C. Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Med. Eng. Phys. 2014, 36, 1134–1139. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Man, T.; Huang, D.; Li, X.; Zhu, H.; Li, Z. High resolution non-invasive intraocular pressure monitoring by use of graphene woven fabrics on contact lens. Microsyst. Nanoeng. 2019, 5, 39. [Google Scholar] [CrossRef]
- Lee, B.; Jeong, J.; Kim, J.; Kim, B.; Chun, K. Cantilever arrayed blood pressure sensor for arterial applanation tonometry. IET Nanobiotechnol. 2014, 8, 37–43. [Google Scholar] [CrossRef]
- Roh, D.; Han, S.; Park, J.; Shin, H. Development of a multi-array pressure sensor module for radial artery pulse wave measurement. Sensors 2019, 20, 33. [Google Scholar] [CrossRef]
- Agnoletti, D.; Millasseau, S.C.; Topouchian, J.; Zhang, Y.; Safar, M.E.; Blacher, J. Pulse wave analysis with two tonometric devices: A comparison study. Physiol. Meas. 2014, 35, 1837–1848. [Google Scholar] [CrossRef]
- Hansen, S.; Staber, M. Oscillometric blood pressure measurement used for calibration of the arterial tonometry method contributes significantly to error. Eur. J. Anaesthesiol. 2006, 23, 781–787. [Google Scholar] [CrossRef]
- Uemura, K.; Kawada, T.; Sugimachi, M. A novel minimally occlusive cuff method utilizing ultrasound vascular imaging for stress-free blood pressure measurement: A-proof-of-concept study. IEEE Trans. Biomed. Eng. 2019, 66, 934–945. [Google Scholar] [CrossRef]
- Saugel, B.; Cecconi, M.; Hajjar, L.A. Noninvasive cardiac output monitoring in cardiothoracic surgery patients: Available methods and future directions. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1742–1752. [Google Scholar] [CrossRef]
- Saugel, B.; Dueck, R.; Wagner, J.Y. Measurement of blood pressure. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 309–322. [Google Scholar] [CrossRef]
- Gerdt, D.W.; Adkins, C.; Baruch, M. Hydrostatic Finger Cuff for Blood Wave Formanalysis And Dagnostic Support. U.S. Patent 20,120,238,887A, 24 August 2012. [Google Scholar]
- Cline, R.L.; Rosthauser, J.W.; Markusch, P.H. Removable Polyurethane Adhesives with Improved Temperature Resistance Properties. U.S. Patent 6,040,028A, 13 January 2000. [Google Scholar]
- Huber, C.; Grüllenberger, R.; Fortin, J. Disposable and Detachable Sensor For Continuous Non-Invasive Arterial Blood Pressure Monitoring. U.S. Patent 1,608,261B1, 1 April 2011. [Google Scholar]
- Westerhof, B.; Schraa, O.; Van Groeningen, C.J.E.; Li, P. Self Closing Finger Cuff. U.S. Patent 2,019,074,692A1, 11 October 2018. [Google Scholar]
- Edwards Lifesciences, ClearSight System. Innovation for Noninvasive Hemodynamic Management; Edwards Lifesciences, Ed.; Edwards Lifesciences: Irvine, CA, USA, 2018. [Google Scholar]
- Hertzman, A.B. The blood supply of various skin areas as estimated by the photoelectric plethysmograph. Am. J. Physiol. Cell Physiol. 1938, 124, 328–340. [Google Scholar] [CrossRef]
- Ding, X.R.; Zhao, N.; Yang, G.Z.; Pettigrew, R.I.; Lo, B.; Miao, F.; Li, Y.; Liu, J.; Zhang, Y.T. Continuous blood pressure measurement from invasive to unobtrusive: Celebration of 200th birth anniversary of Carl Ludwig. IEEE J. Biomed. Health Inform. 2016, 20, 1455–1465. [Google Scholar] [CrossRef]
- Cluff, K.; Becker, R.; Jayakumar, B.; Han, K.; Condon, E.; Dudley, K.; Szatkowski, G.; Pipinos, I.I.; Amick, R.Z.; Patterson, J. Passive wearable skin patch sensor measures limb hemodynamics based on electromagnetic resonance. IEEE Trans. Biomed. Eng. 2018, 65, 847–856. [Google Scholar] [CrossRef]
- Birch, A.A.; Morris, S.L. Do the Finapres and Colin radial artery tonometer measure the same blood pressure changes following deflation of thigh cuffs? Physiol. Meas. 2003, 24, 653–660. [Google Scholar] [CrossRef]
- Hohn, A.; Defosse, J.M.; Becker, S.; Steffen, C.; Wappler, F.; Sakka, S.G. Non-invasive continuous arterial pressure monitoring with Nexfin does not sufficiently replace invasive measurements in critically ill patients. Br. J. Anaesth. 2013, 111, 178–184. [Google Scholar] [CrossRef]
- Berkelmans, G.F.N.; Kuipers, S.; Westerhof, B.E.; Spoelstra-de Man, A.M.E.; Smulders, Y.M. Comparing volume-clamp method and intra-arterial blood pressure measurements in patients with atrial fibrillation admitted to the intensive or medium care unit. J. Clin. Monit. Comput. 2018, 32, 439–446. [Google Scholar] [CrossRef]
- Xiong, G.; Figueroa, C.A.; Xiao, N.; Taylor, C.A. Simulation of blood flow in deformable vessels using subject-specific geometry and spatially varying wall properties. Int. J. Numer. Method Biomed. Eng. 2011, 27, 1000–1016. [Google Scholar] [CrossRef]
- Davies, J.I.; Struthers, A.D. Beyond blood pressure: Pulse wave analysis--a better way of assessing cardiovascular risk? Future Cardiol. 2005, 1, 69–78. [Google Scholar] [CrossRef]
- De Cort, S.C.; Innes, J.A.; Barstow, T.J.; Guz, A. Cardiac output, oxygen consumption and arteriovenous oxygen difference following a sudden rise in exercise level in humans. J. Physiol. 1991, 441, 501–512. [Google Scholar] [CrossRef]
- Alruwaili, F.; Cluff, K.; Griffith, J.; Farhoud, H. Passive self resonant skin patch sensor to monitor cardiac intraventricular stroke volume using electromagnetic properties of blood. IEEE J. Transl. Eng. Health Med. 2018, 6, 1900709. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Task Force, M.; Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar]
- Ma, Y.; Choi, J.; Hourlier-Fargette, A.; Xue, Y.; Chung, H.U.; Lee, J.Y.; Wang, X.; Xie, Z.; Kang, D.; Wang, H.; et al. Relation between blood pressure and pulse wave velocity for human arteries. Proc. Natl. Acad. Sci. USA 2018, 115, 11144–11149. [Google Scholar] [CrossRef]
- Meijboom, W.B.; Van Mieghem, C.A.; van Pelt, N.; Weustink, A.; Pugliese, F.; Mollet, N.R.; Boersma, E.; Regar, E.; van Geuns, R.J.; de Jaegere, P.J.; et al. Comprehensive assessment of coronary artery stenoses: Computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J. Am. Coll. Cardiol. 2008, 52, 636–643. [Google Scholar] [CrossRef]
- Douglas, P.S.; Pontone, G.; Hlatky, M.A.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: The prospective longitudinal trial of FFR(CT): Outcome and resource impacts study. Eur. Heart J. 2015, 36, 3359–3367. [Google Scholar]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Norgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of coronary blood flow using ct angiography: Next steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef]
- Cook, C.M.; Petraco, R.; Shun-Shin, M.J.; Ahmad, Y.; Nijjer, S.; Al-Lamee, R.; Kikuta, Y.; Shiono, Y.; Mayet, J.; Francis, D.P.; et al. Diagnostic Accuracy of computed tomography-derived fractional flow reserve: A systematic review. JAMA Cardiol. 2017, 2, 803–810. [Google Scholar] [CrossRef]
- Finlay, D.D.; Nugent, C.D.; Donnelly, M.P.; McCullagh, P.J.; Black, N.D. Optimal electrocardiographic lead systems: Practical scenarios in smart clothing and wearable health systems. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 433–441. [Google Scholar] [CrossRef]
- Baek, J.-Y.; An, J.-H.; Choi, J.-M.; Park, K.-S.; Lee, S.-H. Flexible polymeric dry electrodes for the long-term monitoring of ECG. Sens. Actuators A Phys. 2008, 143, 423–429. [Google Scholar] [CrossRef]
- Haghdoost, F.; Mottaghitalab, V.; Haghi, A.K. Comfortable textile-based electrode for wearable electrocardiogram. Sens. Rev. 2015, 35, 20–29. [Google Scholar] [CrossRef]
- Chlaihawi, A.A.; Narakathu, B.B.; Emamian, S.; Bazuin, B.J.; Atashbar, M.Z. Development of printed and flexible dry ECG electrodes. Sens. Bio-Sens. Res. 2018, 20, 9–15. [Google Scholar] [CrossRef]
- Pani, D.; Dessi, A.; Saenz-Cogollo, J.F.; Barabino, G.; Fraboni, B.; Bonfiglio, A. fully textile, pedot:pss based electrodes for wearable ecg monitoring systems. IEEE Trans. Biomed. Eng. 2016, 63, 540–549. [Google Scholar] [CrossRef]
- Stauffer, F.; Thielen, M.; Sauter, C.; Chardonnens, S.; Bachmann, S.; Tybrandt, K.; Peters, C.; Hierold, C.; Voros, J. Skin conformal polymer electrodes for clinical ECG and EEG recordings. Adv. Healthcare Mater. 2018, 7, e1700994. [Google Scholar] [CrossRef]
- Chen, Y.H.; Op de Beeck, M.; Vanderheyden, L.; Carrette, E.; Mihajlovic, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; Van Hoof, C. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef]
- Joosten, A.; Desebbe, O.; Suehiro, K.; Murphy, L.S.; Essiet, M.; Alexander, B.; Fischer, M.O.; Barvais, L.; Van Obbergh, L.; Maucort-Boulch, D.; et al. Accuracy and precision of non-invasive cardiac output monitoring devices in perioperative medicine: A systematic review and meta-analysisdagger. Br. J. Anaesth. 2017, 118, 298–310. [Google Scholar] [CrossRef]
- Mukkamala, R.; Hahn, J.O.; Inan, O.T.; Mestha, L.K.; Kim, C.S.; Toreyin, H.; Kyal, S. Toward Ubiquitous Blood Pressure Monitoring via Pulse Transit Time: Theory and Practice. IEEE Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Y.T. Pulse transit time technique for cuffless unobtrusive blood pressure measurement: From theory to algorithm. Biomed. Eng. Lett. 2019, 9, 37–52. [Google Scholar] [CrossRef]
- Ale, I.S.; Maibacht, H.A. Diagnostic approach in allergic and irritant contact dermatitis. Exp. Rev. Clin. Immunol. 2010, 6, 291–310. [Google Scholar] [CrossRef]
- Brasch, J.; Becker, D.; Aberer, W.; Bircher, A.; Kranke, B.; Jung, K.; Przybilla, B.; Biedermann, T.; Werfel, T.; John, S.M.; et al. Guideline contact dermatitis: S1-Guidelines of the German Contact Allergy Group (DKG) of the German Dermatology Society (DDG), the Information Network of Dermatological Clinics (IVDK), the German Society for Allergology and Clinical Immunology (DGAKI), the Working Group for Occupational and Environmental Dermatology (ABD) of the DDG, the Medical Association of German Allergologists (AeDA), the Professional Association of German Dermatologists (BVDD) and the DDG. Allergol. J. Int. 2014, 23, 126–138. [Google Scholar]
- Jin, H.; Abu-Raya, Y.S.; Haick, H. Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthcare Mater. 2017, 6, 11. [Google Scholar] [CrossRef]
- Zink, M.D.; Bruser, C.; Stuben, B.O.; Napp, A.; Stohr, R.; Leonhardt, S.; Marx, N.; Mischke, K.; Schulz, J.B.; Schiefer, J. Unobtrusive nocturnal heartbeat monitoring by a ballistocardiographic sensor in patients with sleep disordered breathing. Sci. Rep. 2017, 7, 13175. [Google Scholar] [CrossRef]
- Lee, K.J.; Roh, J.; Cho, D.; Hyeong, J.; Kim, S. A Chair-based unconstrained/nonintrusive cuffless blood pressure monitoring system using a two-channel ballistocardiogram. Sensors 2019, 19, 595. [Google Scholar] [CrossRef]
- Ha, T.; Tran, J.; Liu, S.; Jang, H.; Jeong, H.; Mitbander, R.; Huh, H.; Qiu, Y.; Duong, J.; Wang, R.L.; et al. A chest-laminated ultrathin and stretchable e-tattoo for the measurement of electrocardiogram, seismocardiogram, and cardiac time intervals. Adv. Sci. (Weinh) 2019, 6, 1900290. [Google Scholar] [CrossRef]
- Lu, N.; Ameri, S.; Ha, T.; Nicolini, L.; Stier, A.; Wang, P. Epidermal Electronic Systems for Sensing and Therapy; SPIE: Bellingham, DC, USA, 2017; Volume 10167. [Google Scholar]
- Pinheiro, E.; Postolache, O.; Girão, P. Study on Ballistocardiogram Acquisition in a Moving Wheelchair with Embedded Sensors. Metrol. Meas. Syst. 2012, 19, 739–750. [Google Scholar] [CrossRef]
- Heise, D.; Rosales, L.; Skubic, M.; Devaney, M.J. Refinement and Evaluation of a Hydraulic Bed Sensor. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 3 August–3 September 2011; pp. 4356–4360. [Google Scholar]
- Chiu, Y.-Y.; Lin, W.-Y.; Wang, H.-Y.; Huang, S.-B.; Wu, M.-H. Development of a piezoelectric polyvinylidene fluoride (PVDF) polymer-based sensor patch for simultaneous heartbeat and respiration monitoring. Sens. Actuators A Phys. 2013, 189, 328–334. [Google Scholar] [CrossRef]
- Marquez, J.C.; Rempfler, M.; Seoane, F.; Lindecrantz, K. Textrode-enabled transthoracic electrical bioimpedance measurements–towards wearable applications of impedance cardiography. J. Electric. Bioimpedance 2019, 4, 45–50. [Google Scholar] [CrossRef]
- Searle, A.; Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000, 21, 271–283. [Google Scholar] [CrossRef]
- Ramasamy, S.; Balan, A. Wearable sensors for ECG measurement: A review. Sens. Rev. 2018, 38, 412–419. [Google Scholar] [CrossRef]
- Merritt, C.R.; Nagle, H.T.; Grant, E. Textile-based capacitive sensors for respiration monitoring. IEEE Sens. J. 2009, 9, 71–78. [Google Scholar] [CrossRef]
- Sharma, P.; Imtiaz, S.A.; Rodriguez-Villegas, E. Acoustic sensing as a novel wearable approach for cardiac monitoring at the wrist. Sci. Rep. 2019, 9, 20079. [Google Scholar] [CrossRef]
- Griffith, J.; Cluff, K.; Eckerman, B.; Aldrich, J.; Becker, R.; Moore-Jansen, P.; Patterson, J. Non-invasive electromagnetic skin patch sensor to measure intracranial fluid-volume shifts. Sensors 2018, 18, 1022. [Google Scholar] [CrossRef]
- Kaniusas, E.; Pfutzner, H.; Mehnen, L.; Kosel, J.; Tellez-Blanco, C.; Varoneckas, G.; Alonderis, A.; Meydan, T.; Vazquez, M.; Rohn, M.; et al. Method for continuous nondisturbing monitoring of blood pressure by magnetoelastic skin curvature sensor and ECG. IEEE Sens. J. 2006, 6, 819–828. [Google Scholar] [CrossRef]
- Pogue, B.W.; Poplack, S.P.; McBride, T.O.; Wells, W.A.; Osterman, K.S.; Osterberg, U.L.; Paulsen, K.D. Quantitative hemoglobin tomography with diffuse near-infrared spectroscopy: Pilot results in the breast. Radiology 2001, 218, 261–266. [Google Scholar] [CrossRef]
- Xing, X.; Sun, M. Optical blood pressure estimation with photoplethysmography and FFT-based neural networks. Biomed. Opt. Express 2016, 7, 3007–3020. [Google Scholar] [CrossRef]
- Kamal, A.A.; Harness, J.B.; Irving, G.; Mearns, A.J. Skin photoplethysmography—A review. Comput. Methods Programs Biomed. 1989, 28, 257–269. [Google Scholar] [CrossRef]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Mendelson, Y.; Ochs, B.D. Noninvasive pulse oximetry utilizing skin reflectance photoplethysmography. IEEE Trans. Biomed. Eng. 1988, 35, 798–805. [Google Scholar] [CrossRef]
- Vazquez, K.; Cota, J.; Sifuentes, E.; Gonzalez, R. High Signal-to-noise ratio phonocardiogram using a shielded pvdf film sensor. IEEE Lat. Am. Trans. 2016, 14, 1139–1145. [Google Scholar] [CrossRef]
- Ding, X.; Dai, W.; Luo, N.; Liu, J.; Zhao, N.; Zhang, Y. A Flexible Tonoarteriography-Based Body Sensor Network for Cuffless Measurement Of Arterial Blood Pressure. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 9–12 June 2015; pp. 1–4. [Google Scholar]
- Eduardo, C.; Octavian, A.; Pedro, S. Calibration and validation of homeostasis parameters estimates produced by a DSP embedded in a wheelchair. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 6–9 May 2013. [Google Scholar]
- Yang, C.; Tavassolian, N. Pulse transit time measurement using seismocardiogram, photoplethysmogram, and acoustic recordings: Evaluation and comparison. IEEE J Biomed. Health Inform. 2018, 22, 733–740. [Google Scholar] [CrossRef]
- Pang, C.; Koo, J.H.; Nguyen, A.; Caves, J.M.; Kim, M.G.; Chortos, A.; Kim, K.; Wang, P.J.; Tok, J.B.; Bao, Z. Highly skin-conformal microhairy sensor for pulse signal amplification. Adv. Mater. 2015, 27, 634–640. [Google Scholar] [CrossRef]
- Luo, N.; Dai, W.; Li, C.; Zhou, Z.; Lu, L.; Poon, C.C.Y.; Chen, S.-C.; Zhang, Y.; Zhao, N. Flexible piezoresistive sensor patch enabling ultralow power cuffless blood pressure measurement. Adv. Funct. Mater. 2016, 26, 1178–1187. [Google Scholar] [CrossRef]
- Rendon, D.B.; Rojas Ojeda, J.L.; Crespo Foix, L.F.; Morillo, D.S.; Fernandez, M.A. Mapping the human body for vibrations using an accelerometer. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 2007, 1671–1674. [Google Scholar]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.S.; Huang, Y.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef]
- Simjanoska, M.; Gjoreski, M.; Gams, M.; Madevska Bogdanova, A. Non-invasive blood pressure estimation from ecg using machine learning techniques. Sensors 2018, 18, 1160. [Google Scholar] [CrossRef]
- Yazdanian, H.; Mahnam, A.; Edrisi, M.; Esfahani, M.A. Design and implementation of a portable impedance cardiography system for noninvasive stroke volume monitoring. J. Med. Sign. Sens. 2016, 6, 47–56. [Google Scholar]
- Xu, H.; Liu, J.; Zhang, J.; Zhou, G.; Luo, N.; Zhao, N. Flexible organic/inorganic hybrid near-infrared photoplethysmogram sensor for cardiovascular monitoring. Adv. Mater. 2017, 29, 31. [Google Scholar] [CrossRef]
- Choong, C.L.; Shim, M.B.; Lee, B.S.; Jeon, S.; Ko, D.S.; Kang, T.H.; Bae, J.; Lee, S.H.; Byun, K.E.; Im, J.; et al. Highly stretchable resistive pressure sensors using a conductive elastomeric composite on a micropyramid array. Adv. Mater. 2014, 26, 3451–3458. [Google Scholar] [CrossRef]
- Stauffer, D. Introduction to Percolation Theory; Taylor & Francis: London, UK, 2014. [Google Scholar]
- Yu, H.; Huang, J. Design and application of a high sensitivity piezoresistive pressure sensor for low pressure conditions. Sensors 2015, 15, 22692–22704. [Google Scholar] [CrossRef]
- Kaidarova, A.; Alsharif, N.; Oliveira, B.N.M.; Marengo, M.; Geraldi, N.R.; Duarte, C.M.; Kosel, J. Laser-Printed, Flexible Graphene Pressure Sensors. Glob. Chall. 2020, 4, 2000001. [Google Scholar] [CrossRef]
- Lu, Y.; Tian, M.; Sun, X.; Pan, N.; Chen, F.; Zhu, S.; Zhang, X.; Chen, S. Highly sensitive wearable 3D piezoresistive pressure sensors based on graphene coated isotropic non-woven substrate. Compos. Part A Appl. Sci. Manufact. 2019, 117, 202–210. [Google Scholar] [CrossRef]
- Herren, B.; Saha, M.C.; Liu, Y. Carbon nanotube-based piezoresistive sensors fabricated by microwave irradiation. Adv. Eng. Mater. 2019, 22, 1901068. [Google Scholar] [CrossRef]
- Hu, J.; Yu, J.; Li, Y.; Liao, X.; Yan, X.; Li, L. Nano carbon black-based high performance wearable pressure sensors. Nanomaterials 2020, 10, 664. [Google Scholar] [CrossRef]
- Chang, X.; Sun, S.; Sun, S.; Liu, T.; Xiong, X.; Lei, Y.; Dong, L.; Yin, Y. ZnO nanorods/carbon black-based flexible strain sensor for detecting human motions. J. Alloys Comp. 2018, 738, 111–117. [Google Scholar] [CrossRef]
- Vuorinen, T.; Laurila, M.-M.; Mangayil, R.; Karp, M.; Mäntysalo, M. High Resolution E-Jet Printed Temperature Sensor on Artificial Skin; Springer: Singapore, 2018; pp. 839–842. [Google Scholar]
- Choi, S.; Han, S.I.; Jung, D.; Hwang, H.J.; Lim, C.; Bae, S.; Park, O.K.; Tschabrunn, C.M.; Lee, M.; Bae, S.Y.; et al. Highly conductive, stretchable and biocompatible Ag-Au core-sheath nanowire composite for wearable and implantable bioelectronics. Nat. Nanotechnol. 2018, 13, 1048–1056. [Google Scholar]
- Wang, Z.; Wang, S.; Zeng, J.; Ren, X.; Chee, A.J.; Yiu, B.Y.; Chung, W.C.; Yang, Y.; Yu, A.C.; Roberts, R.C.; et al. High sensitivity, wearable, piezoresistive pressure sensors based on irregular microhump structures and its applications in body motion sensing. Small 2016, 12, 3827–3836. [Google Scholar] [CrossRef]
- Zhou, Y.; He, J.; Wang, H.; Qi, K.; Nan, N.; You, X.; Shao, W.; Wang, L.; Ding, B.; Cui, S. Highly sensitive, self-powered and wearable electronic skin based on pressure-sensitive nanofiber woven fabric sensor. Sci. Rep. 2017, 7, 12949. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, C.; Huang, H.; Yang, T.; Tian, G.; Xiong, D.; Chen, N.; Chu, X.; Zhong, S.; Deng, W.; et al. Microchannel-confined mxene based flexible piezoresistive multifunctional micro-force sensor. Adv. Funct. Mater. 2020, 30, 1909603. [Google Scholar] [CrossRef]
- Li, L.; Fu, X.; Chen, S.; Uzun, S.; Levitt, A.S.; Shuck, C.E.; Han, W.; Gogotsi, Y. Hydrophobic and stable mxene-polymer pressure sensors for wearable electronics. ACS Appl. Mater. Interfaces 2020, 12, 15362–15369. [Google Scholar] [CrossRef]
- Luo, S.; Yang, J.; Song, X.; Zhou, X.; Yu, L.; Sun, T.; Yu, C.; Huang, D.; Du, C.; Wei, D. Tunable-Sensitivity flexible pressure sensor based on graphene transparent electrode. Solid-State Electron. 2018, 145, 29–33. [Google Scholar] [CrossRef]
- Kang, M.; Kim, J.; Jang, B.; Chae, Y.; Kim, J.H.; Ahn, J.H. Graphene-based three-dimensional capacitive touch sensor for wearable electronics. ACS Nano 2017, 11, 7950–7957. [Google Scholar] [CrossRef]
- Wan, S.; Bi, H.; Zhou, Y.; Xie, X.; Su, S.; Yin, K.; Sun, L. Graphene oxide as high-performance dielectric materials for capacitive pressure sensors. Carbon 2017, 114, 209–216. [Google Scholar] [CrossRef]
- Sahatiya, P.; Badhulika, S. Eraser-based eco-friendly fabrication of a skin-like large-area matrix of flexible carbon nanotube strain and pressure sensors. Nanotechnology 2017, 28, 095501. [Google Scholar] [CrossRef]
- Kwon, D.; Lee, T.I.; Shim, J.; Ryu, S.; Kim, M.S.; Kim, S.; Kim, T.S.; Park, I. Highly sensitive, flexible, and wearable pressure sensor based on a giant piezocapacitive effect of three-dimensional microporous elastomeric dielectric layer. ACS Appl. Mater. Interfaces 2016, 8, 16922–16931. [Google Scholar] [CrossRef]
- Wan, Y.; Qiu, Z.; Huang, J.; Yang, J.; Wang, Q.; Lu, P.; Yang, J.; Zhang, J.; Huang, S.; Wu, Z.; et al. Natural plant materials as dielectric layer for highly sensitive flexible electronic skin. Small 2018, 14, e1801657. [Google Scholar] [CrossRef]
- Chhetry, A.; Yoon, H.; Park, J.Y. A flexible and highly sensitive capacitive pressure sensor based on conductive fibers with a microporous dielectric for wearable electronics. J. Mater. Chem. C 2017, 5, 10068–10076. [Google Scholar] [CrossRef]
- Li, W.; Xiong, L.; Pu, Y.; Quan, Y.; Li, S. High-Performance paper-based capacitive flexible pressure sensor and its application in human-related measurement. Nanoscale Res. Lett. 2019, 14, 183. [Google Scholar] [CrossRef]
- Maity, K.; Garain, S.; Henkel, K.; Schmeißer, D.; Mandal, D. Self-Powered human-health monitoring through aligned pvdf nanofibers interfaced skin-interactive piezoelectric sensor. ACS Appl. Polym. Mater. 2020, 2, 862–878. [Google Scholar] [CrossRef]
- Kim, H.; Kim, G.; Kim, T.; Lee, S.; Kang, D.; Hwang, M.S.; Chae, Y.; Kang, S.; Lee, H.; Park, H.G.; et al. Transparent, flexible, conformal capacitive pressure sensors with nanoparticles. Small 2018, 14, 1703432. [Google Scholar] [CrossRef]
- Guo, Y.; Zhong, M.; Fang, Z.; Wan, P.; Yu, G. A Wearable transient pressure sensor made with mxene nanosheets for sensitive broad-range human-machine interfacing. Nano Lett. 2019, 19, 1143–1150. [Google Scholar] [CrossRef]
- Ramuz, M.; Tee, B.C.; Tok, J.B.; Bao, Z. Transparent, optical, pressure-sensitive artificial skin for large-area stretchable electronics. Adv. Mater. 2012, 24, 3223–3227. [Google Scholar] [CrossRef]
- Koyama, S.; Ishizawa, H. Vital sign measurement using fbg sensor for new wearable sensor development, fiber optic sensing-principle, measurement and applications. Shien-Kuei Liaw 2019, 11340. [Google Scholar] [CrossRef]
- Dziuda, L.; Skibniewski, F.W.; Krej, M.; Baran, P.M. Fiber Bragg grating-based sensor for monitoring respiration and heart activity during magnetic resonance imaging examinations. J. Biomed. Opt. 2013, 18, 57006. [Google Scholar] [CrossRef]
- Ogawa, K.; Koyama, S.; Haseda, Y.; Fujita, K.; Ishizawa, H.; Fujimoto, K. Wireless, portable fiber bragg grating interrogation system employing optical edge filter. Sensors 2019, 19, 3222. [Google Scholar] [CrossRef]
- Nedoma, J.; Kepak, S.; Fajkus, M.; Cubik, J.; Siska, P.; Martinek, R.; Krupa, P. Magnetic resonance imaging compatible non-invasive fibre-optic sensors based on the bragg gratings and interferometers in the application of monitoring heart and respiration rate of the human body: A comparative study. Sensors 2018, 18, 3713. [Google Scholar] [CrossRef]
- Lo Presti, D.; Romano, C.; Massaroni, C.; D’Abbraccio, J.; Massari, L.; Caponero, M.A.; Oddo, C.M.; Formica, D.; Schena, E. Cardio-Respiratory monitoring in archery using a smart textile based on flexible fiber bragg grating sensors. Sensors 2019, 19, 3581. [Google Scholar] [CrossRef]
- Yogeswaran, N.; Navaraj, W.T.; Gupta, S.; Liu, F.; Vinciguerra, V.; Lorenzelli, L.; Dahiya, R. Piezoelectric graphene field effect transistor pressure sensors for tactile sensing. Appl. Phys. Lett. 2018, 113, 014102. [Google Scholar] [CrossRef]
- Kotlowski, C.; Aspermair, P.; Khan, H.U.; Reiner-Rozman, C.; Breu, J.; Szunerits, S.; Kim, J.-J.; Bao, Z.; Kleber, C.; Pelosi, P.; et al. Electronic biosensing with flexible organic transistor devices. Flexib. Print. Electron. 2018, 3, 034003. [Google Scholar] [CrossRef]
- Viola, F.A.; Spanu, A.; Ricci, P.C.; Bonfiglio, A.; Cosseddu, P. Ultrathin, flexible and multimodal tactile sensors based on organic field-effect transistors. Sci. Rep. 2018, 8, 8073. [Google Scholar] [CrossRef]
- Wang, C.; Hwang, D.; Yu, Z.; Takei, K.; Park, J.; Chen, T.; Ma, B.; Javey, A. User-interactive electronic skin for instantaneous pressure visualization. Nat. Mater. 2013, 12, 899–904. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jang, M.; Lee, M.Y.; Kweon, O.Y.; Oh, J.H. Flexible field-effect transistor-type sensors based on conjugated molecules. Chemicals 2017, 3, 724–763. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.L. Reviving vibration energy harvesting and self-powered sensing by a triboelectric nanogenerator. Joule 2017, 1, 480–521. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, J.; Li, X.; Wu, Y.; Wei, W.; Liu, J.; Chen, J.; Yang, J. Large-Scale and washable smart textiles based on triboelectric nanogenerator arrays for self-powered sleeping monitoring. Adv. Funct. Mater. 2018, 28, 1704112. [Google Scholar] [CrossRef]
- Zhang, N.; Tao, C.; Fan, X.; Chen, J. Progress in triboelectric nanogenerators as self-powered smart sensors. J. Mater. Res. 2017, 32, 1628–1646. [Google Scholar] [CrossRef]
- Meng, K.; Zhao, S.; Zhou, Y.; Wu, Y.; Zhang, S.; He, Q.; Wang, X.; Zhou, Z.; Fan, W.; Tan, X.; et al. Wireless textile-based sensor system for self-powered personalized health care. Matter 2020, 2, 896–907. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Su, Y.; Jing, Q.; Li, Z.; Yi, F.; Wen, X.; Wang, Z.; Wang, Z.L. Eardrum-inspired active sensors for self-powered cardiovascular system characterization and throat-attached anti-interference voice recognition. Adv. Mater. 2015, 27, 1316–1326. [Google Scholar] [CrossRef]
- Bai, P.; Zhu, G.; Jing, Q.; Yang, J.; Chen, J.; Su, Y.; Ma, J.; Zhang, G.; Wang, Z.L. Membrane-Based self-powered triboelectric sensors for pressure change detection and its uses in security surveillance and healthcare monitoring. Adv. Funct. Mater. 2014, 24, 5807–5813. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, J.; Li, X.; Zhou, Z.; Meng, K.; Wei, W.; Yang, J.; Wang, Z.L. Triboelectric nanogenerator enabled body sensor network for self-powered human heart-rate monitoring. ACS Nano 2017, 11, 8830–8837. [Google Scholar] [CrossRef]
- Yan, C.; Deng, W.; Jin, L.; Yang, T.; Wang, Z.; Chu, X.; Su, H.; Chen, J.; Yang, W. Epidermis-Inspired ultrathin 3d cellular sensor array for self-powered biomedical monitoring. ACS Appl. Mater. Interfaces 2018, 10, 41070–41075. [Google Scholar] [CrossRef]
- Meng, K.; Chen, J.; Li, X.; Wu, Y.; Fan, W.; Zhou, Z.; He, Q.; Wang, X.; Fan, X.; Zhang, Y.; et al. Flexible weaving constructed self-powered pressure sensor enabling continuous diagnosis of cardiovascular disease and measurement of cuffless blood pressure. Adv. Funct. Mater. 2018, 29, 1806388. [Google Scholar] [CrossRef]
- Lee, J.H.; Hinchet, R.; Kim, S.K.; Kim, S.; Kim, S.-W. Shape memory polymer-based self-healing triboelectric nanogenerator. Energy Environ. Sci. 2015, 8, 3605–3613. [Google Scholar] [CrossRef]
- Ha, M.; Park, J.; Lee, Y.; Ko, H. Triboelectric generators and sensors for self-powered wearable electronics. ACS Nano 2015, 9, 3421–3427. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.R.; Lin, L.; Zhu, G.; Wu, W.; Zhang, R.; Wang, Z.L. Transparent triboelectric nanogenerators and self-powered pressure sensors based on micropatterned plastic films. Nano Lett. 2012, 12, 3109–3114. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, L.; Xie, Y.; Jing, Q.; Niu, S.; Wang, Z.L. Sliding-triboelectric nanogenerators based on in-plane charge-separation mechanism. Nano Lett. 2013, 13, 2226–2233. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, stretchable sensors for wearable health monitoring: Sensing mechanisms, materials, fabrication strategies and features. Sensors 2018, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Bok, B.G.; Ahn, J.H.; Kim, M.S. Recent advances in tactile sensing technology. Micromachines 2018, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Haddara, Y.M.; Howlader, M.M.R. Integration of heterogeneous materials for wearable sensors. Polymers 2018, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jug, L.; Meng, E. High strain biocompatible polydimethylsiloxane-based conductive graphene and multiwalled carbon nanotube nanocomposite strain sensors. Appl. Phys. Lett. 2013, 102, 183511. [Google Scholar] [CrossRef]
- Tao, L.; Wang, D.; Tian, H.; Ju, Z.; Liu, Y.; Chen, Y.; Xie, Q.; Zhao, H.; Yang, Y.; Ren, T. In Tunable and Wearable High Performance Strain Sensors Based on Laser Patterned Graphene Flakes. In Proceedings of the 2016 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 3–7 December 2016; pp. 18.3.1–18.3.4. [Google Scholar]
- Glennon, T.; O’Quigley, C.; McCaul, M.; Matzeu, G.; Beirne, S.; Wallace, G.G.; Stroiescu, F.; O’Mahoney, N.; White, P.; Diamond, D. ‘SWEATCH’: A wearable platform for harvesting and analysing sweat sodium content. Electroanalysis 2016, 28, 1283–1289. [Google Scholar] [CrossRef]
- Munje, R.D.; Muthukumar, S.; Prasad, S. Lancet-free and label-free diagnostics of glucose in sweat using Zinc Oxide based flexible bioelectronics. Sens. Actuators B Chem. 2017, 238, 482–490. [Google Scholar] [CrossRef]
- Kurra, N.; Kulkarni, G.U. Pencil-on-paper: Electronic devices. Lab. Chip 2013, 13, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhou, B.; Lukowicz, P.; Seoane, F.; Varga, M.; Mehmann, A.; Chabrecek, P.; Gaschler, W.; Goenner, K.; Horter, H.; et al. Textile building blocks: Toward simple, modularized, and standardized smart textile. Smart Text. 2017, 303–331. [Google Scholar]
- Guay, P.; Gorgutsa, S.; LaRochelle, S.; Messaddeq, Y. Wearable contactless respiration sensor based on multi-material fibers integrated into textile. Sensors 2017, 17, 1050. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, S.; Lin, Y.; Yuan, X.; Liu, L. Silver nanowires coated on cotton for flexible pressure sensors. J. Mater. Chem. C 2016, 4, 935–943. [Google Scholar] [CrossRef]
- Lei, K.F.; Lee, K.-F.; Lee, M.-Y. Development of a flexible PDMS capacitive pressure sensor for plantar pressure measurement. Microelectron. Eng. 2012, 99, 1–5. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.J.; Zhang, H.M.; Zhao, Z.X.; Dong, S.L.; Wei, S.; Zhao, J.; Wang, Z.L.; Guo, B.; Hu, P.A. Highly sensitive flexible three-axis tactile sensors based on the interface contact resistance of microstructured graphene. Nanoscale 2018, 10, 7387–7395. [Google Scholar] [CrossRef]
- Ponnamma, D.; Sadasivuni, K.K.; Grohens, Y.; Guo, Q.; Thomas, S. Carbon nanotube based elastomer composites – an approach towards multifunctional materials. J. Mater. Chem. C 2014, 2, 8446–8485. [Google Scholar] [CrossRef]
- Wu, X.; Han, Y.; Zhang, X.; Zhou, Z.; Lu, C. Large-Area compliant, low-cost, and versatile pressure-sensing platform based on microcrack-designed carbon black @ polyurethane sponge for human-machine interfacing. Adv. Funct. Mater. 2016, 26, 6246–6256. [Google Scholar] [CrossRef]
- Guo, S.Z.; Qiu, K.; Meng, F.; Park, S.H.; McAlpine, M.C. 3D Printed stretchable tactile sensors. Adv. Mater. 2017, 29, 27. [Google Scholar] [CrossRef]
- Xia, K.; Wang, C.; Jian, M.; Wang, Q.; Zhang, Y. CVD growth of fingerprint-like patterned 3D graphene film for an ultrasensitive pressure sensor. Nano Res. 2017, 11, 1124–1134. [Google Scholar] [CrossRef]
- Zhu, B.; Niu, Z.; Wang, H.; Leow, W.R.; Wang, H.; Li, Y.; Zheng, L.; Wei, J.; Huo, F.; Chen, X. Microstructured graphene arrays for highly sensitive flexible tactile sensors. Small 2014, 10, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Tee, B.C.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Douguet, M.; Picard, C.; Savary, G.; Merlaud, F.; Loubat-Bouleuc, N.; Grisel, M. Spreading properties of cosmetic emollients: Use of synthetic skin surface to elucidate structural effect. Colloids Surf. B Biointerfaces 2017, 154, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, S.; Zhou, X.; Li, J.; Fu, J.; Yang, W.; Wei, D. Flexible, tunable, and ultrasensitive capacitive pressure sensor with microconformal graphene electrodes. ACS Appl. Mater. Interfaces 2019, 11, 14997–15006. [Google Scholar] [CrossRef]
- Chen, M.; Luo, W.; Xu, Z.; Zhang, X.; Xie, B.; Wang, G.; Han, M. An ultrahigh resolution pressure sensor based on percolative metal nanoparticle arrays. Nat. Commun. 2019, 10, 4024. [Google Scholar] [CrossRef]
- Fu, X.; Dong, H.; Zhen, Y.; Hu, W. Solution-processed large-area nanocrystal arrays of metal-organic frameworks as wearable, ultrasensitive, electronic skin for health monitoring. Small 2015, 11, 3351–3356. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Li, L.; Hu, X.; Zou, Z.; Wang, J.; Luo, S.; Gao, Y. A highly flexible and sensitive piezoresistive sensor based on MXene with greatly changed interlayer distances. Nat. Commun. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Lou, Z.; Chen, S.; Wang, L.; Jiang, K.; Shen, G. An ultra-sensitive and rapid response speed graphene pressure sensors for electronic skin and health monitoring. Nano Energy 2016, 23, 7–14. [Google Scholar] [CrossRef]
- Spahr, M.E.; Rothon, R. Carbon Black as a Polymer Filler. In Polymers and Polymeric Composites: A Reference Series; Palsule, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–31. [Google Scholar]
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical properties of carbon nanotube based fibers and their future use in electrical wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Tang, J.; Cao, Q.; Tulevski, G.; Jenkins, K.A.; Nela, L.; Farmer, D.B.; Han, S.-J. Flexible CMOS integrated circuits based on carbon nanotubes with sub-10 ns stage delays. Nat. Electron. 2018, 1, 191–196. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L. Effect of carbon nanotube aspect ratio on the thermal and electrical properties of epoxy nanocomposites. Fuller. Nanotubes Carbon Nanostruct. 2018, 26, 697–704. [Google Scholar] [CrossRef]
- Tewari, A.; Gandla, S.; Bohm, S.; McNeill, C.R.; Gupta, D. Highly exfoliated MWNT-RGO ink-wrapped polyurethane foam for piezoresistive pressure sensor applications. ACS Appl. Mater. Interfaces 2018, 10, 5185–5195. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, J.H.; Kim, J.; Choi, S.; Lee, J.; Park, I.; Hyeon, T.; Kim, D.H. Reverse-micelle-induced porous pressure-sensitive rubber for wearable human-machine interfaces. Adv. Mater. 2014, 26, 4825–4830. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Hong, J.; Ha, M.; Jung, Y.D.; Lim, H.; Kim, S.Y.; Ko, H. Giant tunneling piezoresistance of composite elastomers with interlocked microdome arrays for ultrasensitive and multimodal electronic skins. ACS Nano 2014, 8, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Xia, K.; Wang, Q.; Yin, Z.; Wang, H.; Wang, C.; Xie, H.; Zhang, M.; Zhang, Y. Flexible and highly sensitive pressure sensors based on bionic hierarchical structures. Adv. Funct. Mater. 2017, 27, 1606066. [Google Scholar] [CrossRef]
- Kim, K.H.; Hong, S.K.; Jang, N.S.; Ha, S.H.; Lee, H.W.; Kim, J.M. Wearable resistive pressure sensor based on highly flexible carbon composite conductors with irregular surface morphology. ACS Appl. Mater. Interfaces 2017, 9, 17499–17507. [Google Scholar] [CrossRef]
- Ishikawa, F.N.; Chang, H.K.; Ryu, K.; Chen, P.C.; Badmaev, A.; Gomez De Arco, L.; Shen, G.; Zhou, C. Transparent electronics based on transfer printed aligned carbon nanotubes on rigid and flexible substrates. ACS Nano 2009, 3, 73–79. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.-W. Aligned multi-walled carbon nanotube-reinforced composites: Processing and mechanical characterization. J. Phys. D Appl. Phys. 2002, 35, L77–L80. [Google Scholar] [CrossRef]
- Mikhalchan, A.; Gspann, T.; Windle, A. Aligned carbon nanotube–epoxy composites: The effect of nanotube organization on strength, stiffness, and toughness. J. Mater. Sci. 2016, 51, 10005–10025. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Progress Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Ursaki, V.; Popa, V. Ultra-thin membranes for sensor applications. In Nanocoatings and Ultra-Thin Films; Makhlouf, A.S.H., Tiginyanu, I., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 330–354. [Google Scholar]
- Azizighannad, S.; Mitra, S. Stepwise Reduction of Graphene Oxide (GO) and its effects on chemical and colloidal properties. Sci. Rep. 2018, 8, 10083. [Google Scholar] [CrossRef]
- Pyo, S.; Choi, J.; Kim, J. Flexible, Transparent, Sensitive, and Crosstalk-Free Capacitive Tactile Sensor Array Based on Graphene Electrodes and Air Dielectric. Adv. Electron. Mater. 2018, 4, 1. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, J.; Yang, X.; Zhao, C.; Qin, S.; Cho, J.H.; Zhang, C.; Sun, Q.; Wang, Z.L. Mechanosensation-active matrix based on direct-contact tribotronic planar graphene transistor array. ACS Nano 2018, 12, 9381–9389. [Google Scholar] [CrossRef]
- Shin, S.H.; Ji, S.; Choi, S.; Pyo, K.H.; Wan An, B.; Park, J.; Kim, J.; Kim, J.Y.; Lee, K.S.; Kwon, S.Y.; et al. Integrated arrays of air-dielectric graphene transistors as transparent active-matrix pressure sensors for wide pressure ranges. Nat. Commun. 2017, 8, 14950. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, H.; Ding, H.; Pan, N.; Wang, X. Fabrication of low-cost and highly sensitive graphene-based pressure sensors by direct laser scribing polydimethylsiloxane. ACS Appl. Mater. Interfaces 2019, 11, 6195–6200. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef]
- Yao, H.B.; Ge, J.; Wang, C.F.; Wang, X.; Hu, W.; Zheng, Z.J.; Ni, Y.; Yu, S.H. A flexible and highly pressure-sensitive graphene-polyurethane sponge based on fractured microstructure design. Adv. Mater. 2013, 25, 6692–6698. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Krishna Ghatkesar, M.; Zhang, C.; Janssen, G.C.A.M. Graphene based piezoresistive pressure sensor. Appl. Phys. Lett. 2013, 102, 161904. [Google Scholar] [CrossRef]
- Tao, L.Q.; Zhang, K.N.; Tian, H.; Liu, Y.; Wang, D.Y.; Chen, Y.Q.; Yang, Y.; Ren, T.L. Graphene-Paper pressure sensor for detecting human motions. ACS Nano 2017, 11, 8790–8795. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Mei, D.; Wu, X. Highly sensitive and flexible tactile sensor based on porous graphene sponges for distributed tactile sensing in monitoring human motions. J. Microelectromech. Syst. 2019, 28, 154–163. [Google Scholar] [CrossRef]
- Berger, C.; Phillips, R.; Centeno, A.; Zurutuza, A.; Vijayaraghavan, A. Capacitive pressure sensing with suspended graphene-polymer heterostructure membranes. Nanoscale 2017, 9, 17439–17449. [Google Scholar] [CrossRef]
- Khan, U.; Kim, T.H.; Ryu, H.; Seung, W.; Kim, S.W. Graphene tribotronics for electronic skin and touch screen applications. Adv. Mater. 2017, 29, 1. [Google Scholar] [CrossRef]
- Ge, G.; Cai, Y.; Dong, Q.; Zhang, Y.; Shao, J.; Huang, W.; Dong, X. A flexible pressure sensor based on rGO/polyaniline wrapped sponge with tunable sensitivity for human motion detection. Nanoscale 2018, 10, 10033–10040. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Wei, G.; Su, Z. Reduced graphene oxide-based double network polymeric hydrogels for pressure and temperature sensing. Sensors 2018, 18, 3162. [Google Scholar] [CrossRef]
- Zhang, B.-X.; Hou, Z.-L.; Yan, W.; Zhao, Q.-L.; Zhan, K.-T. Multi-dimensional flexible reduced graphene oxide/polymer sponges for multiple forms of strain sensors. Carbon 2017, 125, 199–206. [Google Scholar] [CrossRef]
- Jia, J.; Huang, G.; Deng, J.; Pan, K. Skin-inspired flexible and high-sensitivity pressure sensors based on rGO films with continuous-gradient wrinkles. Nanoscale 2019, 11, 4258–4266. [Google Scholar] [CrossRef]
- Ai, Y.; Hsu, T.H.; Wu, D.C.; Lee, L.; Chen, J.-H.; Chen, Y.-Z.; Wu, S.-C.; Wu, C.; Wang, Z.M.; Chueh, Y.-L. An ultrasensitive flexible pressure sensor for multimodal wearable electronic skins based on large-scale polystyrene ball@reduced graphene-oxide core–shell nanoparticles. J. Mater. Chem. C 2018, 6, 5514–5520. [Google Scholar] [CrossRef]
- Kim, S.J.; Mondal, S.; Min, B.K.; Choi, C.G. Highly sensitive and flexible strain-pressure sensors with cracked paddy-shaped mos2/graphene foam/ecoflex hybrid nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 36377–36384. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Yue, Y.; Rao, J.; Cheng, F.; Su, J.; Liu, Z.; Gao, Y. Piezoresistive pressure sensor based on synergistical innerconnect polyvinyl alcohol nanowires/wrinkled graphene film. Small 2018, 14, e1704149. [Google Scholar] [CrossRef]
- Kweon, O.Y.; Lee, S.J.; Oh, J.H. Wearable high-performance pressure sensors based on three-dimensional electrospun conductive nanofibers. NPG Asia Mater. 2018, 10, 540–551. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, Q.; Wu, Y.; Wang, Y.; Qing, X.; Li, M.; Liu, K.; Wang, W.; Wang, D. A nanofiber based artificial electronic skin with high pressure sensitivity and 3D conformability. Nanoscale 2016, 8, 12105–12112. [Google Scholar] [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef]
- Li, S.X.; Xia, H.; Xu, Y.S.; Lv, C.; Wang, G.; Dai, Y.Z.; Sun, H.B. Gold nanoparticle densely packed micro/nanowire-based pressure sensors for human motion monitoring and physiological signal detection. Nanoscale 2019, 11, 4925–4932. [Google Scholar] [CrossRef]
- Wang, Y.; Chao, M.; Wan, P.; Zhang, L. A wearable breathable pressure sensor from metal-organic framework derived nanocomposites for highly sensitive broad-range healthcare monitoring. Nano Energy 2020, 70, 1801858. [Google Scholar] [CrossRef]
- Zhao, X.H.; Ma, S.N.; Long, H.; Yuan, H.; Tang, C.Y.; Cheng, P.K.; Tsang, Y.H. Multifunctional sensor based on porous carbon derived from metal-organic frameworks for real time health monitoring. ACS Appl. Mater. Interfaces 2018, 10, 3986–3993. [Google Scholar] [CrossRef]
- Pang, C.; Lee, G.Y.; Kim, T.I.; Kim, S.M.; Kim, H.N.; Ahn, S.H.; Suh, K.Y. A flexible and highly sensitive strain-gauge sensor using reversible interlocking of nanofibres. Nat. Mater. 2012, 11, 795–801. [Google Scholar] [CrossRef]
- Zhuo, B.; Chen, S.; Zhao, M.; Guo, X. High sensitivity flexible capacitive pressure sensor using polydimethylsiloxane elastomer dielectric layer micro-structured by 3-D printed mold. IEEE J. Electron. Dev. Soc. 2017, 5, 219–223. [Google Scholar] [CrossRef]
- Wu, N.; Chen, S.; Lin, S.; Li, W.; Xu, Z.; Yuan, F.; Huang, L.; Hu, B.; Zhou, J. Theoretical study and structural optimization of a flexible piezoelectret-based pressure sensor. J. Mater. Chem. A 2018, 6, 5065–5070. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.; Tee, B.C.; Stoltenberg, R.M.; Chen, C.V.; Barman, S.; Muir, B.V.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef]
- Shuai, X.; Zhu, P.; Zeng, W.; Hu, Y.; Liang, X.; Zhang, Y.; Sun, R.; Wong, C.P. Highly sensitive flexible pressure sensor based on silver nanowires-embedded polydimethylsiloxane electrode with microarray structure. ACS Appl. Mater. Interfaces 2017, 9, 26314–26324. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Eom, J.; Jaisutti, R.; Lee, H.; Lee, W.; Heo, J.S.; Lee, J.Y.; Park, S.K.; Kim, Y.H. Highly sensitive textile strain sensors and wireless user-interface devices using all-polymeric conducting fibers. ACS Appl. Mater. Interfaces 2017, 9, 10190–10197. [Google Scholar] [CrossRef]
- Nandi, A.K.; Mandelkern, L. The influence of chain structure on the equilibrium melting temperature of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1991, 29, 1287–1297. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Analysis Method: FTIR studies of β-phase crystal formation in stretched PVDF films. Polym. Test. 2003, 22, 699–704. [Google Scholar] [CrossRef]
- Giannetti, E. Semi-crystalline fluorinated polymers. Polym. Int. 2001, 50, 10–26. [Google Scholar] [CrossRef]
- Wang, M.; Gurunathan, R.; Imasato, K.; Geisendorfer, N.R.; Jakus, A.E.; Peng, J.; Shah, R.N.; Grayson, M.; Snyder, G.J. A Percolation Model for Piezoresistivity in Conductor–Polymer Composites. Adv. Theory Simul. 2018, 2, 2. [Google Scholar] [CrossRef]
- Leleux, P.; Badier, J.M.; Rivnay, J.; Benar, C.; Herve, T.; Chauvel, P.; Malliaras, G.G. Conducting polymer electrodes for electroencephalography. Adv. Healthcare Mater. 2014, 3, 490–493. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Aagesen, M.; Liu, H. III–V nanowires and nanowire optoelectronic devices. J. Phys. D Appl. Phys. 2015, 48, 463001. [Google Scholar] [CrossRef]
- Signorello, G.; Sant, S.; Bologna, N.; Schraff, M.; Drechsler, U.; Schmid, H.; Wirths, S.; Rossell, M.D.; Schenk, A.; Riel, H. manipulating surface states of iii-v nanowires with uniaxial stress. Nano Lett. 2017, 17, 2816–2824. [Google Scholar] [CrossRef]
- Lee, P.; Lee, J.; Lee, H.; Yeo, J.; Hong, S.; Nam, K.H.; Lee, D.; Lee, S.S.; Ko, S.H. Highly stretchable and highly conductive metal electrode by very long metal nanowire percolation network. Adv. Mater. 2012, 24, 3326–3332. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.D.; Cao, X.W.; Buividas, R.; Wang, X.; Juodkazis, S.; Sun, H.B. Plasmonic nano-printing: Large-area nanoscale energy deposition for efficient surface texturing. Light Sci. Appl. 2017, 6, e17112. [Google Scholar] [CrossRef]
- Pan, L.; Liu, G.; Shi, W.; Shang, J.; Leow, W.R.; Liu, Y.; Jiang, Y.; Li, S.; Chen, X.; Li, R.W. Mechano-regulated metal-organic framework nanofilm for ultrasensitive and anti-jamming strain sensing. Nat. Commun. 2018, 9, 3813. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Pathak, A.; Shen, J.W.; Usman, M.; Wei, L.F.; Mendiratta, S.; Chang, Y.S.; Sainbileg, B.; Ngue, C.M.; Chen, R.S.; Hayashi, M.; et al. Integration of a (-Cu-S-)n plane in a metal-organic framework affords high electrical conductivity. Nat. Commun. 2019, 10, 1721. [Google Scholar] [CrossRef]
- Le Ouay, B.; Boudot, M.; Kitao, T.; Yanagida, T.; Kitagawa, S.; Uemura, T. Nanostructuration of PEDOT in porous coordination polymers for tunable porosity and conductivity. J. Am. Chem. Soc. 2016, 138, 10088–10091. [Google Scholar] [CrossRef]
- Kousik, S.; Velmathi, S. Engineering Metal-organic framework catalysts for C-C and C-X coupling reactions: Advances in reticular approaches from 2014-2018. Chemistry 2019, 25, 16451–16505. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, X.; Gao, H.; Chen, S.; Wang, J. Metal-organic framework-based nanomaterials for biomedical applications. Chin. Chem. Lett. 2019, 9, 3122–3133. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and optical properties of 2D transition metal carbides and nitrides (MXenes). Adv. Mater. 2018, 30, e1804779. [Google Scholar] [CrossRef]
- Luo, J.-Q.; Zhao, S.; Zhang, H.-B.; Deng, Z.; Li, L.; Yu, Z.-Z. Flexible, stretchable and electrically conductive MXene/natural rubber nanocomposite films for efficient electromagnetic interference shielding. Compos. Sci. Technol. 2019, 182, 107754. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Lipatov, A.; Lu, H.; Alhabeb, M.; Anasori, B.; Gruverman, A.; Gogotsi, Y.; Sinitskii, A. Elastic properties of 2D Ti3C2T x MXene monolayers and bilayers. Sci. Adv. 2018, 4, eaat0491. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, G.; Wu, M.; Liu, Z.; Xiang, D.; Wu, C.; Cheng, Y.; Wang, H.; Wang, Z.L.; Li, L. Ionogel infiltrated paper as flexible electrode for wearable all-paper based sensors in active and passive modes. Nano Energy 2019, 66, 104161. [Google Scholar] [CrossRef]
- Kim, K.; Jung, M.; Kim, B.; Kim, J.; Shin, K.; Kwon, O.-S.; Jeon, S. Low-voltage, high-sensitivity and high-reliability bimodal sensor array with fully inkjet-printed flexible conducting electrode for low power consumption electronic skin. Nano Energy 2017, 41, 301–307. [Google Scholar] [CrossRef]
- Fan, X.; Xu, B.; Wang, N.; Wang, J.; Liu, S.; Wang, H.; Yan, F. Highly conductive stretchable all-plastic electrodes using a novel dipping-embedded transfer method for high-performance wearable sensors and semitransparent organic solar cells. Adv. Electron. Mater. 2017, 3, 5. [Google Scholar] [CrossRef]
- Kim, K.; Song, G.; Park, C.; Yun, K.S. Multifunctional woven structure operating as triboelectric energy harvester, capacitive tactile sensor array, and piezoresistive strain sensor array. Sensors 2017, 17, 2582. [Google Scholar] [CrossRef]
- Ding, S.; Jiu, J.; Gao, Y.; Tian, Y.; Araki, T.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; et al. One-Step Fabrication of Stretchable Copper Nanowire Conductors by a Fast Photonic Sintering Technique and Its Application in Wearable Devices. ACS Appl. Mater. Interfaces 2016, 8, 6190–6199. [Google Scholar] [CrossRef]
- Choo, D.C.; Bae, S.K.; Kim, T.W. Flexible, transparent patterned electrodes based on graphene oxide/silver nanowire nanocomposites fabricated utilizing an accelerated ultraviolet/ozone process to control silver nanowire degradation. Sci. Rep. 2019, 9, 5527. [Google Scholar] [CrossRef]
- Hyun, W.J.; Secor, E.B.; Hersam, M.C.; Frisbie, C.D.; Francis, L.F. High-resolution patterning of graphene by screen printing with a silicon stencil for highly flexible printed electronics. Adv. Mater. 2015, 27, 109–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Cui, K.; Ge, S.; Cheng, X.; Yan, M.; Yu, J.; Liu, H. Flexible electronics based on micro/nanostructured paper. Adv. Mater. 2018, 30, e1801588. [Google Scholar] [CrossRef]
- Xu, B.; Gopalan, S.A.; Gopalan, A.I.; Muthuchamy, N.; Lee, K.P.; Lee, J.S.; Jiang, Y.; Lee, S.W.; Kim, S.W.; Kim, J.S.; et al. Functional solid additive modified PEDOT:PSS as an anode buffer layer for enhanced photovoltaic performance and stability in polymer solar cells. Sci. Rep. 2017, 7, 45079. [Google Scholar] [CrossRef]
- Pereni, C.I.; Zhao, Q.; Liu, Y.; Abel, E. Surface free energy effect on bacterial retention. Colloids Surf. B Biointerfaces 2006, 48, 143–147. [Google Scholar] [CrossRef]
- Gusnaniar, N.; van der Mei, H.C.; Qu, W.; Nuryastuti, T.; Hooymans, J.M.M.; Sjollema, J.; Busscher, H.J. Physico-chemistry of bacterial transmission versus adhesion. Adv. Colloid Interface Sci. 2017, 250, 15–24. [Google Scholar] [CrossRef]
- Oh, J.Y.; Rondeau-Gagne, S.; Chiu, Y.C.; Chortos, A.; Lissel, F.; Wang, G.N.; Schroeder, B.C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, 411–415. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Choe, A.; Cho, S.; Kim, J.; Ko, H. Mimicking human and biological skins for multifunctional skin electronics. Adv. Funct. Mater. 2019, 11, 1904523. [Google Scholar] [CrossRef]
- Shimizu, R.; Nonomura, Y. Preparation of artificial skin that mimics human skin surface and mechanical properties. J. Oleo Sci. 2018, 67, 47–54. [Google Scholar] [CrossRef]
- Wang, T.; Si, Y.; Luo, S.; Dong, Z.; Jiang, L. Wettability manipulation of overflow behavior via vesicle surfactant for water-proof surface cleaning. Mater. Horiz. 2019, 6, 294–301. [Google Scholar] [CrossRef]
- Stadlober, B.; Zirkl, M.; Irimia-Vladu, M. Route towards sustainable smart sensors: Ferroelectric polyvinylidene fluoride-based materials and their integration in flexible electronics. Chem. Soc. Rev. 2019, 48, 1787–1825. [Google Scholar] [CrossRef]
- Santarelli, L. Organic Semiconductors-Based Devices Electrical Reliability to Environmental Stress. Ph.D. Thesis, University College London (UCL), London, UK, 2018. [Google Scholar]
- Xu, K.; Lu, Y.; Takei, K. Multifunctional skin-inspired flexible sensor systems for wearable electronics. Adv. Mater. Technol. 2019, 4, 1800628. [Google Scholar] [CrossRef]
- Kenry; Yeo, J.C.; Lim, C.T. Emerging flexible and wearable physical sensing platforms for healthcare and biomedical applications. Microsyst. Nanoeng. 2016, 2, 16043. [Google Scholar] [CrossRef]
- Song, F.; Ren, D. Stiffness of cross-linked poly(dimethylsiloxane) affects bacterial adhesion and antibiotic susceptibility of attached cells. Langmuir 2014, 30, 10354–10362. [Google Scholar] [CrossRef]
- Song, F.; Brasch, M.E.; Wang, H.; Henderson, J.H.; Sauer, K.; Ren, D. How bacteria respond to material stiffness during attachment: A role of escherichia coli flagellar motility. ACS Appl. Mater. Interfaces 2017, 9, 22176–22184. [Google Scholar] [CrossRef]
- Song, F.; Wang, H.; Sauer, K.; Ren, D. Cyclic-di-GMP and oprF are involved in the response of pseudomonas aeruginosa to substrate material stiffness during attachment on polydimethylsiloxane (PDMS). Front. Microbiol. 2018, 9, 110. [Google Scholar] [CrossRef]
- Hia, I.L.; Vahedi, V.; Pasbakhsh, P. Self-healing polymer composites: Prospects, challenges, and applications. Polym. Rev. 2016, 56, 225–261. [Google Scholar] [CrossRef]
- Latif, S.; Amin, S.; Haroon, S.S.; Sajjad, I.A. Self-healing materials for electronic applications: An overview. Mater. Res. Express 2019, 6, 062001. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Dickey, M.D.; Majidi, C. Self-healing materials for soft-matter machines and electronics. NPG Asia Mater. 2019, 11, 21. [Google Scholar] [CrossRef]
- Wang, Y.; Adokoh, C.K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Exp. Opin. Drug Deliv. 2018, 15, 77–91. [Google Scholar] [CrossRef]
- Patrick, J.F.; Robb, M.J.; Sottos, N.R.; Moore, J.S.; White, S.R. Polymers with autonomous life-cycle control. Nature 2016, 540, 363–370. [Google Scholar] [CrossRef]
- Fritzen, C.P.; Kraemer, P. Self-diagnosis of smart structures based on dynamical properties. Mech. Syst. Sign. Process. 2009, 23, 1830–1845. [Google Scholar] [CrossRef]
- Choi, S.-B. The grand challenges in smart materials research. Front. Mater. 2014, 1, 11. [Google Scholar] [CrossRef]
- Song, P.; Yang, G.; Lang, T.; Yong, K.-T. Nanogenerators for wearable bioelectronics and biodevices. J. Phys. D Appl. Phys. 2019, 52, 2. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, I.; Gotsiridze-Columbus, N. Biofilms: Formation, Development and Properties. In Biofilms: Formation, Development and Properties; Bailey, W.C., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 609–620. [Google Scholar]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Obolski, U.; Stein, G.Y.; Hadany, L. antibiotic restriction might facilitate the emergence of multi-drug resistance. PLoS Comput. Biol. 2015, 11, e1004340. [Google Scholar] [CrossRef]
- MacCallum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B.; et al. Liquid-infused silicone as a biofouling-free medical material. ACS Biomater. Sci. Eng. 2014, 1, 43–51. [Google Scholar] [CrossRef]
- Miller-Petrie, M.; Pant, S.; Laxminarayan, R. Drug-Resistant Infections. In Major Infectious Diseases, 3rd ed.; Holmes, K.K., Bertozzi, S., Bloom, B.R., Jha, P., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Kargar, M.; Chang, Y.-R.; Khalili Hoseinabad, H.; Pruden, A.; Ducker, W.A. colloidal crystals delay formation of early stage bacterial biofilms. ACS Biomater. Sci. Eng. 2016, 2, 1039–1048. [Google Scholar] [CrossRef]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Bruchmann, J.; Ruping, J.L.; Levkin, P.A.; Schwartz, T. Biofilm bridges forming structural networks on patterned lubricant-infused surfaces. Adv. Sci. (Weinh) 2019, 6, 1900519. [Google Scholar] [CrossRef]
- Moazzam, P.; Razmjou, A.; Golabi, M.; Shokri, D.; Landarani-Isfahani, A. Investigating the BSA protein adsorption and bacterial adhesion of Al-alloy surfaces after creating a hierarchical (micro/nano) superhydrophobic structure. J. Biomed. Mater. Res. A 2016, 104, 2220–2233. [Google Scholar] [CrossRef]
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A brief recap of microbial adhesion and biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Becker, K. Detachment studies on microfouling in natural biofilms on substrata with different surface tensions. Int. Biodet. Biodegrad. 1998, 41, 93–100. [Google Scholar] [CrossRef]
- Tsibouklis, J.; Stone, M.; Thorpe, A.A.; Graham, P.; Peters, V.; Heerlien, R.; Smith, J.R.; Green, K.L.; Nevell, T.G. Preventing bacterial adhesion onto surfaces: The low-surface-energy approach. Biomaterials 1999, 20, 1229–1235. [Google Scholar] [CrossRef]
- Reche, I.; D’Orta, G.; Mladenov, N.; Winget, D.M.; Suttle, C.A. Deposition rates of viruses and bacteria above the atmospheric boundary layer. ISME J. 2018, 12, 1154–1162. [Google Scholar] [CrossRef]
- Yang, W.; Elankumaran, S.; Marr, L.C. Concentrations and size distributions of airborne influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes. J. R. Soc. Interface 2011, 8, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Sjollema, J.; van der Mei, H.C.; Hall, C.L.; Peterson, B.W.; de Vries, J.; Song, L.; Jong, E.D.; Busscher, H.J.; Swartjes, J. Detachment and successive re-attachment of multiple, reversibly-binding tethers result in irreversible bacterial adhesion to surfaces. Sci. Rep. 2017, 7, 4369. [Google Scholar] [CrossRef] [PubMed]
- Poortinga, A.T.; Bos, R.; Norde, W.; Busscher, H.J. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 2002, 47, 1–32. [Google Scholar] [CrossRef]
- Hong, Y.; Brown, D.G. Electrostatic behavior of the charge-regulated bacterial cell surface. Langmuir 2008, 24, 5003–5009. [Google Scholar] [CrossRef]
- Soni, K.A.; Balasubramanian, A.K.; Beskok, A.; Pillai, S.D. Zeta potential of selected bacteria in drinking water when dead, starved, or exposed to minimal and rich culture media. Curr. Microbiol. 2008, 56, 93–97. [Google Scholar] [CrossRef]
- Samandoulgou, I.; Fliss, I.; Jean, J. Zeta Potential and aggregation of virus-like particle of human norovirus and feline calicivirus under different physicochemical conditions. Food Environ. Virol. 2015, 7, 249–260. [Google Scholar] [CrossRef]
- Tan, M.S.; White, A.P.; Rahman, S.; Dykes, G.A. Role of fimbriae, flagella and cellulose on the attachment of salmonella typhimurium ATCC 14028 to plant cell wall models. PLoS ONE 2016, 11, e0158311. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef]
- Hsu, L.C.; Fang, J.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Effect of micro- and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef]
- Spengler, C.; Nolle, F.; Mischo, J.; Faidt, T.; Grandthyll, S.; Thewes, N.; Koch, M.; Muller, F.; Bischoff, M.; Klatt, M.A.; et al. Strength of bacterial adhesion on nanostructured surfaces quantified by substrate morphometry. Nanoscale 2019, 11, 19713–19722. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Maattanen, A.; Fallarero, A.; Kujala, J.; Ihalainen, P.; Vuorela, P.; Peltonen, J. Printed paper-based arrays as substrates for biofilm formation. AMB Express 2014, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography sensitive bacterial attachment mechanisms: A review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Bos, R.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions-its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef]
- Van der Mei, H.C.; Bos, R.; Busscher, H.J. A reference guide to microbial cell surface hydrophobicity based on contact angles. Colloids Surf. B Biointerfaces 1998, 11, 213–221. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yan, T.; Jiang, Z.; Zhang, X.; Zuo, Y.Y. Quantitatively predicting bacterial adhesion using surface free energy determined with a spectrophotometric method. Environ. Sci. Technol. 2015, 49, 6164–6171. [Google Scholar] [CrossRef]
- Swartjes, J.J.T.M.; Veeregowda, D.H.; van der Mei, H.C.; Busscher, H.J.; Sharma, P.K. Normally oriented adhesion versus friction forces in bacterial adhesion to polymer-brush functionalized surfaces under fluid flow. Adv. Funct. Mater. 2014, 24, 4435–4441. [Google Scholar] [CrossRef]
- Valentin, J.D.P.; Qin, X.H.; Fessele, C.; Straub, H.; van der Mei, H.C.; Buhmann, M.T.; Maniura-Weber, K.; Ren, Q. Substrate viscosity plays an important role in bacterial adhesion under fluid flow. J. Colloid Interface Sci. 2019, 552, 247–257. [Google Scholar] [CrossRef]
- Freschauf, L.R.; McLane, J.; Sharma, H.; Khine, M. Shrink-induced superhydrophobic and antibacterial surfaces in consumer plastics. PLoS ONE 2012, 7, e40987. [Google Scholar] [CrossRef]
- Bonner, M.C.; Tunney, M.M.; Jones, D.S.; Gorman, S.P. Factors affecting in vitro adherence of ureteral stent biofilm isolates to polyurethane. Int. J. Pharm. 1997, 151, 201–207. [Google Scholar] [CrossRef]
- Reid, G.; Beg, H.S.; Preston, C.A.K.; Hawthorn, L.A. Effect of bacterial, urine and substratum surface tension properties on bacterial adhesion to biomaterials. Biofouling 1991, 4, 171–176. [Google Scholar] [CrossRef]
- Dou, X.Q.; Zhang, D.; Feng, C.; Jiang, L. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.A.; Antonelli, P.J.; Brennan, A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94. [Google Scholar] [CrossRef]
- Dundar Arisoy, F.; Kolewe, K.W.; Homyak, B.; Kurtz, I.S.; Schiffman, J.D.; Watkins, J.J. Bioinspired Photocatalytic shark-skin surfaces with antibacterial and antifouling activity via nanoimprint lithography. ACS Appl. Mater. Interfaces 2018, 10, 20055–20063. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Pillet, F.; Formosa-Dague, C.; Baaziz, H.; Dague, E.; Rols, M.P. Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci. Rep. 2016, 6, 19778. [Google Scholar] [CrossRef]
- Zhu, A.; Liu, H.K.; Long, F.; Su, E.; Klibanov, A.M. Inactivation of bacteria by electric current in the presence of carbon nanotubes embedded within a polymeric membrane. Appl. Biochem. Biotechnol. 2015, 175, 666–676. [Google Scholar] [CrossRef]
- Montazerian, H.; Rashidi, A.; Dalili, A.; Najjaran, H.; Milani, A.S.; Hoorfar, M. Graphene-coated spandex sensors embedded into silicone sheath for composites health monitoring and wearable applications. Small 2019, 15, e1804991. [Google Scholar] [CrossRef]
- Yu, L.; Chen, G.Y.; Xu, H.; Liu, X. Substrate-Independent, Transparent oil-repellent coatings with self-healing and persistent easy-sliding oil repellency. ACS Nano 2016, 10, 1076–1085. [Google Scholar] [CrossRef]
- Prosheva, M.; Aboudzadeh, M.A.; Leal, G.P.; Gilev, J.B.; Tomovska, R. High-performance uv protective waterborne polymer coatings based on hybrid graphene/carbon nanotube radicals scavenging filler. Part. Part. Syst. Charact. 2019, 36, 1800555. [Google Scholar] [CrossRef]
- Nuraje, N.; Khan, S.I.; Misak, H.; Asmatulu, R. The Addition of graphene to polymer coatings for improved weathering. ISRN Polym. Sci. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C.; Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-titania nanocomposite coating: Fabrication and corrosion resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Nakamura, A.; Hamanishi, T.; Kawakami, S.; Takeda, M. A piezo-resistive graphene strain sensor with a hollow cylindrical geometry. Mater. Sci. Eng. B 2017, 219, 20–27. [Google Scholar] [CrossRef]
- Maya-Cornejo, J.; Rodríguez-Gómez, F.J.; Molina, G.A.; Galindo-de-la-Rosa, J.; Ledesma-García, J.; Hernández-Martínez, Á.R.; Esparza, R.; Pérez, R.; Estévez, M. Electrochemical study of a hybrid polymethyl methacrylate coating using SIO2 nanoparticles toward the mitigation of the corrosion in marine environments. Materials 2019, 12, 3216. [Google Scholar] [CrossRef]
- Radwan, A.B.; Mohamed, A.M.A.; Abdullah, A.M.; Al-Maadeed, M.A. Corrosion protection of electrospun PVDF–ZnO superhydrophobic coating. Surf. Coatings Technol. 2016, 289, 136–143. [Google Scholar] [CrossRef]
- Xiao, Y.-K.; Ji, W.-F.; Chang, K.-S.; Hsu, K.-T.; Yeh, J.-M.; Liu, W.-R. Sandwich-structured rGO/PVDF/PU multilayer coatings for anti-corrosion application. RSC Adv. 2017, 7, 33829–33836. [Google Scholar] [CrossRef]
- Gao, H.; Minh, P.T.; Wang, H.; Minko, S.; Locklin, J.; Nguyen, T.; Sharma, S. High-performance flexible yarn for wearable piezoelectric nanogenerators. Smart Mater. Struct. 2018, 27, 095018. [Google Scholar] [CrossRef]
- Satyanarayana, N.; Minn, M.; Samad, M.A.; Sinha, S.K. Polymer Tribological Coatings. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.-W., Eds.; Springer: Boston, MA, USA, 2013; pp. 2608–2614. [Google Scholar]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Wang, X.; Yang, Y.; Hu, Z.; Liu, L.; Huang, Y. Highly Conductive PVA/Ag Coating by Aqueous in Situ Reduction and Its Stretchable Structure for Strain Sensor. ACS Appl. Mater. Interfaces 2020, 12, 1427–1435. [Google Scholar] [CrossRef]
- Busuioc, C.; Evanghelidis, A.; Galatanu, A.; Enculescu, I. Direct and contactless electrical control of temperature of paper and textile foldable substrates using electrospun metallic-web transparent electrodes. Sci. Rep. 2016, 6, 34584. [Google Scholar] [CrossRef]
- Chun, S.; Choi, Y.; Park, W. All-graphene strain sensor on soft substrate. Carbon 2017, 116, 753–759. [Google Scholar] [CrossRef]
- Li, X.; Koh, K.H.; Farhan, M.; Lai, K.W.C. An ultraflexible polyurethane yarn-based wearable strain sensor with a polydimethylsiloxane infiltrated multilayer sheath for smart textiles. Nanoscale 2020, 12, 4110–4118. [Google Scholar] [CrossRef] [PubMed]
- Therapeutic Goods Administration. Literature Review on the Safety Of Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens, 1st ed.; Health, D., Ed.; Australian Government Department of Health: Canberra, Australia, 2016; p. 24.
- Dong, C.; Fu, Y.; Zang, W.; He, H.; Xing, L.; Xue, X. Self-powering/self-cleaning electronic-skin basing on PVDF/TiO2 nanofibers for actively detecting body motion and degrading organic pollutants. Appl. Surf. Sci. 2017, 416, 424–431. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Ling, L.; Yan, S.; Pan, D.; Ge, H.; Li, H.; Bian, Z. Enhanced photocatalytic degradation performance by fluid-induced piezoelectric field. Environ. Sci. Technol. 2018, 52, 7842–7848. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Tong, Y.; Zhang, M.; Zhang, Y.; Shao, C. TiO2 nanoparticles immobilized on polyacrylonitrile nanofibers mats: A flexible and recyclable photocatalyst for phenol degradation. RSC Adv. 2013, 3, 7503–7512. [Google Scholar] [CrossRef]
- Marelli, B.; Brenckle, M.A.; Kaplan, D.L.; Omenetto, F.G. Silk fibroin as edible coating for perishable food preservation. Sci. Rep. 2016, 6, 25263. [Google Scholar] [CrossRef]
- Umuhoza, D.; Yang, F.; Long, D.; Hao, Z.; Dai, J.; Zhao, A. Strategies for tuning the biodegradation of silk fibroin-based materials for tissue engineering applications. ACS Biomater. Sci. Eng. 2020, 6, 1290–1310. [Google Scholar] [CrossRef]
- Ling, S.; Qin, Z.; Li, C.; Huang, W.; Kaplan, D.L.; Buehler, M.J. Polymorphic regenerated silk fibers assembled through bioinspired spinning. Nat. Commun. 2017, 8, 1387. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Gao, E.; Jian, M.; Xia, K.; Wang, Q.; Xu, Z.; Ren, T.; Zhang, Y. Carbonized silk fabric for ultrastretchable, highly sensitive, and wearable strain sensors. Adv. Mater. 2016, 28, 6640–6648. [Google Scholar] [CrossRef]
- Li, L.; Bai, Y.; Li, L.; Wang, S.; Zhang, T. A Superhydrophobic smart coating for flexible and wearable sensing electronics. Adv. Mater. 2017, 29, 1702517. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rao, G.V.R.; Ista, L.K.; Wu, Y.; Andrzejewski, B.P.; Sklar, L.A.; Ward, T.L.; López, G.P. Control of molecular transport through stimuli-responsive ordered mesoporous materials. Adv. Mater. 2003, 15, 1262–1266. [Google Scholar] [CrossRef]
- Stumpel, J.E.; Broer, D.J.; Schenning, A.P. Stimuli-responsive photonic polymer coatings. Chem. Commun. (Cambridge) 2014, 50, 15839–15848. [Google Scholar] [CrossRef] [PubMed]
- Cotting, F.; Koebsch, A.; Aoki, I.V. Epoxy self-healing coating by encapsulated epoxy ester resin in poly (urea-formaldehyde-melamine) microcapsules. Front. Mater. 2019, 6, 314. [Google Scholar] [CrossRef]
- Lazauskas, A.; Jucius, D.; Baltrusaitis, V.; Gudaitis, R.; Prosycevas, I.; Abakeviciene, B.; Guobiene, A.; Andrulevicius, M.; Grigaliunas, V. Shape-memory assisted scratch-healing of transparent thiol-ene coatings. Materials 2019, 12, 482. [Google Scholar] [CrossRef]
- Coppola, A.M.; Griffin, A.S.; Sottos, N.R.; White, S.R. Retention of mechanical performance of polymer matrix composites above the glass transition temperature by vascular cooling. Compos. Part A Appl. Sci. Manufact. 2015, 78, 412–423. [Google Scholar] [CrossRef]
- Xu, K.; Lu, Y.; Yamaguchi, T.; Arie, T.; Akita, S.; Takei, K. Highly precise multifunctional thermal management-based flexible sensing sheets. ACS Nano 2019, 13, 14348–14356. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Wang, B.; Yang, H.; Ban, J.; Liu, F.; Li, T. Acute effects of temperature exposure on blood pressure: An hourly level panel study. Environ. Int. 2019, 124, 493–500. [Google Scholar] [CrossRef]
- Halonen, J.I.; Zanobetti, A.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Relationship between outdoor temperature and blood pressure. Occup. Environ. Med. 2011, 68, 296–301. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, J.; Ma, Z.; Yan, K.; Wang, Y.; Wang, H.; Li, S.; Li, Y.; Pan, L.; Shi, Y. Flexible pressure sensor with high sensitivity and low hysteresis based on a hierarchically microstructured electrode. IEEE Electron. Dev. Lett. 2018, 39, 288–291. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Repellent materials. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Fernandez-Blazquez, J.P.; Fell, D.; Bonaccurso, E.; del Campo, A. Superhydrophilic and superhydrophobic nanostructured surfaces via plasma treatment. J. Colloid Interface Sci. 2011, 357, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Kota, A.K.; Mabry, J.M.; Tuteja, A. Superomniphobic surfaces for effective chemical shielding. J. Am. Chem. Soc. 2013, 135, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lin, L.; Zang, D.; Guo, X.; Liu, M. Designing bioinspired anti-biofouling surfaces based on a superwettability strategy. Small 2017, 13, 1503334. [Google Scholar] [CrossRef] [PubMed]
- Hermelin, E.; Petitjean, J.; Lacroix, J.-C.; Chane-Ching, K.I.; Tanguy, J.; Lacaze, P.-C. Ultrafast electrosynthesis of high hydrophobic polypyrrole coatings on a zinc electrode: Applications to the protection against corrosion. Chem. Mater. 2008, 20, 4447–4456. [Google Scholar] [CrossRef]
- Bi; Li; Zhao; Ran; Cao; Guo; Xue, Robust super-hydrophobic coating prepared by electrochemical surface engineering for corrosion protection. Coatings 2019, 9, 7.
- Wang, P.; Sun, B.; Liang, Y.; Han, H.; Fan, X.; Wang, W.; Yang, Z. A stretchable and super-robust graphene superhydrophobic composite for electromechanical sensor application. J. Mater. Chem. A 2018, 6, 10404–10410. [Google Scholar] [CrossRef]
- Rawal, S.P.; Goodman, J.W. Composites for Spacecraft. In Comprehensive Composite Materials; Kelly, A., Zweben, C., Eds.; Pergamon: Oxford, UK, 2000; pp. 279–315. [Google Scholar]
- Ali, N.; Hong, J.-E. Failure detection and prevention for cyber-physical systems using ontology-based knowledge base. Computers 2018, 7, 68. [Google Scholar] [CrossRef]
- Rodgers, M.A.J. Chromic Phenomena: Technological Applications of Color Chemistry. J. Am. Chem. Soc. 2002, 124, 13960. [Google Scholar] [CrossRef]
- Pucci, A. Mechanochromic Fluorescent polymers with aggregation-induced emission features. Sensors 2019, 19, 4969. [Google Scholar] [CrossRef]
- Fu, F.; Hu, L. Temperature sensitive colour-changed composites. In Advanced High Strength Natural Fibre Composites in Construction; Fan, M., Fu, F., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 405–423. [Google Scholar]
- Kong, L.B.; Ruan, S.; Xiao, Z.; Li, X.; Zhou, K.; Su, H.; Wang, C.; Zhang, T. Tin oxide-based electrochromics. In Tin Oxide Materials; Orlandi, M.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 489–517. [Google Scholar]
- Estupiñán, D.; Gegenhuber, T.; Blinco, J.P.; Barner-Kowollik, C.; Barner, L. Self-reporting fluorescent step-growth raft polymers based on nitrile imine-mediated tetrazole-ene cycloaddition chemistry. ACS Macro Lett. 2017, 6, 229–234. [Google Scholar] [CrossRef]
- Kim, T.A.; Robb, M.J.; Moore, J.S.; White, S.R.; Sottos, N.R. Mechanical reactivity of two different spiropyran mechanophores in polydimethylsiloxane. Macromolecules 2018, 51, 9177–9183. [Google Scholar] [CrossRef]
- Gossweiler, G.R.; Brown, C.L.; Hewage, G.B.; Sapiro-Gheiler, E.; Trautman, W.J.; Welshofer, G.W.; Craig, S.L. Mechanochemically Active Soft Robots. ACS Appl. Mater. Interfaces 2015, 7, 22431–22435. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hisano, K.; Zhu, M.; Toyoshi, T.; Pan, M.; Okada, S.; Tsutsumi, O.; Kawamura, S.; Bowen, C. Flexible multifunctional sensors for wearable and robotic applications. Adv. Mater. Technol. 2019, 4, 1800626. [Google Scholar] [CrossRef]
- Beiermann, B.A.; Kramer, S.L.B.; Moore, J.S.; White, S.R.; Sottos, N.R. Role of mechanophore orientation in mechanochemical reactions. ACS Macro Lett. 2011, 1, 163–166. [Google Scholar] [CrossRef]
- Bowser, B.H.; Craig, S.L. Empowering mechanochemistry with multi-mechanophore polymer architectures. Polym. Chem. 2018, 9, 3583–3593. [Google Scholar] [CrossRef]
- Li, J.; Nagamani, C.; Moore, J.S. Polymer mechanochemistry: From destructive to productive. Acc. Chem. Res. 2015, 48, 2181–2190. [Google Scholar] [CrossRef]
- Kean, Z.S.; Niu, Z.; Hewage, G.B.; Rheingold, A.L.; Craig, S.L. Stress-responsive polymers containing cyclobutane core mechanophores: Reactivity and mechanistic insights. J. Am. Chem. Soc. 2013, 135, 13598–13604. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Khusnutdinova, J.R. Dynamic phosphorescent probe for facile and reversible stress sensing. Adv. Mater. 2017, 29, 1700563. [Google Scholar] [CrossRef]
- Schenzel, A.M.; Moszner, N.; Barner-Kowollik, C. Self-reporting dynamic covalent polycarbonate networks. Polym. Chem. 2017, 8, 414–420. [Google Scholar] [CrossRef]
- Peterson, G.I.; Larsen, M.B.; Ganter, M.A.; Storti, D.W.; Boydston, A.J. 3D-printed mechanochromic materials. ACS Appl. Mater. Interfaces 2015, 7, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, G.; Ouyang, M.; Hu, B.; Song, Q.; Sun, J.; Zhang, C.; Gu, C.; Xu, Y.; Ma, Y. Mechanochromic and thermochromic fluorescent properties of cyanostilbene derivatives. Dyes Pigments 2013, 98, 486–492. [Google Scholar] [CrossRef]
- Toivola, R.; Jang, S.-H.; Baker, S.; Jen, A.K.-Y.; Flinn, B.D. Thermochromic polymer film sensors for detection of incipient thermal damage in carbon fiber–epoxy composites. Sensors 2018, 18, 1362. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Nguyen, A.; Chortos, A.; To, J.W.; Lu, C.; Mei, J.; Kurosawa, T.; Bae, W.G.; Tok, J.B.; Bao, Z. A chameleon-inspired stretchable electronic skin with interactive colour changing controlled by tactile sensing. Nat. Commun. 2015, 6, 8011. [Google Scholar] [CrossRef]
- Chung, D.D.L. Damage detection using self-sensing concepts. Proc. Inst. Mech. Eng. Part G J. Aerospace Eng. 2007, 221, 509–520. [Google Scholar] [CrossRef]
- Gao, L.; Thostenson, E.T.; Zhang, Z.; Chou, T.-W. Coupled carbon nanotube network and acoustic emission monitoring for sensing of damage development in composites. Carbon 2009, 47, 1381–1388. [Google Scholar] [CrossRef]
- Odom, S.A.; Tyler, T.P.; Caruso, M.M.; Ritchey, J.A.; Schulmerich, M.V.; Robinson, S.J.; Bhargava, R.; Sottos, N.R.; White, S.R.; Hersam, M.C.; et al. Autonomic restoration of electrical conductivity using polymer-stabilized carbon nanotube and graphene microcapsules. Appl. Phys. Lett. 2012, 101, 043106. [Google Scholar] [CrossRef][Green Version]
- Baltopoulos, A.; Polydorides, N.; Pambaguian, L.; Vavouliotis, A.; Kostopoulos, V. Damage identification in carbon fiber reinforced polymer plates using electrical resistance tomography mapping. J. Compos. Mater. 2012, 47, 3285–3301. [Google Scholar] [CrossRef]
- Hu, M.; Peil, S.; Xing, Y.; Döhler, D.; Caire da Silva, L.; Binder, W.H.; Kappl, M.; Bannwarth, M.B. Monitoring crack appearance and healing in coatings with damage self-reporting nanocapsules. Mater. Horiz. 2018, 5, 51–58. [Google Scholar] [CrossRef]
- Robb, M.J.; Li, W.; Gergely, R.C.; Matthews, C.C.; White, S.R.; Sottos, N.R.; Moore, J.S. A Robust damage-reporting strategy for polymeric materials enabled by aggregation-induced emission. ACS Cent. Sci. 2016, 2, 598–603. [Google Scholar] [CrossRef]
- Kang, J.; Son, D.; Wang, G.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv. Mater. 2018, 30, e1706846. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: From old chemistry to modern day innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, S.M.; Eom, Y.; Koo, J.M.; Cho, H.W.; Lee, T.J.; Lee, K.G.; Park, H.J.; Kim, Y.K.; Yoo, H.J.; et al. Extremely Fast Self-Healable Bio-Based Supramolecular Polymer for Wearable Real-Time Sweat-Monitoring Sensor. ACS Appl. Mater. Interfaces 2019, 11, 46165–46175. [Google Scholar] [CrossRef]
- Cao, S.; Tong, X.; Dai, K.; Xu, Q. A super-stretchable and tough functionalized boron nitride/PEDOT:PSS/poly(N-isopropylacrylamide) hydrogel with self-healing, adhesion, conductive and photothermal activity. J. Mater. Chem. A 2019, 7, 8204–8209. [Google Scholar] [CrossRef]
- Park, S.; Shin, B.G.; Jang, S.; Chung, K. Three-dimensional self-healable touch sensing artificial skin device. ACS Appl. Mater. Interfaces 2020, 12, 3953–3960. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A healable supramolecular polymer blend based on aromatic π−π stacking and hydrogen-bonding interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef]
- Keller, M.W. CHAPTER 2. Encapsulation-Based Self-Healing Polymers and Composites. In Healable Polymer Systems; The Royal Society of Chemistry: London, UK, 2013; pp. 16–61. [Google Scholar]
- Tian, F.; Zhong, Z.; Pan, Y. A chemo-mechanically coupled model for capsule-based self-healing polymer materials. Int. J. Damage Mech. 2018, 28, 1075–1094. [Google Scholar] [CrossRef]
- Li, P.; Liu, G.; Liu, Y.; Huang, J. Microvascular network optimization of self-healing materials using non-dominated sorting genetic algorithm II and experimental validation. Sci. Progr. 2020, 103, 36850419883541. [Google Scholar] [CrossRef] [PubMed]
- Corten, C.C.; Urban, M.W. Repairing polymers using oscillating magnetic field. Adv. Mater. 2009, 21, 5011–5015. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Lopez, C.S.; Vinu Mohan, A.M.; Yin, L.; Kumar, R.; Wang, J. All-printed magnetically self-healing electrochemical devices. Sci. Adv. 2016, 2, e1601465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Li, J.; Ling, L.; Zhang, G.; Sun, R.; Wong, C.-P. UV-triggered self-healing polyurethane with enhanced stretchability and elasticity. Polymer 2019, 172, 187–195. [Google Scholar] [CrossRef]
- Fang, Y.; Du, X.; Du, Z.; Wang, H.; Cheng, X. Light- and heat-triggered polyurethane based on dihydroxyl anthracene derivatives for self-healing applications. J. Mater. Chem. A 2017, 5, 8010–8017. [Google Scholar] [CrossRef]
- Luo, H.; Wang, H.; Zhou, H.; Zhou, X.; Hu, J.; Yi, G.; Hao, Z.; Lin, W. Shape memory-enhanced electrical self-healing of stretchable electrodes. Appl. Sci. 2018, 8, 392. [Google Scholar] [CrossRef]
- Michal, B.T.; McKenzie, B.M.; Felder, S.E.; Rowan, S.J. Metallo-, thermo-, and photoresponsive shape memory and actuating liquid crystalline elastomers. Macromolecules 2015, 48, 3239–3246. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Y.; Wei, Y.; Chen, S.; Zhu, J.; Liu, L. A high performance self-healing strain sensor with synergetic networks of poly(ɛ-caprolactone) microspheres, graphene and silver nanowires. Compos. Sci. Technol. 2017, 146, 110–118. [Google Scholar] [CrossRef]
- Ye, F.; Li, M.; Ke, D.; Wang, L.; Lu, Y. Ultrafast self-healing and injectable conductive hydrogel for strain and pressure sensors. Adv. Mater. Technol. 2019, 4, 1900346. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Haick, H. Self-healing, fully functional, and multiparametric flexible sensing platform. Adv. Mater. 2016, 28, 138–143. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, L.; Gao, Y.; Fang, X.; Lu, T.; Pan, L.; Xuan, F. Highly stretchable and self-healable mxene/polyvinyl alcohol hydrogel electrode for wearable capacitive electronic skin. Adv. Electron. Mater. 2019, 5, 1900285. [Google Scholar] [CrossRef]
- Kee, S.; Haque, M.A.; Corzo, D.; Alshareef, H.N.; Baran, D. Self-healing and stretchable 3D-printed organic thermoelectrics. Adv. Funct. Mater. 2019, 29, 1905426. [Google Scholar] [CrossRef]
- Zou, Z.; Zhu, C.; Li, Y.; Lei, X.; Zhang, W.; Xiao, J. Rehealable, fully recyclable, and malleable electronic skin enabled by dynamic covalent thermoset nanocomposite. Sci. Adv. 2018, 4, eaaq0508. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.F.; Hart, K.R.; Krull, B.P.; Diesendruck, C.E.; Moore, J.S.; White, S.R.; Sottos, N.R. Continuous self-healing life cycle in vascularized structural composites. Adv. Mater. 2014, 26, 4302–4308. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, E.; Barg, S.; Ni, N.; Rocha, V.G.; Saiz, E. Self-healing graphene-based composites with sensing capabilities. Adv. Mater. 2015, 27, 4788–4794. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Ramanujan, R.V. Magnetic field triggered multicycle damage sensing and self healing. Sci. Rep. 2015, 5, 13773. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, A.; Wu, H.; Wu, B.; Li, C.; Tang, W. Electrochemistry-assisted microstructuring of reduced graphene oxide-based microarrays with adjustable electrical behavior. Electrochem. Commun. 2014, 48, 86–90. [Google Scholar] [CrossRef]
- Haubner, K.; Murawski, J.; Olk, P.; Eng, L.M.; Ziegler, C.; Adolphi, B.; Jaehne, E. The route to functional graphene oxide. Chemphyschem 2010, 11, 2131–2139. [Google Scholar] [CrossRef]
- Wang, Q.; Ling, S.; Liang, X.; Wang, H.; Lu, H.; Zhang, Y. Self-healable multifunctional electronic tattoos based on silk and graphene. Adv. Funct. Mater. 2019, 29, 1808695. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, Y.-T.; Xie, X.-M. Self-healable, super tough graphene oxide–poly(acrylic acid) nanocomposite hydrogels facilitated by dual cross-linking effects through dynamic ionic interactions. J. Mater. Chem. B 2015, 3, 4001–4008. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, Q.; Chen, Z.; Zhang, X.; Lu, C.; Cao, J.; Zheng, Z. Scalable Manufactured Self-Healing Strain Sensors Based on Ion-Intercalated Graphene Nanosheets and Interfacial Coordination. ACS Appl. Mater. Interfaces 2019, 11, 23527–23534. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Bernal, M.M.; Grande, A.M.; Zhong, N.; van der Zwaag, S.; García, S.J. Effect of graphene content on the restoration of mechanical, electrical and thermal functionalities of a self-healing natural rubber. Smart Mater. Struct. 2017, 26, 085010. [Google Scholar] [CrossRef]
- Yao, Z.; Kane, C.L.; Dekker, C. High-field electrical transport in single-wall carbon nanotubes. Phys. Rev. Lett. 2000, 84, 2941–2944. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wu, X.; Zhang, X.; Lu, C. Self-healing, highly sensitive electronic sensors enabled by metal-ligand coordination and hierarchical structure design. ACS Appl. Mater. Interfaces 2017, 9, 20106–20114. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zou, S.; Gosselin, F.P.; Therriault, D.; Heuzey, M.-C. 3D printing of a self-healing nanocomposite for stretchable sensors. J. Mater. Chem. C 2018, 6, 12180–12186. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Qian, K.; Chen, J.; Li, S.; Lee, P.S. Extremely stretchable strain sensors based on conductive self-healing dynamic cross-links hydrogels for human-motion detection. Adv. Sci. 2017, 4, 1600190. [Google Scholar] [CrossRef]
- Liu, X.; Lu, C.; Wu, X.; Zhang, X. Self-healing strain sensors based on nanostructured supramolecular conductive elastomers. J. Mater. Chem. A 2017, 5, 9824–9832. [Google Scholar] [CrossRef]
- Bailey, B.M.; Leterrier, Y.; Garcia, S.J.; van der Zwaag, S.; Michaud, V. Electrically conductive self-healing polymer composite coatings. Progress Org. Coatings 2015, 85, 189–198. [Google Scholar] [CrossRef]
- Al-Mansoori, T.; Norambuena-Contreras, J.; Garcia, A. Effect of capsule addition and healing temperature on the self-healing potential of asphalt mixtures. Mater. Struct. 2018, 51, 53. [Google Scholar] [CrossRef]
- Zamal, H.H.; Barba, D.; Aissa, B.; Haddad, E.; Rosei, F. Recovery of electro-mechanical properties inside self-healing composites through microencapsulation of carbon nanotubes. Sci. Rep. 2020, 10, 2973. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, F.; Xue, Z.; Luan, Y.; Yan, X.; Guo, Z.; Wang, Z. Synergistic effect of different graphene-CNT heterostructures on mechanical and self-healing properties of thermoplastic polyurethane composites. Mater. Des. 2018, 137, 438–445. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, K.; Song, Y.; Han, J.; Yue, Y.; Biswas, S.K.; Wu, Q.; Xiao, H. A Skin-inspired stretchable, self-healing and electro-conductive hydrogel with a synergistic triple network for wearable strain sensors applied in human-motion detection. Nanomaterials 2019, 9, 1737. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, Y.; Li, B.; Sun, C.; Wu, Z.; Yan, H.; Xing, L.; Qi, S.; Li, Y.; Liu, H.; et al. Flexible sandwich structural strain sensor based on silver nanowires decorated with self-healing substrate. Macromol. Mater. Eng. 2019, 304, 1900074. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes stretch hydrogel sensor performance to new limits. Sci. Adv. 2018, 4, 6. [Google Scholar] [CrossRef]
- The Platform for Accelerating the Circular Economy (PACE). The E-Waste Coalition A New Circular Vision for Electronics; World Economic Forum: Cologny, Switzerland, 2019. [Google Scholar]
- Sartor, F.; Gelissen, J.; van Dinther, R.; Roovers, D.; Papini, G.B.; Coppola, G. Wrist-worn optical and chest strap heart rate comparison in a heterogeneous sample of healthy individuals and in coronary artery disease patients. BMC Sports Sci. Med. Rehabil. 2018, 10, 10. [Google Scholar] [CrossRef]
- Abt, G.; Bray, J.; Benson, A.C. The validity and inter-device variability of the Apple Watch for measuring maximal heart rate. J. Sports Sci. 2018, 36, 1447–1452. [Google Scholar] [CrossRef]
- Lee, H.; Chung, H.; Ko, H.; Lee, J. Wearable multichannel photoplethysmography framework for heart rate monitoring during intensive exercise. IEEE Sens. J. 2018, 18, 2983–2993. [Google Scholar] [CrossRef]
- Winston, F.K.; Yan, A.C. Wearable health device dermatitis: A case of acrylate-related contact allergy. Cutis 2017, 100, 97–99. [Google Scholar]
- Ilankoon, I.; Ghorbani, Y.; Chong, M.N.; Herath, G.; Moyo, T.; Petersen, J. E-waste in the international context - A review of trade flows, regulations, hazards, waste management strategies and technologies for value recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef]
- Fries, R. Biomaterials-The Intersection of Biology and Materials Science. (Temenoff, J.S. et al.; 2008) [Book reviews]. IEEE Eng. Med. Biol. Mag. 2009, 28, 100. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. biodegradable polymeric materials in degradable electronic devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shou, W.; Mahajan, B.K.; Huang, X.; Pan, H. Materials, processes, and facile manufacturing for bioresorbable electronics: A review. Adv. Mater. 2018, 30, e1707624. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.M.; Kaizawa, Y.; Schroeder, B.C.; Chortos, A.; Legrand, A.; Wang, Z.; Chang, J.; Fox, P.; Bao, Z. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 2018, 1, 314–321. [Google Scholar] [CrossRef]
- Salvatore, G.A.; Sülzle, J.; Dalla Valle, F.; Cantarella, G.; Robotti, F.; Jokic, P.; Knobelspies, S.; Daus, A.; Büthe, L.; Petti, L.; et al. Biodegradable and highly deformable temperature sensors for the internet of things. Adv. Funct. Mater. 2017, 27, 1702390. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kuroda, E.; Hirai, T.; Tsutsumi, Y.; Ishii, K.J. Allergic Responses Induced by the Immunomodulatory Effects of Nanomaterials upon Skin Exposure. Front. Immunol. 2017, 8, 169. [Google Scholar] [CrossRef]
- Tabei, Y.; Sonoda, A.; Nakajima, Y.; Biju, V.; Makita, Y.; Yoshida, Y.; Horie, M. In vitro evaluation of the cellular effect of indium tin oxide nanoparticles using the human lung adenocarcinoma A549 cells. Metallomics 2015, 7, 816–827. [Google Scholar] [CrossRef]
- Olgun, N.S.; Morris, A.M.; Barber, T.L.; Stefaniak, A.B.; Kashon, M.L.; Schwegler-Berry, D.; Cummings, K.J.; Leonard, S.S. Comparison of the toxicity of sintered and unsintered indium-tin oxide particles in murine macrophage and epidermal cells. Toxicol. Appl. Pharmacol. 2017, 331, 85–93. [Google Scholar] [CrossRef]
- Ng, A.M.; Guo, M.Y.; Leung, Y.H.; Chan, C.M.; Wong, S.W.; Yung, M.M.; Ma, A.P.; Djurisic, A.B.; Leung, F.C.; Leung, K.M.; et al. Metal oxide nanoparticles with low toxicity. J Photochem. Photobiol. B 2015, 151, 17–24. [Google Scholar] [CrossRef]
- Swarts, N. Material safety data sheet (MSDS): Indium tin oxide. In Indium Corporation of America; Indium Corporation of America: Clinton, NY, USA, 2018; Volume 13323, p. 7. [Google Scholar]
- Crosera, M.; Bovenzi, M.; Maina, G.; Adami, G.; Zanette, C.; Florio, C.; Filon Larese, F. Nanoparticle dermal absorption and toxicity: A review of the literature. Int. Arch. Occup. Environ. Health 2009, 82, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Larese, F.F.; D’Agostin, F.; Crosera, M.; Adami, G.; Renzi, N.; Bovenzi, M.; Maina, G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 2009, 255, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.K.; Pal, S.; Naoghare, P.K.; Rangasamy, S.; Song, J.M. Shape-Dependent Skin Penetration of Silver Nanoparticles: Does It Really Matter? Sci. Rep. 2015, 5, 16908. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Kong, N.; Ji, X.; Zhang, Y.; Sharma, A.; Ouyang, J.; Qi, B.; Wang, J.; Xie, N.; Kang, C.; et al. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 2019, 48, 2891–2912. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zhu, Z.; Yin, K.; Su, S.; Bi, H.; Xu, T.; Zhang, H.; Shi, Z.; He, L.; Sun, L. A Highly skin-conformal and biodegradable graphene-based strain sensor. Small Methods 2018, 2, 1700374. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, L.-Q.; Wang, D.-Y.; Zhang, T.-Y.; Yang, Y.; Ren, T.-L. Flexible, highly sensitive pressure sensor with a wide range based on graphene-silk network structure. Appl. Phys. Lett. 2017, 110, 123508. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Porosity, density and mechanical properties of the paper of steam exploded bamboo microfibers controlled by nanofibrillated cellulose. J. Mater. Res. Technol. 2019, 8, 3612–3622. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Jiang, K.; Shen, G. Bio-Multifunctional Smart Wearable Sensors for Medical Devices. Adv. Intell. Syst. 2019, 1, 1900040. [Google Scholar] [CrossRef]
- González, I.; Alcalà, M.; Chinga-Carrasco, G.; Vilaseca, F.; Boufi, S.; Mutjé, P. From paper to nanopaper: Evolution of mechanical and physical properties. Cellulose 2014, 21, 2599–2609. [Google Scholar] [CrossRef]
- Petritz, A.; Wolfberger, A.; Fian, A.; Griesser, T.; Irimia-Vladu, M.; Stadlober, B. Cellulose-derivative-based gate dielectric for high-performance organic complementary inverters. Adv. Mater. 2015, 27, 7645–7656. [Google Scholar] [CrossRef]
- Koga, H.; Nagashima, K.; Huang, Y.; Zhang, G.; Wang, C.; Takahashi, T.; Inoue, A.; Yan, H.; Kanai, M.; He, Y.; et al. Paper-based disposable molecular sensor constructed from oxide nanowires, cellulose nanofibers, and pencil-drawn electrodes. ACS Appl. Mater. Interfaces 2019, 11, 15044–15050. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications - A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Vaicekauskaite, J.; Mazurek, P.; Vudayagiri, S.; Skov, A.L. Mapping the mechanical and electrical properties of commercial silicone elastomer formulations for stretchable transducers. J. Mater. Chem. C 2020, 8, 1273–1279. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, J.; Ding, H.; Zhang, W.; Wu, D.; Xu, J.; Shi, Z.; Xu, T.; Tian, Y.; Li, X. Ultra-highly sensitive, low hysteretic and flexible pressure sensor based on porous MWCNTs/Ecoflex elastomer composites. J. Mater. Sci. Mater. Electron. 2018, 29, 20978–20983. [Google Scholar] [CrossRef]
- Baek, S.; Jang, H.; Kim, S.Y.; Jeong, H.; Han, S.; Jang, Y.; Kim, D.H.; Lee, H.S. Flexible piezocapacitive sensors based on wrinkled microstructures: Toward low-cost fabrication of pressure sensors over large areas. RSC Adv. 2017, 7, 39420–39426. [Google Scholar] [CrossRef]
- Cholleti, E.R.; Stringer, J.; Assadian, M.; Battmann, V.; Bowen, C.; Aw, K. Highly stretchable capacitive sensor with printed carbon black electrodes on barium titanate elastomer composite. Sensors 2018, 19, 42. [Google Scholar] [CrossRef]
- Evlashin, S.; Dyakonov, P.; Tarkhov, M.; Dagesyan, S.; Rodionov, S.; Shpichka, A.; Kostenko, M.; Konev, S.; Sergeichev, I.; Timashev, P.; et al. Flexible Polycaprolactone and Polycaprolactone/Graphene Scaffolds for Tissue Engineering. Materials 2019, 12, 2991. [Google Scholar] [CrossRef]
- Khalid, M.A.U.; Ali, M.; Soomro, A.M.; Kim, S.W.; Kim, H.B.; Lee, B.-G.; Choi, K.H. A highly sensitive biodegradable pressure sensor based on nanofibrous dielectric. Sens. Actuators A Phys. 2019, 294, 140–147. [Google Scholar] [CrossRef]
- Cameron, R.E.; Kamvari-Moghaddam, A. Synthetic bioresorbable polymers. In Durability and Reliability of Medical Polymers; Jenkins, M., Stamboulis, A., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 96–118. [Google Scholar]
- Kalenov, D.S.; Meriakri, V.V.; Parkhomenko, M.P.; Zhou, S. Dielectric properties of biocompatible and biodegradable polycaprolone and polylactide and their nanocomposites in the millimeter wave band. AIP Conf. Proc. 2012, 1459, 77–79. [Google Scholar]
- Kim, T.K.; Kim, J.K.; Jeong, O.C. Measurement of nonlinear mechanical properties of PDMS elastomer. Microelectron. Eng. 2011, 88, 1982–1985. [Google Scholar] [CrossRef]
- Peterson, S.L.; McDonald, A.; Gourley, P.L.; Sasaki, D.Y. Poly(dimethylsiloxane) thin films as biocompatible coatings for microfluidic devices: Cell culture and flow studies with glial cells. J. Biomed. Mater. Res. A 2005, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, Y.; Nomani, M.W.; Wen, X.; Hsia, T.-Y.; Koley, G. A highly sensitive pressure sensor using a Au-patterned polydimethylsiloxane membrane for biosensing applications. J. Micromech. Microeng. 2013, 23, 025022. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, B.; Duan, J.; Guo, H.; Tang, J. Flexible pressure sensor with ag wrinkled electrodes based on PDMS substrate. Sensors 2016, 16, 2131. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, S.; Yu, J.; Cui, M.; He, J.; Li, L.; Wang, X.; Chou, X. Flexible piezoelectric nanofibers/polydimethylsiloxane-based pressure sensor for self-powered human motion monitoring. Energy Technol. 2020, 8, 1901242. [Google Scholar] [CrossRef]
- Chen, J.; Guo, H.; He, X.; Liu, G.; Xi, Y.; Shi, H.; Hu, C. Enhancing performance of triboelectric nanogenerator by filling high dielectric nanoparticles into sponge PDMS film. ACS Appl. Mater. Interfaces 2016, 8, 736–744. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Borovanska, I.; Todorov, P.; Ivanov, E.; Menseidov, D.; Chakraborty, S.; Bhattacharjee, C. tensile and surface mechanical properties of polyethersulphone (PES) and polyvinylidene fluoride (PVDF) membranes. J. Theor. Appl. Mech. 2018, 48, 85–99. [Google Scholar] [CrossRef]
- Azadbakht, M.; Madaeni, S.S.; Sahebjamee, F. Biocompatibility of polyethersulfone membranes for cell culture systems. Eng. Life Sci. 2011, 11, 629–635. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Doi, Y. Biodegradable poly(ethylene succinate) (PES). 1. Crystal growth kinetics and morphology. Biomacromolecules 2000, 1, 704–712. [Google Scholar] [CrossRef]
- Hu, L.; Zhong, J.; Tian, Y.; Zheng, X.; Cheng, J.; Pu, Z. Study on properties of barium titanate/polyethersulfone dielectric composites prepared by physical dispersion method. J. Mater. Sci.: Mater. Electron. 2018, 30, 221–229. [Google Scholar] [CrossRef]
- Dardmeh, N.; Khosrowshahi, A.; Almasi, H.; Zandi, M. Study on effect of the polyethylene terephthalate/nanoclay nanocomposite film on the migration of terephthalic acid into the yoghurt drinks simulant. J. Food Process Eng. 2017, 40, e12324. [Google Scholar] [CrossRef]
- Samavedi, S.; Poindexter, L.K.; Van Dyke, M.; Goldstein, A.S. Synthetic Biomaterials for Regenerative Medicine Applications. In Regenerative Medicine Applications in Organ Transplantation; Orlando, G., Lerut, J., Soker, S., Stratta, R.J., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 81–99. [Google Scholar]
- Loh, K.J.; Lynch, J.P.; Shim, B.S.; Kotov, N.A. Tailoring piezoresistive sensitivity of multilayer carbon nanotube composite strain sensors. J. Intell. Mater. Syst. Struct. 2007, 19, 747–764. [Google Scholar] [CrossRef]
- Yoon, J.I.; Choi, K.S.; Chang, S.P. A novel means of fabricating microporous structures for the dielectric layers of capacitive pressure sensor. Microelectron. Eng. 2017, 179, 60–66. [Google Scholar] [CrossRef]
- Yaqoob, U.; Phan, D.-T.; Uddin, A.S.M.I.; Chung, G.-S. Highly flexible room temperature NO2 sensor based on MWCNTs-WO3 nanoparticles hybrid on a PET substrate. Sens. Actuators B Chem. 2015, 221, 760–768. [Google Scholar] [CrossRef]
- Sundriyal, P.; Bhattacharya, S. Inkjet-printed sensors on flexible substrates. In Environmental, Chemical and Medical Sensors; Bhattacharya, S., Agarwal, A.K., Chanda, N., Pandey, A., Sen, A.K., Eds.; Springer: Singapore, 2018; pp. 89–113. [Google Scholar]
- Dai, W.; Yu, J.; Wang, Y.; Song, Y.; Bai, H.; Nishimura, K.; Liao, H.; Jiang, N. Enhanced thermal and mechanical properties of polyimide/graphene composites. Macromol. Res. 2014, 22, 983–989. [Google Scholar] [CrossRef]
- Constantin, C.P.; Aflori, M.; Damian, R.F.; Rusu, R.D. Biocompatibility of polyimides: A mini-review. Materials 2019, 12, 3166. [Google Scholar] [CrossRef]
- Lin, R.-H.; Wang, W.-M.; Chen, Y.-H.; Ho, T.-H. Preparation and characterization of biodegradable condensation polyimide. Polym. Degrad. Stab. 2012, 97, 1534–1544. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Afsarimanesh, N.; Akhter, F.; Liu, H.; Sapra, S.; Mukhopadhyay, S.; Xu, Y. Gold/polyimide-based resistive strain sensors. Electronics 2019, 8, 565. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Karimi, A.; Navidbakhsh, M. Mechanical properties of PVA material for tissue engineering applications. Mater. Technol. 2013, 29, 90–100. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly (vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Progress Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Nawaz, A.; Hümmelgen, I.A. Poly (vinyl alcohol) gate dielectric in organic field-effect transistors. J. Mater. Sci. Mater. Electron. 2019, 30, 5299–5326. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Lau, K.-T.; Ho, M.-P.; Mosallam, A. Study on the mechanical properties of different silkworm silk fibers. J. Compos. Mater. 2009, 43, 2521–2531. [Google Scholar] [CrossRef]
- Zhu, Z.; Ling, S.; Yeo, J.; Zhao, S.; Tozzi, L.; Buehler, M.J.; Omenetto, F.; Li, C.; Kaplan, D.L. High-strength, durable all-silk fibroin hydrogels with versatile processability toward multifunctional applications. Adv. Funct. Mater. 2018, 28, 1704757. [Google Scholar] [CrossRef] [PubMed]
- Brenckle, M.A.; Partlow, B.; Tao, H.; Applegate, M.B.; Reeves, A.; Paquette, M.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Methods and Applications of Multilayer Silk Fibroin Laminates Based on Spatially Controlled Welding in Protein Films. Adv. Funct. Mater. 2016, 26, 44–50. [Google Scholar] [CrossRef]
- Wu, R.; Ma, L.; Hou, C.; Meng, Z.; Guo, W.; Yu, W.; Yu, R.; Hu, F.; Liu, X.Y. silk composite electronic textile sensor for high space precision 2d combo temperature-pressure sensing. Small 2019, 15, e1901558. [Google Scholar] [CrossRef]
- He, F.; You, X.; Gong, H.; Yang, Y.; Bai, T.; Wang, W.; Guo, W.; Liu, X.; Ye, M. Stretchable, biocompatible, and multifunctional silk fibroin-based hydrogels toward wearable strain/pressure sensors and triboelectric nanogenerators. ACS Appl. Mater. Interfaces 2020, 12, 6442–6450. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsieh, C.Y.; Hwang, J.C. Flexible organic thin-film transistors with silk fibroin as the gate dielectric. Adv. Mater. 2011, 23, 1630–1634. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef]
- Gao, J.; Li, K.; Xu, J.; Zhang, W.; Ma, J.; Liu, L.; Sun, Y.; Zhang, H.; Li, K. Unexpected Rheological Behavior of a Hydrophobic Associative Shellac-Based Oligomeric Food Thickener. J. Agric. Food Chem. 2018, 66, 6799–6805. [Google Scholar] [CrossRef]
- Leick, S.; Kott, M.; Degen, P.; Henning, S.; Pasler, T.; Suter, D.; Rehage, H. Mechanical properties of liquid-filled shellac composite capsules. Phys. Chem. Chem. Phys. 2011, 13, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Irimia-Vladu, M.; Głowacki, E.D.; Schwabegger, G.; Leonat, L.; Akpinar, H.Z.; Sitter, H.; Bauer, S.; Sariciftci, N.S. Natural resin shellac as a substrate and a dielectric layer for organic field-effect transistors. Green Chem. 2013, 15, 1473–1476. [Google Scholar] [CrossRef]
- Baek, S.W.; Ha, J.W.; Yoon, M.; Hwang, D.H.; Lee, J. Shellac films as a natural dielectric layer for enhanced electron transport in polymer field-effect transistors. ACS Appl. Mater. Interfaces 2018, 10, 18948–18955. [Google Scholar] [CrossRef] [PubMed]
- Irimia-Vladu, M.; Troshin, P.A.; Reisinger, M.; Shmygleva, L.; Kanbur, Y.; Schwabegger, G.; Bodea, M.; Schwödiauer, R.; Mumyatov, A.; Fergus, J.W.; et al. Biocompatible and Biodegradable Materials for Organic Field-Effect Transistors. Adv. Funct. Mater. 2010, 20, 4069–4076. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, D.S.; Kim, I.G.; Sohn, D.W.; Park, J.Y.; Choi, B.K.; Kim, S.W. Evaluation of the biocompatibility of a coating material for an implantable bladder volume sensor. Kaohsiung J. Med. Sci. 2012, 28, 123–129. [Google Scholar] [CrossRef]
- Park, S.; Mondal, K.; Treadway, R.M., 3rd; Kumar, V.; Ma, S.; Holbery, J.D.; Dickey, M.D. Silicones for Stretchable and Durable Soft Devices: Beyond Sylgard-184. ACS Appl. Mater. Interfaces 2018, 10, 11261–11268. [Google Scholar] [CrossRef]
- Yun, Y.S.; Kim, D.H.; Kim, B.; Park, H.H.; Jin, H.-J. Transparent conducting films based on graphene oxide/silver nanowire hybrids with high flexibility. Synth. Metals 2012, 162, 1364–1368. [Google Scholar] [CrossRef]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef]
- Jung, J.; Cho, H.; Yuksel, R.; Kim, D.; Lee, H.; Kwon, J.; Lee, P.; Yeo, J.; Hong, S.; Unalan, H.E.; et al. Stretchable/flexible silver nanowire Electrodes for energy device applications. Nanoscale 2019, 11, 20356–20378. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, R.; Zhai, H.; Sun, J. Stretchable electronic skin based on silver nanowire composite fiber electrodes for sensing pressure, proximity, and multidirectional strain. Nanoscale 2017, 9, 3834–3842. [Google Scholar] [CrossRef]
- Popov, A.M.; Lozovik, Y.E.; Fiorito, S.; Yahia, L. Biocompatibility and applications of carbon nanotubes in medical nanorobots. Int. J. Nanomed. 2007, 2, 361–372. [Google Scholar]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of carbon nanotubes for biomedical applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, L.; Zhou, B.; Zhu, Y.; Jiang, X. The pristine graphene produced by liquid exfoliation of graphite in mixed solvent and its application to determination of dopamine. J. Colloid Interface Sci. 2018, 513, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Banerjee, S.; Datta, A.; Chakravorty, D. A brief review on graphene/inorganic nanostructure composites: Materials for the future. Ind. J. Phys. 2016, 90, 1019–1032. [Google Scholar] [CrossRef]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-based sensors for human health monitoring. Front. Chem. 2019, 7, 399. [Google Scholar] [CrossRef]
- Pena-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef]
- Ali, A.; Pothu, R.; Siyal, S.H.; Phulpoto, S.; Sajjad, M.; Thebo, K.H. Graphene-based membranes for CO2 separation. Mater. Sci. Energy Technol. 2019, 2, 83–88. [Google Scholar] [CrossRef]
- El Beqqali, O.; Zorkani, I.; Rogemond, F.; Chermette, H.; Chaabane, R.B.; Gamoudi, M.; Guillaud, G. Electrical properties of molybdenum disulfide MoS2. Experimental study and density functional calculation results. Synth. Metals 1997, 90, 165–172. [Google Scholar] [CrossRef]
- Shah, P.; Narayanan, T.N.; Li, C.Z.; Alwarappan, S. Probing the biocompatibility of MoS2 nanosheets by cytotoxicity assay and electrical impedance spectroscopy. Nanotechnology 2015, 26, 315102. [Google Scholar] [CrossRef]
- Yang, T.; Xiang, H.; Qin, C.; Liu, Y.; Zhao, X.; Liu, H.; Li, H.; Ouzounian, M.; Hong, G.; Chen, H.; et al. Highly sensitive 1T-MOS pressure sensor with wide linearity based on hierarchical microstructures of leaf vein as spacer. Adv. Electron. Mater. 2019, 6, 1900916. [Google Scholar] [CrossRef]
- Yi, N.; Abidian, M.R. Conducting polymers and their biomedical applications. In Biosynthetic Polymers for Medical Applications; Poole-Warren, L., Martens, P., Green, R., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 243–276. [Google Scholar]
- Humpolicek, P.; Kasparkova, V.; Pachernik, J.; Stejskal, J.; Bober, P.; Capakova, Z.; Radaszkiewicz, K.A.; Junkar, I.; Lehocky, M. The biocompatibility of polyaniline and polypyrrole: A comparative study of their cytotoxicity, embryotoxicity and impurity profile. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ziadan, K.M.; Saadon, W.T. Study of the electrical characteristics of polyaniline prepeared by electrochemical polymerization. Energy Proc. 2012, 19, 71–79. [Google Scholar] [CrossRef][Green Version]
- He, X.X.; Li, J.T.; Jia, X.S.; Tong, L.; Wang, X.X.; Zhang, J.; Zheng, J.; Ning, X.; Long, Y.Z. facile fabrication of multi-hierarchical porous polyaniline composite as pressure sensor and gas sensor with adjustable sensitivity. Nanoscale Res. Lett. 2017, 12, 476. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, K.; Zhou, Z.; Yan, X.; Meng, X.; Tang, H.; Qu, K.; Gao, Y.; Li, Y.; Yu, J.; Li, L. Polyaniline nanofiber wrapped fabric for high performance flexible pressure sensors. Polymers 2019, 11, 1120. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Sun, L.; Lee, D.; Lee, S.; Wang, M.; Zhao, J.; Shao-Horn, Y.; Dinca, M.; Palacios, T.; et al. High electrical conductivity and carrier mobility in oCVD PEDOT thin films by engineered crystallization and acid treatment. Sci. Adv. 2018, 4, eaat5780. [Google Scholar] [CrossRef]
- Asplund, M.; Thaning, E.; Lundberg, J.; Sandberg-Nordqvist, A.C.; Kostyszyn, B.; Inganas, O.; von Holst, H. Toxicity evaluation of PEDOT/biomolecular composites intended for neural communication electrodes. Biomed. Mater. 2009, 4, 045009. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.-Y.; Ho, K.-C.; Chu, C.-W. Highly conductive PEDOT: PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Stöcker, T.; Köhler, A.; Moos, R. Why does the electrical conductivity in PEDOT: PSS decrease with PSS content? A study combining thermoelectric measurements with impedance spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 976–983. [Google Scholar] [CrossRef]
- Tessarolo, M.; Possanzini, L.; Campari, E.G.; Bonfiglioli, R.; Violante, F.S.; Bonfiglio, A.; Fraboni, B. Adaptable pressure textile sensors based on a conductive polymer. Flex. Print. Electron. 2018, 3, 3. [Google Scholar] [CrossRef]
- Miao, J.; Liu, H.; Li, Y.; Zhang, X. Biodegradable Transparent Substrate Based on Edible Starch-Chitosan Embedded with Nature-Inspired Three-Dimensionally Interconnected Conductive Nanocomposites for Wearable Green Electronics. ACS Appl. Mater. Interfaces 2018, 10, 23037–23047. [Google Scholar] [CrossRef]
- Lv, B.; Chen, X.; Liu, C. A Highly sensitive piezoresistive pressure sensor based on graphene oxide/polypyrrole @ polyurethane sponge. Sensors 2020, 20, 1219. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, C.; Han, W. Extending the pressure sensing range of porous polypyrrole with multiscale microstructures. Nanoscale 2020, 12, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xiong, P.; Liu, J.; Gao, S.; Xi, T.; Cheng, Y. A silk-based coating containing GREDVY peptide and heparin on Mg–Zn–Y–Nd alloy: Improved corrosion resistance, hemocompatibility and endothelialization. J. Mater. Chem. B 2018, 6, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, C.; Sangwan, P.; Yu, L.; Tong, Z. Accelerating the degradation of polyolefins through additives and blending. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Lin, C.L.; Lai, Y.T.; Yang, Y.J. A polymer-based capacitive sensing array for normal and shear force measurement. Sensors 2010, 10, 10211–10225. [Google Scholar] [CrossRef]
- Nie, B.; Huang, R.; Yao, T.; Zhang, Y.; Miao, Y.; Liu, C.; Liu, J.; Chen, X. Textile-Based wireless pressure sensor array for human-interactive sensing. Adv. Funct. Mater. 2019, 29, 1808786. [Google Scholar] [CrossRef]
- Tian, X.; Lee, P.M.; Tan, Y.J.; Wu, T.L.Y.; Yao, H.; Zhang, M.; Li, Z.; Ng, K.A.; Tee, B.C.K.; Ho, J.S. Wireless body sensor networks based on metamaterial textiles. Nat. Electron. 2019, 2, 243–251. [Google Scholar] [CrossRef]
- Chen, S.; Wu, N.; Lin, S.; Duan, J.; Xu, Z.; Pan, Y.; Zhang, H.; Xu, Z.; Huang, L.; Hu, B.; et al. Hierarchical elastomer tuned self-powered pressure sensor for wearable multifunctional cardiovascular electronics. Nano Energy 2020, 70, 104460. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, K.Y.; Cheong, O.J.; Kim, J.H.; Jang, J. Highly sensitive and multifunctional tactile sensor using free-standing ZnO/PVDF thin film with graphene electrodes for pressure and temperature monitoring. Sci. Rep. 2015, 5, 7887. [Google Scholar] [CrossRef]
- Kim, D.I.; Trung, T.Q.; Hwang, B.U.; Kim, J.S.; Jeon, S.; Bae, J.; Park, J.J.; Lee, N.E. A Sensor array using multi-functional field-effect transistors with ultrahigh sensitivity and precision for bio-monitoring. Sci. Rep. 2015, 5, 12705. [Google Scholar] [CrossRef]
- Fan, W.; He, Q.; Meng, K.; Tan, X.; Zhou, Z.; Zhang, G.; Yang, J.; Wang, Z.L. Machine-knitted washable sensor array textile for precise epidermal physiological signal monitoring. Sci. Adv. 2020, 6, eaay2840. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xiao, P.; Lu, W.; Shi, J.; Zhang, L.; Liang, Y.; Pan, C.; Kuo, S.-W.; Chen, T. A Universal high accuracy wearable pulse monitoring system via high sensitivity and large linearity graphene pressure sensor. Nano Energy 2019, 59, 422–433. [Google Scholar] [CrossRef]
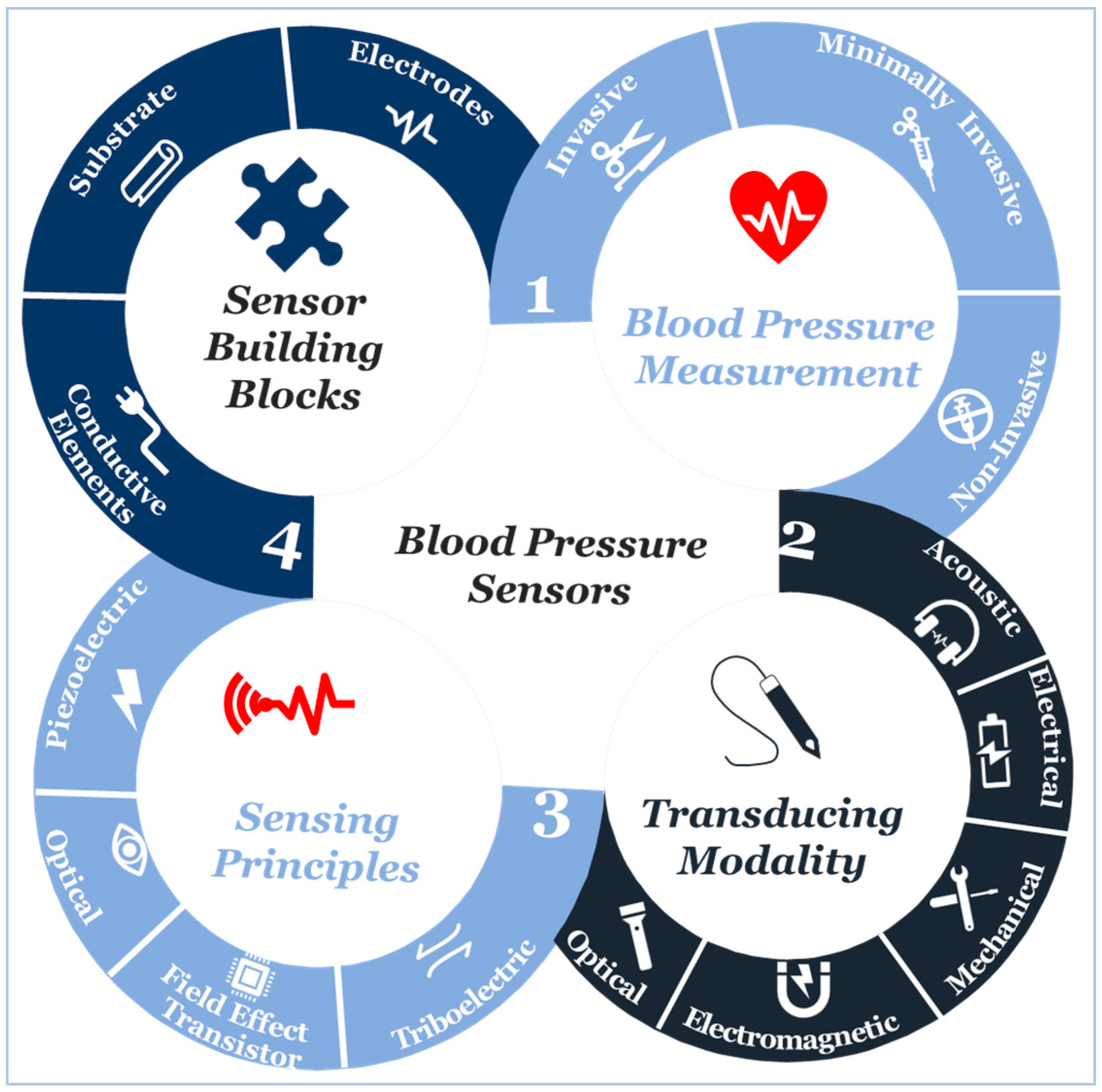
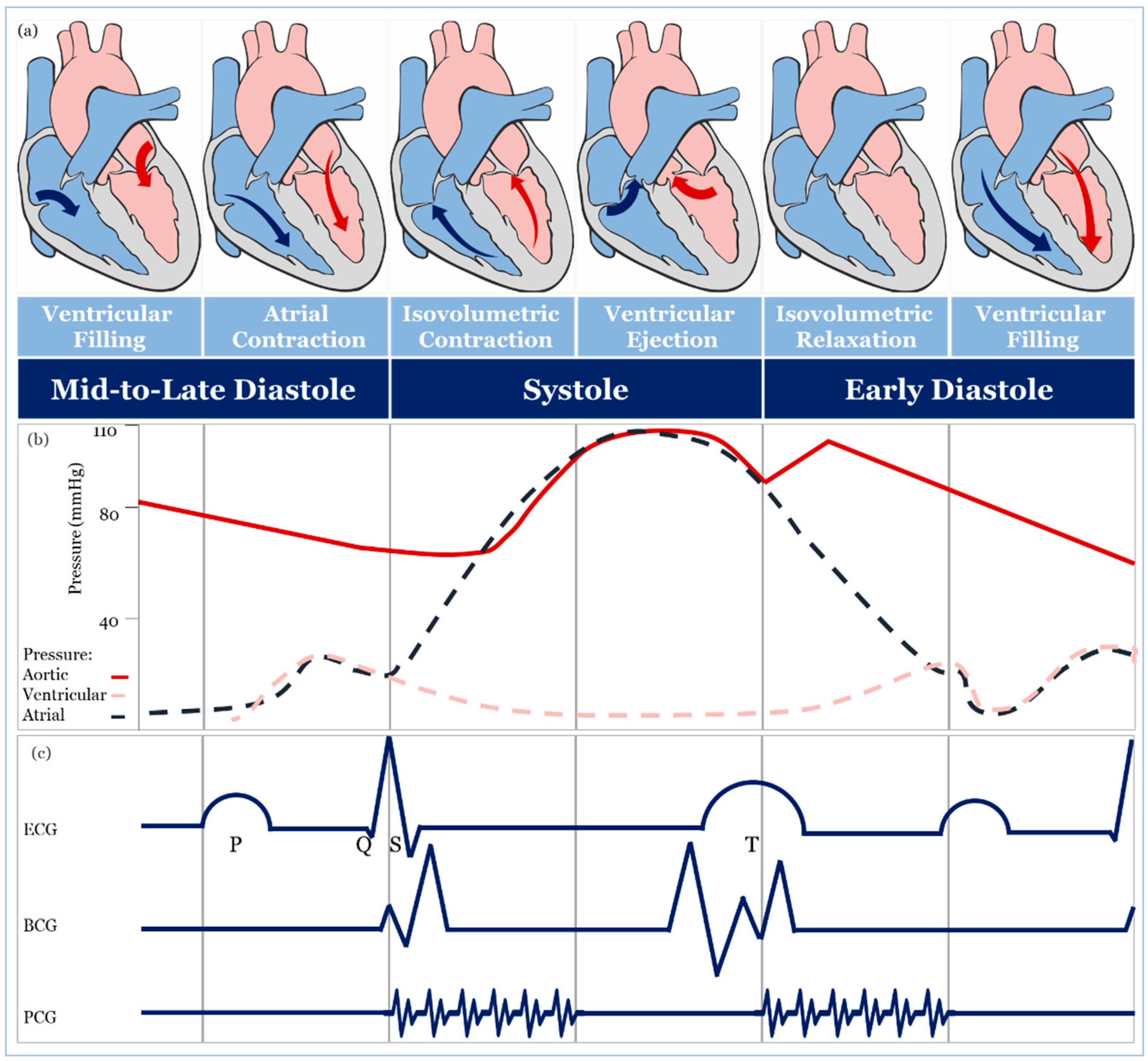
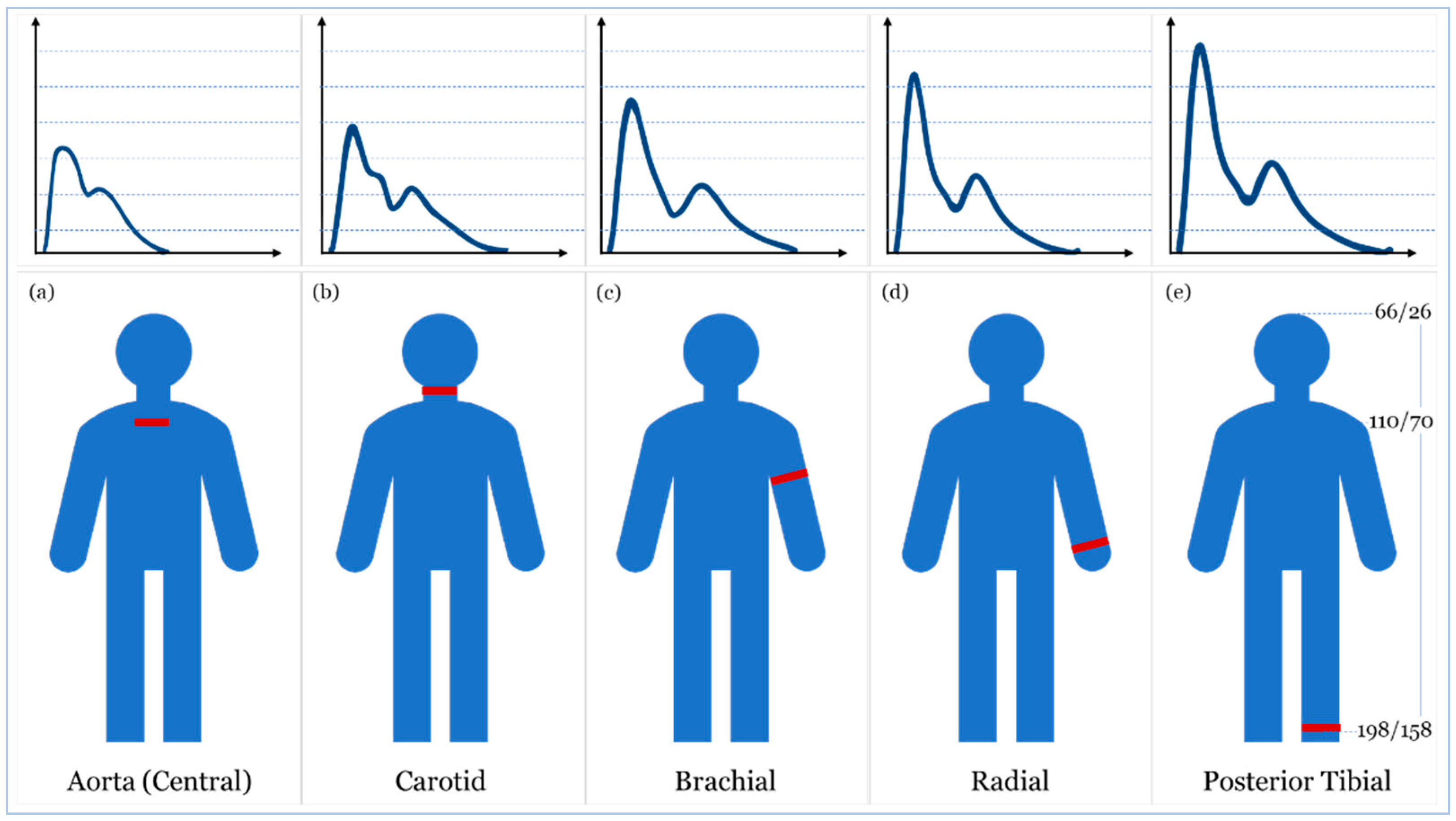
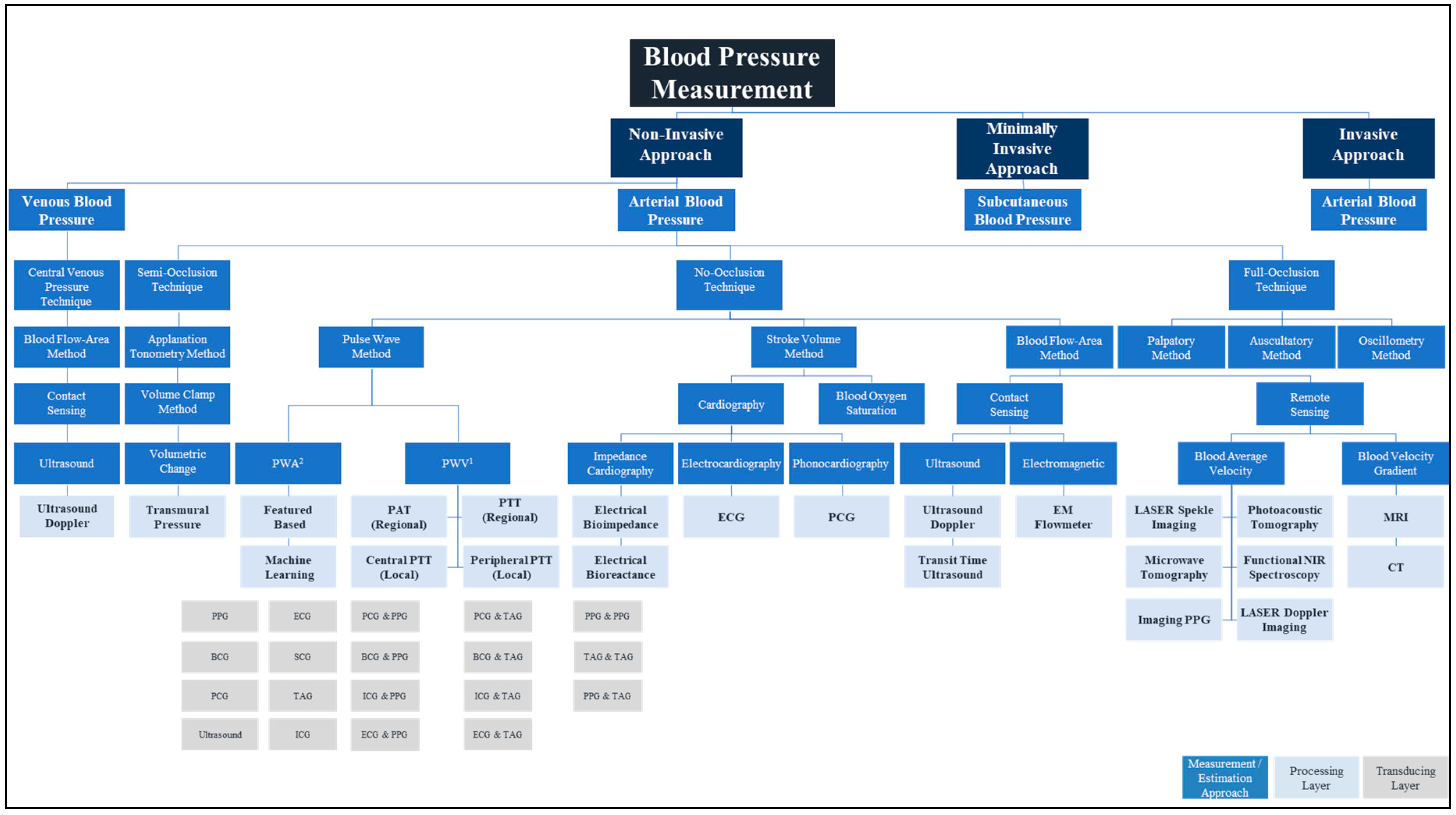
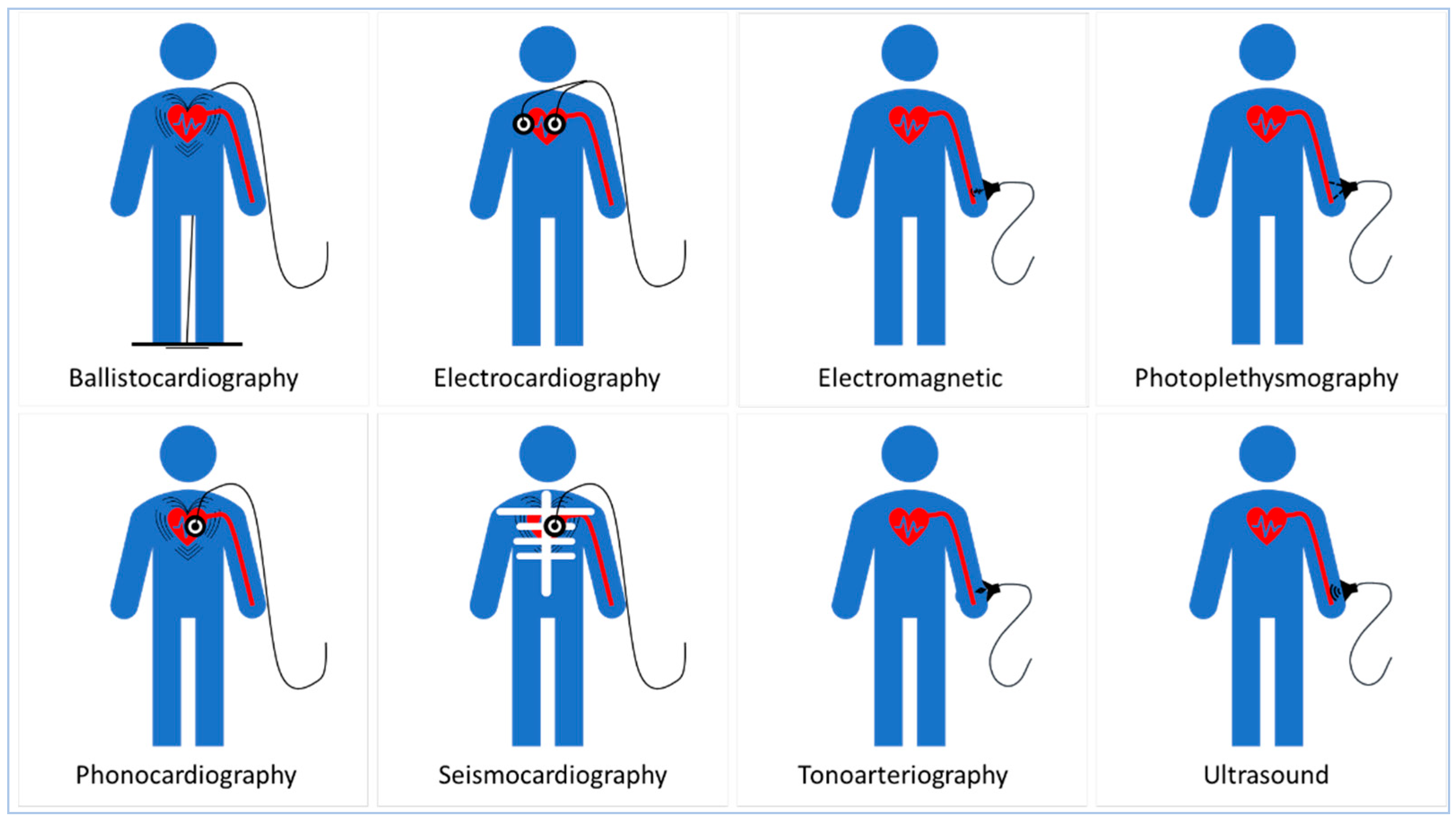
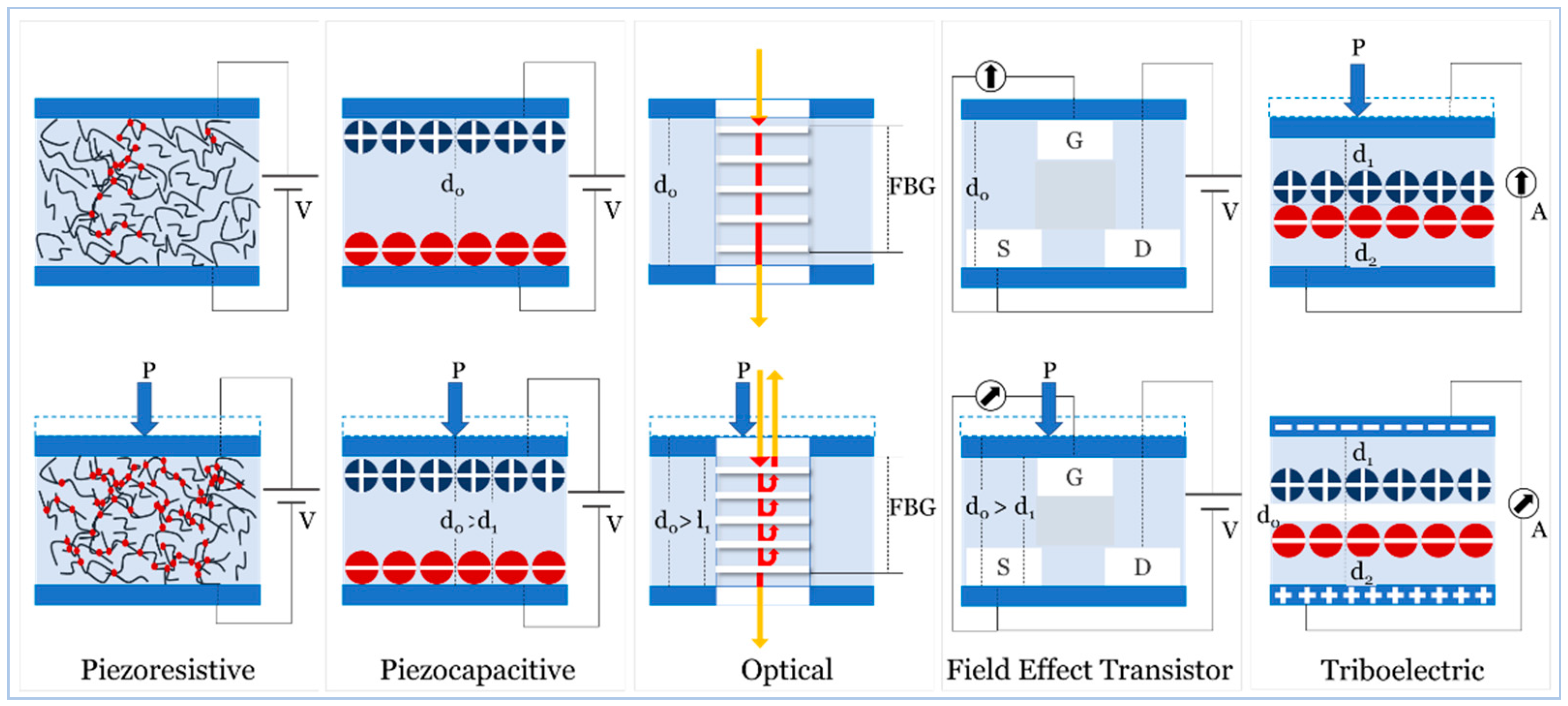
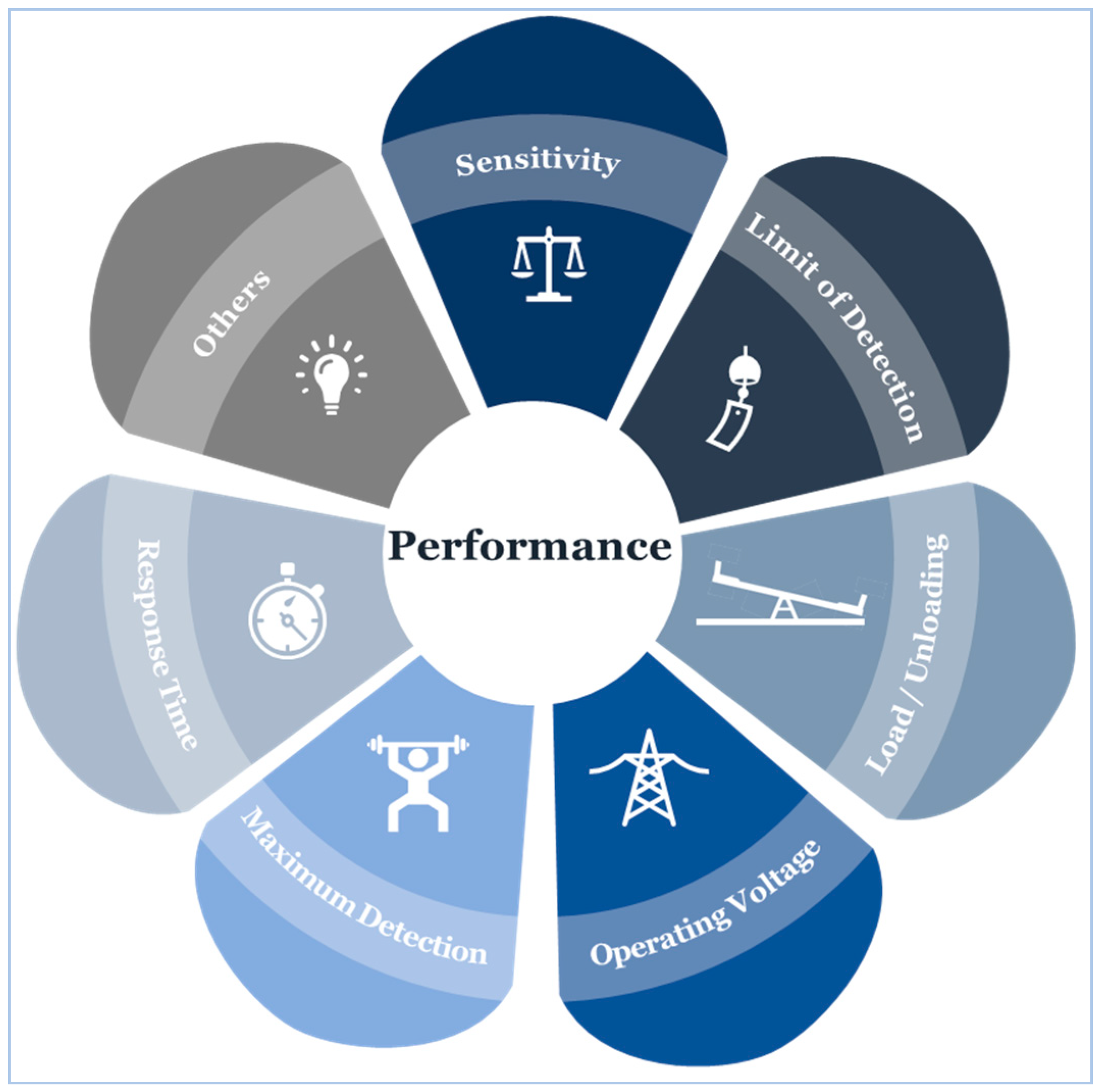
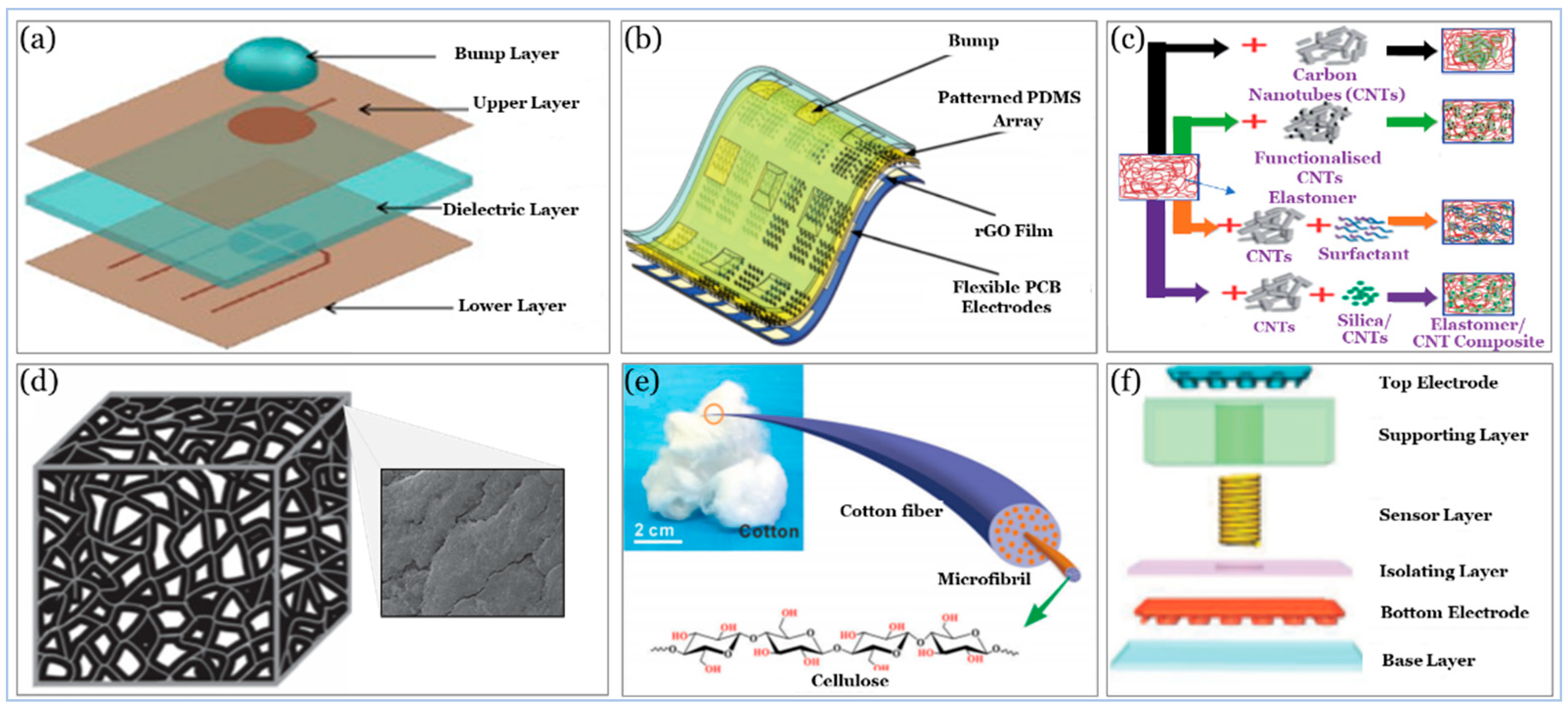
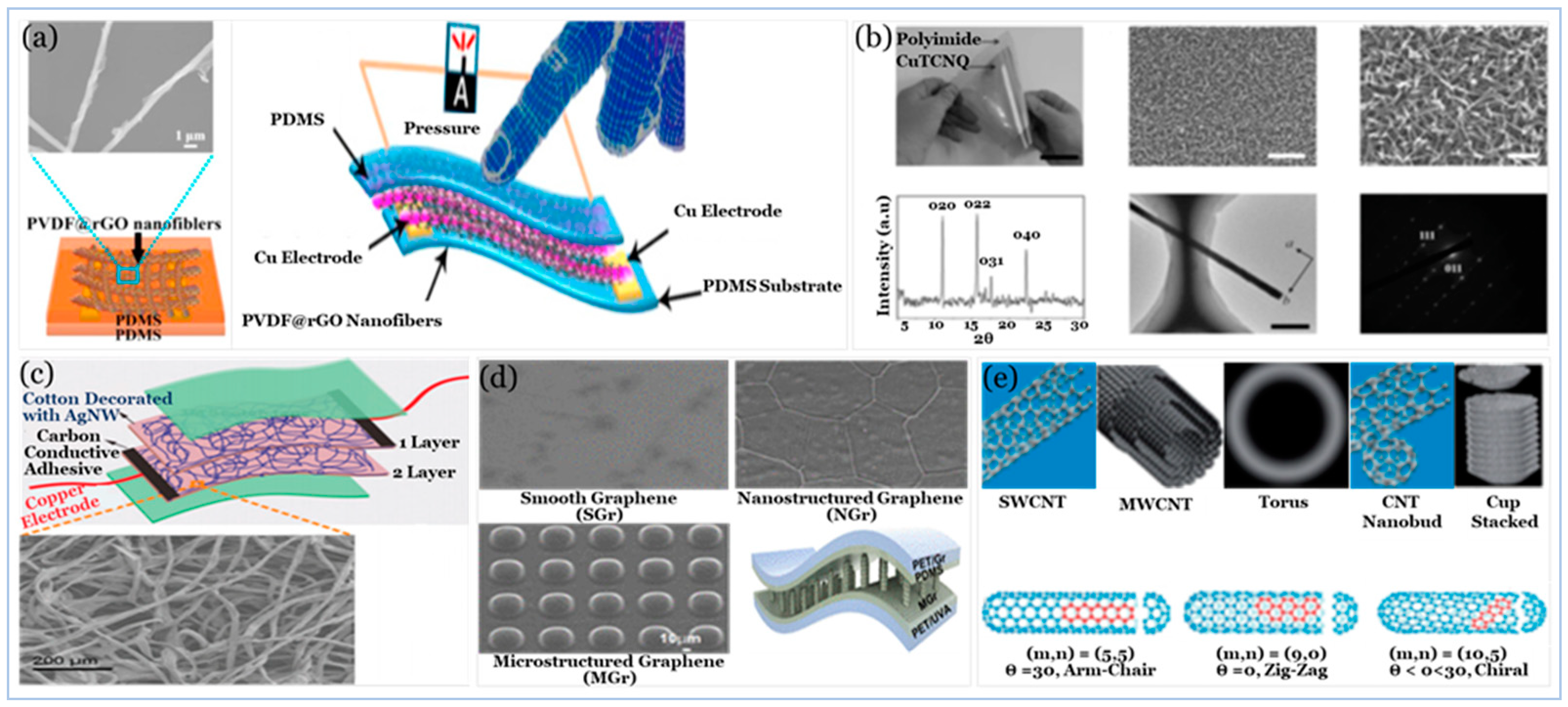
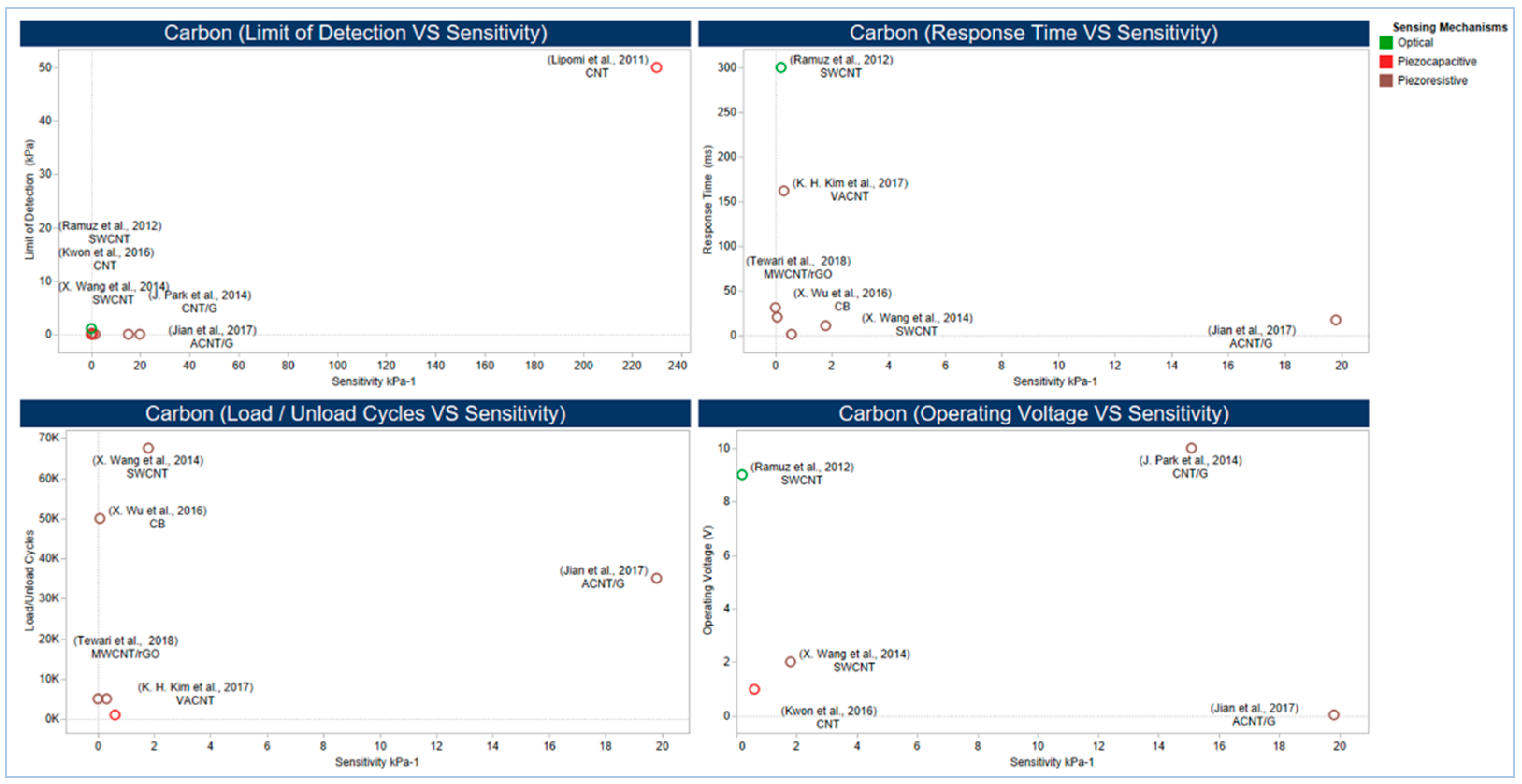
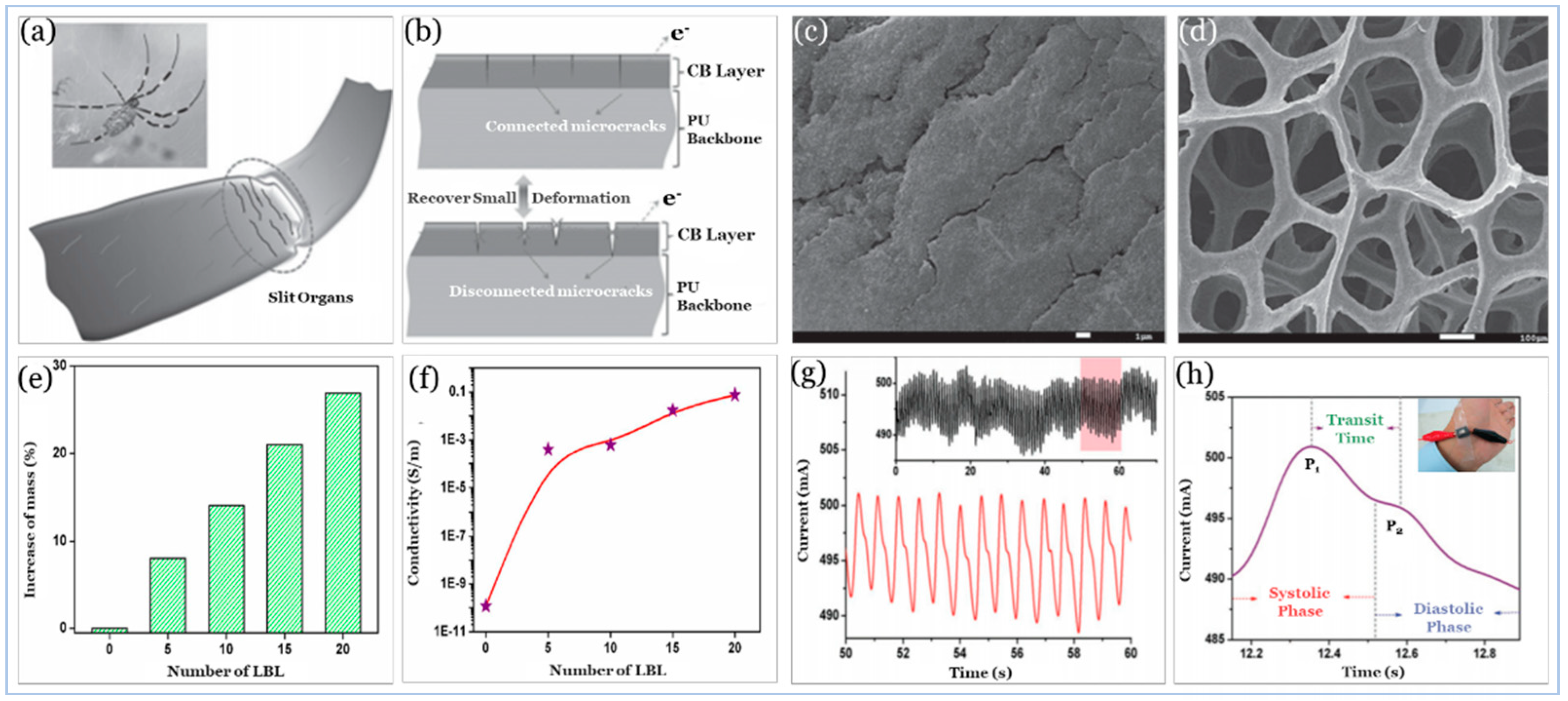
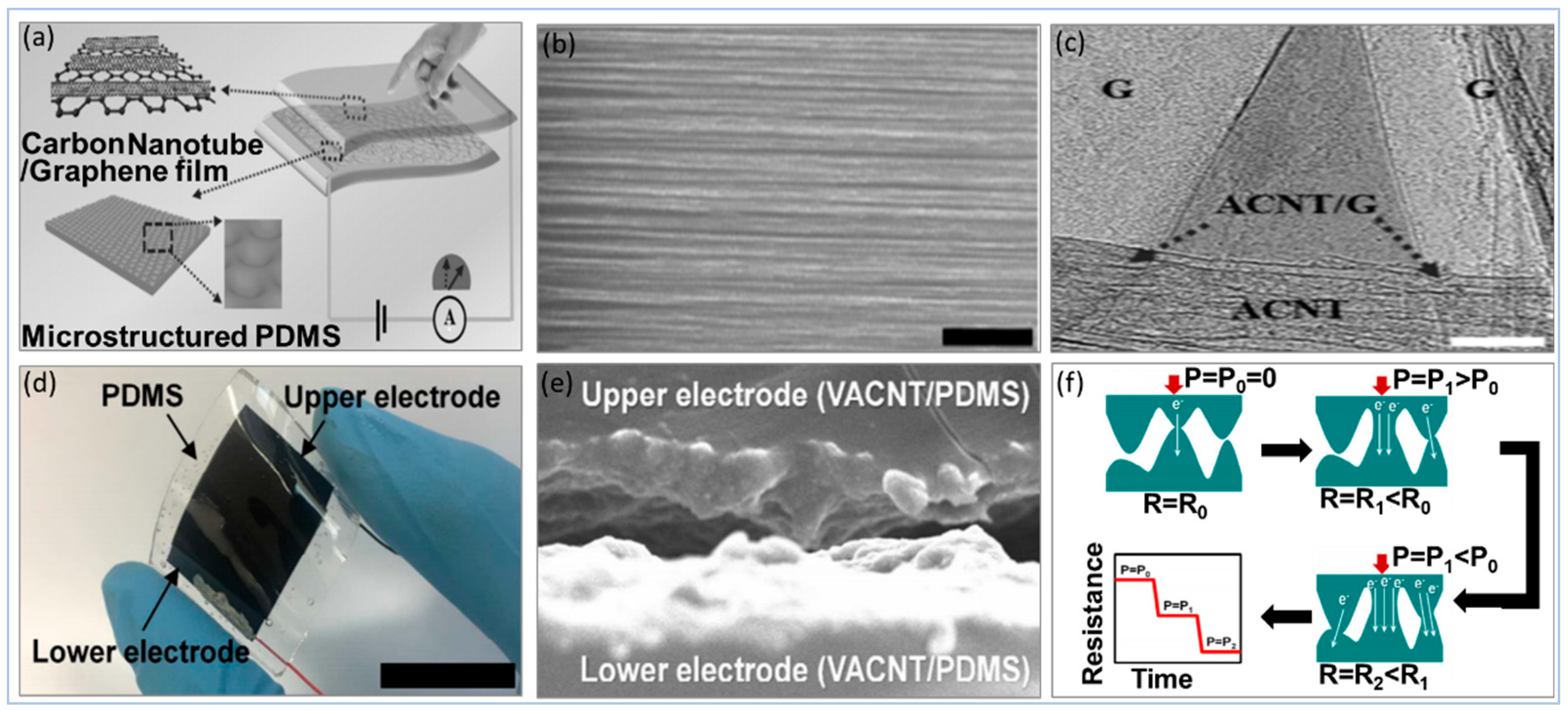
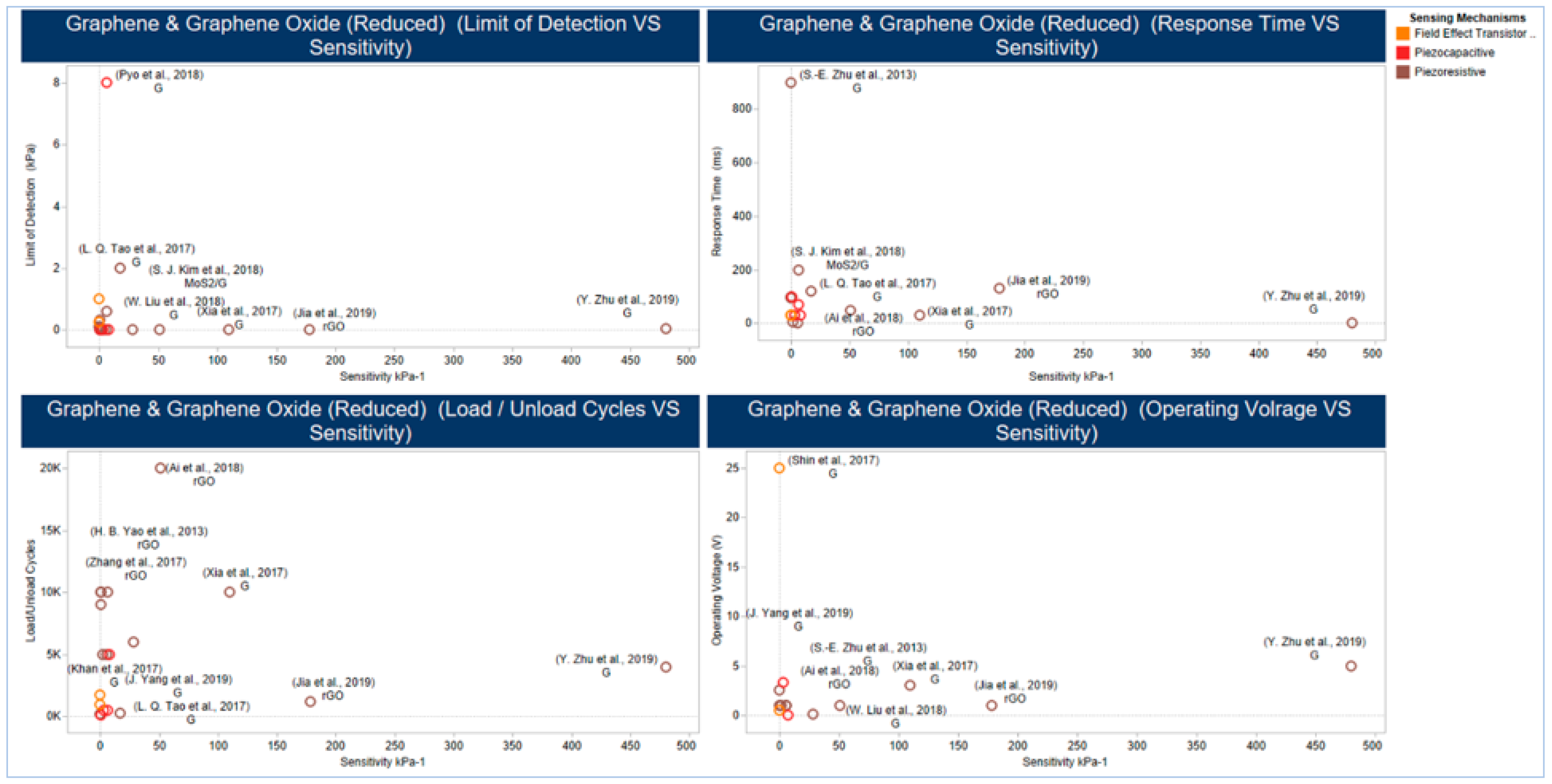
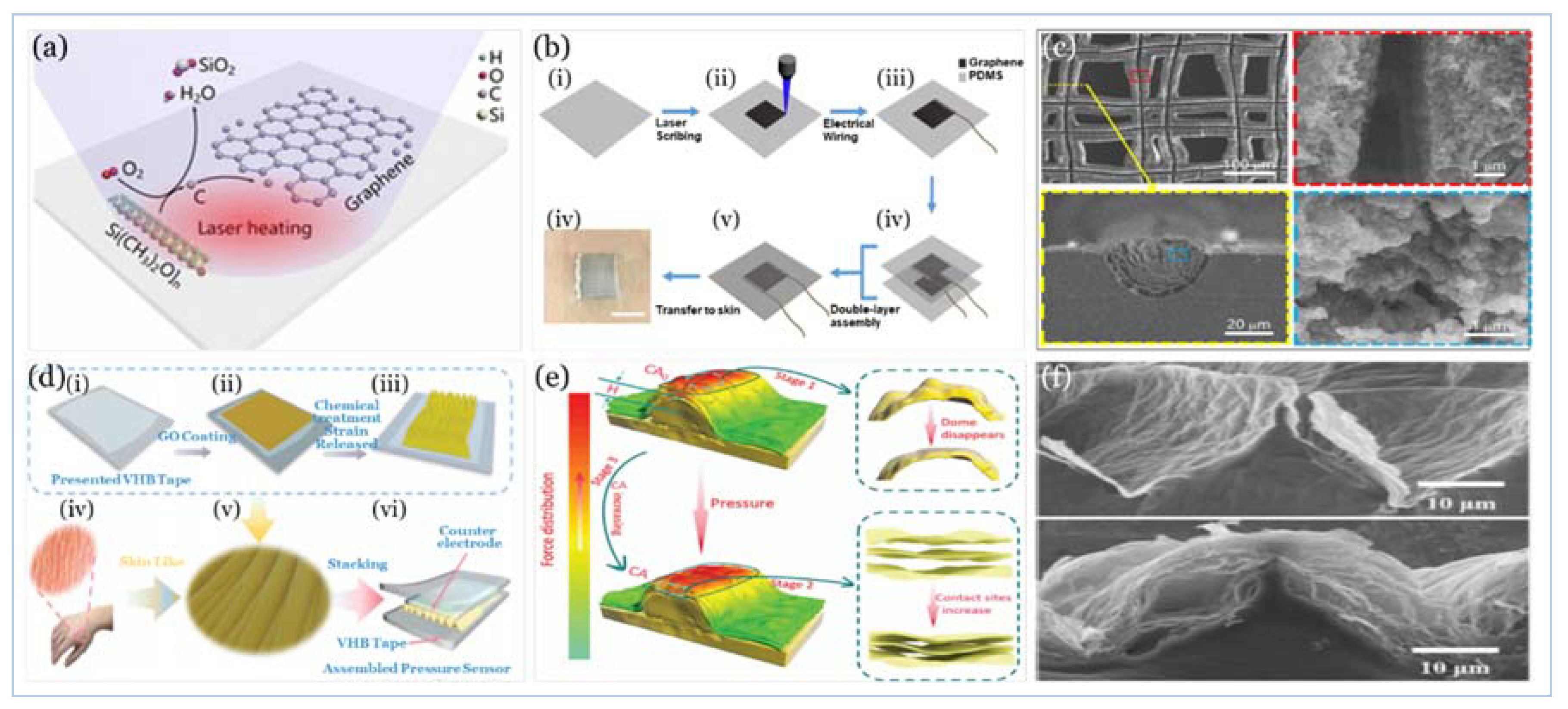
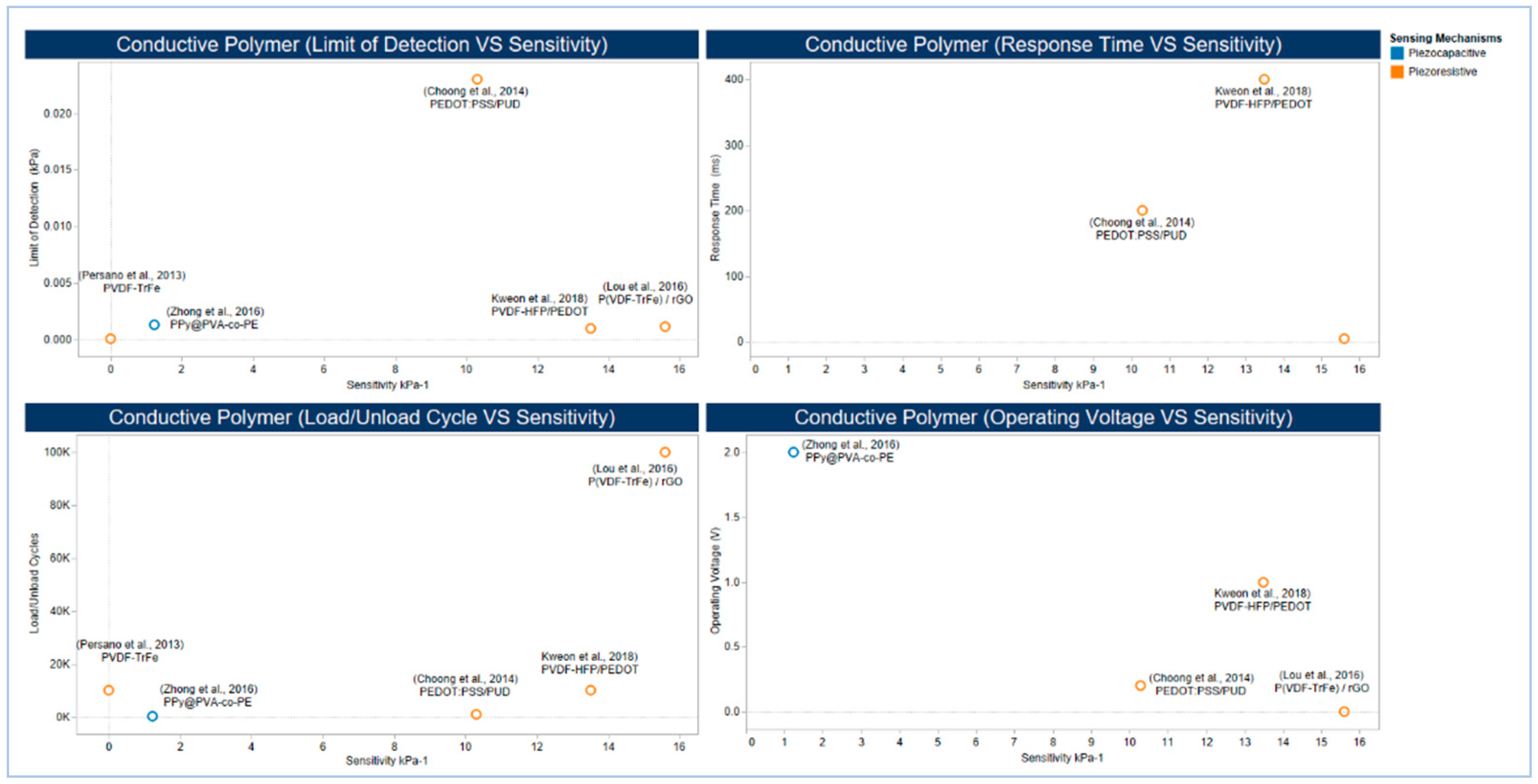
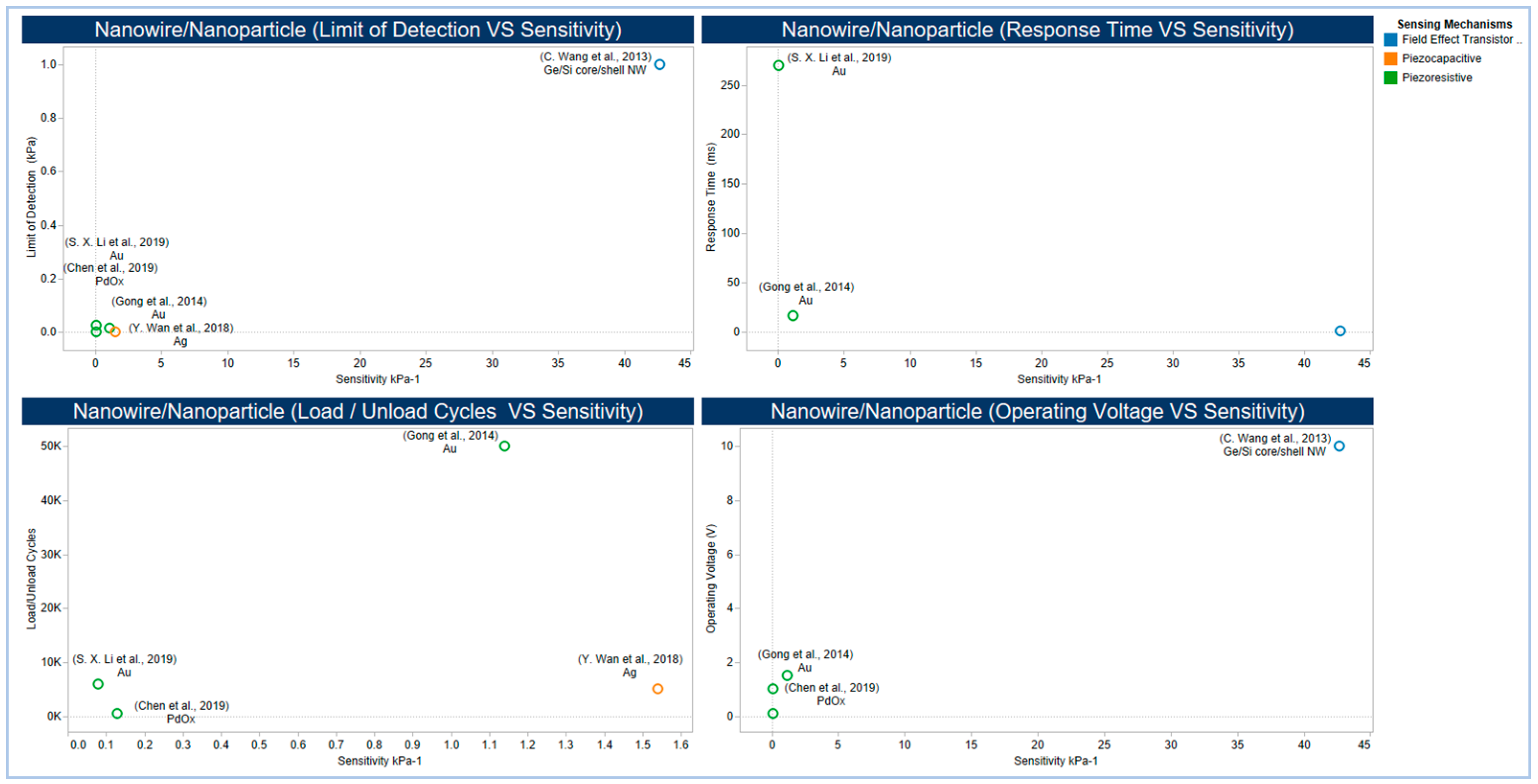
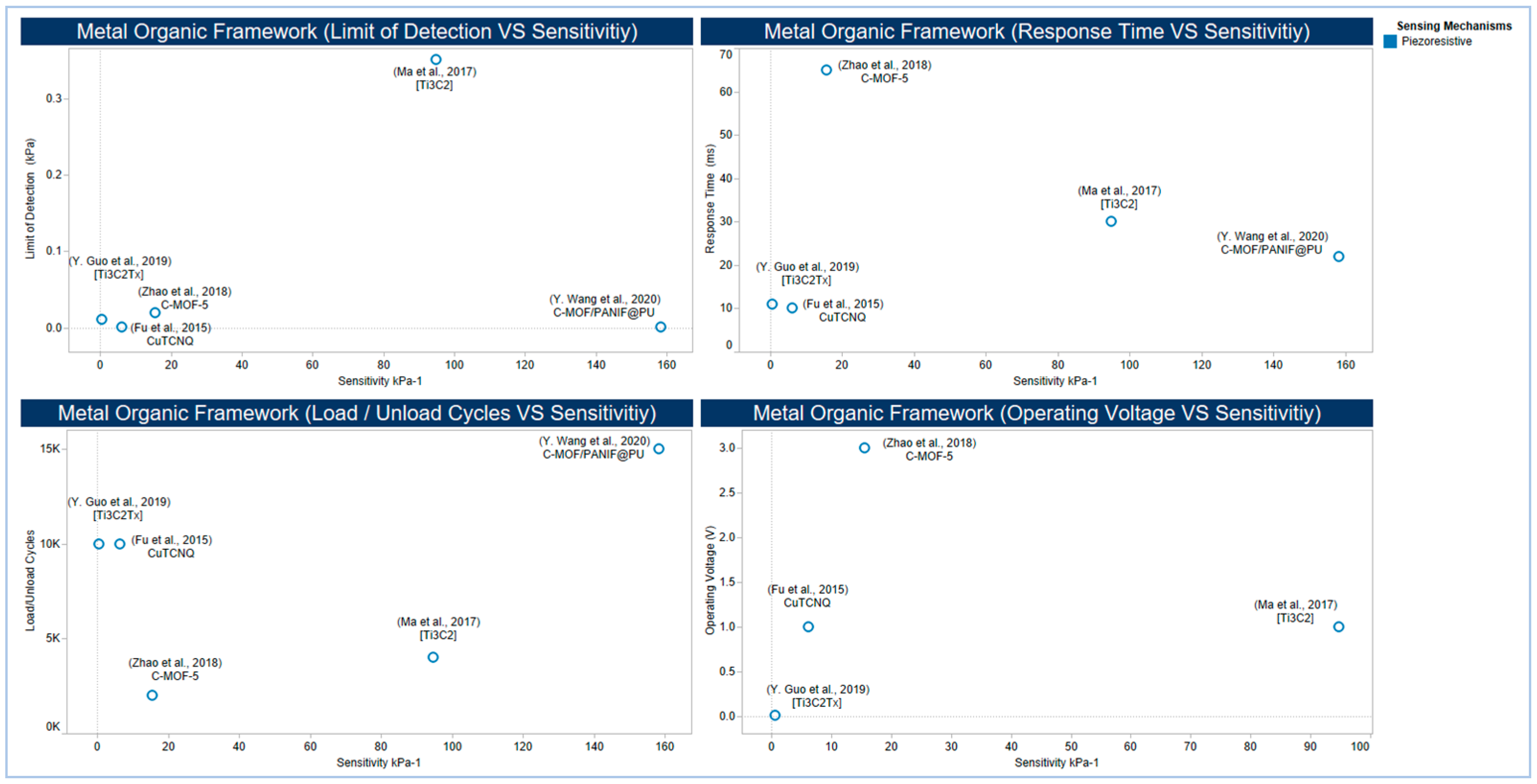
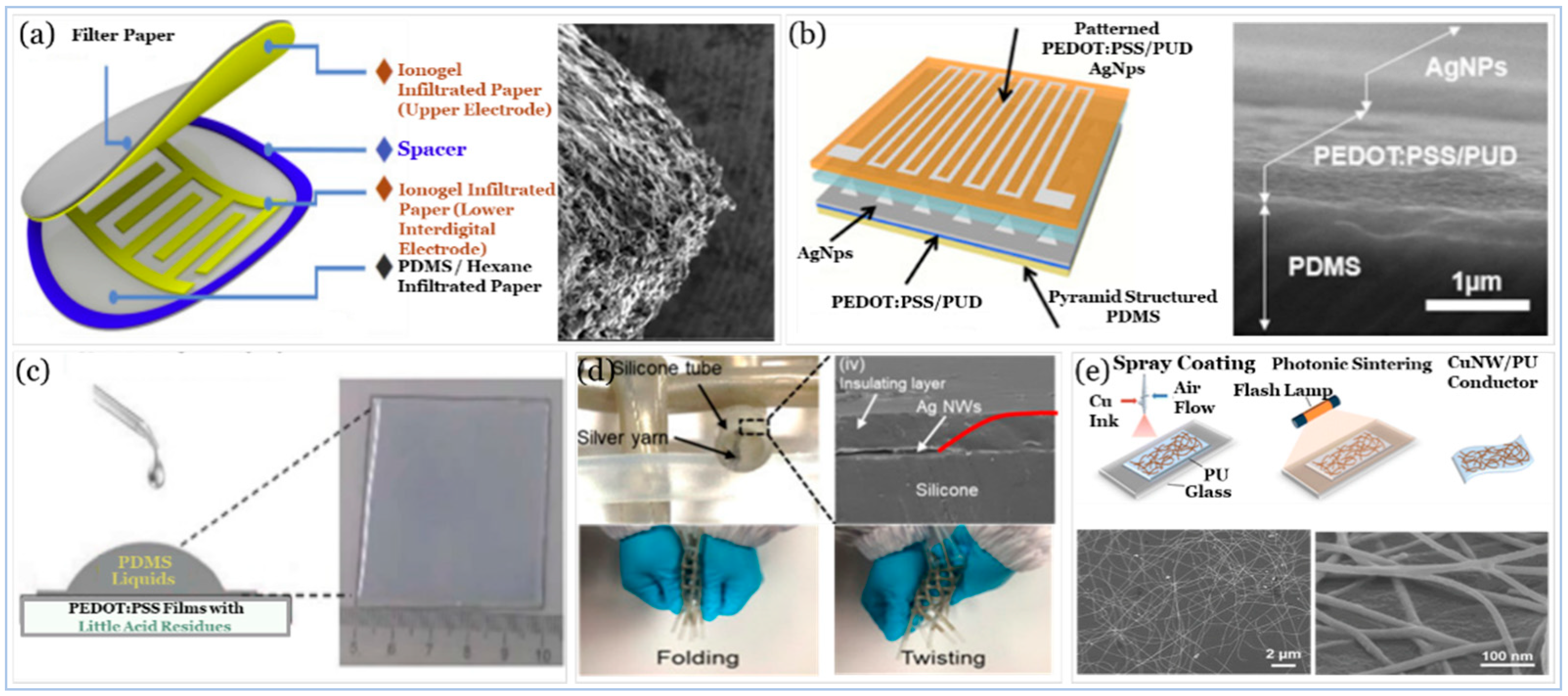
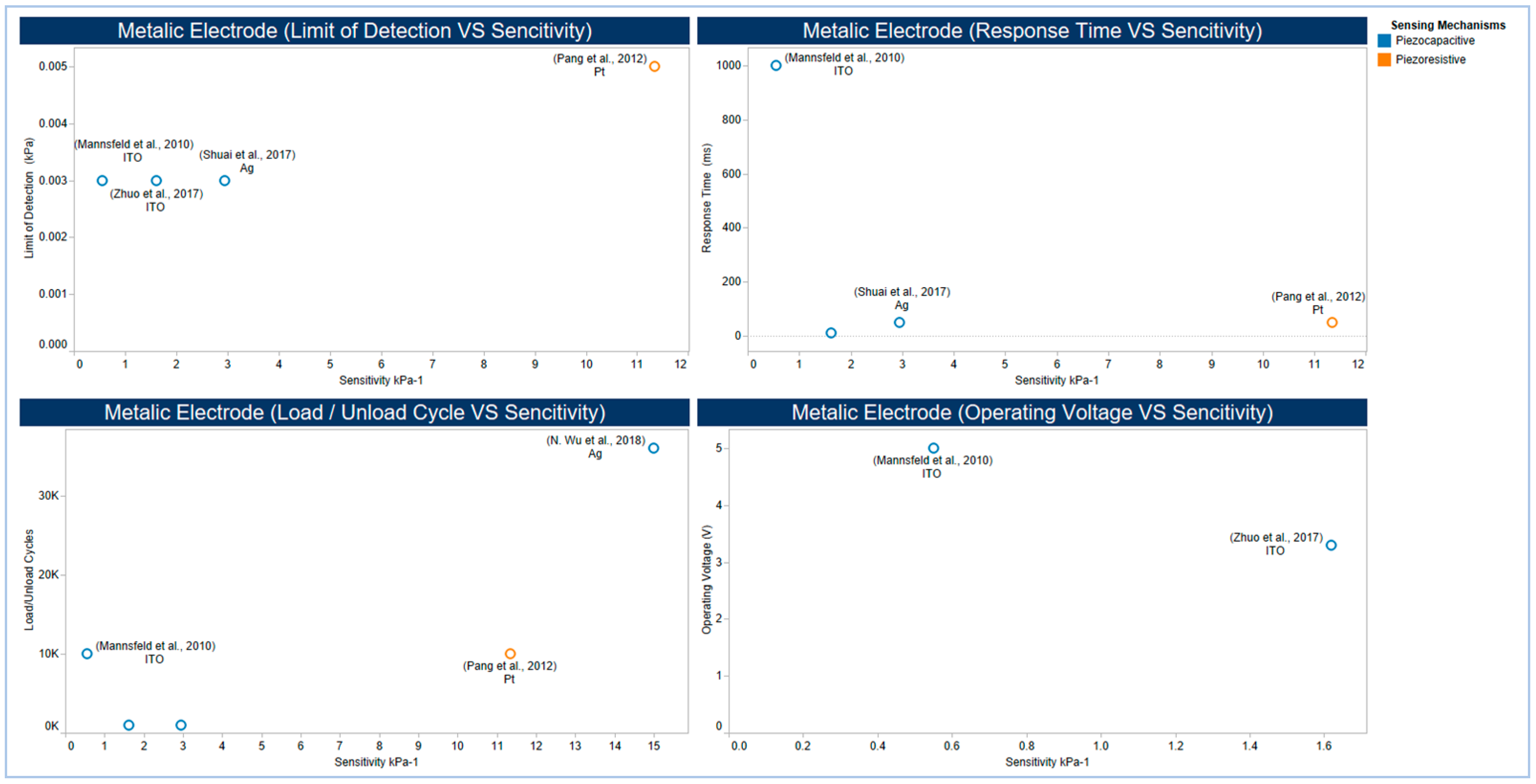
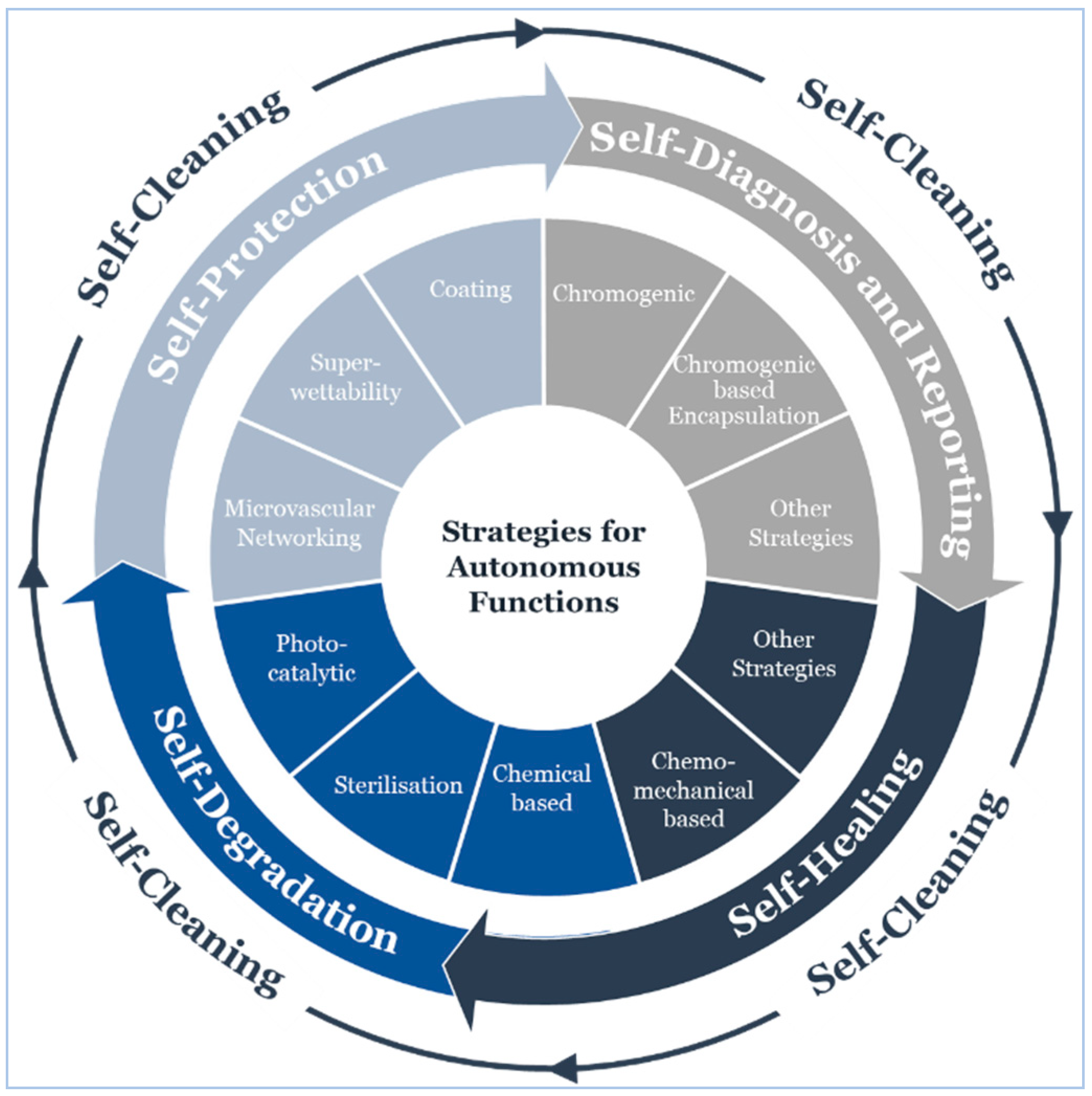
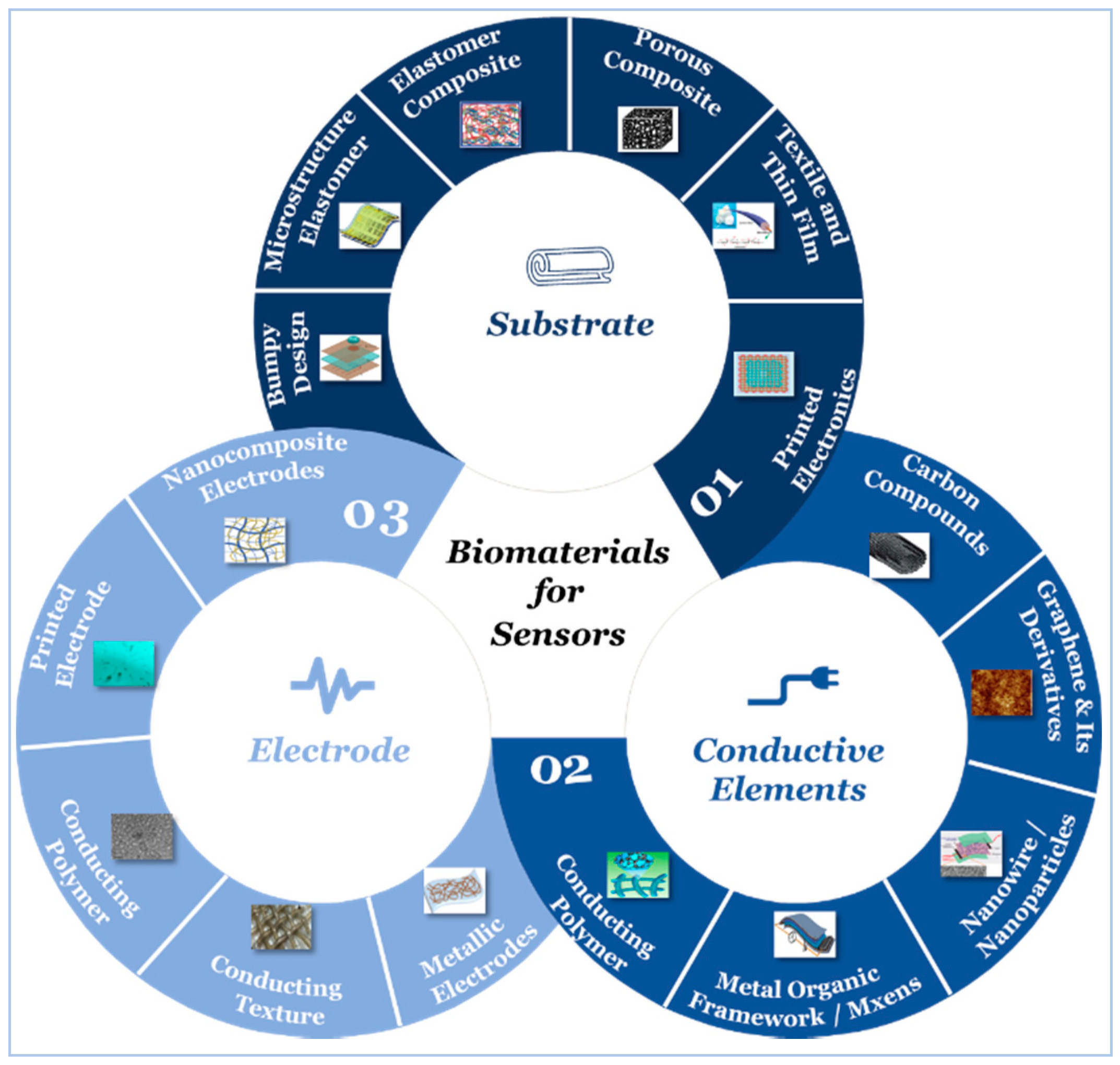
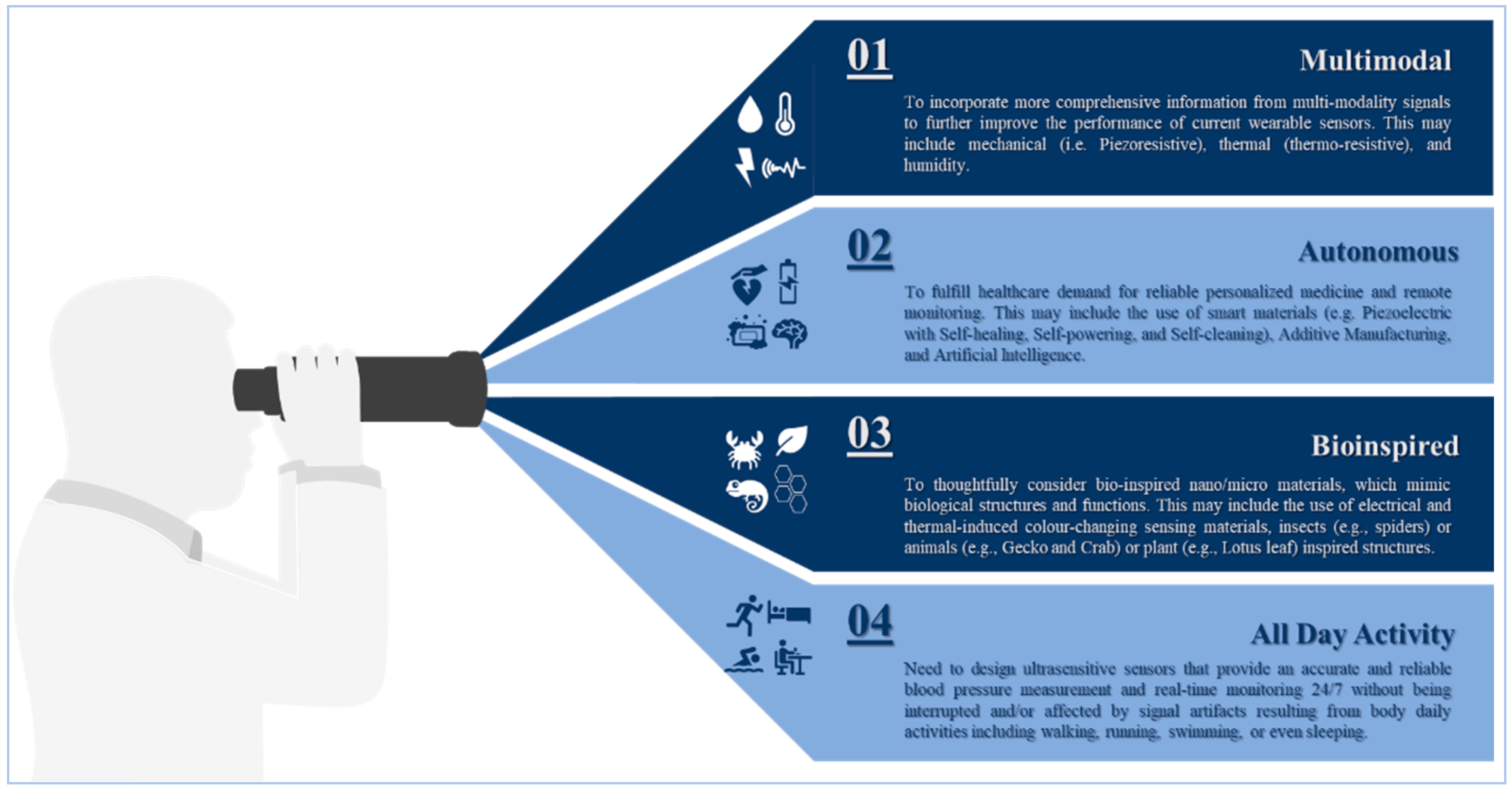
| SN | Approach | Technique | Method | Clinical Compliance | All-Day Activity 1 | Remarks/Usability | ||
|---|---|---|---|---|---|---|---|---|
| Periodicity 2 | Accuracy 3 | Wearable | Wireless | |||||
| 1.0 | Invasive | Single/Multisensory | Arterial Catheter | Continuous 4 [52,53] | Gold Standard [67,68] | N/A 5 | N/A |
|
| 2.0 | Minimally Invasive | Subcutaneous Blood Pressure | Subcutaneous implantable PPG | Beat by Beat 6 [69] | Controversial [75] | N/A | N/A |
|
| 3.0 | Non-invasive | Full Occlusion | Auscultatory | Intermittent 7 [80] | Gold Standard [78] | Can Be 8 | Can Be | • Operator Bias and White Coat Effect [6]. |
| Oscillometry | Intermittent [80] | Good [76] | Can Be | Can Be | • Affected by Artery Stiffness and Age [76]. | |||
| Palpatory | Intermittent [79] | Poor 9 [79] | Can Be | Can Be | • Operator Bias (i.e., Radial Pulse) [79] | |||
| Semi Occlusion | Applanation Tonometry | Continuous [105,106] | Poor 10 [105,106] | Can Be | Can Be |
| ||
| Volume Clamp | Continuous [107,108,109] | Controversial [110,111,112,113] | Yes | Can Be | • Complex Calibration may Lead to Overestimation of Blood Pressure [118]. | |||
| Blood Flow | Beat by Beat [107] | Controversial [107] | Yes | Yes | • Use of Contact Ultrasound Sensor [107] | |||
| Non-Occlusion | Pulse Wave | Beat by Beat [122] | Controversial [141] | Yes 11 | Yes | • Lack of changes in physiological factors [e.g., Blood Viscosity] [142]. | ||
| Stroke Volume | Beat by Beat 12 [123] | Controversial [139] | Yes | Yes | • Complex Calibration Due to Physiological Artefacts [108,140]. | |||
| Blood Flow 13 | Beat by Beat [121] | Controversial [127,128] | Yes | Yes | • Needs to be further developed [127,128] | |||
| SN | Transducer Category | Transducing Modality | Sensing Principles | |||
|---|---|---|---|---|---|---|
| Potential | Capacitive | Piezoelectricity | Piezoresistivity | |||
| 1.0 | Acoustic | PCG | - | * | Piezoelectric Accelerometer [171] | - |
| Ultrasound | - | LZT Sensor [172] | - | |||
| 2.0 | Electrical | ECG | Lead Electrode [173] | CCEs [156] | - | - |
| ICG | Lead Electrode [174] | - | - | - | ||
| 3.0 | Electromagnetic | EM | - | PRF S-R Sensor [124] | - | - |
| 4.0 | Mechanical | BCG | - | Electromechanical Film Sensor [167] | - | - |
| SCG | - | - | MEMS Accelerometer & Gyroscope [168] | |||
| TAG | - | Au/PEN [169] | - | Flexible Piezoresistance [170] | ||
| 5.0 | Optical | PPG | - | - | - | DPP-DTT: PCBM [175] |
| SN | Category | Substrate Material | Chemical Structure | Properties | Reference | Notes |
|---|---|---|---|---|---|---|
| 1.0 | Synthesis | PDMS | [C2H6OSi]n |
| [229] | • Lack of biodegradability. |
| Ecoflex® Silicone Elastomer | - |
| [230] | • Lack of biodegradability. | ||
| PET | [C10H8O4]n |
| [231] | • Relatively high modulus of elasticity (about 2~4 GPa). | ||
| PI | C35H28N2O7 |
| [232] | • Lack of biodegradability. | ||
| 2.0 | Natural | Cellulose Paper | [C6H10O5]n |
| [176,233] | • Durability and stability are still amongst the biggest challenges for enhancing its properties. |
| Smart Textile * | - | • Flexible, inexpensive, and biocompatible. | [234,235,236] |
|
| SN | Active Material (Structure) | Sensing Principles | Limit of Detection (kPa) 1 | Maximum Detection (kPa) 2 | Sensitivity (kPa−1) 3 | Reference | Notes |
|---|---|---|---|---|---|---|---|
| 1.0 | CNT/PDMS (Porous Structure) | Piezoresistive | 0.25 | 100.0 | 0.588 4 | [257] |
|
| 2.0 | CNT@EcoFlex (Buckled Structure) | Piezocapacitive | 50.0 | 1000 | 230 | [244] |
|
| 3.0 | ACNT-Graphene /PDMS (CVD) | Piezoresistive | 0.0006 | 0.3 | 19.8 | [259] |
|
| 4.0 | CNT/PDMS (Patterned Microstructure) | Piezoresistive | 0.0002 | 59.0 | 15.1 | [258] |
|
| 5.0 | VACNT/PDMS (T-CVD) | Piezoresistive | 0.002 | 10.0 | 0.3 and up to 0.7 | [260] |
|
| 6.0 | SWCNTs/PDMS (Silk Molded Microstructure) | Piezoresistive | 0.0006 | 1.2 | 1.8 | [272] |
|
| 7.0 | CB@PU Sponge | Piezoresistive | 0.091 | 16.4 | 0.068 | [240] |
|
| 8.0 | MWCNT-rGO@PU Foam | Piezoresistive | 0.0035 | 2.7 | 0.022 | [256] |
|
| 9.0 | SWCNTs/PDMS | Optical | 1.0 | - | 0.2 | [201] |
|
| 10.0 | CNT/3D Microporous Elastomeric Dielectric Layer | Piezocapacitive | 0.0001 | 130 | 0.601 | [194] |
|
| 11.0 | Graphene@PU | Piezoresistive | 0.009 | 10.0 | 0.26 | [273] |
|
| 12.0 | Graphene (MEMS) | Piezoresistive | 0.1 | - | 3.4 × 10−6 | [274] |
|
| 13.0 | Graphene Paper | Piezocapacitive | 2.0 | 20.0 | 17.2 | [275] |
|
| 14.0 | Graphene Electrode (T-CVD) | Piezocapacitive | 4.4 × 10−5 (1 mg) | - | 3.19 | [247] |
|
| 15.0 | Graphene (Porous GS) | Piezoresistive | 0.3 | 10.0 | 0.046 | [276] |
|
| 16.0 | Graphene Electrode | Piezocapacitive | 8.0 | - | 6.55 | [268] |
|
| 17.0 | Suspended Graphene /Polymer (Heterostructure Membranes) | Piezocapacitive | 80.0 | - | 123ZF | [277] |
|
| 18.0 | Graphene Tribotronics | FET | 1.0 | - | 0.02 | [278] |
|
| 19.0 | rGO/PANI Wrapped Sponge | Piezoresistive | 0.1 | 27.0 | 0.152 | [279] |
|
| 20.0 | PNIPAm/CMC/ rGO DN Hydrogel | Thermo-resisitive | - | 800.0 | - | [280] |
|
| 21.0 | rGO/PU Sponge | Piezoresistive | 4.84 × 10−5 (1.1 mg) | - | 0.21 | [281] |
|
| 22.0 | rGO/PDMS Film (Pattered Micropyramid) | Piezoresistive | 0.0015 | 1.4 | 5.5 | [243] |
|
| 23.0 | rGO Films with Continuous Gradient Wrinkles | Piezoresistive | 0.0042 | 3.0 | 178.0 | [282] |
|
| 24.0 | Large-Scale Polystyrene Ball@rGO Core Shell NPs | Piezoresistive | 0.003 | 3.0 | 50.9 | [283] |
|
| 25.0 | Graphene (Electrode Microconformal) | Piezocapacitive | 4.4 × 10−5 (1 mg) | - | 7.68 | [190] |
|
| 26.0 | Integrated Arrays of Air-Dielectric Graphene Transistors | FET | 0.25 | 3000 | 2.05 × 10−4 | [270] |
|
| 27.0 | Graphene Transistor Array (Direct-Contact Tribotronic Planar) | FET | 0.16 mm−1 | - | - | [269] |
|
| 28.0 | Graphene (Direct Laser Scribing PDMS) | Piezoresistive | 0.028 | - | 480.0 | [271] |
|
| 29.0 | 3D Graphene Film (Fingerprint Like Patterned) | Piezoresistive | 0.0002 | 75.0 | 110.0 | [242] |
|
| 30.0 | GO (Spray Coating through a Stencil Mask) | Piezocapacitive | 0.24 × 10−3 | - | 0.8 | [192] |
|
| 31.0 | MoS2/GPN /Ecoflex (T-CVD) | Piezoresistive | 0.6 | 25.4 | 6.06 | [284] |
|
| 32.0 | PVA NWs/ Wrinkled Graphene Film | Piezoresistive | 0.00224 | - | 28.34 | [285] |
|
| 33.0 | rGO Film/ PDMS Arrays | Piezoresistive | 0.0013 | 225.0 | 1.71 | [238] |
|
| 34.0 | P(VDF-TrFe) /rGO | Piezoresistive | 0.0012 | - | 15.6 | [251] |
|
| 35.0 | PEDOT:PSS /PUD (Pattered Micropyramid) | Piezoresistive | 0.023 | 8.0 | 10.3 | [176] |
|
| 36.0 | PVDF-HFP/PEDOT (3D Electrospun Nanofibers) | Piezoresistive | 0.001 | 30.0 | 13.5 | [286] |
|
| 37.0 | P(VDF-TrFe) (Electrospun Nanofiber) | Piezoresistive | 0.0001 | 0.012 | 0.00041 | [287] |
|
| 38.0 | [PPy@PVA-co-PE] and POE Nanofibers | Piezoresistive | 0.0013 | 7.0 | 1.24 | [288] |
|
| 39.0 | Au NWs/Tissue Paper (Dip-Coating) | Piezoresistive | 0.013 | - | 1.14 | [289] |
|
| 40.0 | Au NP Densely Packed µNW based Pressure | Piezoresistive | 0.025 | 0.0801 | [290] |
| |
| 41.0 | Ag IDEs and PdOx NP (Percolative Metal NP Arrays) | Piezoresistive | 0.0005 | 1.0 | 0.13 | [248] |
|
| 42.0 | AG NWs (Ag NW Flower) | Piezocapacitive | 0.0006 | 115.0 | 1.54 | [195] |
|
| 43.0 | Ge/Si Core/shell NW PSR (OLED) | FET | 1.0 | - | 42.7 | [210] |
|
| 44.0 | MOF (CuTCNQ) | Piezoresistive | 0.00073 | 3.0 | 6.25 | [249] |
|
| 45.0 | C-MOF/PANIF @PU Sponge | Piezoresistive | 0.001 | 60.0 | 158.26 | [291] |
|
| 46.0 | C-MOF-5 Derived Porous Carbon | Piezoresistive | 0.02 | 1.0 | 15.63 | [292] |
|
| 47.0 | MXenes Nanosheets (Ti3C2Tx) | Piezoresistive | 0.0102 | 30.0 | 0.55 | [200] |
|
| 48.0 | MXenes Nanosheets (Ti3C2) | Piezoresistive | - | 0.351 | 7.5 | [250] |
|
| 49.0 | Pt-coated Polymeric Nanofibers (Nanohair) | Piezoresistive | 0.005 | - | 11.35 | [293] |
|
| 50.0 | ITO (3D Printed Mold) | Piezocapacitive | 0.003 | 4.0 | 1.62 | [294] |
|
| 51.0 | Ag Flexible Piezoelectret-Based Pressure Sensor | Piezoelectric | - | 2.5 | 15.0 | [295] |
|
| 52.0 | ITO/PDMS (Pattered micro-pyramid) | Piezocapacitive | 0.003 | 20.0 | 0.55 | [296] |
|
| 53.0 | Ag NWs (Embedded PDMS Electrode with Microarray Structure) | Piezocapacitive | 0.003 | 5.0 | 2.94 | [297] |
|
| SN | Structure Design | Sensor Type | Self-Healing Mechanism | Self-Healing Material/Agent | Self-Healing Time (h) | Note |
|---|---|---|---|---|---|---|
| 1.0 | (f-BN NS)/PEDOT: PSS/PNIPam Hydrogel [469] | Pressure | Chemical bond interaction-based (Hydrogen-bond) | - | 6.0 |
|
| 2.0 | Ag NWs/rGO@m-PCL Microspheres onto PDMS [481] | Strain | Solid Microsphere | Ag NWs/rGO@m-PCL Microspheres | 0.05 (3.0 min) |
|
| 3.0 | MWCNT-PEDOT-PAM-PVA [482] | Pressure | Chemical bond interaction-based (Hydrogen-bond) | - | ~1 s |
|
| 4.0 | Au NP/Sh-crl-PU [483] | Gas analytes, pressure, strain, and temperature | Chemical bond interaction-based (Hydrogen-bond) | - | 48.0 |
|
| 5.0 | (Ti3C2Tx)/PV) hydrogel [484] | Strain | Chemical bond interaction-based (Hydrogen-bond) | - | ~0.15 s |
|
| 6.0 | PAA slightly crosslinked with PEG Ionic Conductive Ink [470] | Strain | Chemical bond interaction-based (Ionic Interaction) | - | 0.5 |
|
| 7.0 | Ternary Composite DMSO-mixed PEDOT: PSS with Triton X-100 Wearable thermoelectric generators [485] | Strain | Chemical bond interaction-based (Hydrogen-bond) | Triton X-100 >(C14H22O(C2H4On) | 1.0 s |
|
| 8.0 | PETMP-TTT Thiol-Ene Coatings [424] | - | Other Strategies (Shape memory assisted with heat) | - | 0.083 (5.0 min) |
|
| 9.0 | Conductive Polyimine Film (Dynamic Covalent Thermoset Polyimine with Ag NPs) [486] | Flow, humidity, tactile, and temperature | Other Strategies (Chemical Reaction assisted with heat and pressure) | Re-healing agent (TAA-DETA-TREN with EToH and Ag NPs) | 4.0 |
|
| 10.0 | Stacked textile reinforcement with dual-channel [487] | - | 3D Micro-vascular Networks | A mixture of DGEBA and Aliphatic amido-TETA | 48.0 |
|
| 11.0 | rGO based Composite [488] | Pressure and flexion | Encapsulation | PBS confined in rGO networks with microscopic porosity | 24.0 |
|
| 12.0 | Self-healing magnet-polymer composite [489] | Strain | Other Strategies (Shape memory assisted with a magnet) | 0.167 10.0 min |
|
| SN | Material | Material Category | Chemical Structure | Young Modulus (MPa)/ Elongation (%) | Bio-compatible | Bio-degradable | Note |
|---|---|---|---|---|---|---|---|
| 1.0 | Cellulose Paper | Organic | - | 17.6 ± 0.7/14.0 ± 0.4 [532] | Yes [533] | Yes [534] |
|
| 2.0 | Ecoflex Silicone Elastomer 00-30 | Organic | - | 0.1/835 [538] | Yes [230,539] | - |
|
| 3.0 | PCL | Organic | [C6H10O2]n | 325±115/650± 350 [537] | Yes [542,543] | Yes [544] |
|
| 4.0 | PDMS | Organic | [C2H6OSi]n | Hyperplastic [546] | Yes [547,548] | - | |
| 5.0 | PES | Organic | [C12H8O3S]n | 3.76/47.66 [552] | Yes [553] | - | |
| 6.0 | PET | Organic | [C10H8O4]n | 19.59±0.22/1.87 ±0.03 [556] | Yes [557] | - |
|
| 7.0 | PI | Organic | C35H28N2O7 | 2010/27.5 [562] | Yes [563] | - | |
| 8.0 | PLGA | Organic | C5H8O5 | - | Yes [543,566] | Yes [566] |
|
| 9.0 | PVA | Organic | [C2H4O]n | Hyperplastic [567] | Yes [568] | Yes [569] |
|
| 10.0 | Silk | Organic | - | 6100/19.55 [571] | Yes [572,573] | Yes [574] |
|
| 11.0 | Shellac | Organic | - | Rheologic [578,579] | Yes [580] | Yes [581] | |
| 12.0 | Sylgard Elastomer (184) | Organic | - | 2.4/135 [538] | Yes [583] | - |
|
| SN | Material | Material Category | Chemical Structure | Conductivity (S/m) | Bio-compatible | Bio-degradable | Note |
|---|---|---|---|---|---|---|---|
| 1.0 | Ag NWs | Inorganic | - | 6.3 × 107 [585] | Yes [586] | - | |
| 2.0 | CNTs | Organic | - | 103 − 6.7 × 106 [553] | Yes [589] | Yes [590] |
|
| 3.0 | Graphene | Inorganic | - | 7.095 × 104 [591] | Yes [592] | - |
|
| 4.0 | MoS2 | Inorganic | - | 107 [596] | Yes [597] | - | |
| 5.0 | PANI (doped) | Organic | - | 103–104 [599] | Yes [600] | - |
|
| 6.0 | PEDOT (doped) | Organic | - | (6.259 ± 1.468) × 105 [604] | Yes [605] | - | |
| 7.0 | PPy (doped) | Organic | - | 103–105 [599] | Yes [600] | - |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qatatsheh, A.; Morsi, Y.; Zavabeti, A.; Zolfagharian, A.; Salim, N.; Z. Kouzani, A.; Mosadegh, B.; Gharaie, S. Blood Pressure Sensors: Materials, Fabrication Methods, Performance Evaluations and Future Perspectives. Sensors 2020, 20, 4484. https://doi.org/10.3390/s20164484
Al-Qatatsheh A, Morsi Y, Zavabeti A, Zolfagharian A, Salim N, Z. Kouzani A, Mosadegh B, Gharaie S. Blood Pressure Sensors: Materials, Fabrication Methods, Performance Evaluations and Future Perspectives. Sensors. 2020; 20(16):4484. https://doi.org/10.3390/s20164484
Chicago/Turabian StyleAl-Qatatsheh, Ahmed, Yosry Morsi, Ali Zavabeti, Ali Zolfagharian, Nisa Salim, Abbas Z. Kouzani, Bobak Mosadegh, and Saleh Gharaie. 2020. "Blood Pressure Sensors: Materials, Fabrication Methods, Performance Evaluations and Future Perspectives" Sensors 20, no. 16: 4484. https://doi.org/10.3390/s20164484
APA StyleAl-Qatatsheh, A., Morsi, Y., Zavabeti, A., Zolfagharian, A., Salim, N., Z. Kouzani, A., Mosadegh, B., & Gharaie, S. (2020). Blood Pressure Sensors: Materials, Fabrication Methods, Performance Evaluations and Future Perspectives. Sensors, 20(16), 4484. https://doi.org/10.3390/s20164484










