Development of Nanoporous AAO Membrane for Nano Filtration Using the Acoustophoresis Method
Abstract
1. Introduction
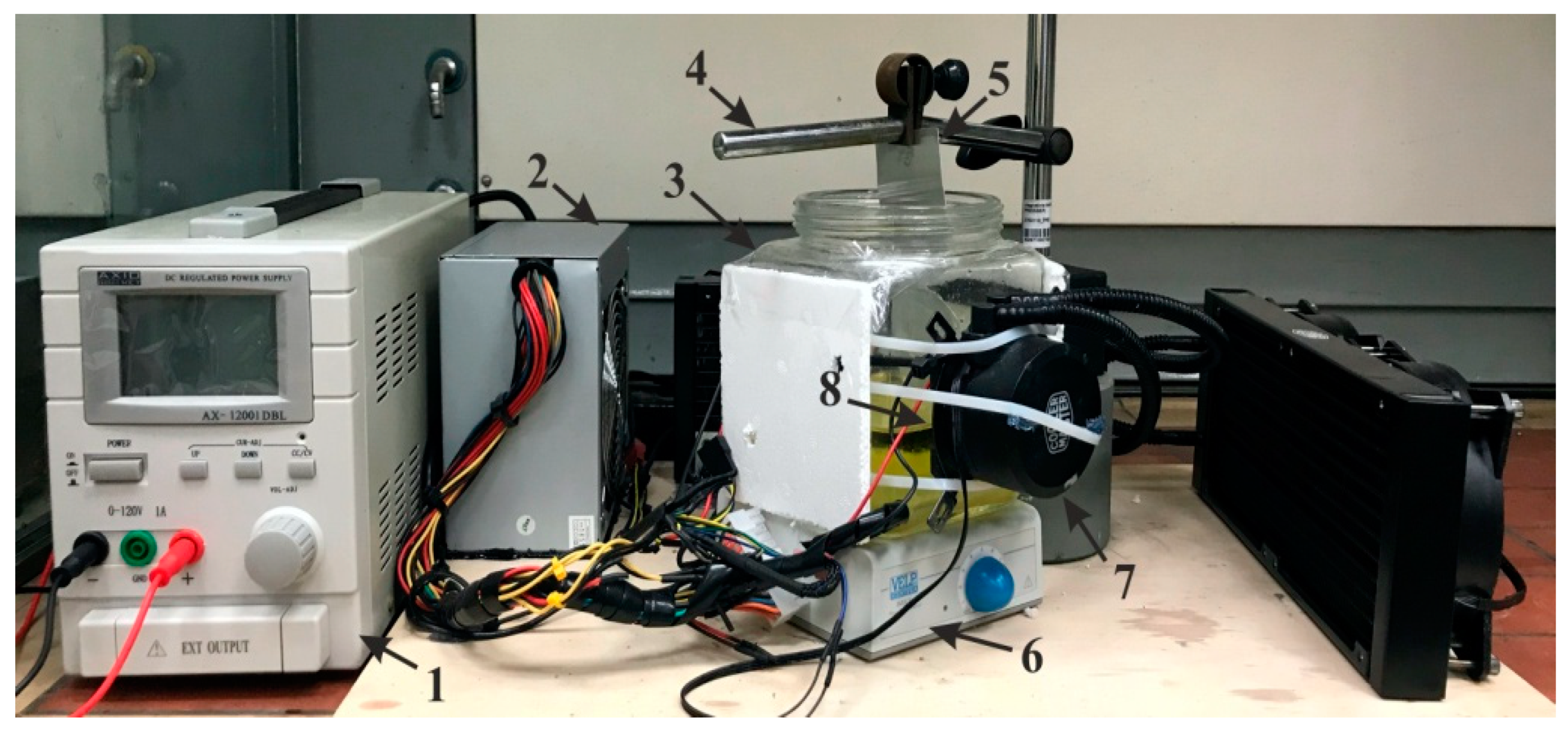
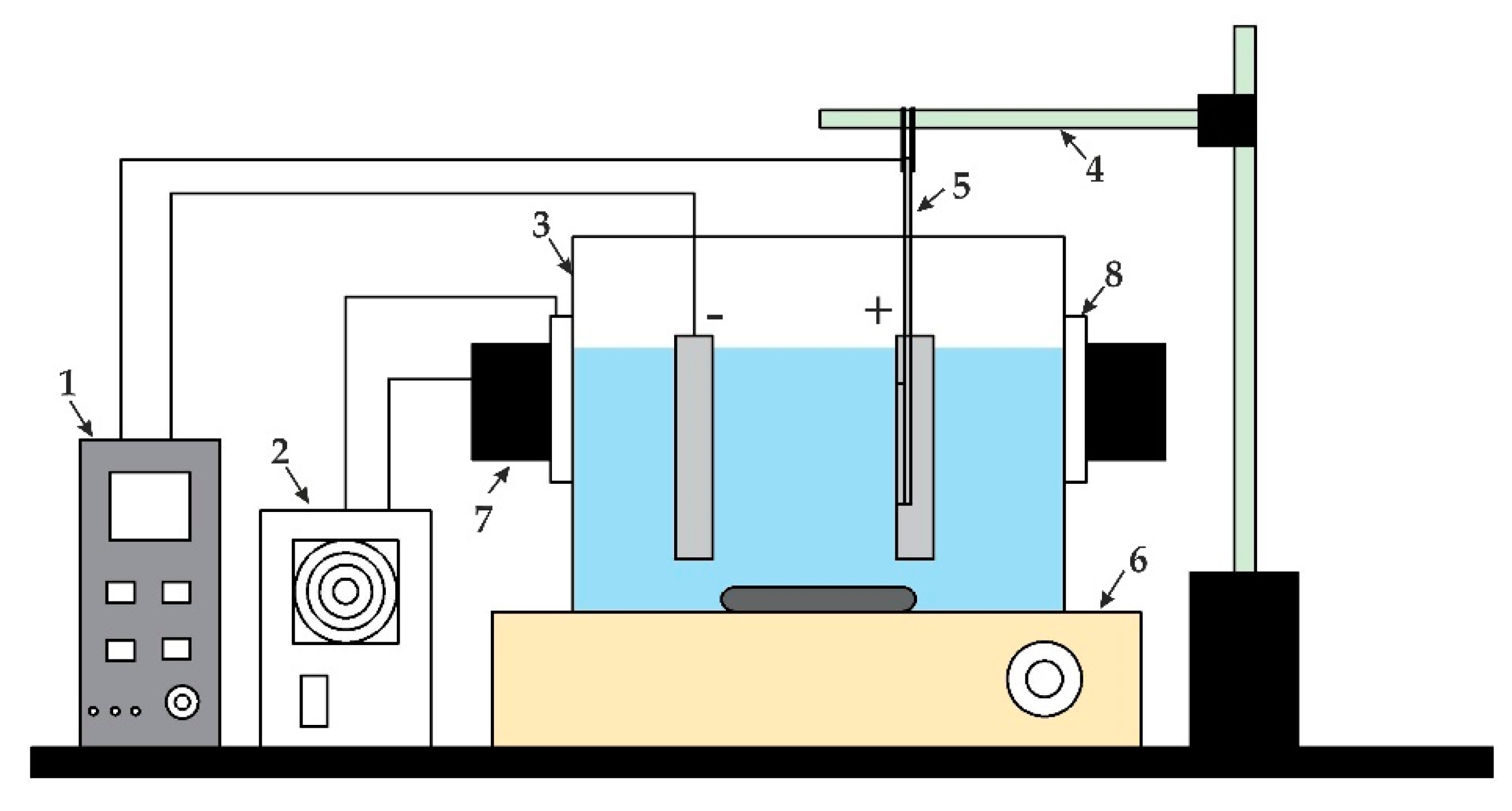
2. Materials and Fabrication
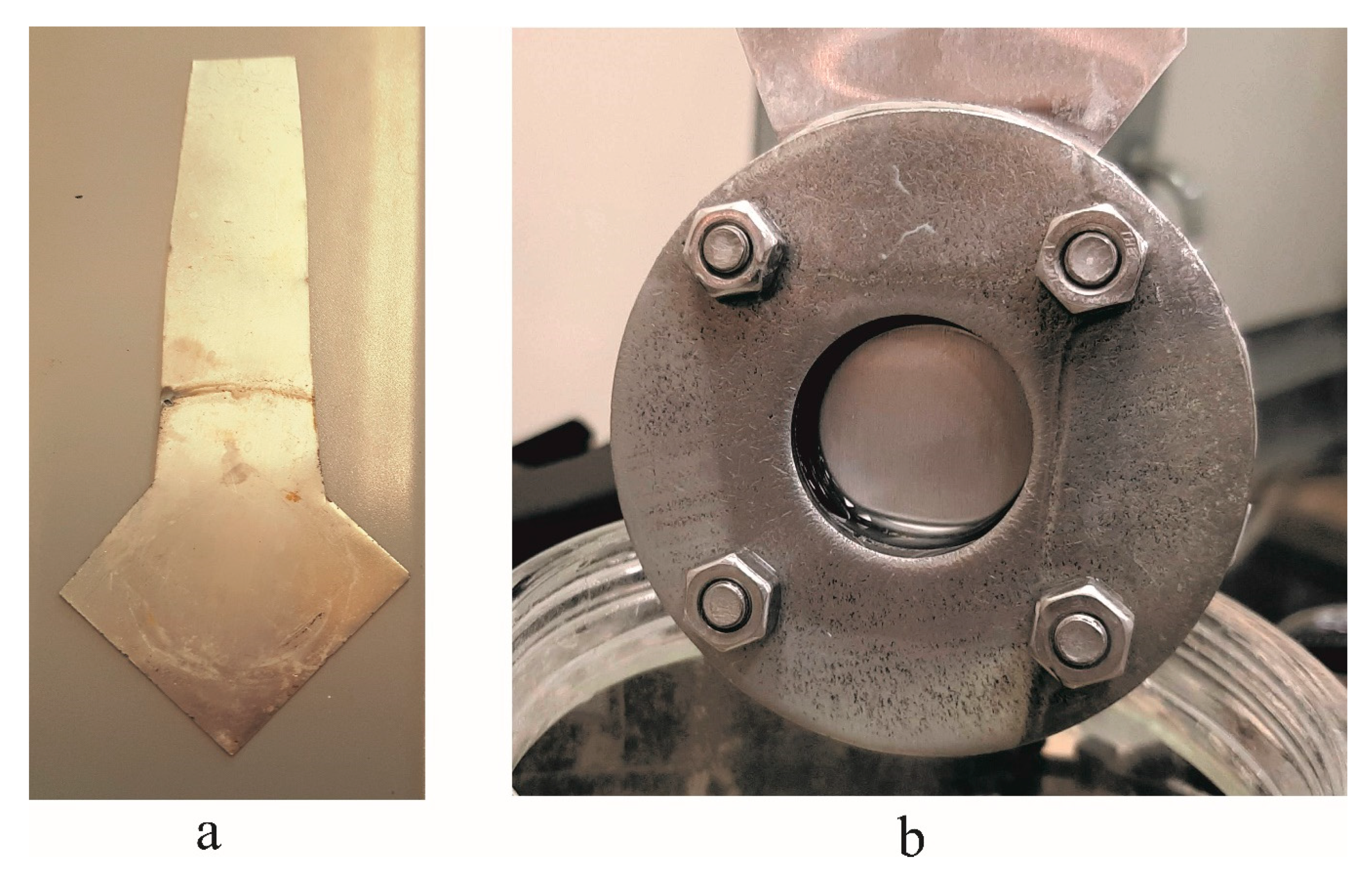
Fabrication of Nanoporous AAO Membrane
3. Experimental Results of Nanoporous AAO Membrane
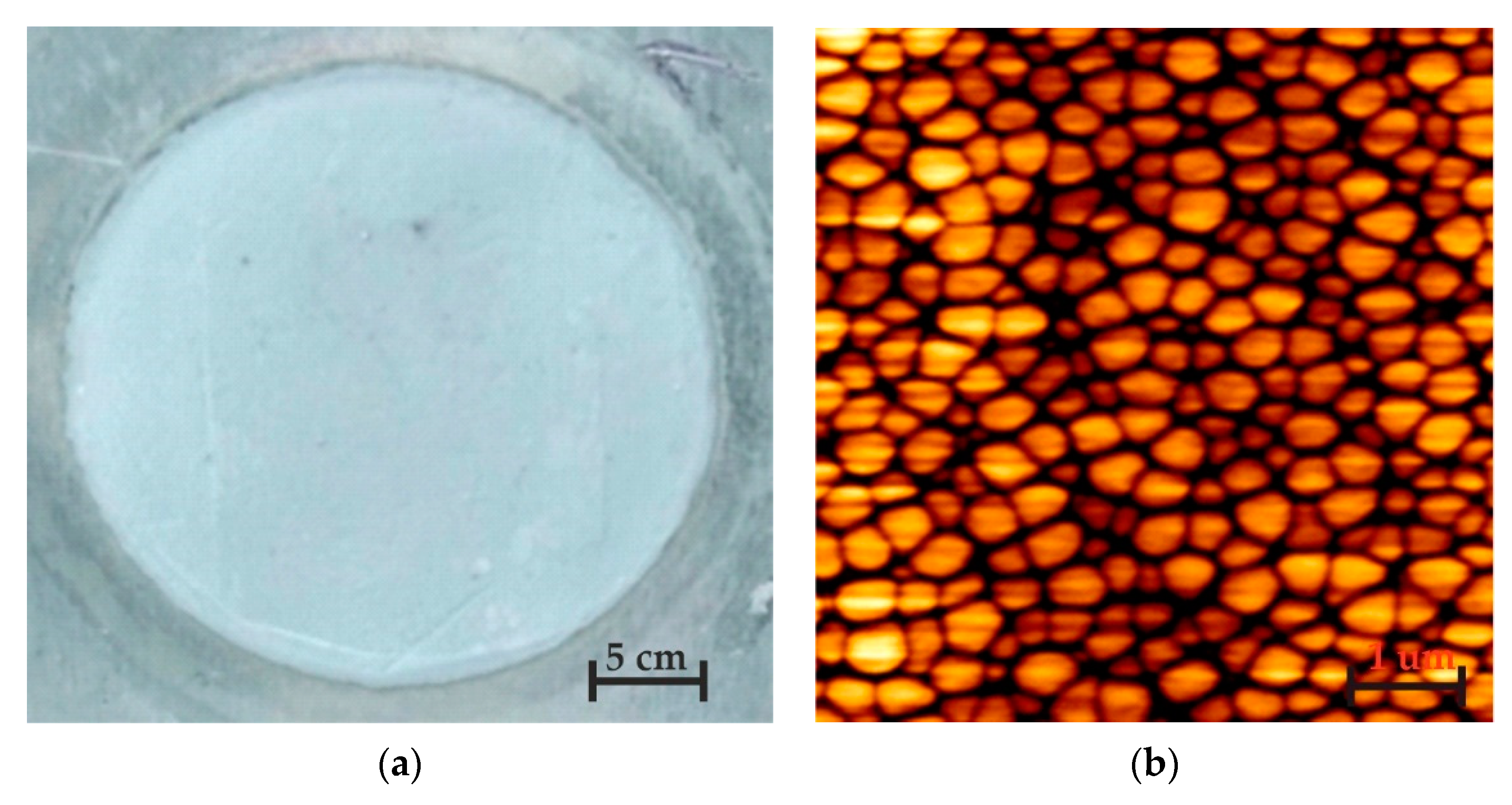
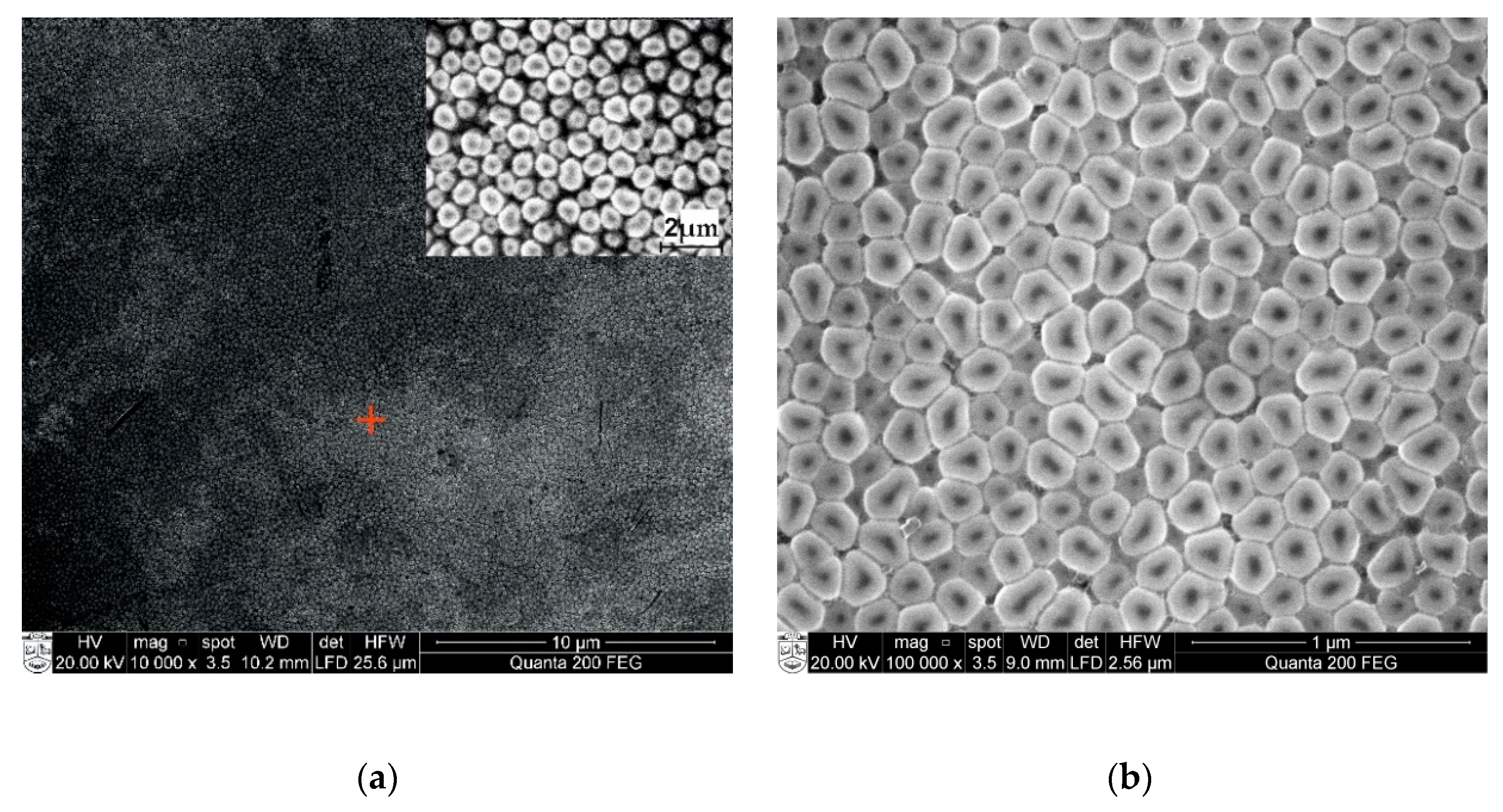
3.1. Surface Morphology of Nanoporous AAO Membrane
3.2. SEM Based EDS Analysis of Fabricated Aluminum Oxide Membrane
3.3. Hydrophobicity Analysis of Nanoporous AAO Membrane
4. Numerical Simulation and Experiment Analysis of Nanoporous AAO Membrane Actuation for Nanofiltration
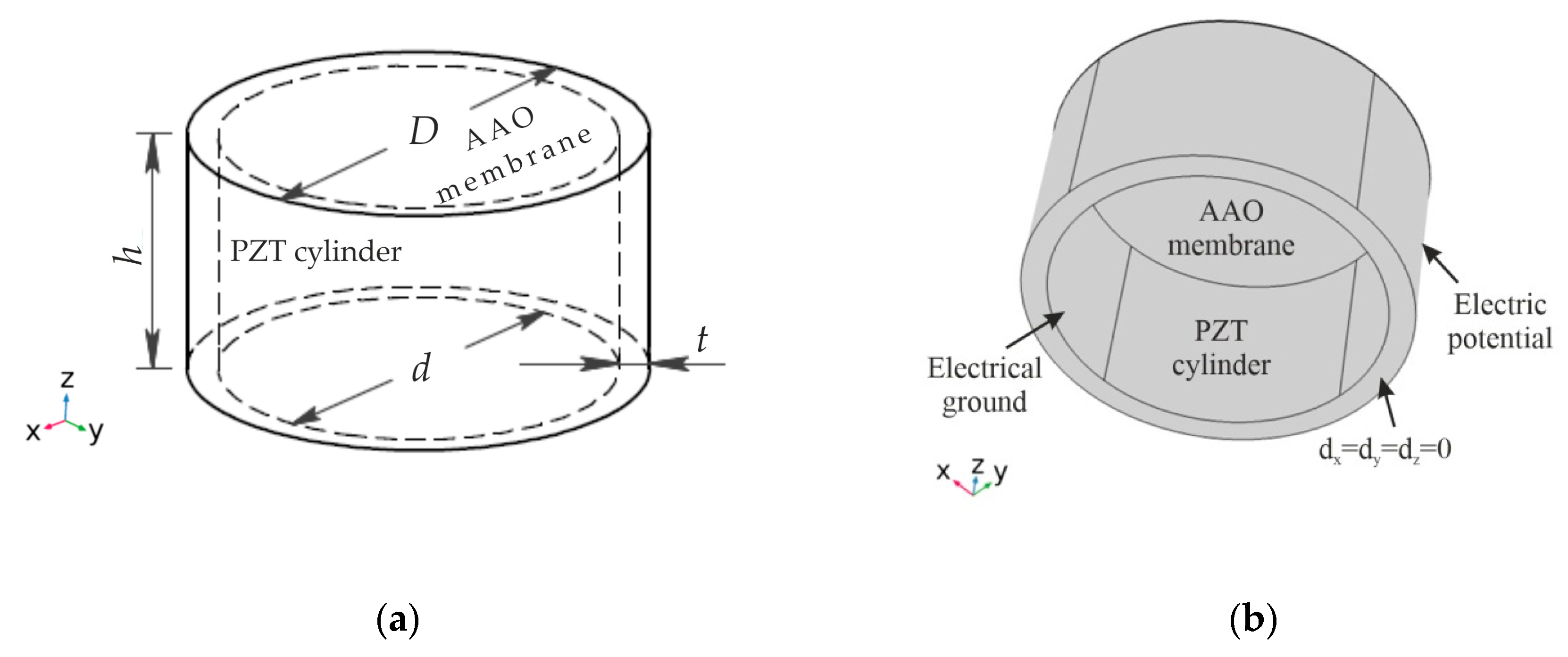
4.1. Numerical Simulation for Nanoporous AAO Membrane Actuation
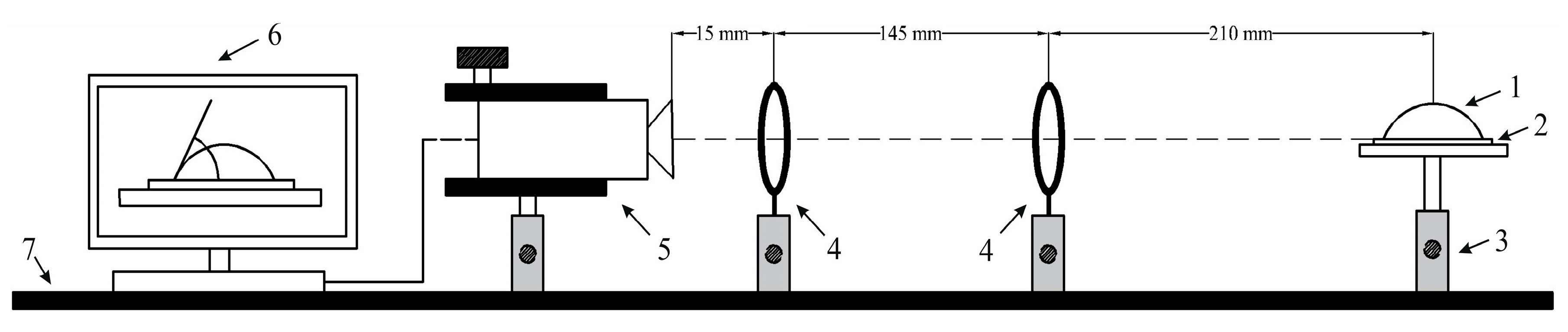
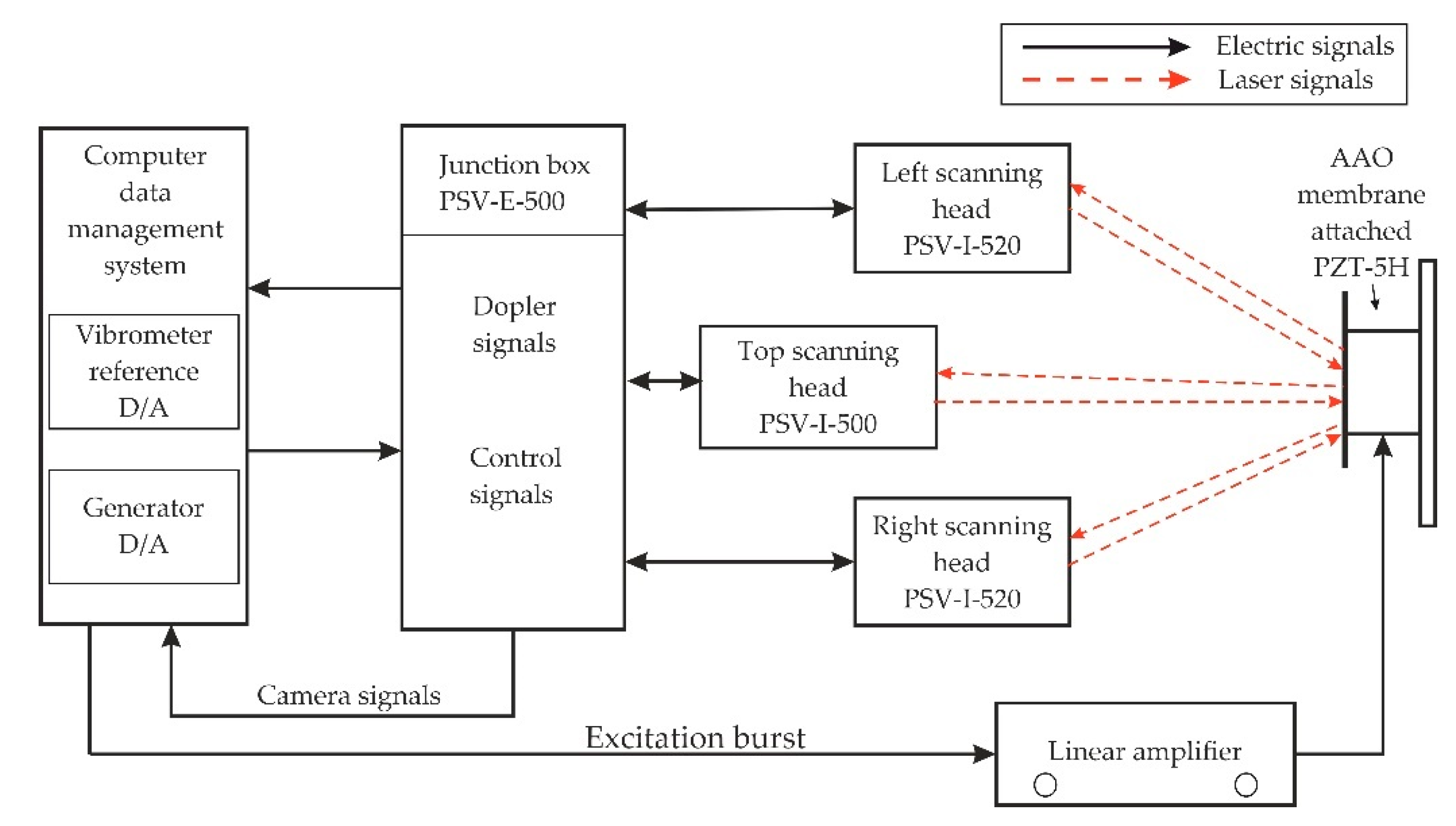
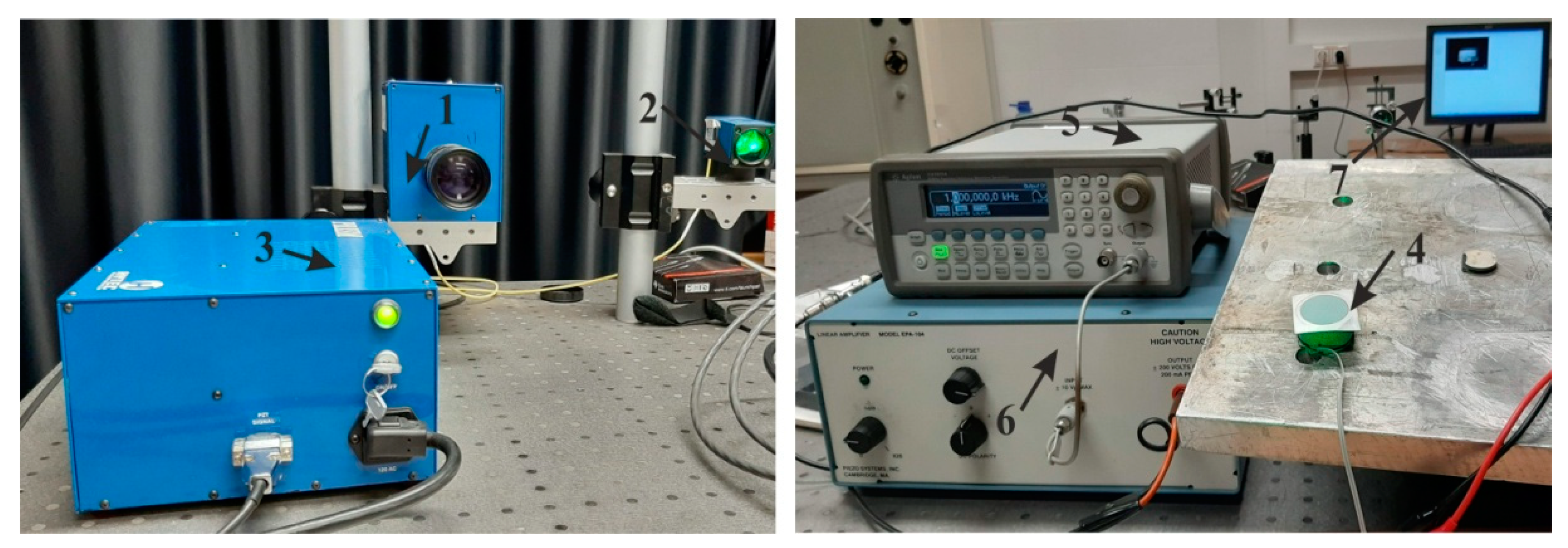
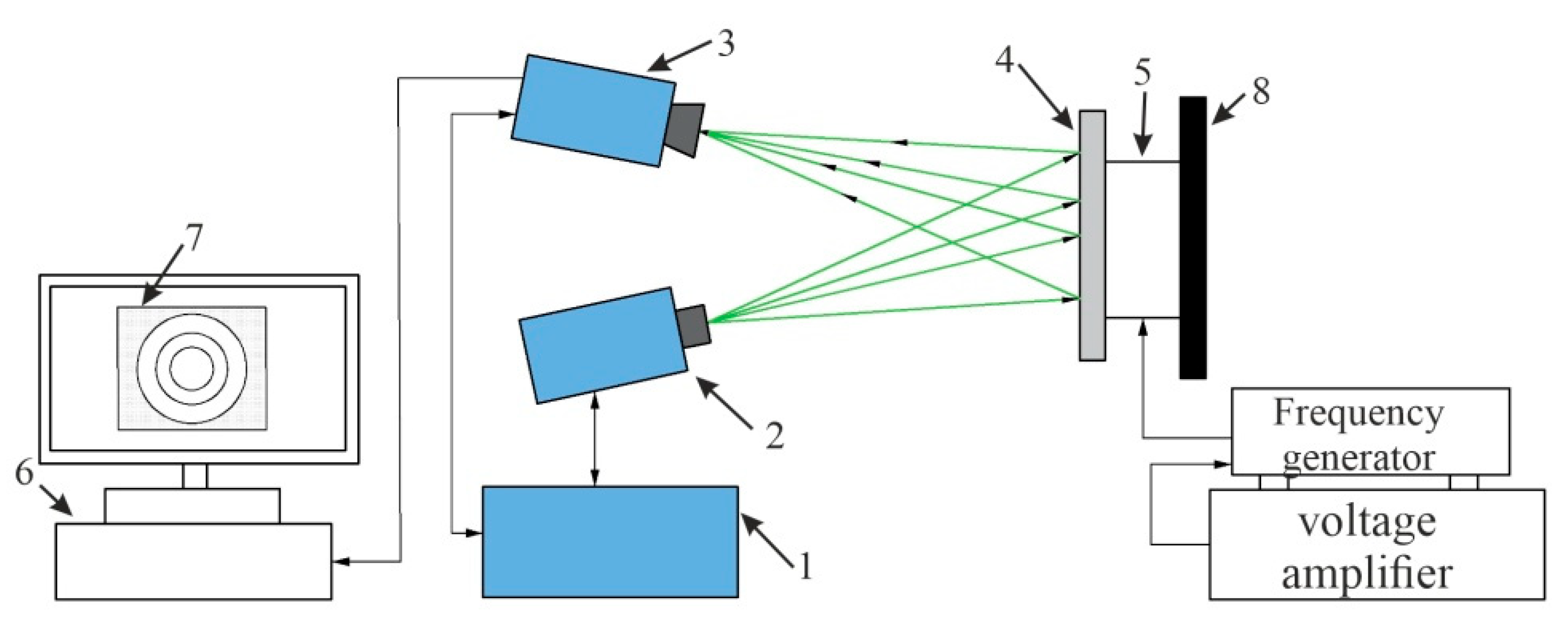
4.2. Experimental Analysis of Nanoporous AAO Membrane
5. Conclusions
- The nanoporous AAO membrane was successfully fabricated using two-step anodization method (MA and HA) in 0.3 M oxalic acid at 60 V with a hexagonal structure having a thickness of 120 µm with 70 nm pore diameter, 110 nm interpore distance, 36.72% porosity, and 4.771 cm−1 × 109 pore density.
- Fourier-transform infrared spectroscopy (FTIR) results confirmed that nanoporous structures were obtained in AAO membrane. SEM based EDS analysis confirmed that the nanoporous honeycomb hexagonal structure was formed on the membrane and microanalysis using EDS spectrum showed the dominance of aluminum (36.7%) and oxygen (59.5%) in the fabricated AAO membrane. Other peaks showed the smaller quantity of carbon (2.8%), sulfur (0.55%), and phosphorus (0.45%).
- The hydrophobic/hydrophilic properties were analyzed by measuring the water contact angle on the surface of the AAO. The contact angle was formed with the water droplet at 71.88° ± 1.25°. This indicated that the formed membrane is a hydrophilic material with water (since the water contact angle was <90). The contact angle measured for glycerin was 76.74° ± 1.85° and for spirit, it was 22.93° ± 0.4°, i.e., less than 90°, meaning that this membrane has a hydrophilic surface. Additionally, a contact angle time dependence study was conducted after 30 s and 60 s, and a gradual decrement of approximately 1° was observed in both cases. This indicated the seeping of the liquid droplet into the AAO membrane pores.
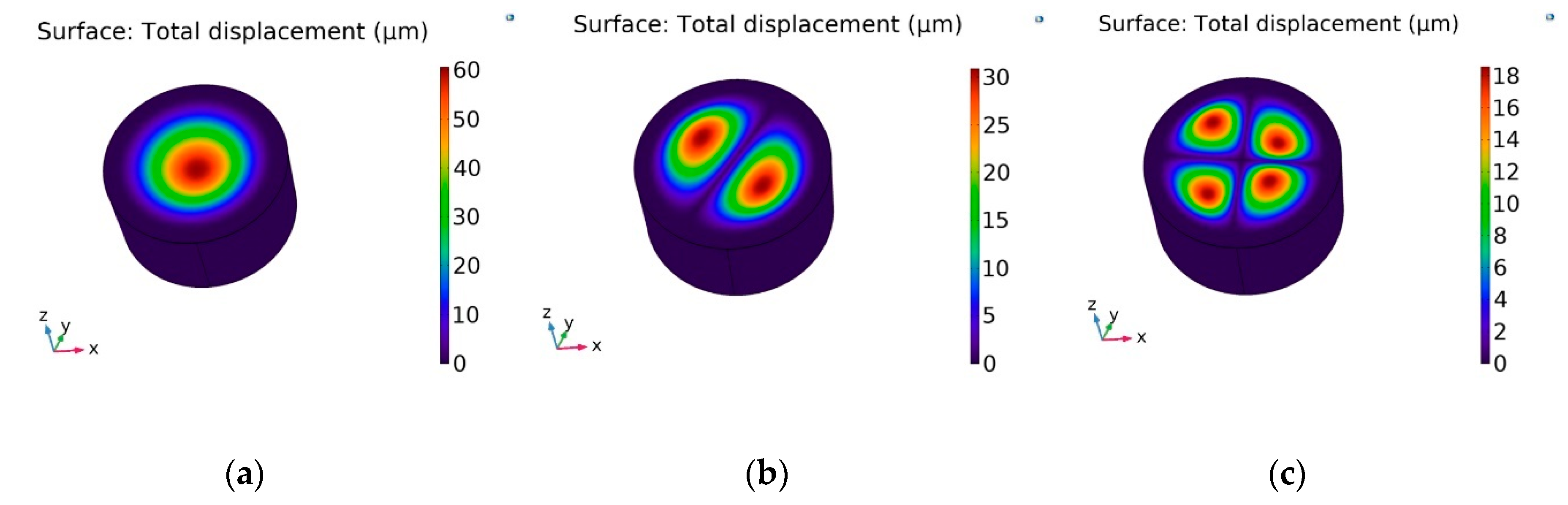
- A simulation of acoustic wave distribution was done with a COMSOL Multiphysics 5.4. The different forms of wave formation on the surface of the nanomembrane were observed: a first mode at 3.50 kHz, a second mode 4.94 kHz, and a third mode 7.89 kHz. This phenomenon enables the internal geometry to be controlled using standing surface acoustic waves of the nanoporous membrane and allows the nanoparticles to be concentrated in the center of the nanotubes.
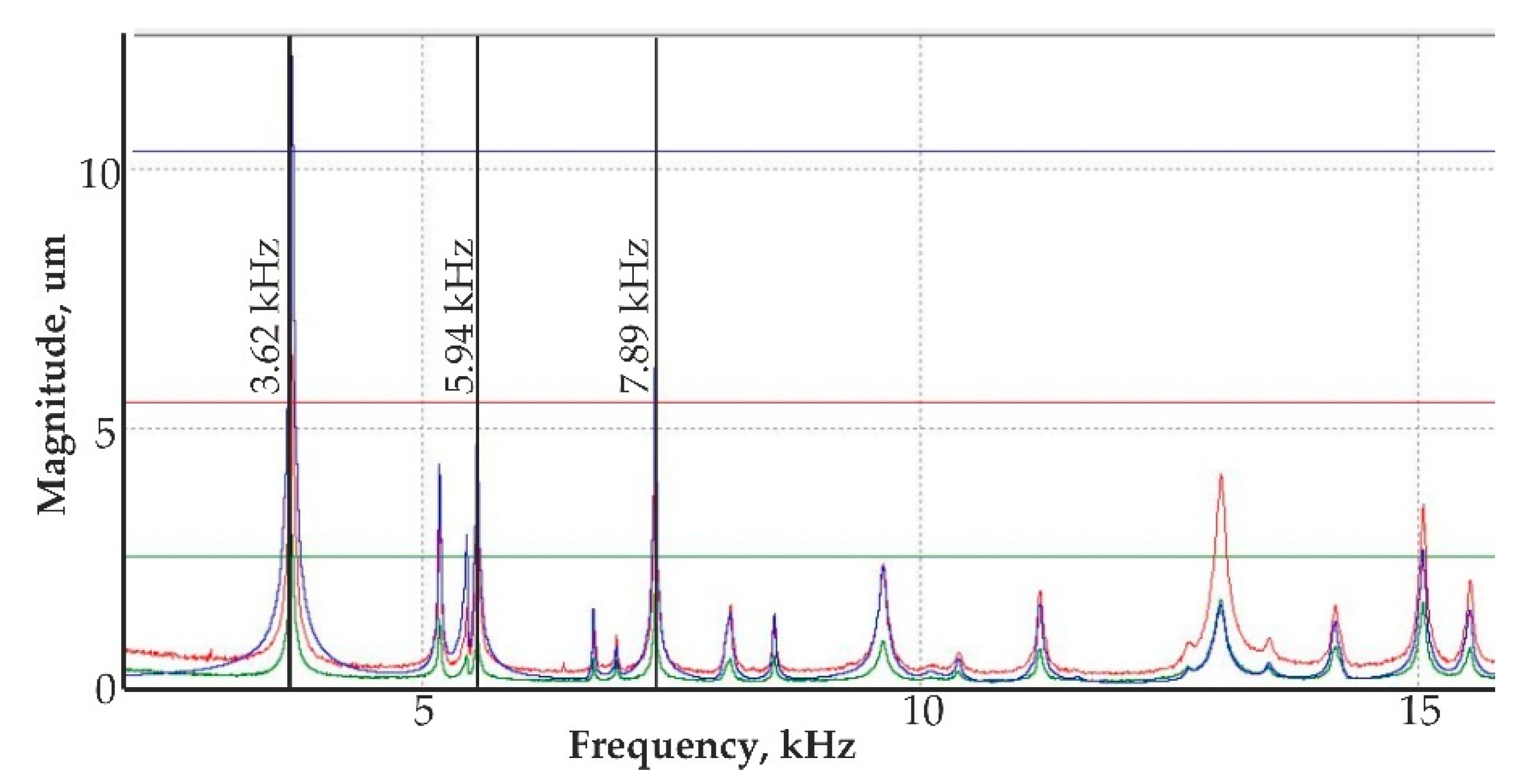
- Theoretical results of the actuated nanomembrane were verified with experimental results. Similar vibration modes using a 3D vibrometer and holographic interferometry PRISM system were obtained at the following frequencies: a first mode at 3.62 kHz and 3.8 kHz, a second mode at 5.94 kHz and 5.18 kHz, and a third mode at 7.89 kHz and 8.06 kHz, respectively, for the simulation and experimental results. An error occurred in the simulation and experimental results because of the glued layer between the PZT 5H cylinder and nanoporous membrane.
- The simulation results for single nanotube for acoustic pressure distribution showed that the lowest pressure is in the center of the channel at 900 MHz. It allows the bioparticle to pass through a nano tube/channel without friction with the walls because of the lower acoustic pressure acting in the center of nanotube. Moreover, it takes 0.27 s to focus the nanoparticle into the center of channel, reducing the possibility of damage to the cell membrane.
- A model for the nanoparticle filtration, using standing surface acoustic waves generated by a PZT 5H cylinder (actuator), was proposed. This model could be implemented as a vibro-active nano filter in a biomedical micro hydraulic mechanical system.
Author Contributions
Funding
Conflicts of Interest
References
- Baik, J.M.; Schierhorn, M.; Moskovits, M. Fe nanowires in nanoporous alumina: Geometric effect versus influence of pore walls. J. Phys. Chem. C 2018, 112, 2252–2255. [Google Scholar] [CrossRef]
- Chen, W.; Wu, J.-S.; Xia, X.-H. Porous Anodic Alumina with Continuously Manipulated Pore/Cell Size. ACS Nano 2008, 2, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Long, Y.; Sun, B. Fabrication of highly ordered porous anodic alumina membrane with ultra-large pore intervals in ethylene glycol-modified citric acid solution. J. Porous Mater. 2013, 20, 785–788. [Google Scholar] [CrossRef]
- Zaraska, L.; Stȩpniowski, W.J.; Jaskuła, M.; Sulka, G.D. Analysis of nanopore arrangement of porous alumina layers formed by anodizing in oxalic acid at relatively high temperatures. Appl. Surf. Sci. 2014, 305, 650–657. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Choi, J.; Okamoto, K.; Tak, Y.; Lee, J. Cu2O Nanowires in an Alumina Template: Electrochemical Conditions for the Synthesis and Photoluminescence Characteristics. ChemPhysChem 2006, 7, 1505–1509. [Google Scholar] [CrossRef]
- Ostasevicius, V.; Janusas, G.; Palevicius, A.; Gaidys, R.; Jurenas, V. Biomechanical Microsystems: Design, Processing and Applications; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Palevičius, A.; Janušas, G.; Čekas, E.; Patel, Y.R. Composite Piezoelectric Material for Biomedical Micro Hydraulic System; Springer: Basel, Switzerland, 2018; pp. 49–58. ISSN 0302-9743. [Google Scholar]
- Tsuru, T. Inorganic Porous Membranes for Liquid Phase Separation. Sep. Purif. Methods 2001, 30, 191–220. [Google Scholar] [CrossRef]
- Kuwahara, M.; Nakano, T.; Tominaga, J.; Lee, M.B.; Atoda, N. High-Speed Optical Near-Field Photolithography by Super Resolution Near-Field Structure. Jpn. J. Appl. Phys. 1999, 38, L1079. [Google Scholar] [CrossRef]
- Tu, F.; Späth, A.; Drost, M.; Vollnhals, F.; Krick Calderon, S.; Fink, R.H.; Marbach, H. Exploring the fabrication of Co and Mn nanostructures with focused soft X-ray beam induced deposition. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2017, 35, 031601. [Google Scholar] [CrossRef]
- Peinado, P.; Sangiao, S.; De Teresa, J.M. Focused Electron and Ion Beam Induced Deposition on Flexible and Transparent Polycarbonate Substrates. ACS Nano 2015, 9, 6139–6146. [Google Scholar] [CrossRef]
- Bae, C.; Shin, H.; Nielsch, K. Surface modification and fabrication of 3D nanostructures by atomic layer deposition. MRS Bull. 2011, 36, 887–897. [Google Scholar] [CrossRef]
- Adiga, S.P.; Jin, C.; Curtiss, L.A.; Monteiro-Riviere, N.A.; Narayan, R.J. Nanoporous membranes for medical and biological applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Kelly, D.N.; Wakabayashi, R.H.; Stacy, A.M. A Modified Sol–Gel Technique for Pore Size Control in Porous Aluminum Oxide Nanowire Templates. ACS Appl. Mater. Interfaces 2014, 6, 20122–20129. [Google Scholar] [CrossRef] [PubMed]
- Hourdakis, E.; Nassiopoulou, A.G. Electronic devices using porous anodic aluminum oxide. Phys. Chem. Appl. Nanostructures 2011, 26, 512–518. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials 2009, 30, 4094–4103. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Lohrengel, M.M. Thin anodic oxide layers on aluminium and other valve metals: High field regime. Mater. Sci. Eng. R Rep. 1993, 11, 243–294. [Google Scholar] [CrossRef]
- Wendorff, J.H.; Agarwal, S.; Greiner, A. Electrospinning—Some Technical Aspects. In Electrospinning: Materials, Processing, and Application; John Wiley & Sons: Weinheim, Germany, 2012; Chapter 5; pp. 127–142. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Schmuki, P. Thick self-organized porous zirconium oxide formed in H2SO4/NH4F electrolytes. Electrochem. Commun. 2004, 6, 1131–1134. [Google Scholar] [CrossRef]
- Macias, G.; Hernández-Eguía, L.P.; Ferré-Borrull, J.; Pallares, J.; Marsal, L.F. Gold-Coated Ordered Nanoporous Anodic Alumina Bilayers for Future Label-Free Interferometric Biosensors. ACS Appl. Mater. Interfaces 2013, 5, 8093–8098. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Richter, C.; Menon, L. A Study of Anodization Process during Pore Formation in Nanoporous Alumina Templates. J. Electrochem. Soc. 2007, 154, E8. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, K.; Purkait, M.K. Membrane Technologies and Applications, 1st ed.; Mohanty, K., Purkait, M.K., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Lee, W. The anodization of aluminum for nanotechnology applications. JOM 2010, 62, 57–63. [Google Scholar] [CrossRef]
- Vorozhtsova, M.; Drbohlavova, J.; Hubalek, J. Microsensors with Ordered Nanostructures. In Microsensors; Minin, I., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef][Green Version]
- Shimizu, K.; Habazaki, H.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Comparison of depth profiling analysis of a thick, electrolytically-colored porous alumina film by EPMA and GDOES. Surf. Interface Anal. 1999, 27, 1046–1049. [Google Scholar] [CrossRef]
- See, Y.H. Fabrication of Tungsten Oxide Nanostructured Films Using Anodic Porous Alumina and Application in Gas Sensing. Master’s Thesis, National University of Singapore, Singapore, 2005. [Google Scholar]
- Vida-Simiti, I.; Nemes, D.; Jumate, N.; Thalmaier, G.; Sechel, N. Self-Ordered Nanoporous Alumina Templates Formed by Anodization of Aluminum in Oxalic Acid. JOM 2012, 64, 1143–1147. [Google Scholar] [CrossRef]
- Bocchetta, P.; Santamaria, M.; Di Quarto, F. Electrosynthesis of Ce–Co Mixed Oxide Nanotubes with High Aspect Ratio and Tunable Composition. Electrochem. Solid State Lett. 2008, 11, K27. [Google Scholar] [CrossRef]
- Santamaria, M.; Bocchetta, P.; Di Quarto, F. Room temperature electrodeposition of photoactive Cd(OH)2 nanowires. Electrochem. Commun. 2009, 11, 580–584. [Google Scholar] [CrossRef]
- Chahrour, K.M.; Ahmed, N.M.; Hashim, M.R.; Elfadill, N.G.; Maryam, W.; Ahmad, M.A.; Bououdina, M. Effects of the voltage and time of anodization on modulation of the pore dimensions of AAO films for nanomaterials synthesis. Superlattices Microstruct. 2015, 88, 489–500. [Google Scholar] [CrossRef]
- Zaraska, L.; Brudzisz, A.; Wierzbicka, E.; Sulka, G.D. The effect of electrolyte change on the morphology and degree of nanopore order of porous alumina formed by two-step anodization. Electrochim. Acta 2016, 198, 259–267. [Google Scholar] [CrossRef]
- Jones, T.B.; Gunji, M.; Washizu, M.; Feldman, M.J. Dielectrophoretic liquid actuation and nanodroplet formation. J. Appl. Phys. 2001, 89, 1441–1448. [Google Scholar] [CrossRef]
- Zhao, Y.; Yi, U.C.; Cho, S.K. Highly efficient in-droplet particle concentration and separation by twDEP and EWOD for digital microfluidics. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Hyogo, Japan, 21–25 January 2007; pp. 537–540. [Google Scholar] [CrossRef]
- Lee, J.; Moon, H.; Fowler, J.; Schoellhammer, T.; Kim, C.-J. Electrowetting and electrowetting-on-dielectric for microscale liquid handling. Sens. Actuators A Phys. 2002, 95, 259–268. [Google Scholar] [CrossRef]
- Darinskii, A.N.; Weihnacht, M.; Schmidt, H. Acoustomicrofluidic application of quasi-shear surface waves. Ultrasonics 2017, 78, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kang, Y.; Ai, Y. Radiation dominated acoustophoresis driven by surface acoustic waves. J. Colloid Interface Sci. 2015, 455, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sazan, H.; Piperno, S.; Layani, M.; Magdassi, S.; Shpaisman, H. Directed assembly of nanoparticles into continuous microstructures by standing surface acoustic waves. J. Colloid Interface Sci. 2019, 536, 701–709. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, C.; Xu, C.; Hu, Q.; Wei, S. Patterning microparticles into a two-dimensional pattern using one column standing surface acoustic waves. Sens. Actuators A Phys. 2018, 284, 168–171. [Google Scholar] [CrossRef]
- Ang, K.M.; Yeo, L.Y.; Hung, Y.M.; Tan, M.K. Acoustially-mediated microfluidic nanofiltration through graphene films. Nanoscale 2017, 9, 6497–6508. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, J.; Kanno, I.; Kotera, H.; Wasa, K.; Suzuki, T. Development of liquid pumping devices using vibrating microchannel walls. Sens. Actuators A 2009, 152, 211–218. [Google Scholar] [CrossRef]
- Cazorla, P.-H.; Fuchs, O.; Cochet, M.; Maubert, S.; Le Rhun, G.; Fouillet, Y.; Defay, E. Integration of PZT thin films on a microfluidic complex system. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 491–494. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Gu, Y.; Li, P.; Ding, X.; Wang, L.; McCoy, J.P.; Levine, S.J.; Huang, T.J. Continuous enrichment of low-abundance cell samples using standing surface acoustic waves (SSAW). Lab Chip 2014, 14, 924–930. [Google Scholar] [CrossRef]
- Zhou, Z.; Nonnenmann, S.S. Progress in nanoporous templates: Beyond anodic aluminum oxide and towards functional complex materials. Materials 2019, 12, 2535. [Google Scholar] [CrossRef] [PubMed]
- Mankotia, D.; Singh, P.S.; Kaur, M. Review of Anodic Porous Alumina Membrane Development. SSRG Int. J. Hum. Soc. Sci. 2015, 1, 48–53. [Google Scholar]
- Prashanth, P.A.; Raveendra, R.S.; Hari Krishna, R.; Ananda, S.; Bhagya, N.P.; Nagabhushana, B.M.; Lingaraju, K.; Raja Naika, H. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Gangwar, J.; Gupta, B.K.; Tripathi, S.K.; Srivastava, A.K. Phase dependent thermal and spectroscopic responses of Al2O3 nanostructures with different morphogenesis. Nanoscale 2015, 7, 13313–13344. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cao, X.; Feng, X.; Ma, Y.; Zou, H. Fabrication of super-hydrophobic film from PMMA with intrinsic water contact angle below 90. Polymer 2007, 48, 7455–7460. [Google Scholar] [CrossRef]
- Kumar, P.; Khan, N.; Kumar, D. Polyvinyl Butyral (PVB), Versetile Template for Designing Nanocomposite/Composite Materials: A Review. Green Chem. Technol. Lett. 2016, 2, 185. [Google Scholar] [CrossRef]
- Ragulskis, K.; Naginevicius, V.; Palevicius, A.; Palevicius, R. Analysis of the dynamics of the vibratory tabular valve. In Proceedings of the SPIE Active and Passive Smart Structures and Integrated Systems 2008, San Diego, CA, USA, 9–13 March 2008. [Google Scholar] [CrossRef]











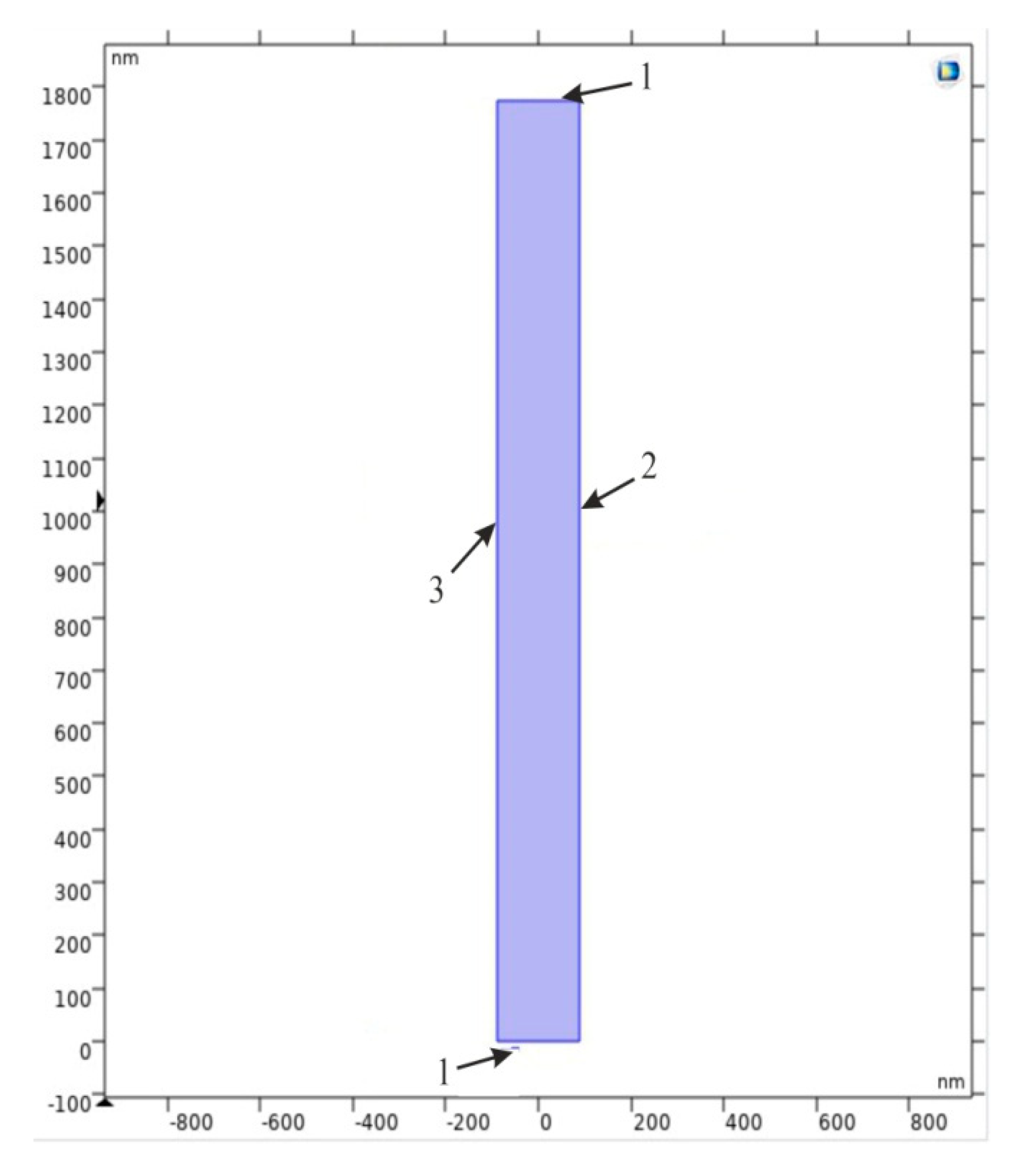


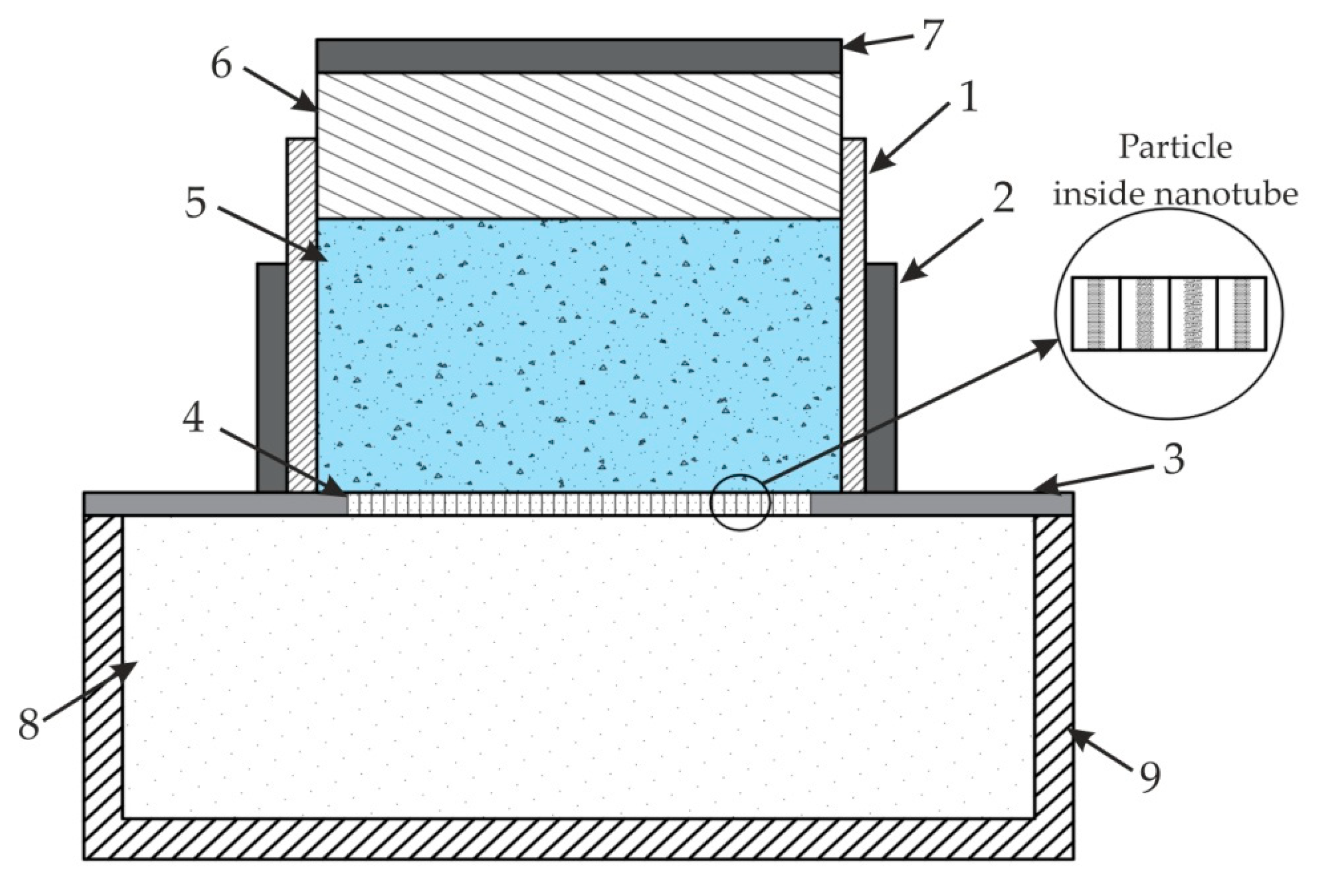
| Anodizing Potential (V) | Mean Pore Diameter, Dp (nm) | Interpore Distance, Dc (nm) | Porosity (%) | Pore Density (cm−1 × 109) |
|---|---|---|---|---|
| 60 | 70 ± 20 | 110 ± 10 | 36.72 | 4.771 |
| Chemical Element | Normalized Concentration in Weight Percentage (norm.wt., %) | Normalized Concentration in Atomic Percentage (norm. at., %) |
|---|---|---|
| Carbon | 2.8 | 4.5 |
| Oxygen | 59.5 | 69.55 |
| Aluminium | 36.7 | 25.39 |
| Sulfur | 0.55 | 0.29 |
| Phosphorus | 0.45 | 0.27 |
| Property (Symbol), Unit | Value |
|---|---|
| Coupling coefficient (k33) | 0.75 |
| Displacement coefficient (d33), m/V | 650 × 10−12 |
| Voltage coefficient (g33), V m/N | 19 × 10−3 |
| Density, kg/m3 | 7800 |
| Young’s modulus, N/m2 | 5.5 × 1010 |
| Poisson’s Ratio | 0.34 |
| Mechanical Q factor | 32 |
| Parameter | Values |
|---|---|
| Driving frequency, Hz | 5.8 × 109 |
| Speed of sound, m/s | 343 |
| Wavelength, m | 5.9138 × 10−8 |
| Transducer diameter, m | 1.1828 × 10−7 |
| Reflector diameter, m | 1.7741 × 10−7 |
| Height, m | 1.7741 × 10−6 |
| Viscous boundary layer thickness, m | 2.8887 × 10−8 |
| Particle diameter, m | 2.3655 × 10−8 |
| Particle density, kg/m3 | 500 |
| Normal acceleration of transducer, m/s2 | 1.5 × 10−8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, Y.; Janusas, G.; Palevicius, A.; Vilkauskas, A. Development of Nanoporous AAO Membrane for Nano Filtration Using the Acoustophoresis Method. Sensors 2020, 20, 3833. https://doi.org/10.3390/s20143833
Patel Y, Janusas G, Palevicius A, Vilkauskas A. Development of Nanoporous AAO Membrane for Nano Filtration Using the Acoustophoresis Method. Sensors. 2020; 20(14):3833. https://doi.org/10.3390/s20143833
Chicago/Turabian StylePatel, Yatinkumar, Giedrius Janusas, Arvydas Palevicius, and Andrius Vilkauskas. 2020. "Development of Nanoporous AAO Membrane for Nano Filtration Using the Acoustophoresis Method" Sensors 20, no. 14: 3833. https://doi.org/10.3390/s20143833
APA StylePatel, Y., Janusas, G., Palevicius, A., & Vilkauskas, A. (2020). Development of Nanoporous AAO Membrane for Nano Filtration Using the Acoustophoresis Method. Sensors, 20(14), 3833. https://doi.org/10.3390/s20143833






