Dynamics of the Prefrontal Cortex during Chess-Based Problem-Solving Tasks in Competition-Experienced Chess Players: An fNIR Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedure
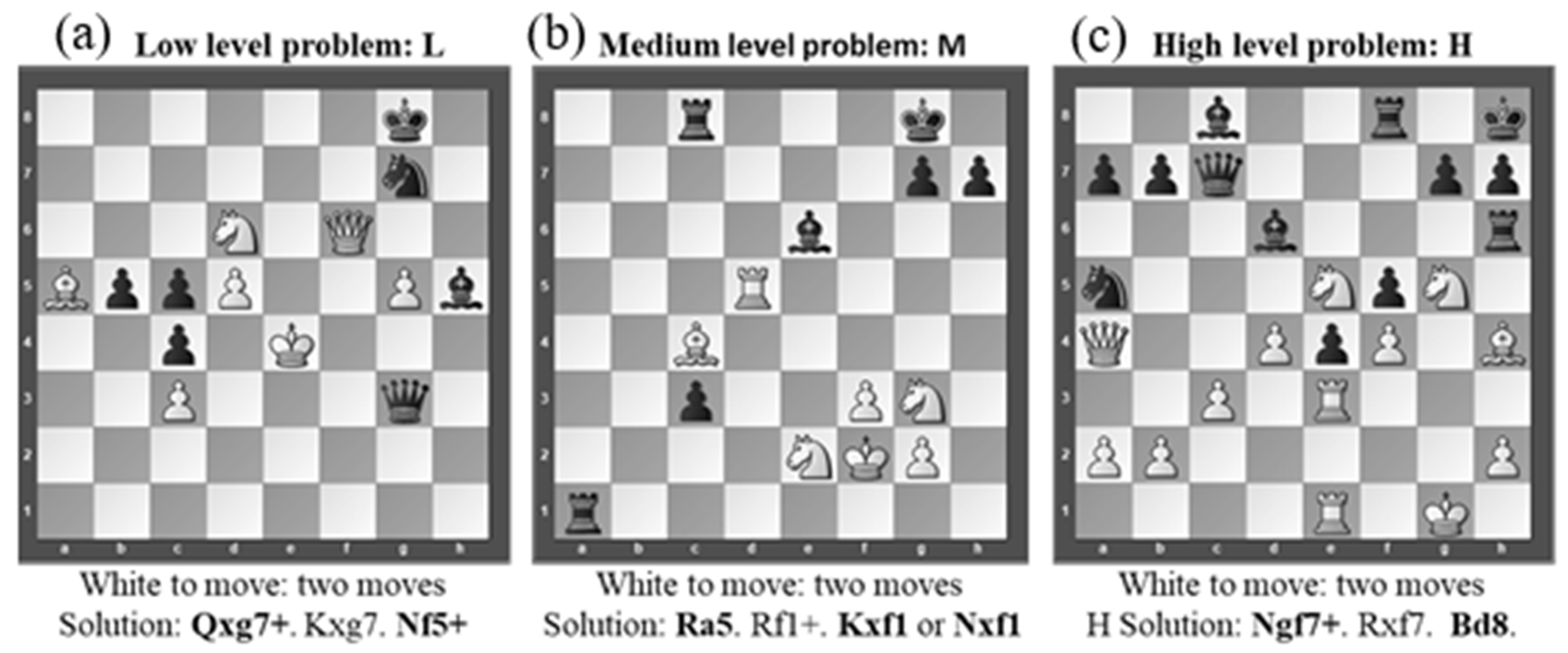
2.3. Chess Problems
2.4. Functional Brain Imaging—fNIRS
2.5. Data Processing
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chayer, C.; Freedman, M. Frontal lobe functions. Curr. Neurol. Neurosci. 2001, 1, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.; Grafman, J. Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi. Neuropsychologia 1995, 33, 623–642. [Google Scholar] [CrossRef]
- Koenraadt, K.L.M.; Roelofsen, E.G.J.; Duysens, J.; Keijsers, N.L.W. Cortical control of normal gait and precision stepping: An fNIRS study. NeuroImage 2014, 85, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E. The Executive Brain: Frontal Lobes and the Civilized Mind; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Fuster, J.n.M. The prefrontal cortex—An update: Time is of the essence. Neuron 2001, 30, 319–333. [Google Scholar] [CrossRef]
- Robbins, T.W. Dissociating executive functions of the prefrontal cortex. Philos. T. R. Soc. B. 1996, 351, 1463–1471. [Google Scholar]
- Damasio, A.R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. T. R. Soc. B. 1996, 351, 1413–1420. [Google Scholar]
- Newman, S.D.; Carpenter, P.A.; Varma, S.; Just, M.A. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia 2003, 41, 1668–1682. [Google Scholar] [CrossRef]
- Delis, D.C.; Squire, L.R.; Bihrle, A.; Massman, P. Componential analysis of problem-solving ability: Performance of patients with frontal lobe damage and amnesic patients on a new sorting test. Neuropsychologia 1992, 30, 683–697. [Google Scholar] [CrossRef]
- Amidzic, O.; Riehle, H.J.; Fehr, T.; Wienbruch, C.; Elbert, T. Pattern of focal γ-bursts in chess players. Nature 2001, 412, 603. [Google Scholar] [CrossRef]
- Bilalić, M.; McLeod, P.; Gobet, F. Specialization effect and its influence on memory and problem solving in expert chess players. Cogn. Sci. 2009, 33, 1117–1143. [Google Scholar] [CrossRef]
- Gobet, F.; Waters, A.J. The role of constraints in expert memory. J. Exp. Psychol. Learn. 2003, 29, 1082. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, L.-Y.; Shewokis, P.A.; Getchell, N. Brain activation in the prefrontal cortex during motor and cognitive tasks in adults. J. Behav. Brain. Sci. 2016, 6, 463–474. [Google Scholar] [CrossRef]
- Bilalić, M.; Langner, R.; Erb, M.; Grodd, W. Mechanisms and neural basis of object and pattern recognition: A study with chess experts. J. Exp. Psychol. Gen. 2010, 139, 728. [Google Scholar] [CrossRef] [PubMed]
- Bilalić, M.; Langner, R.; Ulrich, R.; Grodd, W. Many faces of expertise: Fusiform face area in chess experts and novices. J. Neurosci. 2011, 31, 10206–10214. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liao, W.; Liang, D.; Qiu, L.; Gao, Q.; Liu, C.; Gong, Q.; Chen, H. Large-scale brain networks in board game experts: Insights from a domain-related task and task-free resting state. PLoS ONE 2012, 7, e32532. [Google Scholar] [CrossRef]
- Caballero, A.; Granberg, R.; Tseng, K.Y. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci. Biobehav. R. 2016, 70, 4–12. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Holmes, C.J.; Jernigan, T.L.; Toga, A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999, 2, 859–861. [Google Scholar] [CrossRef]
- Kanwal, J.; Jung, Y.; Zhang, M. Brain plasticity during adolescence: Effects of stress, sleep, sex and sounds on decision making. Anat. Physiol. Curr. Res. 2016, 6, e135. [Google Scholar] [CrossRef]
- Fuster, J.M. Frontal lobe and cognitive development. J. Neurocytol. 2002, 31, 373–385. [Google Scholar] [CrossRef]
- Fuentes-Garcia, J.P.; Pereira, T.; Castro, M.A.; Carvalho Santos, A.; Villafaina, S. Psychophysiological stress response of adolescent chess players during problem-solving tasks. Physiol. Behav. 2019, 209, 112609. [Google Scholar] [CrossRef]
- Ayaz, H.; Shewokis, P.A.; Curtin, A.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B. Using MazeSuite and functional near infrared spectroscopy to study learning in spatial navigation. J. Vis. Exp. 2011, 56, 3443. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, H.; Izzetoglu, M.; Shewokis, P.A.; Onaral, B. Sliding-window motion artifact rejection for functional near-infrared spectroscopy. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; IEEE: New York, NY, USA, 2010. [Google Scholar]
- Izzetoglu, M.; Chitrapu, P.; Bunce, S.; Onaral, B. Motion artifact cancellation in NIR spectroscopy using discrete Kalman filtering. Biomed. Eng. Online 2010, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.C.; Cromheeke, S.; Siugzdaite, R.; Nicolas Boehler, C. Evidence for the triadic model of adolescent brain development: Cognitive load and task-relevance of emotion differentially affect adolescents and adults. Dev. Cogn. Neurosci. 2017, 26, 91–100. [Google Scholar] [CrossRef]
- Silvers, J.A.; Shu, J.; Hubbard, A.D.; Weber, J.; Ochsner, K.N. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev. Sci. 2015, 18, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Hare, T.A.; Tottenham, N.; Galvan, A.; Voss, H.U.; Glover, G.H.; Casey, B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry 2008, 63, 927–934. [Google Scholar] [CrossRef]
- Elo, A.E. The Rating of Chessplayers, Past and Present; Ishi Press: New York, NY, USA, 2008. [Google Scholar]
- Di Fatta, G.; Haworth, G.M.; Regan, K.W.; IEEE. Skill Rating by Bayesian Inference. In Proceedings of the 2009 IEEE Symposium on Computational Intelligence and Data Mining, Nashville, TN, USA, 30 March–2 April 2009; IEEE: New York, NY, USA, 2009. [Google Scholar]
- Ayaz, H.; Onaral, B. Analytical Software and Stimulus-Presentation Platform to Utilize, Visualize and Analyze Near-Infrared Spectroscopy Measures. Master Thesis, Drexel University, Philadelphia, PA, USA, 2005. [Google Scholar]
- Blokh, M. Combinational Motifs, 1st ed.; Hannaco Enterprises: Moscow, Russia, 2003. [Google Scholar]
- Regan, K.W.; Biswas, T.; Zhou, J. Human and computer preferences at chess. In Proceedings of the Workshops at the Twenty-Eighth AAAI Conference on Artificial Intelligence, Québec City, QC, Canada, 27–31 July 2014; AAAI Press: Palo Alto, CA, USA, 2014. [Google Scholar]
- Laureano-Cruces, A.L.; Hernández-González, D.E.; Mora-Torres, M.; Ramírez-Rodríguez, J. Application of a cognitive model of emotional appraisal to the board evaluation function of a program that plays chess. Rev. de Matemática Teoría y Apl. 2012, 19, 211–237. [Google Scholar]
- Fuentes, J.P.; Villafaina, S.; Collado-Mateo, D.; de la Vega, R.; Gusi, N.; Clemente-Suarez, V.J. Use of Biotechnological Devices in the Quantification of Psychophysiological Workload of Professional Chess Players. J. Med. Syst. 2018, 42, 40. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.P.; Villafaina, S.; Collado-Mateo, D.; de la Vega, R.; Olivares, P.R.; Clemente-Suárez, V.J. Differences between high vs low performance chess players in heart rate variability during chess problems. Front. Psychol. 2019, 10, 409. [Google Scholar] [CrossRef]
- Villafaina, S.; Collado-Mateo, D.; Cano-Plasencia, R.; Gusi, N.; Fuentes, J.P. Electroencephalographic response of chess players in decision-making processes under time pressure. Physiol. Behav. 2019, 198, 140–143. [Google Scholar] [CrossRef]
- Gateau, T.; Durantin, G.; Lancelot, F.; Scannella, S.; Dehais, F. Real-time state estimation in a flight simulator using fNIRS. PLoS ONE 2015, 10, e0121279. [Google Scholar] [CrossRef]
- Durantin, G.; Gagnon, J.F.; Tremblay, S.; Dehais, F. Using near infrared spectroscopy and heart rate variability to detect mental overload. Behav. Brain Res. 2014, 259, 16–23. [Google Scholar] [CrossRef]
- Izzetoglu, M.; Bunce, S.C.; Izzetoglu, K.; Onaral, B.; Pourrezaei, K. Functional brain imaging using near-infrared technology. IEEE Eng. Med. Biol. 2007, 26, 38. [Google Scholar] [CrossRef] [PubMed]
- Maidan, I.; Bernad-Elazari, H.; Gazit, E.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: An fNIRS study of transient motor-cognitive failures. J. Neurol. 2015, 262, 899–908. [Google Scholar] [CrossRef]
- Miyai, I.; Tanabe, H.C.; Sase, I.; Eda, H.; Oda, I.; Konishi, I.; Tsunazawa, Y.; Suzuki, T.; Yanagida, T.; Kubota, K. Cortical mapping of gait in humans: A near-infrared spectroscopic topography study. Neuroimage 2001, 14, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Kobayashi, N.; Tamura, M. Interpretation of near-infrared spectroscopy signals: A study with a newly developed perfused rat brain model. J. Appl. Physiol. 2001, 90, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Gobet, F.; Simon, H.A. Expert chess memory: Revisiting the chunking hypothesis. Memory 1998, 6, 225–255. [Google Scholar] [CrossRef]
- Chase, W.G.; Simon, H.A. Perception in chess. Cogn. Psychol. 1973, 4, 55–81. [Google Scholar] [CrossRef]
- Verdière, K.J.; Roy, R.N.; Dehais, F. Detecting pilot’s engagement using fNIRS connectivity features in an automated vs. manual landing scenario. Front. Hum. Neurosci. 2018, 12, 6. [Google Scholar]


| Total (n = 30) | Adults (n = 15) | Adolescents (n = 15) | Ƥ | |
|---|---|---|---|---|
| Age (years) | 24.2 ± 12.8 | 32.7 ± 13.5 | 15.6 ± 1.7 | <0.001 |
| Chess playing (years) | 14.0 ± 10.3 | 19.6 ± 12.2 | 08.4 ± 2.0 | 0.003 |
| Chess competition (years) | 10.9 ± 7.8 | 14.7 ± 9.6 | 07.2 ± 2.2 | 0.010 |
| Chess practicing habits | ||||
| Days/week | 2.5 ± 2.2 | 2.5 ± 2.3 | 2.5 ± 2.2 | 0.968 |
| Hours/Day | 1.7 ± 1.3 | 1.8 ± 1.3 | 1.6 ± 1.4 | 0.689 |
| Hours/week | 5.2 ± 6.3 | 6.1 ± 7.3 | 4.4 ± 5.1 | 0.470 |
| ELO | 1677 ± 332 | 1825 ± 249 | 1529 ± 347 | 0.012 |
| Number of problem-solving chess tasks solved | ||||
| Low difficulty | 22 (73.3%) | 12 (80%) | 10 (66.7%) | 0.409 |
| Medium difficulty | 15 (50%) | 7 (46.7%) | 8 (53.3%) | 0.715 |
| High difficulty | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| Low Difficulty | Medium Difficulty | High Difficulty | F | P | ηp2 | ||
|---|---|---|---|---|---|---|---|
| ∆HbO2 (μmol/L) | L-PFC | 0.33 ± 0.19 | 0.50 ± 0.22 | 0.77 ± 0.28 | 71.656 | <0.001 | 0.712 |
| R-PFC | 0.36 ± 0.26 | 0.50 ± 0.34 | 0.41 ± 0.34 | 1.547 | 0.221 | 0.051 | |
| LM-PFC | 0.34 ± 0.22 | 0.38 ± 0.29 | 0.41 ± 0.29 | 0.753 | 0.476 | 0.025 | |
| RM-PFC | 0.41 ± 0.27 | 0.43 ± 0.34 | 0.49 ± 0.37 | 0.563 | 0.572 | 0.019 | |
| ∆HHb (μmol/L) | L-PFC | −0.51 ± 0.35 | −0.48 ± 0.25 | −0.69 ± 0.32 | 3.901 | 0.026 | 0.119 |
| R-PFC | −0.39 ± 0.25 | −0.39 ± 0.24 | −0.33 ± 0.24 | 1.049 | 0.357 | 0.035 | |
| LM-PFC | −0.43 ± 0.25 | −0.48 ± 0.29 | 0.34 ± 0.27 | 2.409 | 0.099 | 0.077 | |
| RM-PFC | −0.37 ± 0.33 | −0.45 ± 0.34 | −0.38 ± 0.31 | 3723 | 0.490 | 0.024 | |
| ∆HbT (μmol/L) | L-PFC | −0.19 ± 0.42 | 0.01 ± 0.26 | 0.08 ± 0.35 | 5.865 | 0.005 | 0.168 |
| R-PFC | −0.3 ± 0.35 | 0.11 ± 0.27 | 0.09 ± 039 | 1.325 | 0.274 | 0.044 | |
| LM-PFC | −0.10 ± 0.29 | −0.10 ± 0.32 | 0.07 ± 0.36 | 2.727 | 0.074 | 0.086 | |
| RM-PFC | 0.04 ± 0.42 | −0.02 ± 0.40 | 0.11 ± 0.39 | 0.939 | 0.397 | 0.031 | |
| ∆oxy (μmol/L) | L-PFC | 0.84 ± 0.38 | 0.98 ± 0.39 | 1.50 ± 0.48 | 23.777 | <0.001 | 0.451 |
| R-PFC | 0.74 ± 0.38 | 0.90 ± 0.53 | 0.74 ± 0.45 | 1.150 | 0.229 | 0.049 | |
| LM-PFC | 0.77 ± 0.39 | 0.87 ± 0.49 | 0.75 ± 0.43 | 0.898 | 0.413 | 0.030 | |
| RM-PFC | 0.78 ± 0.43 | 0.87 ± 0.56 | 0.87 ± 0.56 | 0.438 | 0.648 | 0.015 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, T.; Castro, M.A.; Villafaina, S.; Carvalho Santos, A.; Fuentes-García, J.P. Dynamics of the Prefrontal Cortex during Chess-Based Problem-Solving Tasks in Competition-Experienced Chess Players: An fNIR Study. Sensors 2020, 20, 3917. https://doi.org/10.3390/s20143917
Pereira T, Castro MA, Villafaina S, Carvalho Santos A, Fuentes-García JP. Dynamics of the Prefrontal Cortex during Chess-Based Problem-Solving Tasks in Competition-Experienced Chess Players: An fNIR Study. Sensors. 2020; 20(14):3917. https://doi.org/10.3390/s20143917
Chicago/Turabian StylePereira, Telmo, Maria António Castro, Santos Villafaina, António Carvalho Santos, and Juan Pedro Fuentes-García. 2020. "Dynamics of the Prefrontal Cortex during Chess-Based Problem-Solving Tasks in Competition-Experienced Chess Players: An fNIR Study" Sensors 20, no. 14: 3917. https://doi.org/10.3390/s20143917
APA StylePereira, T., Castro, M. A., Villafaina, S., Carvalho Santos, A., & Fuentes-García, J. P. (2020). Dynamics of the Prefrontal Cortex during Chess-Based Problem-Solving Tasks in Competition-Experienced Chess Players: An fNIR Study. Sensors, 20(14), 3917. https://doi.org/10.3390/s20143917









