Differential Sensing of Saccharides Based on an Array of Fluorinated Benzosiloxaborole Receptors
Abstract
1. Introduction
2. Materials and Methods
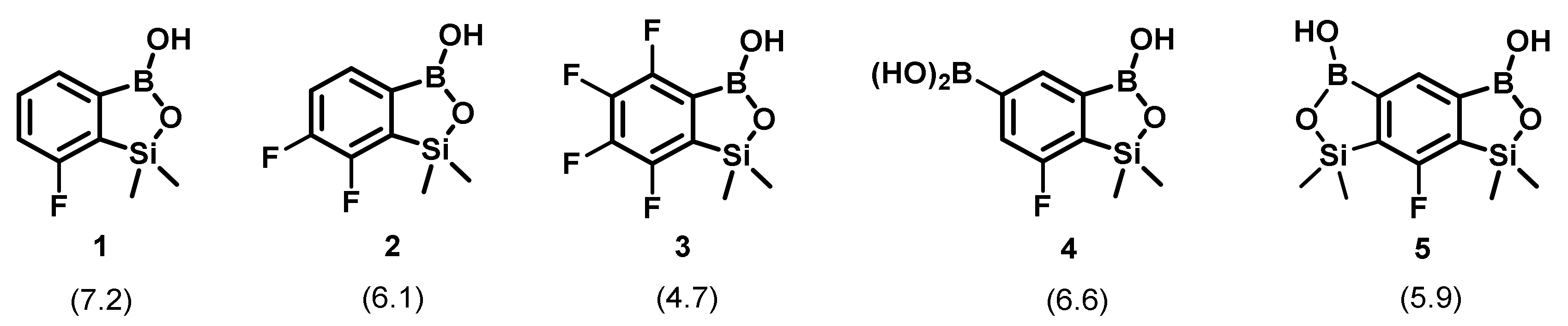
2.1. Structures of Fluorinated Benzosiloxaboroles Receptors 1–5
2.2. Determination of the Stability Constants of Benzosiloxaborole Complexes
2.3. Determination of the Stoichiometry of Formed Complexes
2.4. Chemometric Analysis
2.5. Computational and NMR Spectroscopy Studies
3. Results and Discussion
3.1. Binding Affinity of Benzosiloxaboroles 1–5 Towards Saccharides
3.2. Stoichiometry of the Complexes Formed by Benzosiloxaborole 4 and 5
3.3. Theoretical and NMR Spectroscopy Insight into the Mechanism of Complexation of Benzosiloxaboroles with Diols
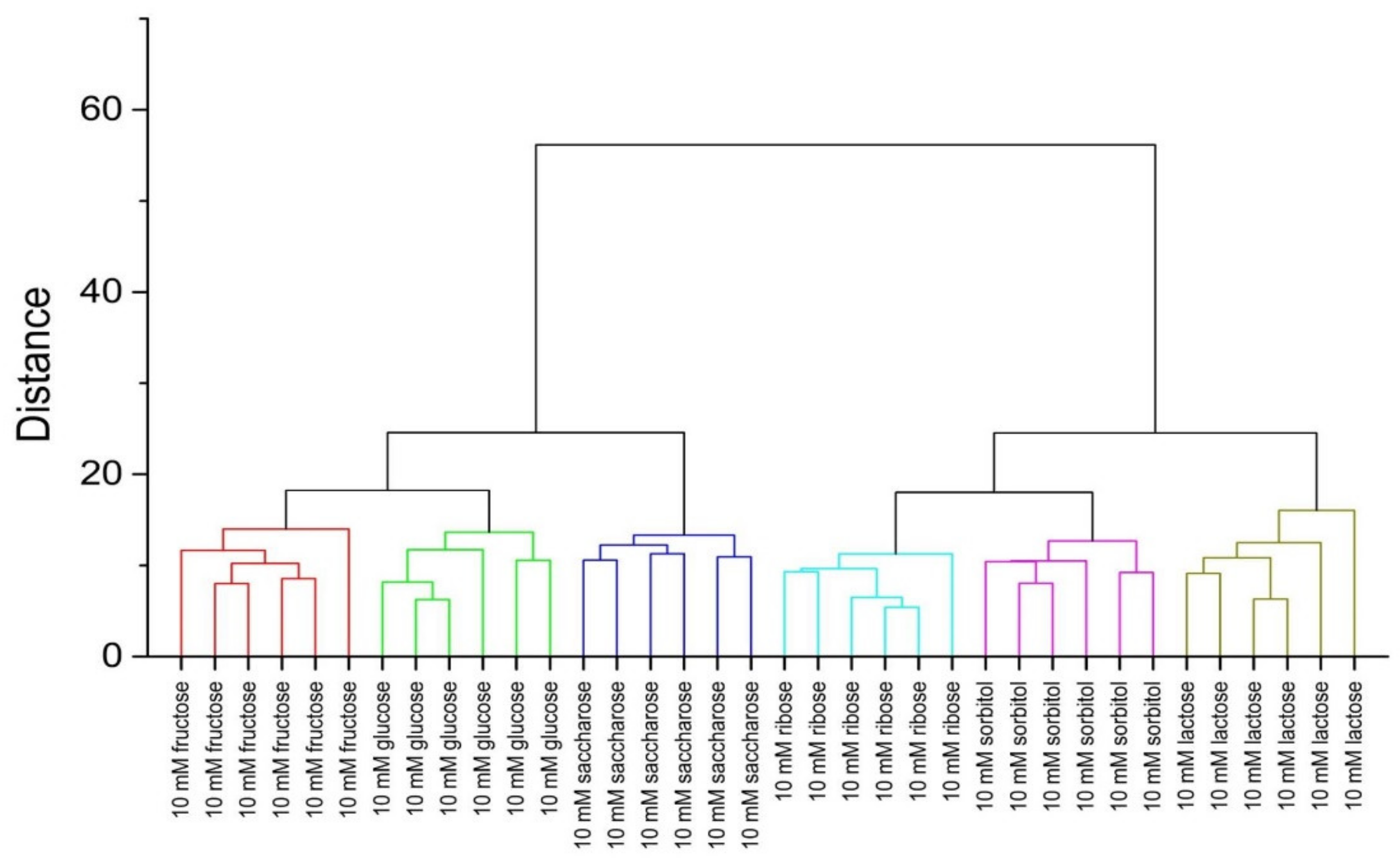
3.4. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torssell, K. Arylboronic acids. III. Bromination of tolylboronic acids according to Wohl-Ziegler. Ark Kemi 1957, 10, 507–511. [Google Scholar]
- Snyder, H.R.; Reedy, A.J.; Lennarz, W.J. Synthesis of aromatic boronic acids. Aldehydo boronic acids and a boronic acid analog of tyrosine. J. Am. Chem. Soc. 1958, 80, 835–838. [Google Scholar] [CrossRef]
- Baker, S.J.; Zhang, Y.K.; Akama, T.; Lau, A.; Zhou, H.C.; Hernandez, V.; Mao, W.M.; Alley, M.R.K.; Sanders, V.; Plattner, J.J. Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690), for the potential treatment of Onychomycosis. J. Med. Chem. 2006, 49, 4447–4450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, M.-Y.; Lin, Y.-N.; Zhou, H.-C. The synthesis of benzoxaboroles and their applications in medicinal chemistry. Sci. China Chem. 2013, 56, 1372–1381. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T.; Winum, J.-Y. Benzoxaborole compounds for therapeutic uses: A patent review (2010–2018). Exp. Opin. Ther. Pat. 2018, 28, 493–504. [Google Scholar] [CrossRef]
- Tomsho, J.W.; Pal, A.; Hall, D.G.; Benkovic, S.J. Ring structure and aromatic substituent effects on the pKa of the benzoxaborole pharmacophore. ACS Med. Chem. Lett. 2012, 3, 48–52. [Google Scholar] [CrossRef]
- Dowlut, M.; Hall, D.G. An improved class of sugar-binding boronic acids; soluble and capable of complexing glycosides in neutral water. J. Am. Chem. Soc. 2006, 128, 4226–4227. [Google Scholar] [CrossRef]
- Bérubé, M.; Dowlut, M.; Hall, D.G. Benzoboroxoles as efficient glycopyranoside-binding agents in physiological conditions: Structure and selectivity of complex formation. J. Org. Chem. 2008, 73, 6471. [Google Scholar] [CrossRef]
- Steciuk, I.; Durka, K.; Gontarczyk, K.; Dąbrowski, M.; Luliński, S.; Woźniak, K. Nitrogen-boron coordination versus OH…N hydrogen bonding in pyridoxaboroles—Aza analogues of benzoxaboroles. Dalton Trans. 2015, 44, 16534–16546. [Google Scholar] [CrossRef]
- Brzozowska, A.; Ćwik, P.; Durka, K.; Kliś, T.; Laudy, A.E.; Luliński, S.; Serwatowski, J.; Tyski, S.; Urban, M.; Wróblewski, W. Benzosiloxaboroles—Silicon benzoxaborole congeners with improved Lewis acidity; high diol affinity and potent bioactivity. Organometallics 2015, 34, 2924–2932. [Google Scholar] [CrossRef]
- Czub, M.; Durka, K.; Luliński, S.; Łosiewicz, J.; Serwatowski, J.; Urban, M.; Woźniak, K. Synthesis and transformations of functionalized benzosiloxaboroles. Eur. J. Org. Chem. 2017, 818–826. [Google Scholar] [CrossRef]
- Durka, K.; Laudy, A.E.; Charzewski, Ł.; Urban, M.; Stępień, K.; Tyski, S.; Krzyśko, K.A.; Luliński, S. Antimicrobial and KPC/AmpC inhibitory activity of functionalized benzosiloxaboroles. Eur. J. Med. Chem. 2019, 171, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.T.; Anslyn, E.V. Differential receptor arrays and assays for solution-based molecular recognition. Chem. Soc. Rev. 2006, 35, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Askim, J.R.; Mahmoudia, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The optoelectronic nose: Colorimetric and fluorometric sensor arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Umali, A.P.; Anslyn, E.V. A general approach to differential sensing using synthetic molecular receptors. Curr. Opin. Chem. Biol. 2010, 14, 685–692. [Google Scholar] [CrossRef]
- Edwards, N.Y.; Sager, T.W.; McDevitt, J.T.; Anslyn, E.V. Boronic acid based peptidic receptors for pattern-based saccharide sensing in neutral aqueous media, an application in real-life samples. J. Am. Chem. Soc. 2007, 129, 13575–13583. [Google Scholar] [CrossRef]
- Musto, C.J.; Suslick, K.S. Differential sensing of sugars by colorimetric arrays. Curr. Opin. Chem. Biol. 2010, 14, 758–766. [Google Scholar] [CrossRef]
- Liang, X.; Bonizzoni, M. Boronic acid-modified poly(amidoamine) dendrimers as sugar-sensing materials in water. J. Mater. Chem. B 2016, 4, 3094–3103. [Google Scholar] [CrossRef]
- Schiller, A.; Wessling, R.A.; Singaram, B. A fluorescent sensor array for saccharides based on boronic acid appended bipyridinium salts. Angew. Chem. Int. Ed. 2007, 46, 6457–6459. [Google Scholar] [CrossRef]
- Resendez, A.; Panescu, P.; Zuniga, R.; Banda, I.; Joseph, J.; Webb, D.-L.; Singaram, B. Multiwell assay for the analysis of sugar gut permeability markers: Discrimination of sugar alcohols with a fluorescent probe array based on boronic acid appended viologens. Anal. Chem. 2016, 88, 5444–5452. [Google Scholar] [CrossRef]
- Zhang, X.; You, L.; Anslyn, E.V.; Qian, X. Discrimination and classification of ginsenosides and ginsengs using bis-boronic acid receptors in dynamic multicomponent indicator displacement sensor arrays. Chem. Eur. J. 2012, 18, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Durka, K.; Urban, M.; Czub, M.; Dąbrowski, M.; Tomaszewski, P.; Luliński, S. An intramolecular ortho-assisted activation of the silicon–hydrogen bond in arylsilanes: An experimental and theoretical study. Dalton Trans. 2018, 47, 3705–3716. [Google Scholar] [CrossRef] [PubMed]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid–diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-consistent molecular-orbital methods. 22. Small split-valence basis sets for second-row elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Tomsho, J.W.; Benkovic, S.J. Examination of the reactivity of benzoxaboroles and related compounds with a cis-diol. J. Org. Chem. 2012, 77, 11200–11209. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aguirre, M.A.; Flores-Alamo, M.; Medrano, F.; Yatsimirsky, A.K. Examination of pinanediol–boronic acid ester formation in aqueous media: Relevance to the relative stability of trigonal and tetrahedral boronate esters. Org. Biomol. Chem. 2020, 18, 2716–2726. [Google Scholar] [CrossRef]
- Martínez-Aguirre, M.A.; Villamil-Ramos, R.; Guerrero-Alvarez, J.A.; Yatsimirsky, A.K. Substituent effects and pH profiles for stability constants of arylboronic acid diol esters. J. Org. Chem. 2013, 78, 4674–4684. [Google Scholar] [CrossRef]
- Roy, C.D.; Brown, H.C. Stability of boronic esters—Structural effects on the relative rates of transesterification of 2-(phenyl)-1,3,2-dioxaborolane. J. Organomet. Chem. 2007, 692, 784–790. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Diaz-Dussan, D.; Peng, Y.-Y.; Chen, Y.; Narain, R.; Hall, D.G. In situ forming, dual-crosslink network, self-healing hydrogel enabled by a bioorthogonal nopoldiol–benzoxaborolate click reaction with a wide pH range. Chem. Mater. 2019, 31, 4092–4102. [Google Scholar] [CrossRef]
- Wong, S.-F.; Khor, S.M. State-of-the-art of differential sensing techniques in analytical sciences. Trends Anal. Chem. 2019, 114, 108–125. [Google Scholar] [CrossRef]





| ΔG/kJ mol−1 | BB | BFB | BSB | BFSB |
|---|---|---|---|---|
| A | −55.8 | −86.3 | −59.4 | −89.6 |
| B | 14.0 | 20.1 | 14.2 | 29.3 |
| C | −30.5 | −32.1 | −27.9 | −27.9 |
| D | −100.3 | −138.5 | −97.5 | −142.8 |
| E | −60.6 | −77.1 | −62.2 | −74.7 |
| F | 39.7 | 61.4 | 35.3 | 68.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćwik, P.; Ciosek-Skibińska, P.; Zabadaj, M.; Luliński, S.; Durka, K.; Wróblewski, W. Differential Sensing of Saccharides Based on an Array of Fluorinated Benzosiloxaborole Receptors. Sensors 2020, 20, 3540. https://doi.org/10.3390/s20123540
Ćwik P, Ciosek-Skibińska P, Zabadaj M, Luliński S, Durka K, Wróblewski W. Differential Sensing of Saccharides Based on an Array of Fluorinated Benzosiloxaborole Receptors. Sensors. 2020; 20(12):3540. https://doi.org/10.3390/s20123540
Chicago/Turabian StyleĆwik, Paweł, Patrycja Ciosek-Skibińska, Marcin Zabadaj, Sergiusz Luliński, Krzysztof Durka, and Wojciech Wróblewski. 2020. "Differential Sensing of Saccharides Based on an Array of Fluorinated Benzosiloxaborole Receptors" Sensors 20, no. 12: 3540. https://doi.org/10.3390/s20123540
APA StyleĆwik, P., Ciosek-Skibińska, P., Zabadaj, M., Luliński, S., Durka, K., & Wróblewski, W. (2020). Differential Sensing of Saccharides Based on an Array of Fluorinated Benzosiloxaborole Receptors. Sensors, 20(12), 3540. https://doi.org/10.3390/s20123540







