Broadband Electrical Sensing of a Live Biological Cell with In Situ Single-Connection Calibration
Abstract
1. Introduction
2. Experimental Preparation
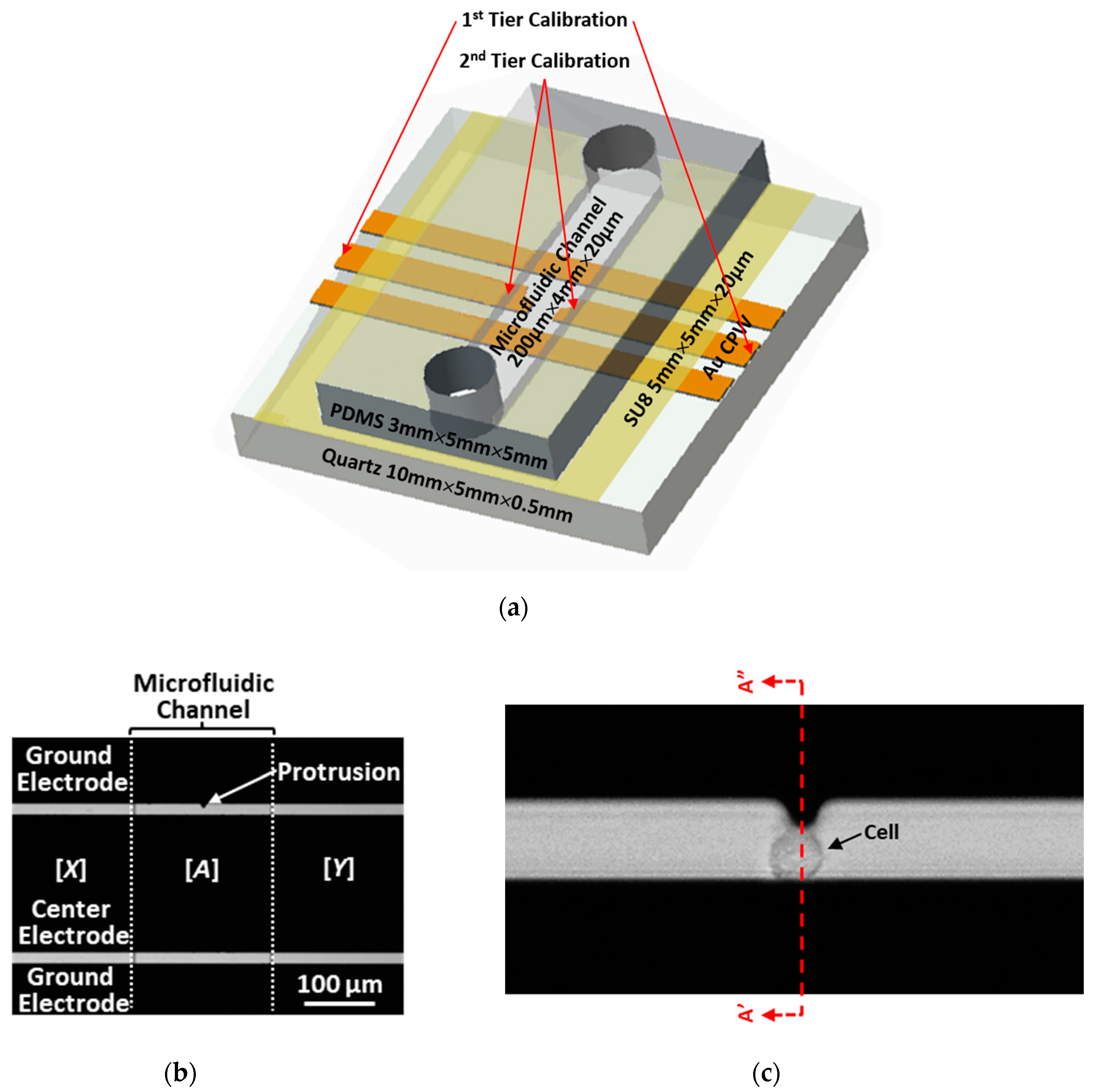
2.1. Test Chip Design
2.2. Electrical Measurement Setup
2.3. Liquid Standards Preparation
2.4. Cell Preparation Protocol
3. Calibration Technique and Standards
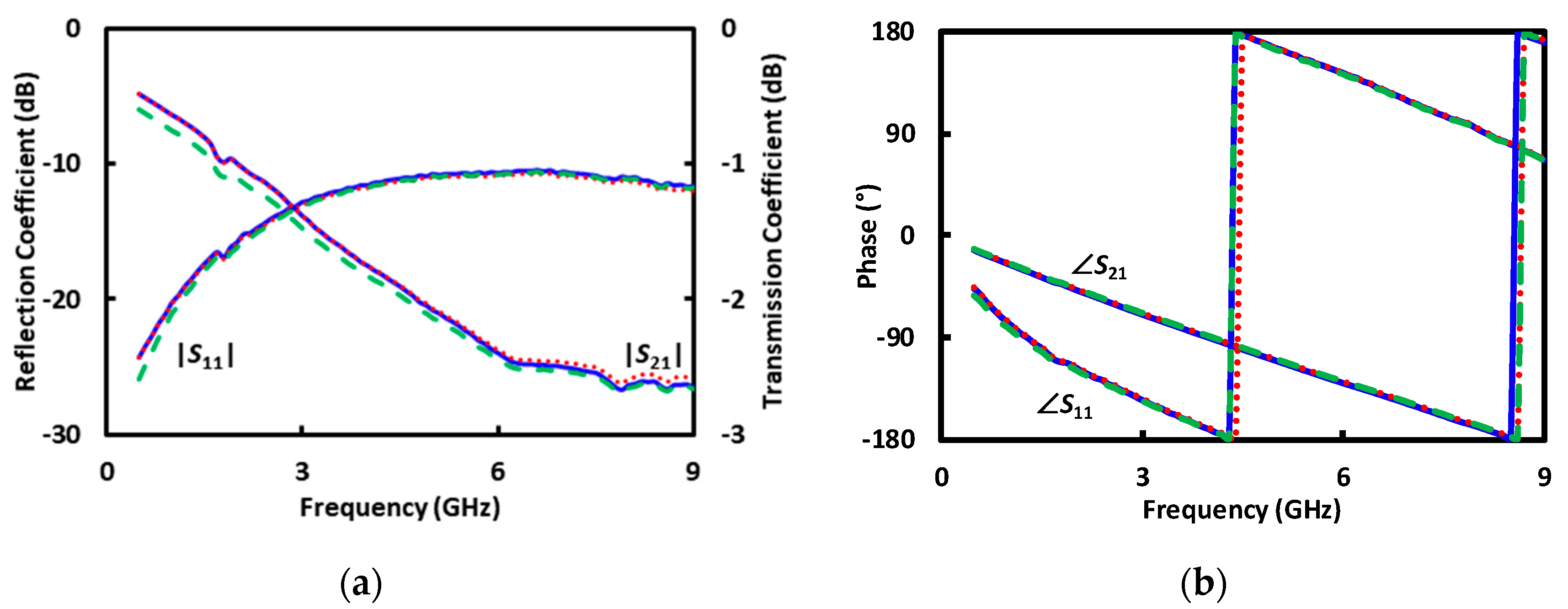
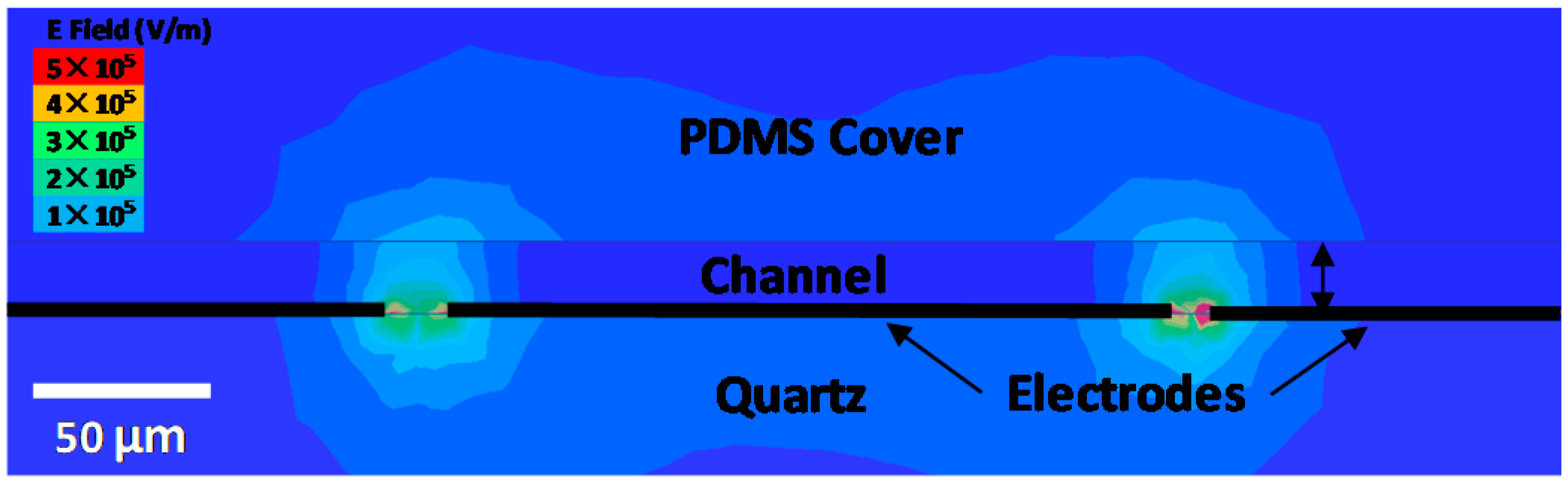
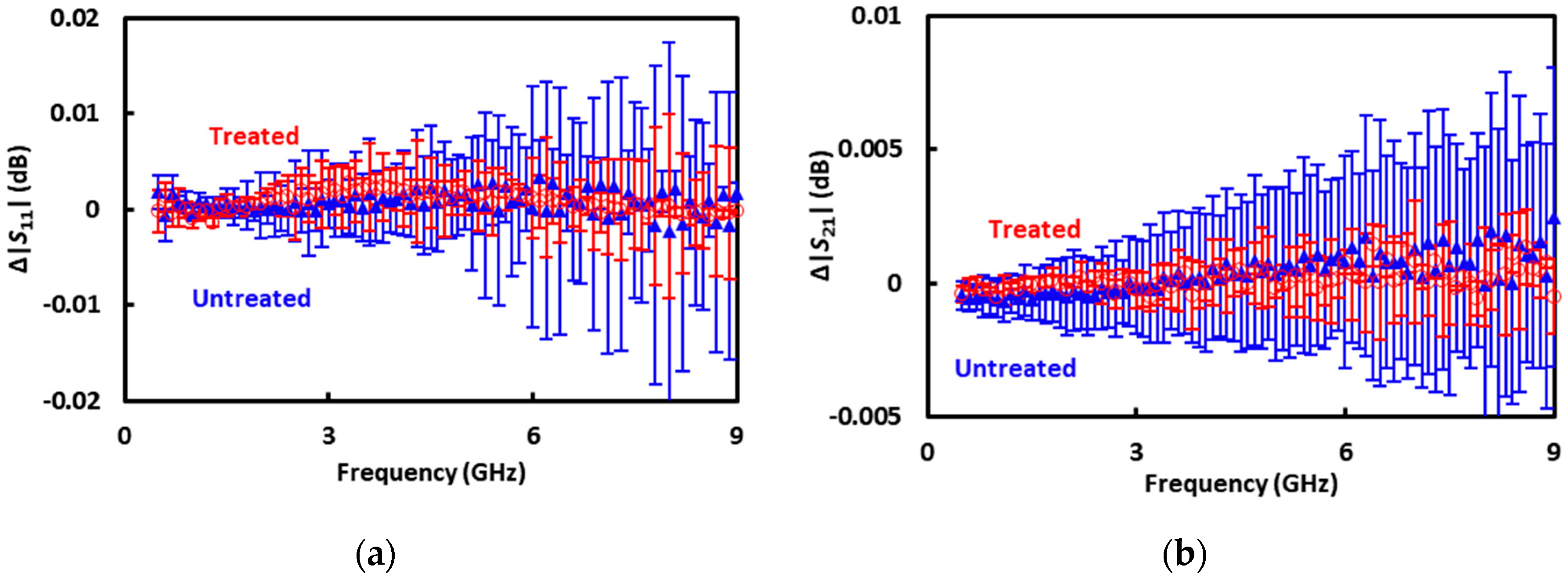
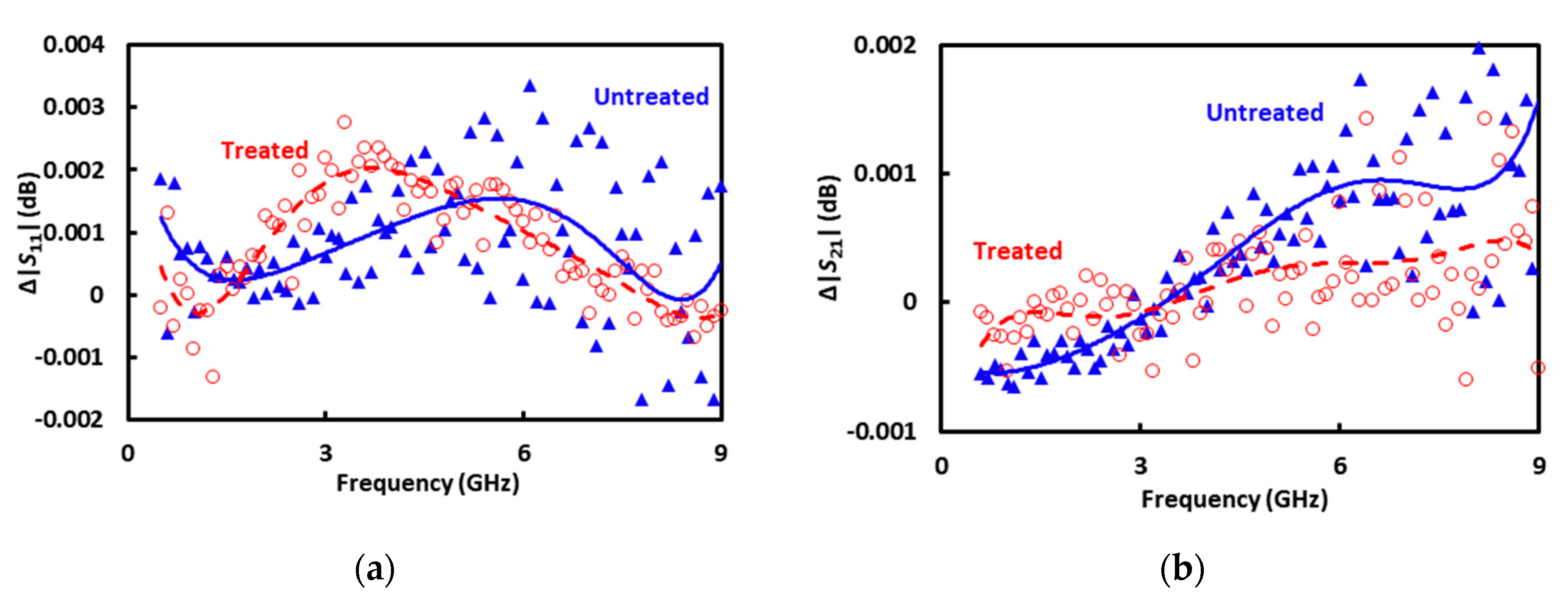
4. Result and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ning, Y.; Multari, C.; Luo, X.; Palego, C.; Cheng, X.; Hwang, J.C.; Denzi, A.; Merla, C.; Apollonio, F.; Liberti, M. Broadband electrical detection of individual biological cells. IEEE Trans. Microw. Theory Tech. 2014, 62, 1905–1911. [Google Scholar] [CrossRef]
- Chien, J.-C.; Ameri, A.; Yeh, E.-C.; Anwar, M.; Killilea, A.N.; Niknejad, A.M. A high-throughput flow cytometry-on-a-CMOS platform for single-cell dielectric spectroscopy at microwave frequencies. Lab Chip 2018, 18, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Grenier, K.; Dubuc, D.; Poleni, P.-E.; Kumemura, M.; Toshiyoshi, H.; Fujii, T.; Fujita, H. Integrated Broadband Microwave and Microfluidic Sensor Dedicated to Bioengineering. IEEE Trans. Microw. Theory Tech. 2009, 57, 3246–3253. [Google Scholar] [CrossRef]
- Booth, J.C.; Orloff, N.D.; Mateu, J.; Janezic, M.; Rinehart, M.; Beall, J.A. Quantitative Permittivity Measurements of Nanoliter Liquid Volumes in Microfluidic Channels to 40 GHz. IEEE Trans. Instrum. Meas. 2010, 59, 3279–3288. [Google Scholar] [CrossRef]
- Liu, S.; Ocket, I.; Barmuta, P.; Markovič, T.; Lewandowski, A.; Schreurs, D.; Nauwelaers, B. Broadband dielectric spectroscopy calibration using calibration liquids with unknown permittivity. In Proceedings of the 84th ARFTG Microwave Measurement Conference, Boulder, CO, USA, 4–5 December 2014; pp. 1–5. [Google Scholar]
- Padmanabhan, S.; Dunleavy, L.; Daniel, J.; Rodriguez, A.; Kirby, P. Broadband Space Conservative On-Wafer Network Analyzer Calibrations with More Complex Load and Thru Models. IEEE Trans. Microw. Theory Tech. 2006, 54, 3583–3593. [Google Scholar] [CrossRef]
- Williams, D.; Marks, R. LRM probe-tip calibrations using nonideal standards. IEEE Trans. Microw. Theory Tech. 1995, 43, 466–469. [Google Scholar] [CrossRef]
- Williams, D.F.; Walker, D.K. Series-Resistor Calibration. In Proceedings of the 50th ARFTG Conference Digest, Portland, OR, USA, 4–5 December 1997; pp. 131–137. [Google Scholar]
- Williams, D.F.; Marks, R.B.; Davidson, A. Comparison of On-Wafer Calibrations. In Proceedings of the 38th ARFTG Conference Digest, San Diego, CA, USA, 5–6 December 1991; pp. 68–81. [Google Scholar]
- Ma, X.; Orloff, N.D.; Little, C.D.; Long, C.J.; Hanemann, I.E.; Liu, S.; Mateu, J.; Booth, J.C.; Hwang, J.C.M. A Multistate Single-Connection Calibration for Microwave Microfluidics. IEEE Trans. Microw. Theory Tech. 2017, 66, 1099–1107. [Google Scholar] [CrossRef]
- Li, H.; Multari, C.; Palego, C.; Ma, X.; Du, X.; Ning, Y.; Buceta, J.; Hwang, J.C.M.; Cheng, X. Differentiation of live and heat-killed E. coli by microwave impedance spectroscopy. Sens. Actuators B Chem. 2018, 255, 1614–1622. [Google Scholar] [CrossRef]
- Du, X.; Ma, X.; Li, H.; Li, L.; Cheng, X.; Hwang, J.C.M. Validation of Clausius–Mossotti Function in Wideband Single-Cell Dielectrophoresis. IEEE J. Electromagn. RF Microw. Med. Boil. 2019, 3, 127–133. [Google Scholar] [CrossRef]
- Ma, X.; Du, X.; Li, H.; Cheng, X.; Hwang, J.C.M. Ultra-Wideband Impedance Spectroscopy of a Live Biological Cell. IEEE Trans. Microw. Theory Tech. 2018, 66, 3690–3696. [Google Scholar] [CrossRef]
- Du, X.; Ladegard, C.; Ma, X.; Cheng, X.; Hwang, J.C.M. Ultra-wideband Electrical Sensing of Nucleus Size in a Live Cell. In Proceedings of the European Microwave Conference (EuMC), Paris, France, 1–3 October 2019; pp. 1–4. [Google Scholar]
- Jevtić, P.; Edens, L.J.; Vuković, L.D.; Levy, D.L. Sizing and shaping the nucleus: Mechanisms and significance. Curr. Opin. Cell Boil. 2014, 28, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Al-Abbadi, M.A. Basics of cytology. Avicenna J. Med. 2011, 1, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, X.; Du, X.; Li, L.; Cheng, X.; Hwang, J.C.M.; Xiao, M. Correlation between Optical Fluorescence and Microwave Transmission during Single-Cell Electroporation. IEEE Trans. Biomed. Eng. 2019, 66, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Ma, X.; Li, L.; Li, H.; Cheng, X.; Hwang, J.C. Ultra-wideband characterization, dielectrophoresis, and electroporation of a live biological cell using the same vector network analyzer. In Proceedings of the IEEE/MTT-S International Microwave Symposium—IMS, Philadelphia, PA, USA, 10–15 June 2018; pp. 1–4. [Google Scholar]
- Buchner, R.; Barthel, J.; Stauber, J. The dielectric relaxation of water between 0 °C and 35 °C. Chem. Phys. Lett. 1999, 306, 57–63. [Google Scholar] [CrossRef]
- Chowdhury, I.; Xu, W.; Stiles, J.K.; Zeleznik, A.; Yao, X.; Matthews, R.; Thomas, K.; Thompson, W.E. Apoptosis of rat granulosa cells after staurosporine and serum withdrawal is suppressed by adenovirus-directed overexpression of prohibition. Endocrinology 2007, 148, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Denzi, A.; Merla, C.; Palego, C.; Paffi, A.; Ning, Y.; Multari, C.R.; Cheng, X.; Apollonio, F.; Hwang, J.C.M.; Liberti, M. Assessment of Cytoplasm Conductivity by Nanosecond Pulsed Electric Fields. IEEE Trans. Biomed. Eng. 2015, 62, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Denzi, A.; Ma, X.; Du, X.; Ning, Y.; Cheng, X.; Apollonio, F.; Liberti, M.; Hwang, J.C.M. Distributed Effect in High-Frequency Electroporation of Biological Cells. IEEE Trans. Microw. Theory Tech. 2017, 65, 3503–3511. [Google Scholar] [CrossRef]
- Simons, R.N. Coplanar Waveguide Circuits, Components, and Systems; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Ye, D.; Islam, S.; Yu, G.; Wang, P. A Single-Line Single-Channel Method with Closed-Form Formulas for the Characterization of Dielectric Liquids. IEEE Trans. Microw. Theory Tech. 2019, 67, 2443–2450. [Google Scholar] [CrossRef]
- Ma, X.; Du, X.; Li, L.; Li, H.; Cheng, X.; Hwang, J.C.M. Sensitivity Analysis for Ultra-Wideband 2-Port Impedance Spectroscopy of a Live Cell. IEEE J. Electromagn. RF Microw. Med. Boil. 2020, 4, 37–44. [Google Scholar] [CrossRef]
- Suzuki, Y.; Taki, M.; Saito, H.; Taguchi, T. Measurement of complex permittivity for biological cells by waveguide penetration method. In Proceedings of the 2009 International Symposium on Electromagnetic Compatibility, Kyoto International Conference Center, Tokyo, Japan, 20–24 July 2009; pp. 317–320. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Du, X.; Li, L.; Ladegard, C.; Cheng, X.; Hwang, J.C.M. Broadband Electrical Sensing of a Live Biological Cell with In Situ Single-Connection Calibration. Sensors 2020, 20, 3844. https://doi.org/10.3390/s20143844
Ma X, Du X, Li L, Ladegard C, Cheng X, Hwang JCM. Broadband Electrical Sensing of a Live Biological Cell with In Situ Single-Connection Calibration. Sensors. 2020; 20(14):3844. https://doi.org/10.3390/s20143844
Chicago/Turabian StyleMa, Xiao, Xiaotian Du, Lei Li, Caroline Ladegard, Xuanhong Cheng, and James C. M. Hwang. 2020. "Broadband Electrical Sensing of a Live Biological Cell with In Situ Single-Connection Calibration" Sensors 20, no. 14: 3844. https://doi.org/10.3390/s20143844
APA StyleMa, X., Du, X., Li, L., Ladegard, C., Cheng, X., & Hwang, J. C. M. (2020). Broadband Electrical Sensing of a Live Biological Cell with In Situ Single-Connection Calibration. Sensors, 20(14), 3844. https://doi.org/10.3390/s20143844





