Real-Time Water Quality Monitoring with Chemical Sensors
Abstract
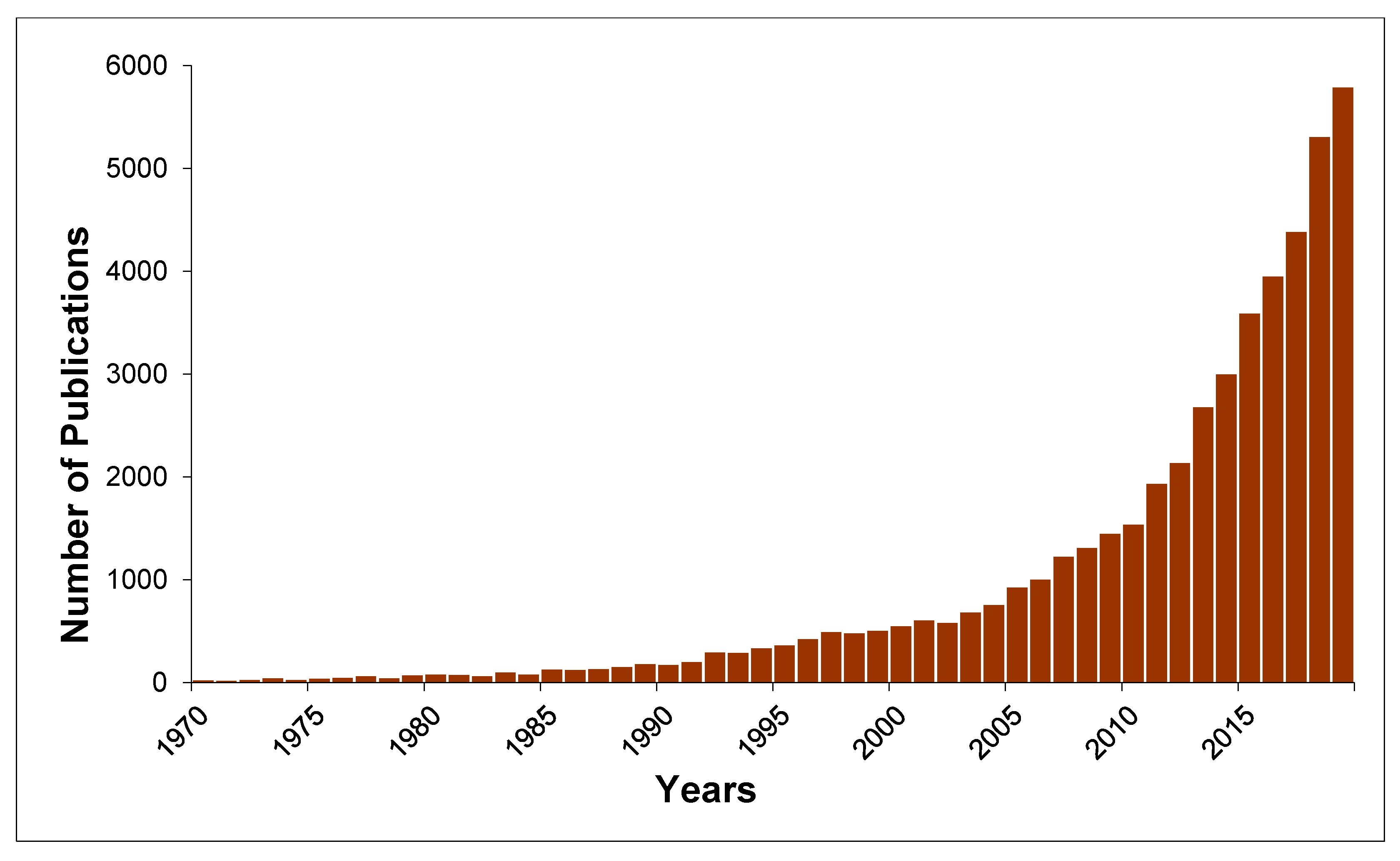
1. Introduction
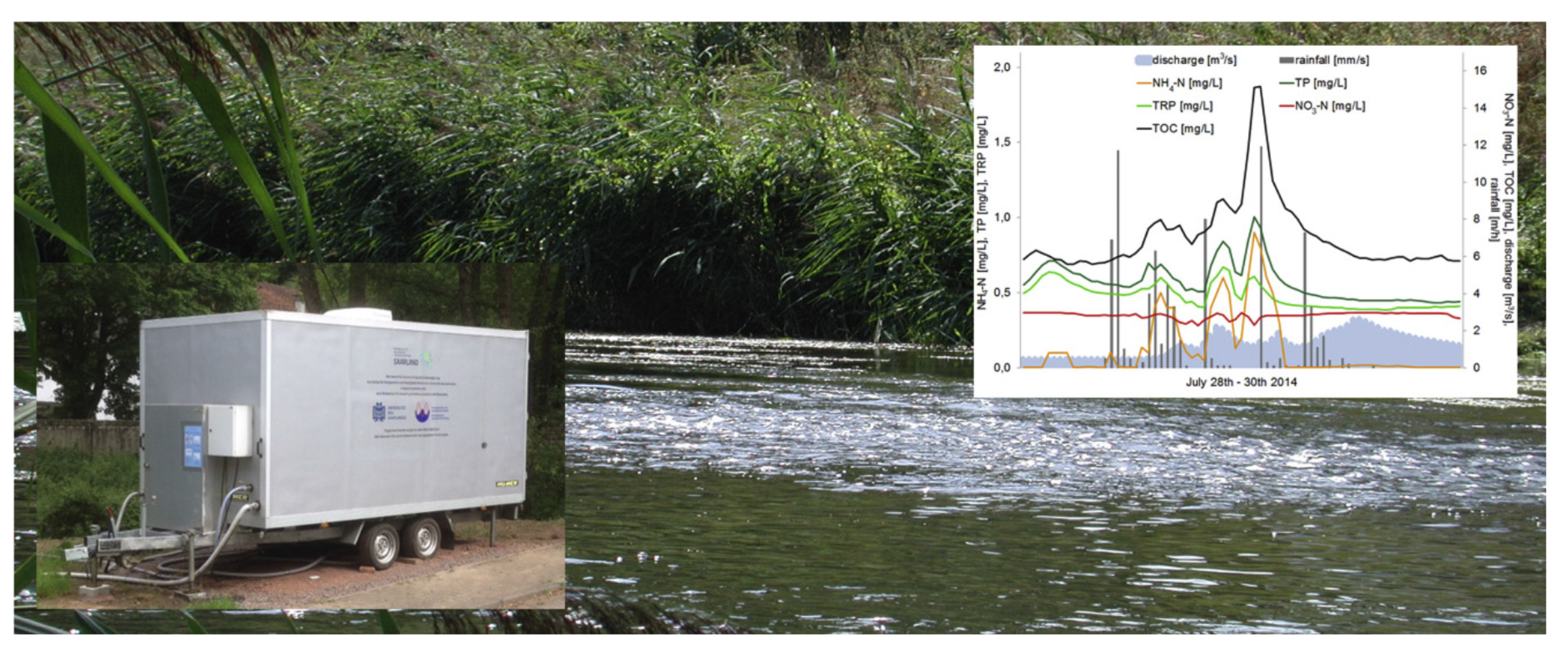
2. Current Situation with Online Water Analysis
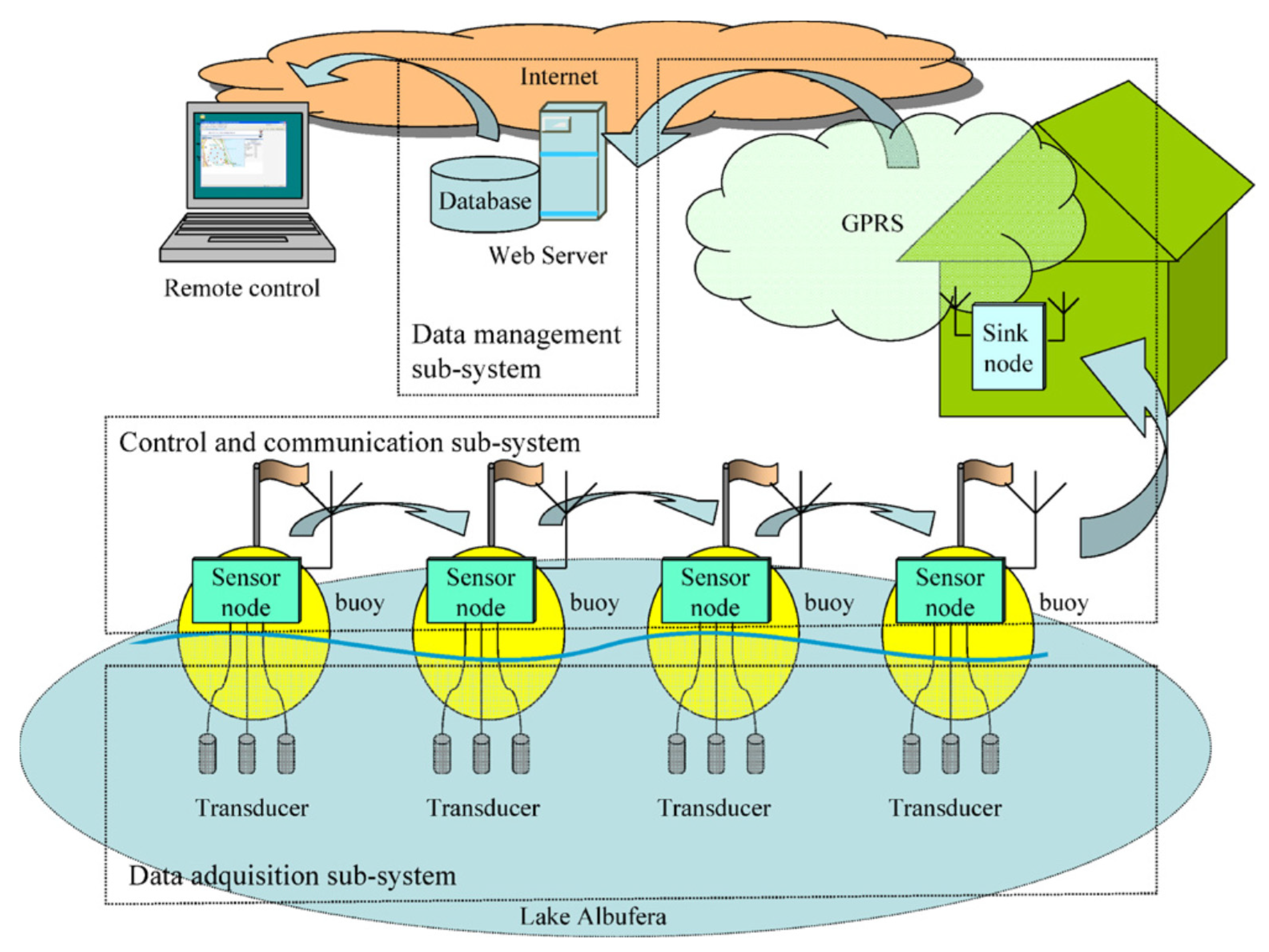
3. Water Quality Monitoring Systems
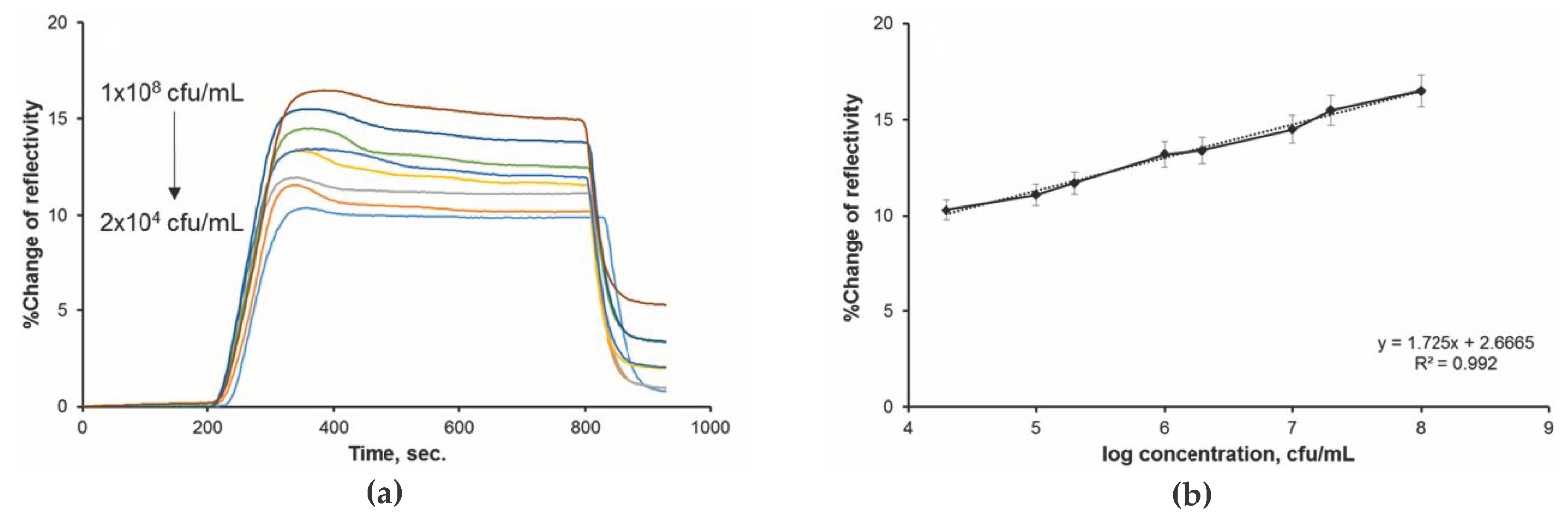
4. Application of Biosensors and Optical Sensors for Water Quality Assessment
5. Biomimetic Approaches for Sensing Water Quality
5.1. Chemical Sensors for Sensing in “Real-Life Environments”
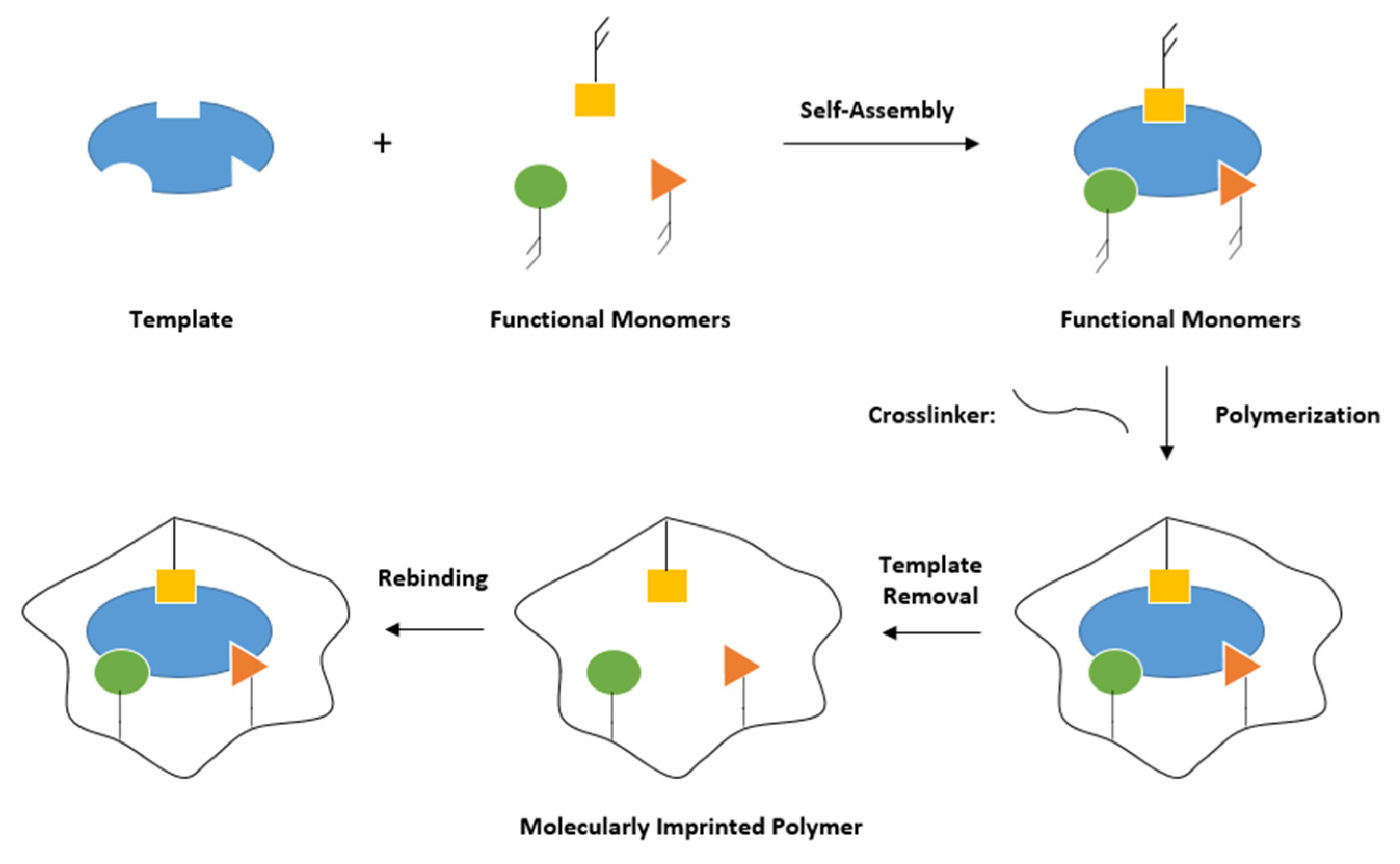
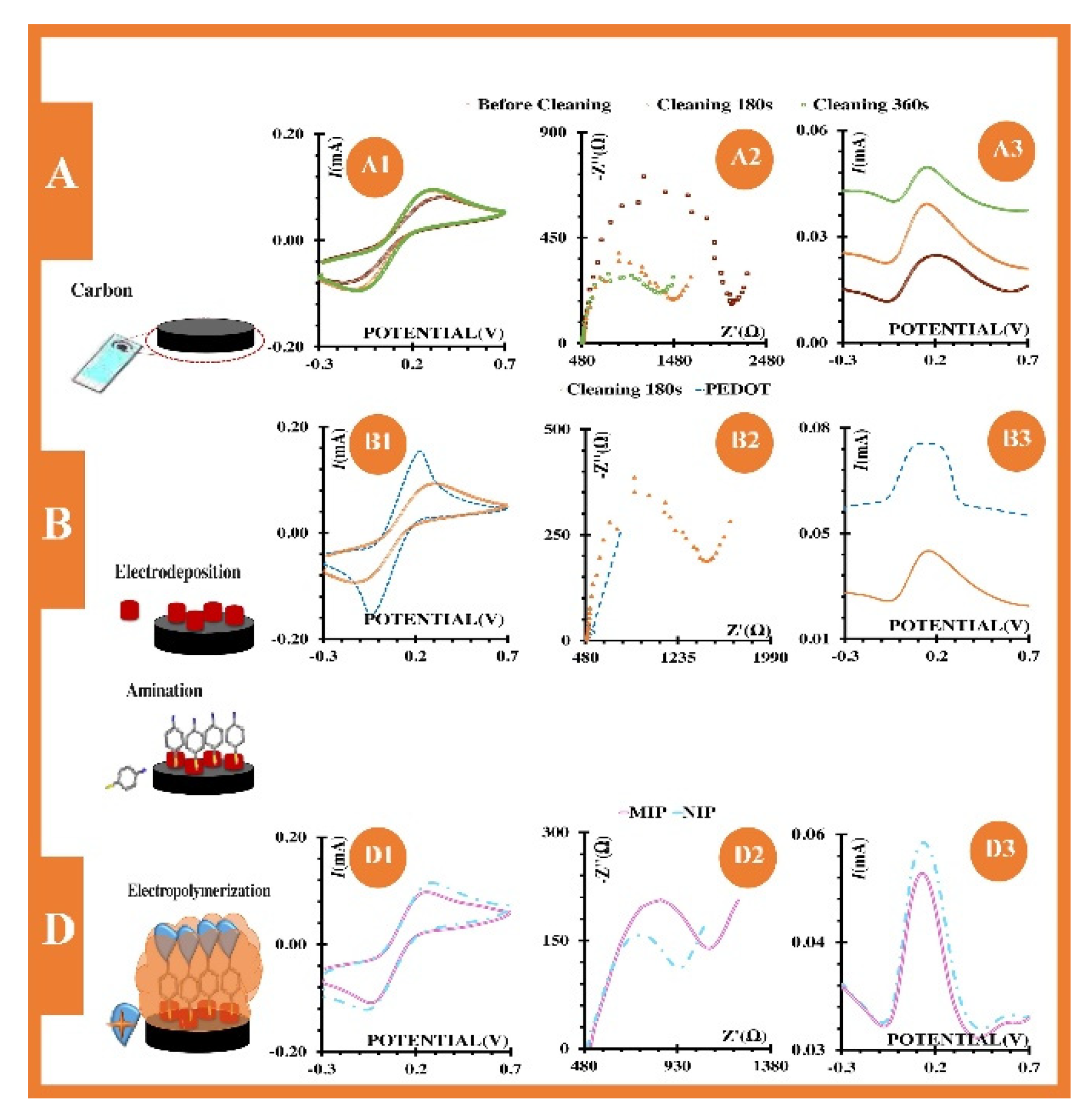
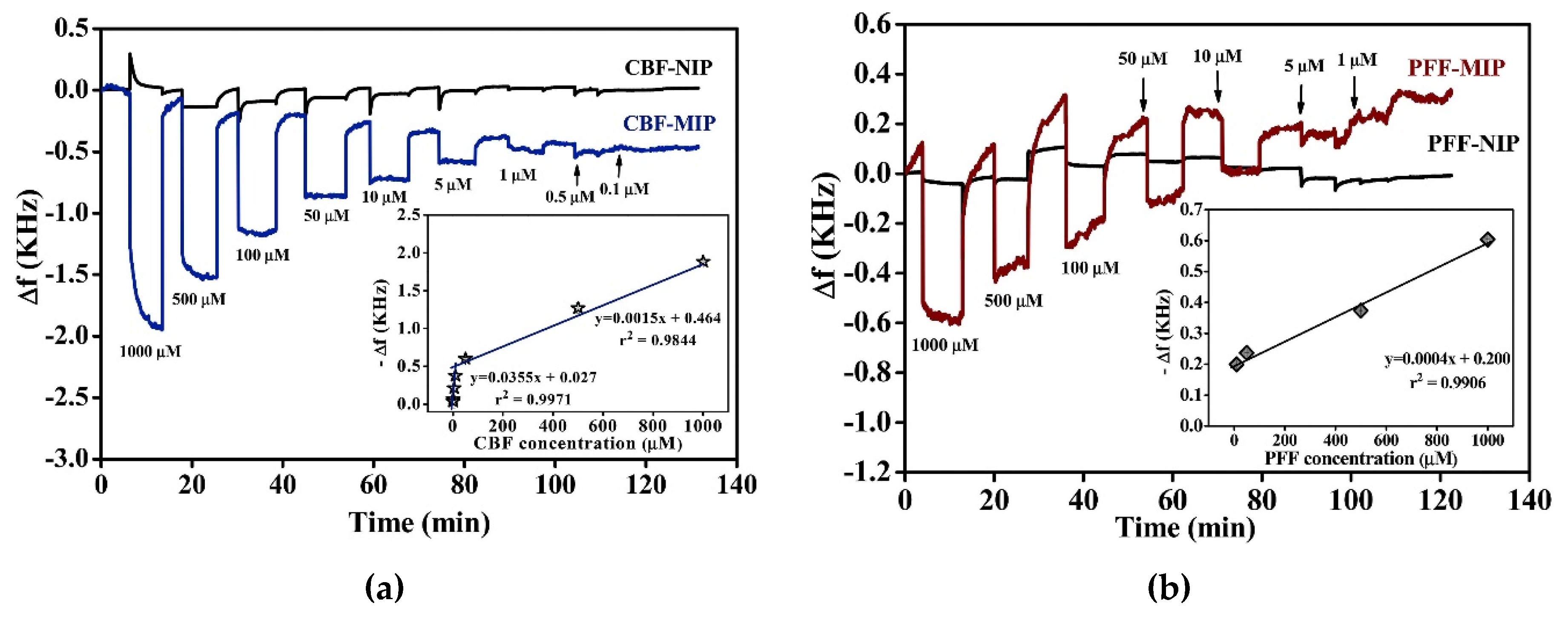
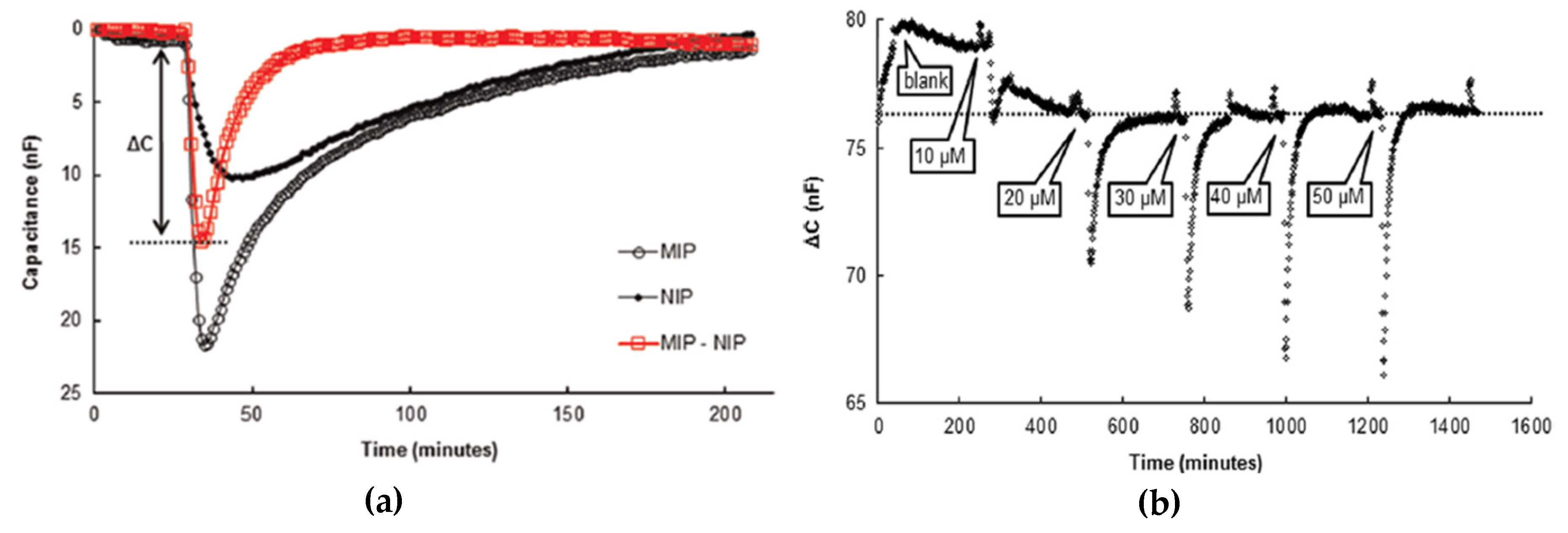
5.2. MIP-Based Sensors for Water Analyses
5.3. MIP-Based Sensors for On-Site Applications
5.4. Mass Production of MIP-Based Sensor Systems
6. Functionalised Electromagnetic Wave Sensors
6.1. Microwave Spectroscopy and Water Analysis
6.2. Progresses and Challenges in Microwave Spectroscopy
6.3. Microwave and Materials Integration
6.4. F-EM Sensors for Toxic Metals Analysis
6.5. F-EM Sensors for On-Site and In Situ Applications
7. New Trends in Water Quality Monitoring
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The United Nations World Water Development Report 4: Managing Water under Uncertainty and Risk, Executive Summary. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000217175 (accessed on 19 October 2019).
- Environment. Available online: http://ec.europa.eu/environment/water/water-drink/legislation_en.html (accessed on 19 October 2019).
- Methods Approved to Analyze Drinking Water Samples to Ensure Compliance with Regulations. Available online: https://www.epa.gov/dwanalyticalmethods (accessed on 19 October 2019).
- Meyer, A.M.; Klein, C.; Fünfrocken, E.; Kautenburger, R.; Beck, H.P. Real-time monitoring of water quality to identify pollution pathways in small and middle scale rivers. Sci. Total. Environ. 2019, 651, 2323–2333. [Google Scholar] [CrossRef]
- Lvova, L.; Di Natale, C.; Paolesse, R. Chemical Sensors for Water Potability Assessment. In Bottled Packaged Water; Grumezescu, A., Holban, A.M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2019; Volume 7, pp. 177–208. [Google Scholar]
- Legin, A.V.; Bychkov, E.A.; Seleznev, B.L.; Vlasov, Y.G. Development and analytical evaluation of a multisensor system for water quality monitoring. Sens. Actuators. B Chem. 1995, 27, 377–379. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Ehlert, A.; Legin, A.; Vlasov, Y.G.; Büttgenbach, S. Multisensor system on the basis of an array of non-specific chemical sensors and artificial neural networks for determination of inorganic pollutants in a model groundwater. Talanta 2001, 55, 425–431. [Google Scholar] [CrossRef]
- Krantz-Rülcker, C.; Stenberg, M.; Winquist, F.; Lundström, I. Electronic tongues for environmental monitoring based on sensor arrays and pattern recognition: A review. Anal. Chim. Acta 2001, 426, 217–226. [Google Scholar] [CrossRef]
- Martınez-Manez, R.; Soto, J.; Garcıa-Breijo, E.; Gil, L.; Ibanez, J.; Gadea, E. A multisensor in thick-film technology for water quality control. Sens. Actuators. A Phys. 2005, 120, 589–595. [Google Scholar] [CrossRef]
- Men, H.; Zou, S.; Li, Y.; Wang, Y.; Ye, X.; Wang, P. A novel electronic tongue combined MLAPS with stripping voltammetry for environmental detection. Sens. Actuators B Chem. 2005, 110, 350–357. [Google Scholar] [CrossRef]
- Yang, Y.J.; Haught, R.C.; Goodrich, J.A. Real-time contaminant detection and classification in a drinking water pipe using conventional water quality sensors: Techniques and experimental results. J. Env. Manag. 2009, 90, 2494–2506. [Google Scholar] [CrossRef] [PubMed]
- Capella, J.V.; Bonastre, A.; Ors, R.; Peris, M. A Wireless Sensor Network approach for distributed in-line chemical analysis of water. Talanta 2010, 80, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Mimendia, A.; Gutierrez, J.M.; Leija, L.; Hernandez, P.R.; Favari, L.; Munoz, R.; del Valle, M. A review of the use of the potentiometric electronic tongue in the monitoring of environmental systems. Environ. Modell. Softw. 2010, 25, 1023–1030. [Google Scholar] [CrossRef]
- Bourgeois, W.; Gardey, G.; Servieres, M.; Stuetz, R.M. A chemical sensor array based system for protecting wastewater treatment plants. Sens. Actuators. B Chem. 2003, 91, 109–116. [Google Scholar] [CrossRef]
- Valdes-Ramirez, G.; Gutierrez, M.; del Valle, M.; Ramirez-Silva, M.T.; Fournier, D.; Marty, J.-L. Automated resolution of dichlorvos and methylparaoxon pesticide mixtures employing a Flow Injection system with an inhibition electronic tongue. Biosens. Bioelectron. 2009, 24, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Czolkos, I.; Dock, E.; Tønning, E.; Christensen, J.; Winther-Nielsen, M.; Carlsson, C.; Mojzíková, R.; Skládal, P.; Wollenberger, U.; Nørgaard, L.; et al. Prediction of wastewater quality using amperometric bioelectronic tongues. Biosens. Bioelectron. 2016, 75, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.H.A.; Gurung, A.; Kang, W.-G.; Shin, B.-S.; Rahimnejad, M.; Jeon, B.-H.; Kim, J.R.; Oh, S.-E. Real-time monitoring of water quality of stream water using sulfur oxidizing bacteria as bio-indicator. Chemosphere 2019, 223, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Attivissimo, F.; Carducci, C.G.C.; Lanzolla, A.M.L.; Massaro, A.; Vadrucci, M.R. A portable optical sensor for sea quality monitoring. IEEE Sens. J. 2015, 15, 146–153. [Google Scholar] [CrossRef]
- Murphy, K.; Heery, B.; Sullivan, T.; Zhang, D.; Paludetti, L.; Lau, K.T.; Diamond, D.; Costa, E.; O׳Connor, N.; Regan, F. A low-cost autonomous optical sensor for water quality monitoring. Talanta 2015, 132, 520–527. [Google Scholar] [CrossRef]
- Skouteris, G.; Webb, D.P.; Shin, K.L.F.; Rahimifard, S. Assessment of the capability of an optical sensor for in-line real time wastewater quality analysis in food manufacturing. Water. Resour. Ind. 2018, 20, 75–81. [Google Scholar] [CrossRef]
- Vaughan, A.A.; Narayanaswamy, R. Optical fibre reflectance sensors for the detection of heavy metal ions based on immobilised Br-PADAP. Sens. Actuators. B Chem. 1998, 51, 368–376. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Dickert, F.L. Sensor technology and its application in environmental analysis. Anal. Bioanal. Chem. 2007, 387, 237–247. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Latif, U.; Qian, J.; Can, S.; Dickert, F.L. Biomimetic receptors for bioanalyte detection by quartz crystal microbalances—From molecules to cells. Sensors 2014, 14, 23419–23438. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing - Synthesis, characterisation and application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecular 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Kubisch, R.; Bohrn, U.; Fleischer, M.; Stütz, E. Cell-based sensor system using L6 cells for broad band continuous pollutant monitoring in aquatic environments. Sensors 2012, 12, 3370–3393. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, C.; Fuchs, K.; Bohrn, U.; Stütz, E.; Wölfl, S. Simultaneous detection of multiple bioactive pollutants using a multiparametric biochip for water quality monitoring. Biosens. Bioelectron. 2015, 72, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Furchner, A.; Syritski, V. Hybrid molecularly imprinted polymer for amoxicillin detection. Biosens. Bioelectron. 2018, 118, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.R.; Tavares, A.P.M.; Sales, M.G.F. In-situ generated molecularly imprinted material for chloramphenicol electrochemical sensing in waters down to the nanomolar level. Sens Actuators B Chem. 2018, 256, 420–428. [Google Scholar] [CrossRef]
- Erdem, Ö.; Saylan, Y.; Cihangir, N.; Denizli, A. Molecularly imprinted nanoparticles based plasmonic sensors for real-time Enterococcus faecalis detection. Biosens. Bioelectron. 2019, 126, 608–614. [Google Scholar] [CrossRef]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Rahimi Foroushani, A.; Ganjali, M.R.; Shahtaheri, S.J.; Yarahmadi, R. Modification of Carbon Paste Electrode Based on Molecularly Imprinted Polymer for Electrochemical Determination of Diazinon in Biological and Environmental Samples. Electroanalysis 2017, 29, 708–715. [Google Scholar] [CrossRef]
- Sroysee, W.; Chunta, S.; Amatatongchai, M.; Lieberzeit, P.A. Molecularly imprinted polymers to detect profenofos and carbofuran selectively with QCM sensors. Phys. Med. 2019, 7, 100016. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Montaseri, H.; Forbes, P.B.C. Advances in the application of nanomaterial-based sensors for detection of polycyclic aromatic hydrocarbons in aquatic systems. Trends Anal. Chem. 2019, 115, 52–69. [Google Scholar] [CrossRef]
- Warwick, C.; Guerreiro, A.; Gomez-Caballero, A.; Wood, E.; Kitson, J.; Robinson, J.; Soares, A. Conductance based sensing and analysis of soluble phosphates in wastewater. Biosens. Bioelectron. 2014, 52, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Warwick, C.; Guerreiro, A.; Wood, E.; Kitson, J.; Robinson, J.; Soares, A. A molecular imprinted polymer based sensor for measuring phosphate in wastewater samples. Water Sci. Technol. 2014, 69, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Lenain, P.; De Saeger, S.; Mattiasson, B.; Hedström, M. Affinity sensor based on immobilised molecular imprinted synthetic recognition elements. Biosens. Bioelectron. 2015, 69, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Arcadio, F.; Perri, C.; Zeni, L.; Sequeira, F.; Bilro, L.; Nogueira, R.; D’Agostino, G.; Porto, G.; Biasiolo, A. Water monitoring in smart cities exploiting plastic optical fibers and molecularly imprinted polymers. In Proceedings of the 2019 IEEE International Symposium on Measurements & Networking (M&N), Catania, Italy, 8–10 July 2019. [Google Scholar]
- Aikio, S.; Zeilinger, M.; Hiltunen, J.; Hakalahti, L.; Hiitola-Keinänen, J.; Hiltunen, M.; Kontturi, V.; Siitonen, S.; Puustinen, J.; Lieberzeit, P.; et al. Disposable (bio)chemical integrated optical waveguide sensors implemented on roll-to-roll produced platforms. RSC Adv. 2016, 6, 50414–50422. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nadaraja, A.V.; Roberts, D.J.; Zarifi, M.H. Real-time and hazard-free water quality monitoring based on microwave planar resonator sensor. Sens. Actuators A Phys. 2020, 303, 111663. [Google Scholar] [CrossRef]
- Zhang, K.; Amineh, R.K.; Dong, Z.; Nadler, D. Microwave sensing of water quality. IEEE Access 2019, 7, 69481–69493. [Google Scholar] [CrossRef]
- Korostynska, O.; Mason, A.; Al-Shamma’a, A.I. Flexible microwave sensors for real-time analysis of water contaminants. J. Electromagn. Waves Appl. 2013, 27, 2075–2089. [Google Scholar] [CrossRef]
- Teng, K.H.; Shaw, A.; Ateeq, M.; Al-Shamma’a, A.; Wylie, S.; Kazi, S.N.; Chew, B.T.; Kot, P. Design and implementation of a non-invasive real-time microwave sensor for assessing water hardness in heat exchangers. J. Electromagn. Waves Appl. 2018, 32, 797–811. [Google Scholar] [CrossRef]
- Cashman, S.; Korostynska, O.; Shaw, A.; Lisboa, P.; Conroy, L. Detecting the presence and concentration of nitrate in water using microwave spectroscopy. IEEE Sens. J. 2017, 17, 4092–4099. [Google Scholar] [CrossRef]
- Ateeq, M.; Shaw, A.; Garrett, R.; Dickson, P. A proof of concept study on utilising a non-invasive microwave analysis technique to characterise silver based materials in aqueous solution. Sens. Imaging 2017, 18, 13. [Google Scholar] [CrossRef]
- Kapilevich, B.; Litvak, B. Microwave Sensor for Accurate Measurements of Water Solution Concentrations. In Proceedings of the 2007 Asia-Pacific Microwave Conference, Bangkok, Thailand, 11–14 December 2007. [Google Scholar]
- Mason, A.; Abdullah, B.; Muradov, M.; Korostynska, O.; Al-Shamma’a, A.; Bjarnadottir, S.G.; Lunde, K.; Alvseike, O. Theoretical basis and application for measuring pork loin drip loss using microwave spectroscopy. Sensors 2016, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Gennarelli, G.; Soldovieri, F. A non-specific microwave sensor for water quality monitoring. Int. Water Technol. J. 2013, 3, 70–77. [Google Scholar]
- Korostynska, O.; Ortoneda-Pedrola, M.; Mason, A.; Al-Shamma’A, A.I. Flexible electromagnetic wave sensor operating at GHz frequencies for instantaneous concentration measurements of NaCl, KCl, MnCl2and CuCl solutions. Meas. Sci. Technol. 2014, 25, 065105. [Google Scholar] [CrossRef]
- Mason, A.; Soprani, M.; Korostynska, O.; Amirthalingam, A.; Cullen, J.; Muradov, M.; Carmona, E.N.; Sberveglieri, G.; Sberveglieri, V.; Al-Shamma’a, A. Real-time microwave, dielectric, and optical sensing of lincomycin and tylosin antibiotics in water: Sensor fusion for environmental safety. J. Sens. 2018, 2018, 7976105. [Google Scholar] [CrossRef]
- Moejes, K.; Sherif, R.; Dürr, S.; Conlan, S.; Mason, A.; Korostynska, O. Real-time monitoring of tetraselmis suecica in a saline environment as means of early water pollution detection. Toxics 2018, 6, 57. [Google Scholar] [CrossRef]
- Frau, I.; Korostynska, O.; Mason, A.; Byrne, P. Comparison of electromagnetic wave sensors with optical and low-frequency spectroscopy methods for real-time monitoring of lead concentrations in mine water. Mine Water Environ. 2018, 37, 617–624. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Daneshmand, M. Liquid sensing in aquatic environment using high quality planar microwave resonator. Sens. Actuators B Chem. 2016, 225, 517–521. [Google Scholar] [CrossRef]
- Al-Kizwini, M.A.; Wylie, S.R.; Al-Khafaji, D.A.; Al-Shamma’a, A.I. The monitoring of the two phase flow-annular flow type regime using microwave sensor technique. Measurement 2013, 46, 45–51. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Review of recent metamaterial microfluidic sensors. Sensors 2018, 18, 232. [Google Scholar] [CrossRef]
- Amirian, M.; Karimi, G.; Wiltshire, B.D.; Zarifi, M.H. Differential narrow bandpass microstrip filter design for material and liquid purity interrogation. IEEE Sens. J. 2019, 19, 10545–10553. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A.; Charoen-In, U.; Siritaratiwat, A. Planar microwave sensor for detection and discrimination of aqueous organic and inorganic solutions. Sens. Actuators B Chem. 2018, 271, 300–305. [Google Scholar] [CrossRef]
- Harnsoongnoen, S.; Wanthong, A.; Charoen-In, U.; Siritaratiwat, A. Microwave sensor for nitrate and phosphate concentration sensing. IEEE Sens. J. 2019, 19, 2950–2955. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Farsinezhad, S.; Abdolrazzaghi, M.; Daneshmand, M.; Shankar, K. Selective microwave sensors exploiting the interaction of analytes with trap states in tio2 nanotube arrays. Nanoscale 2016, 8, 7466–7473. [Google Scholar] [CrossRef] [PubMed]
- Afsarimanesh, N.; Mukhopadhyay, S.C.; Kruger, M. Performance assessment of interdigital sensor for varied coating thicknesses to detect ctx-i. IEEE Sens J. 2018, 18, 3924–3931. [Google Scholar] [CrossRef]
- Li, F.; Zheng, Y.; Hua, C.; Jian, J. Gas sensing by microwave transduction: Review of progress and challenges. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Azmi, A.; Azman, A.A.; Kaman, K.K.; Ibrahim, S.; Mukhopadhyay, S.C.; Nawawi, S.W.; Yunus, M.A.M. Performance of coating materials on planar electromagnetic sensing array to detect water contamination. IEEE Sens. J. 2017, 17, 5244–5251. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Abbott, D. High-sensitivity metamaterial-inspired sensor for microfluidic dielectric characterisation. IEEE Sens. J. 2014, 14, 1345–1351. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Sun, H. Metamaterials application in sensing. Sensors 2012, 12, 2742. [Google Scholar] [CrossRef]
- Frau, I.; Wylie, S.; Byrne, P.; Cullen, J.; Korostynska, O.; Mason, A. New sensing system based on electromagnetic waves and functionalised em sensors for continuous monitoring of zn in freshwater. In Proceedings of the 11th ICARD | IMWA | MWD Conference—“Risk to Opportunity”, Pretoria, South Africa, 10–14 September 2018. [Google Scholar]
- Frau, I.; Wylie, S.; Byrne, P.; Cullen, J.; Korostynska, O.; Mason, A. Detection of zn in water using novel functionalised planar microwave sensors. Mater. Sci. Eng. B 2019, 247, 114382. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoci, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 2015, 63, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wanekaya, A.K. Applications of nanoscale carbon-based materials in heavy metal sensing and detection. Analysis 2011, 136, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.K.; SenGupta, A.K. Toxic metal sensing through novel use of hybrid inorganic and polymeric ion-exchangers. Solvent Extr. Ion Exch. 2011, 29, 398–420. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Bhatia, M.; Satish Babu, R.; Sonawane, S.H.; Gogate, P.R.; Girdhar, A.; Reddy, E.R.; Pola, M. Application of nanoadsorbents for removal of lead from water. Int. J. Environ. Sci. Technol. 2017, 14, 1135–1154. [Google Scholar] [CrossRef]
- Frau, I.; Wylie, S.R.; Byrne, P.; Cullen, J.D.; Korostynska, O.; Mason, A. Functionalised microwave sensors for real-time monitoring of copper and zinc concentration in mining-impacted water. Int. J. Environ. Sci. Technol. 2019, 17, 1861–1876. [Google Scholar] [CrossRef]
- Vélez, P.; Muñoz-Enano, J.; Gil, M.; Mata-Contreras, J.; Martín, F. Differential microfluidic sensors based on dumbbell-shaped defect ground structures in microstrip technology: Analysis, optimisation, and applications. Sensors 2019, 19, 3189. [Google Scholar] [CrossRef]
- Banna, M.H.; Najjaran, H.; Sadiq, R.; Imran, S.A.; Rodriguez, M.J.; Hoorfar, M. Miniaturized water quality monitoring pH and conductivity sensors. Sens. Actuators. B Chem. 2014, 193, 434–441. [Google Scholar] [CrossRef]
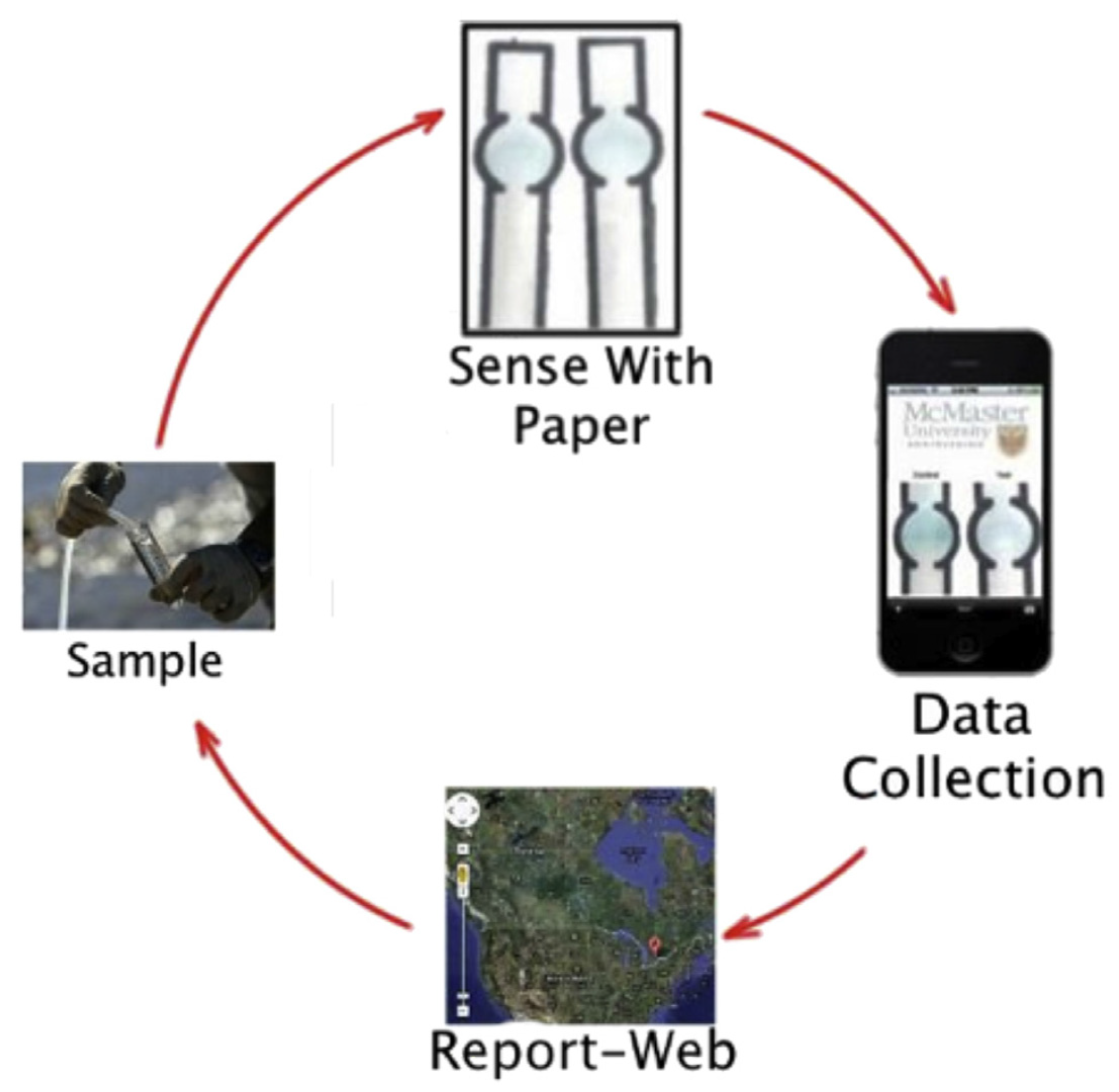
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D.M. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [Google Scholar] [CrossRef]
- Belikova, V.; Panchuk, V.; Legin, E.; Melenteva, A.; Kirsanov, D.; Legin, A. Continuous monitoring of water quality at aeration plant with potentiometric sensor array. Sens. Actuators. B Chem. 2019, 282, 854–860. [Google Scholar] [CrossRef]
- Legin, E.; Zadorozhnaya, O.; Khaydukova, M.; Kirsanov, D.; Rybakin, V.; Zagrebin, A.; Ignatyeva, N.; Ashina, J.; Sarkar, S.; Mukherjee, S.; et al. Rapid Evaluation of Integral Quality and Safety of Surface and Wastewater s by a Multisensor System (Electronic Tongue). Sensors 2019, 19, 2019. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Water Quality—Fresh Water Algal Growth Inhibition Test with Scenedesmus Subspicatus and Selenastrum Capricornutum, ISO 8692; International Organization for Standardization: Geneva, Switzerland, 1989; p. 12. [Google Scholar]
- International Organization for Standardization. Water Quality—Determination of The Inhibitory Effect of Water Samples on The Light Emission of Vibrio Fischeri (Luminescent bacteria test). ISO 11348-3; International Organization for Standardization: Geneva, Switzerland, 1998; p. 13. [Google Scholar]
- Zadorozhnaya, O.; Kirsanov, D.; Buzhinsky, I.; Tsarev, F.; Abramova, N.; Bratov, A.; Muñoz, F.J.; Ribó, J.; Bori, J.; Riva, M.C.; et al. Water pollution monitoring by an artificial sensory system performing in terms of Vibrio fischeri bacteria. Sens. Actuators. B Chem. 2015, 207, 1069–1075. [Google Scholar] [CrossRef]
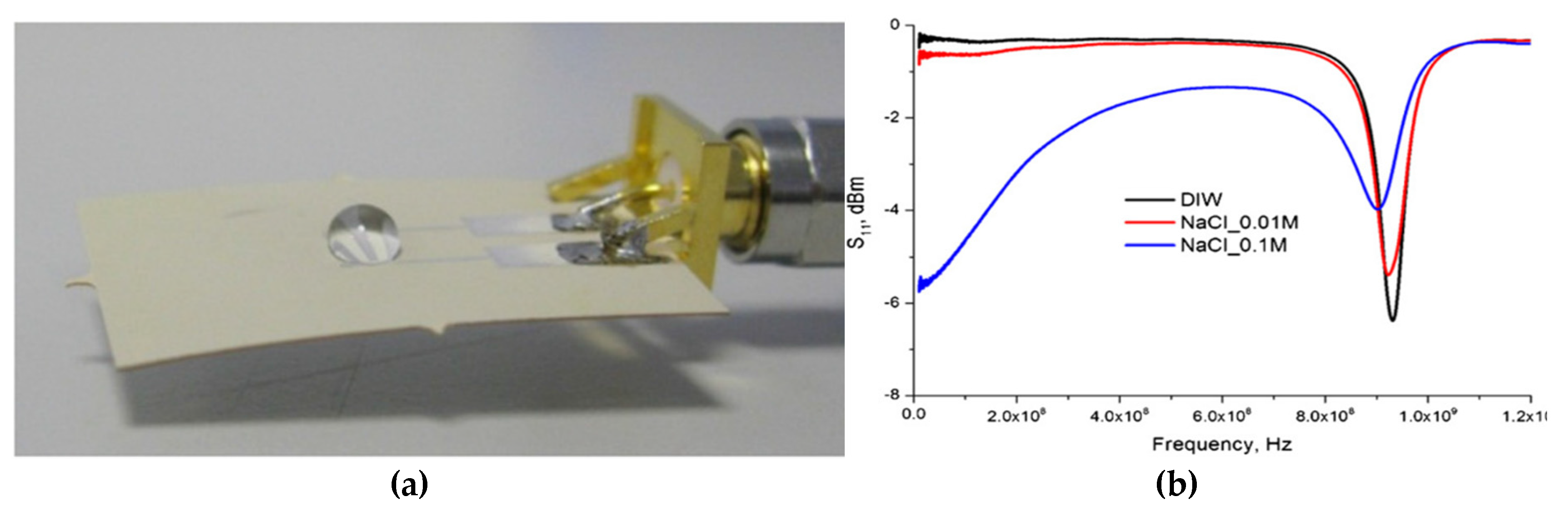
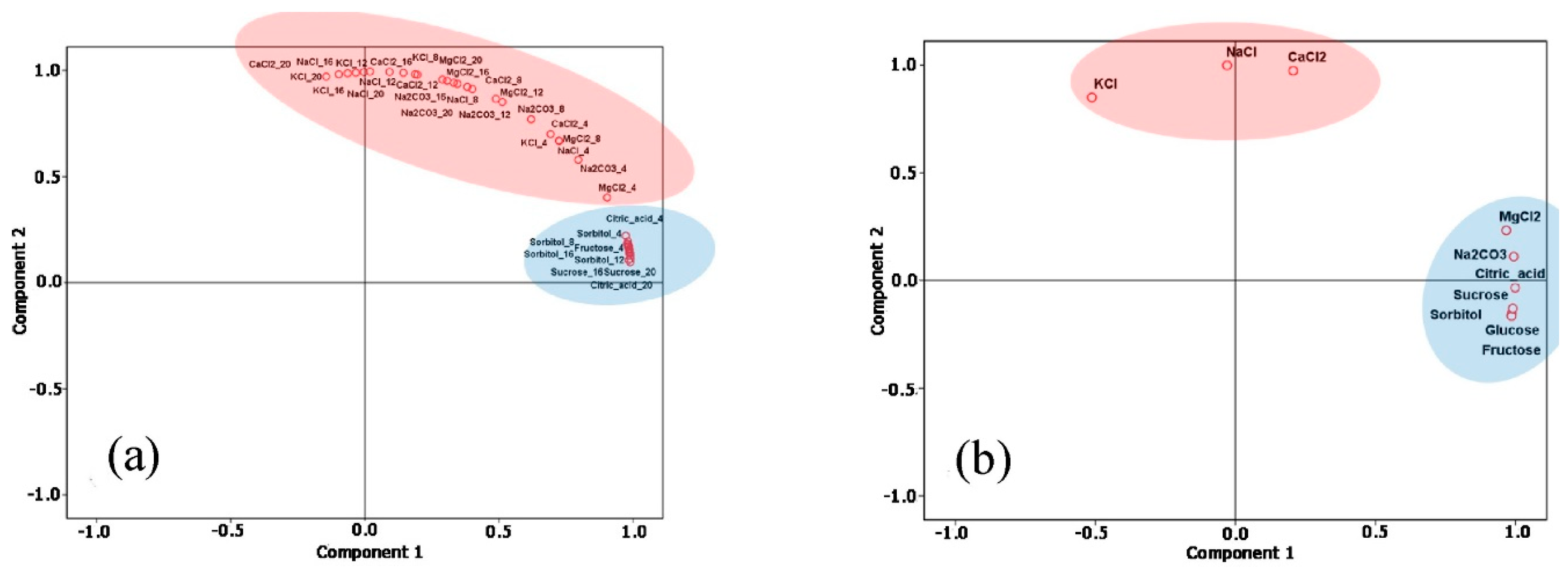
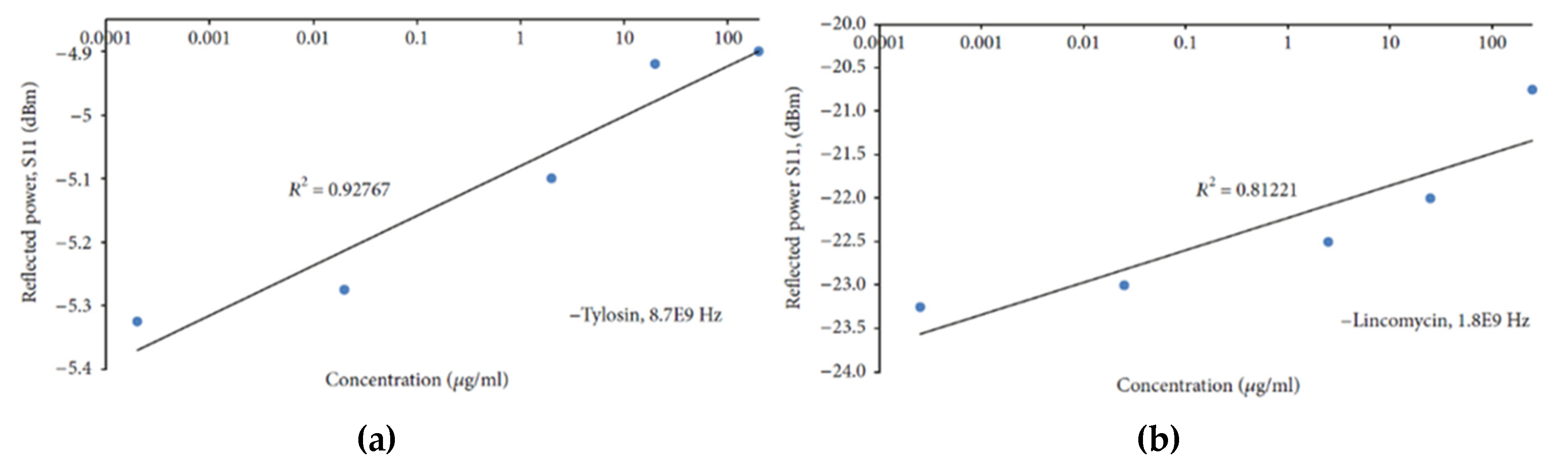
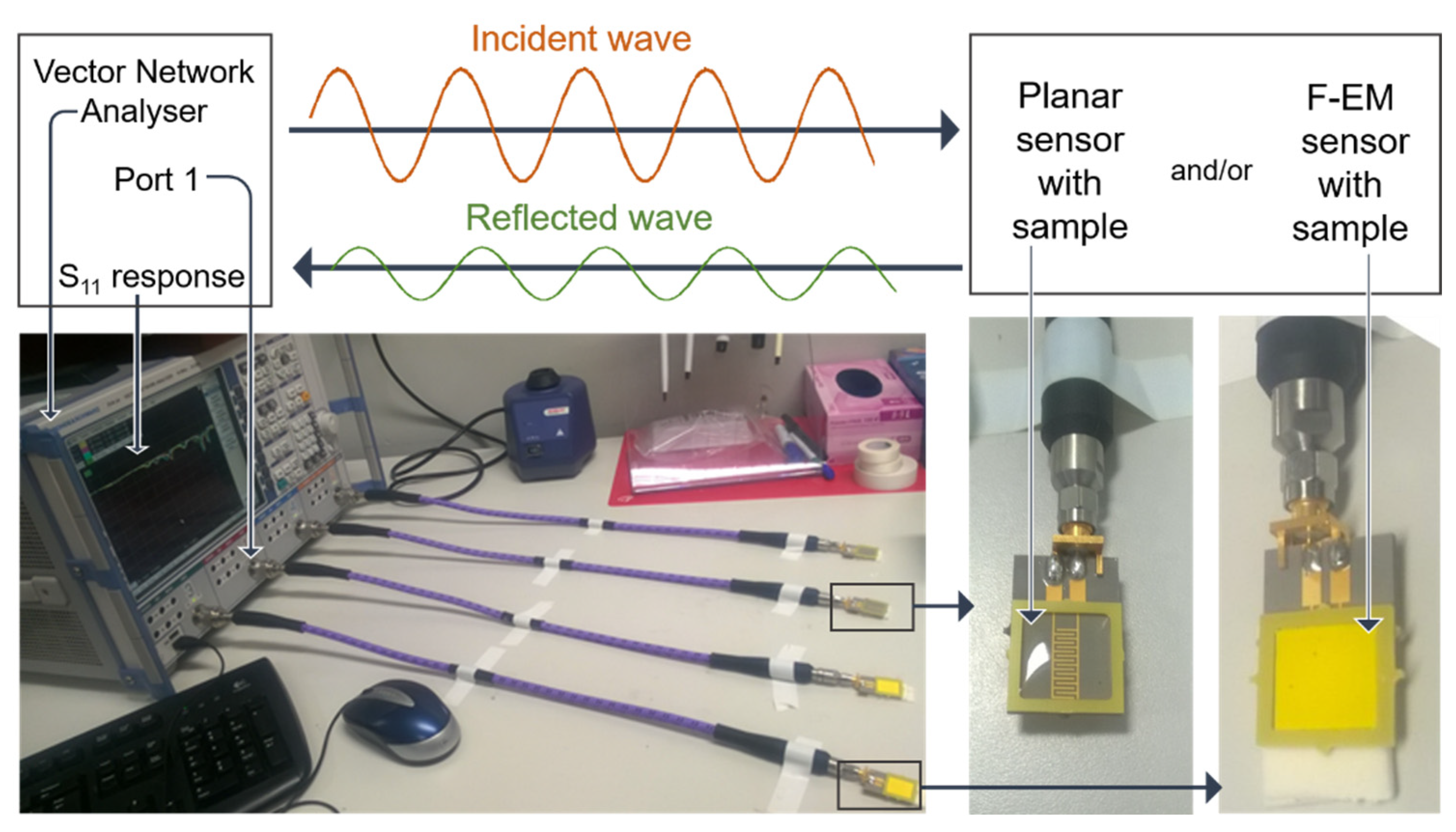
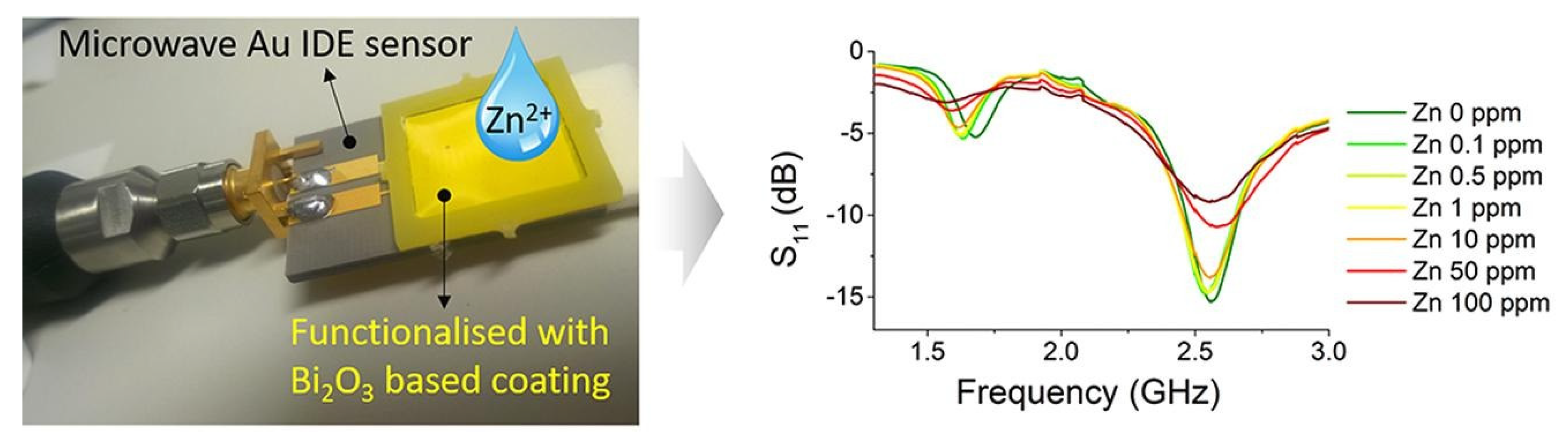
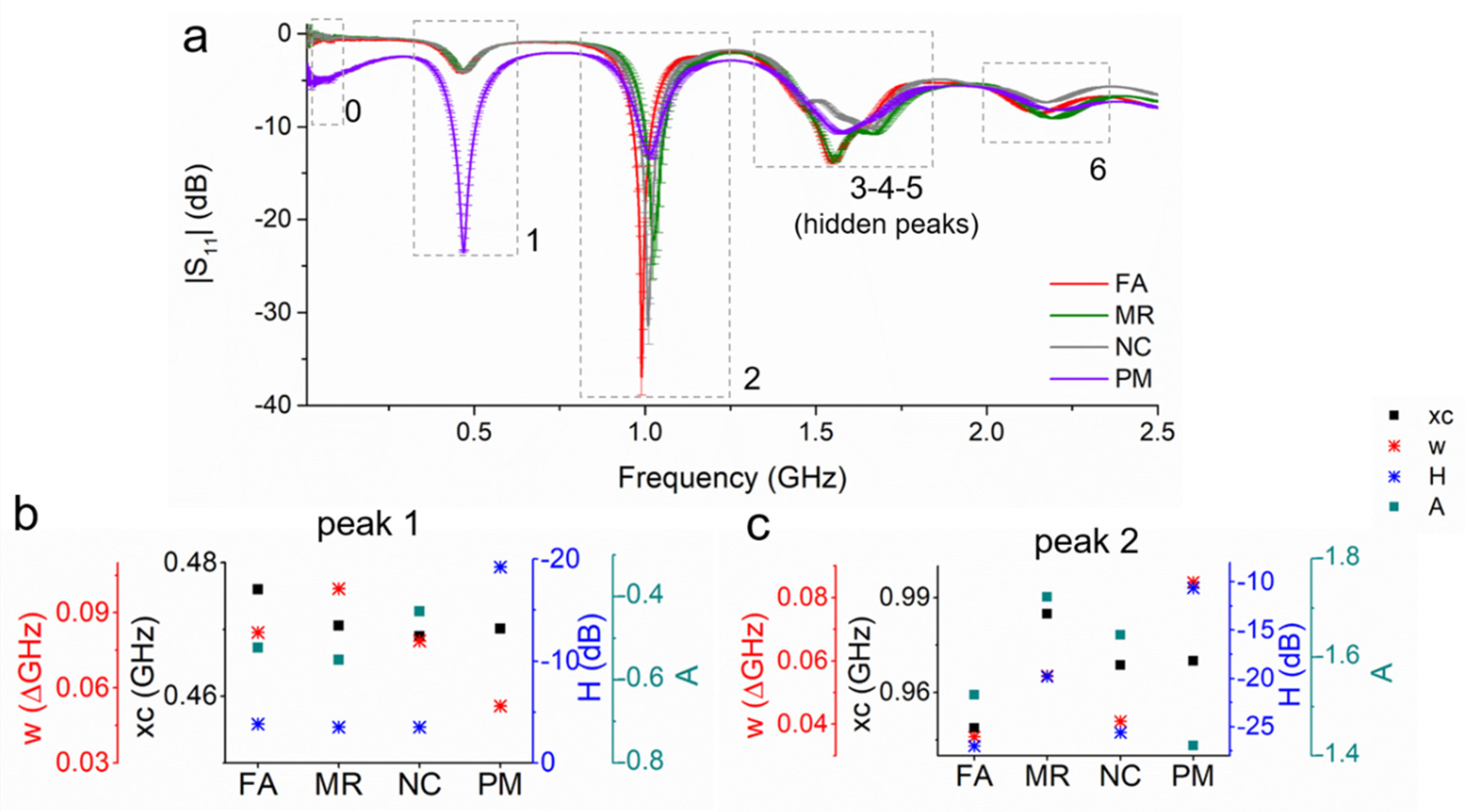

| R2 | CV (dB) | Sensitivity (ΔdB/mg/L) | LoD (mg/L) | Q-Factor | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UNC | f-EM | UNC | f-EM | UNC | f-EM | UNC | f-EM | UNC | f-EM | |
| Peak 0 | 0.970 | 0.928 | 0.20 | 0.25 | 0.362 | 0.222 | 0.194 | 0.379 | / | / |
| Peak 1 | 0.963 | 0.981 | 0.02 | 0.03 | 0.354 | 0.260 | 0.146 | 0.409 | 2.60 | 6.57 |
| Peak 2 | 0.888 | 0.983 | 0.01 | 0.02 | 0.824 | 1.651 | 0.083 | 0.036 | 30.71 | 135.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaroshenko, I.; Kirsanov, D.; Marjanovic, M.; Lieberzeit, P.A.; Korostynska, O.; Mason, A.; Frau, I.; Legin, A. Real-Time Water Quality Monitoring with Chemical Sensors. Sensors 2020, 20, 3432. https://doi.org/10.3390/s20123432
Yaroshenko I, Kirsanov D, Marjanovic M, Lieberzeit PA, Korostynska O, Mason A, Frau I, Legin A. Real-Time Water Quality Monitoring with Chemical Sensors. Sensors. 2020; 20(12):3432. https://doi.org/10.3390/s20123432
Chicago/Turabian StyleYaroshenko, Irina, Dmitry Kirsanov, Monika Marjanovic, Peter A. Lieberzeit, Olga Korostynska, Alex Mason, Ilaria Frau, and Andrey Legin. 2020. "Real-Time Water Quality Monitoring with Chemical Sensors" Sensors 20, no. 12: 3432. https://doi.org/10.3390/s20123432
APA StyleYaroshenko, I., Kirsanov, D., Marjanovic, M., Lieberzeit, P. A., Korostynska, O., Mason, A., Frau, I., & Legin, A. (2020). Real-Time Water Quality Monitoring with Chemical Sensors. Sensors, 20(12), 3432. https://doi.org/10.3390/s20123432








