A Multimodal Analysis Combining Behavioral Experiments and Survey-Based Methods to Assess the Cognitive Effect of Video Game Playing: Good or Evil?
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
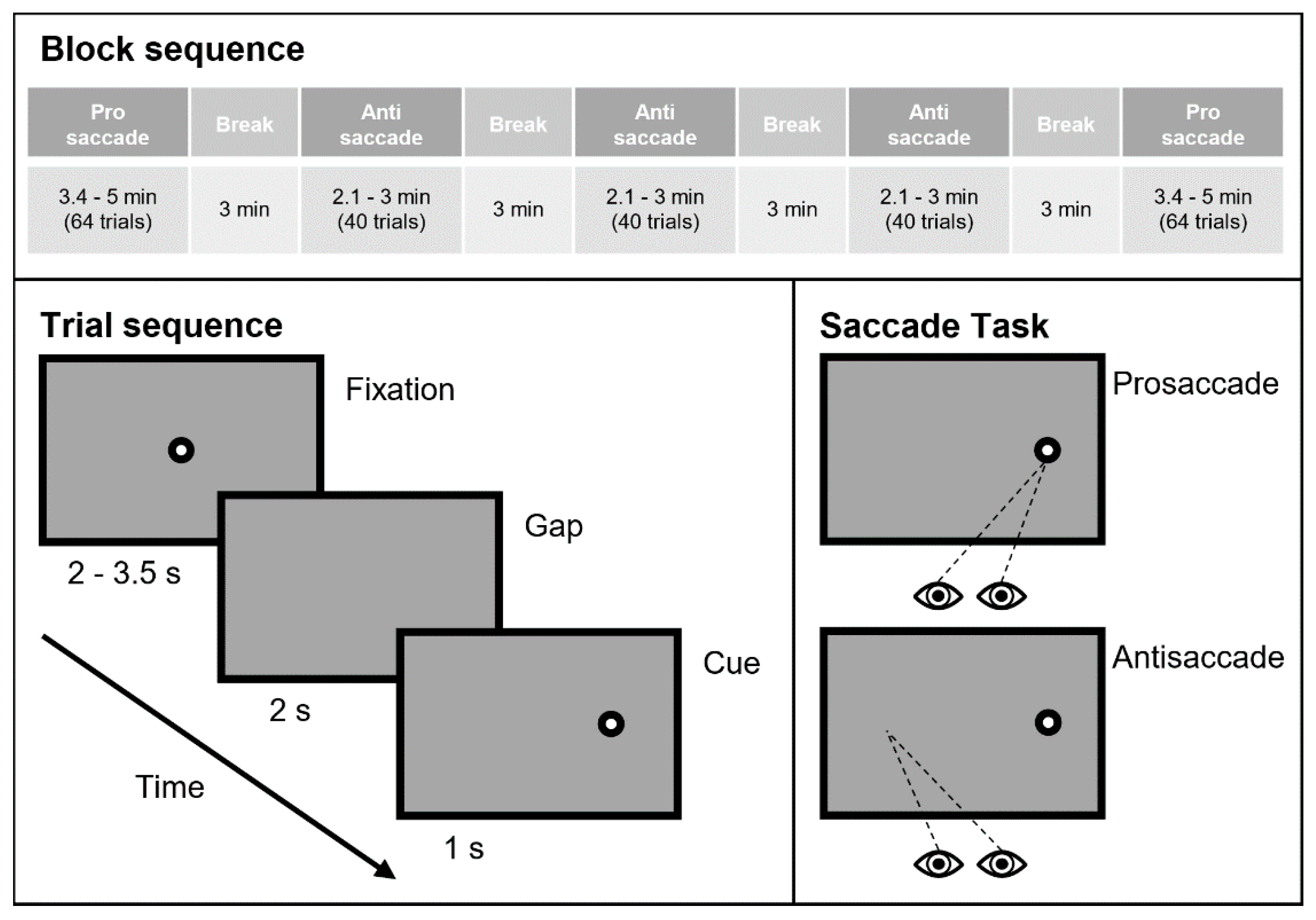
2.2. Saccade Experiment
2.2.1. Experimental Setup
2.2.2. Experimental Design
2.2.3. Data Processing
2.3. Self-Report Surveys
3. Results
3.1. Behavioral Experiment Performance Measurements
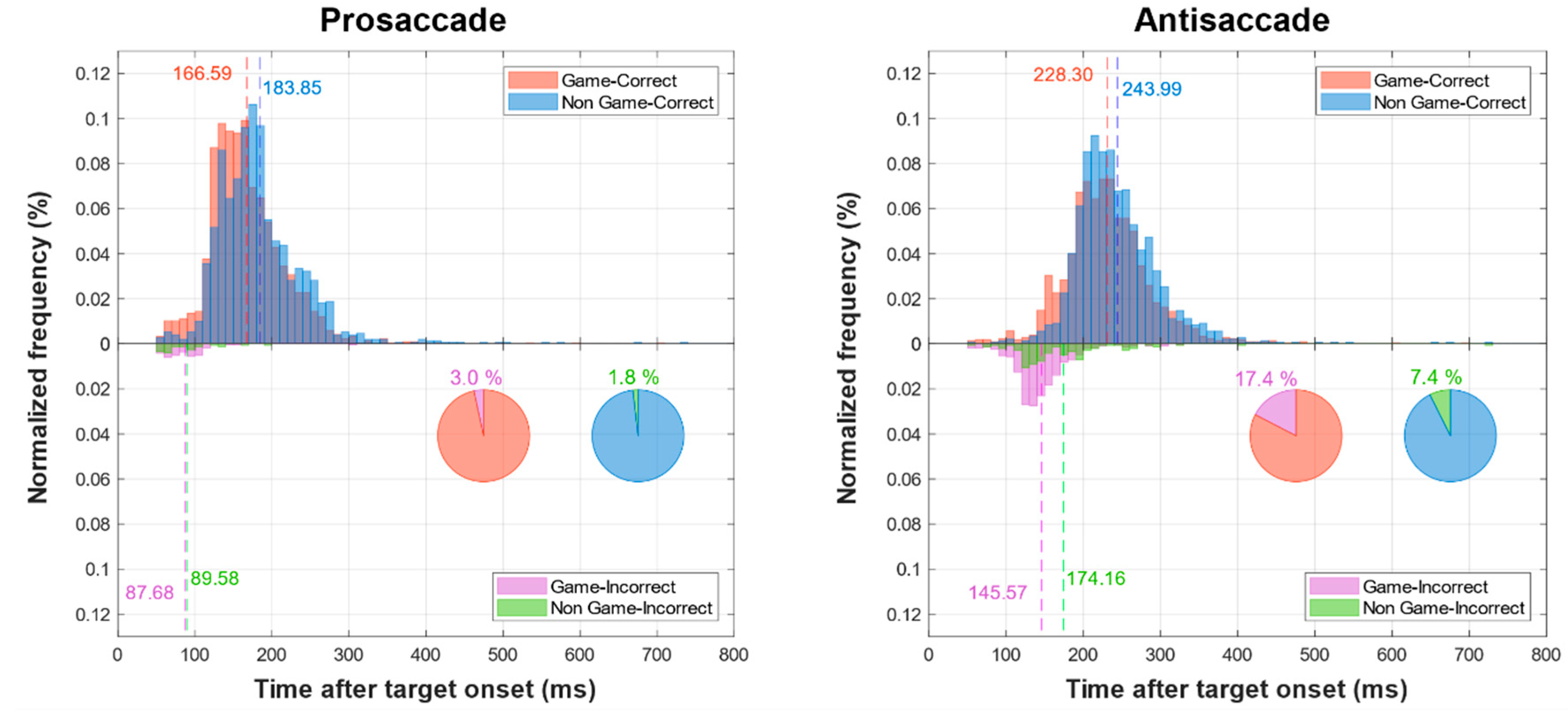
3.1.1. Reaction Time (RT)
3.1.2. Error Rate
3.2. Self-Report Surveys
3.2.1. Brief Self-Control Scale (BSCS)
3.2.2. Behavioral Activation System and Behavioral Inhibition System (BIS/BAS)
3.2.3. Barratt Impulsiveness Scale (BIS-11-R)
3.2.4. Beck Depression Inventory (BDI-II)
3.2.5. Beck Anxiety Inventory (BAI)
3.3. Correlation Analysis of the Behavioral Experiment Performance Measurements and the Self-Report Surveys
4. Discussion and Conclusions
4.1. Behavioral Experiment Analysis on the Cognitive Effect of Video Game Playing
4.2. Self-Report Survey Analysis on the Cognitive Effect of Video Game Playing
4.3. Correlation Analysis between Behavioral-Experiment-Based and Self-Report-Based Analysis
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization Home Page. Available online: http://www.who.int/en/ (accessed on 27 May 2020).
- Billieux, J.; King, D.L.; Higuchi, S.; Achab, S.; Bowden-Jones, H.; Hao, W.; Long, J.; Lee, H.K.; Potenza, M.N.; Saunders, J.B.; et al. Functional impairment matters in the screening and diagnosis of gaming disorder. J. Behav. Addict. 2017, 6, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.B.; Hao, W.; Long, J.; King, D.L.; Mann, K.; Fauth-Bühler, M.; Rumpf, H.-J.; Bowden-Jones, H.; Rahimi-Movaghar, A.; Chung, T.; et al. Gaming disorder: Its delineation as an important condition for diagnosis, management, and prevention. J. Behav. Addict. 2017, 6, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, H.-J.; Achab, S.; Billieux, J.; Bowden-Jones, H.; Carragher, N.; Demetrovics, Z.; Higuchi, S.; King, D.L.; Mann, K.; Potenza, M.; et al. Including gaming disorder in the ICD-11: The need to do so from a clinical and public health perspective. J. Behav. Addict. 2018, 7, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Achab, S.; Nicolier, M.; Mauny, F.; Monnin, J.; Trojak, B.; Vandel, P.; Sechter, D.; Gorwood, P.; Haffen, E. Massively multiplayer online role-playing games: Comparing characteristics of addict vs non-addict online recruited gamers in a French adult population. BMC Psychiatry 2011, 11, 156. [Google Scholar] [CrossRef]
- Ko, C.H.; Hsieh, T.J.; Wang, P.W.; Lin, W.C.; Yen, C.F.; Chen, C.S.; Yen, J.Y. Altered gray matter density and disrupted functional connectivity of the amygdala in adults with Internet gaming disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2015, 57, 185–192. [Google Scholar] [CrossRef]
- Weinstein, A.; Livny, A.; Weizman, A. New developments in brain research of internet and gaming disorder. Neurosci. Biobehav. Rev. 2017, 75, 314–330. [Google Scholar] [CrossRef]
- Müller, K.W.; Beutel, M.E.; Egloff, B.; Wölfling, K. Investigating risk factors for internet gaming disorder: A comparison of patients with addictive gaming, pathological gamblers and healthy controls regarding the big five personality traits. Eur. Addict. Res. 2014, 20, 129–136. [Google Scholar] [CrossRef]
- Zadra, S.; Bischof, G.; Besser, B.; Bischof, A.; Meyer, C.; John, U.; Rumpf, H.-J. The association between Internet addiction and personality disorders in a general population-based sample. J. Behav. Addict. 2016, 5, 691–699. [Google Scholar] [CrossRef]
- Griffiths, M.D.; van Rooij, A.J.; Kardefelt-Winther, D.; Starcevic, V.; Király, O.; Pallesen, S.; Müller, K.; Dreier, M.; Carras, M.; Prause, N.; et al. Working towards an international consensus on criteria for assessing internet gaming disorder: A critical commentary on Petry et al. (2014). Addiction 2016, 111, 167–175. [Google Scholar] [CrossRef]
- Aarseth, E.; Bean, A.M.; Boonen, H.; Colder Carras, M.; Coulson, M.; Das, D.; Deleuze, J.; Dunkels, E.; Edman, J.; Ferguson, C.J.; et al. Scholars’ open debate paper on the World Health Organization ICD-11 Gaming Disorder proposal. J. Behav. Addict. 2017, 6, 267–270. [Google Scholar] [CrossRef]
- Bean, A.M.; Nielsen, R.K.L.; van Rooij, A.J.; Ferguson, C.J. Video game addiction: The push to pathologize video games. Prof. Psychol. Res. Pr. 2017, 48, 378–389. [Google Scholar] [CrossRef]
- van Rooij, A.J.; Ferguson, C.J.; Colder Carras, M.; Kardefelt-Winther, D.; Shi, J.; Aarseth, E.; Bean, A.M.; Bergmark, K.H.; Brus, A.; Coulson, M.; et al. A weak scientific basis for gaming disorder: Let us err on the side of caution. J. Behav. Addict. 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Palo, V.; Monacis, L.; Sinatra, M.; Griffiths, M.D.; Pontes, H.; Petro, M.; Miceli, S. Measurement Invariance of the Nine-Item Internet Gaming Disorder Scale (IGDS9-SF) Across Albania, USA, UK, and Italy. Int. J. Ment. Health Addict. 2019, 17, 935–946. [Google Scholar] [CrossRef]
- Pontes, H.M.; Stavropoulos, V.; Griffiths, M.D. Emerging insights on internet gaming disorder: Conceptual and measurement issues. Addict. Behav. Rep. 2019, 11, 100242. [Google Scholar] [CrossRef]
- Kardefelt-Winther, D. A conceptual and methodological critique of internet addiction research: Towards a model of compensatory internet use. Comput. Hum. Behav. 2014, 31, 351–354. [Google Scholar] [CrossRef]
- Snodgrass, J.G.; Lacy, M.G.; Dengah, F.; Eisenhauer, S.; Batchelder, G.; Cookson, R.J. A vacation from your mind: Problematic online gaming is a stress response. Comput. Hum. Behav. 2014, 38, 248–260. [Google Scholar] [CrossRef]
- Gentile, D.A.; Choo, H.; Liau, A.; Sim, T.; Li, D.; Fung, D.; Khoo, A. Pathological Video Game Use Among Youths: A Two-Year Longitudinal Study. Pediatrics 2011, 127, e319–e329. [Google Scholar] [CrossRef]
- Swing, E.L.; Gentile, D.A.; Anderson, C.A.; Walsh, D.A. Television and Video Game Exposure and the Development of Attention Problems. Pediatrics 2010, 126, 214–221. [Google Scholar] [CrossRef]
- Rho, M.J.; Lee, H.; Lee, T.H.; Cho, H.; Jung, D.J.; Kim, D.J.; Choi, I.Y. Risk factors for internet gaming disorder: Psychological factors and internet gaming characteristics. Int. J. Env. Res. Public Health 2018, 15, 40. [Google Scholar] [CrossRef]
- Na, E.; Choi, I.; Lee, T.-H.; Lee, H.; Rho, M.J.; Cho, H.; Jung, D.J.; Kim, D.-J. The influence of game genre on Internet gaming disorder. J. Behav. Addict. 2017, 6, 248–255. [Google Scholar] [CrossRef]
- Müller, K.W.; Psych, D.; Dreier, M.; Soz, D.; Duven, E.; Giralt, S.; Beutel, M.E.; Wölfling, K. Adding clinical validity to the statistical power of large-scale epidemiological surveys on internet addiction in adolescence: A combined approach to investigate psychopathology and development-specific personality traits associated with internet addictio. J. Clin. Psychiatry 2017, 78, e244–e251. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Leung, L. Are you addicted to Candy Crush Saga? An exploratory study linking psychological factors to mobile social game addiction. Telemat. Inform. 2016, 33, 1155–1166. [Google Scholar] [CrossRef]
- Beutel, M.E.; Hoch, C.; Wölfling, K.; Müller, K.W. Klinische merkmale der computerspiel- und internetsucht am beispiel der inanspruchnehmer einer spielsuchtambulanz. Z. Psychosom. Med. Psychother. 2011, 57, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Bargeron, A.H.; Hormes, J.M. Psychosocial correlates of internet gaming disorder: Psychopathology, life satisfaction, and impulsivity. Comput. Hum. Behav. 2017, 68, 388–394. [Google Scholar] [CrossRef]
- Thorens, G.; Achab, S.; Billieux, J.; Khazaal, Y.; Khan, R.; Pivin, E.; Gupta, V.; Zullino, D. Characteristics and treatment response of self-identified problematic Internet users in a behavioral addiction outpatient clinic. J. Behav. Addict. 2014, 3, 78–81. [Google Scholar] [CrossRef]
- Sprong, M.E.; Griffiths, M.D.; Lloyd, D.P.; Paul, E.; Buono, F.D. Comparison of the video game functional assessment-revised (VGFA-R) and internet gaming disorder test (IGD-20). Front. Psychol. 2019, 10, 310. [Google Scholar] [CrossRef]
- Buono, F.D.; Sprong, M.E.; Lloyd, D.P.; Cutter, C.J.; Printz, D.M.B.; Sullivan, R.M.; Moore, B.A. Delay Discounting of Video Game Players: Comparison of Time Duration among Gamers. Cyberpsychology Behav. Soc. Netw. 2017, 20, 104–108. [Google Scholar] [CrossRef]
- Buono, F.D.; Upton, T.D.; Griffiths, M.D.; Sprong, M.E.; Bordieri, J. Demonstrating the validity of the Video Game Functional Assessment-Revised (VGFA-R). Comput. Hum. Behav. 2016, 54, 501–510. [Google Scholar] [CrossRef]
- Anguera, J.A.; Boccanfuso, J.; Rintoul, J.L.; Al-Hashimi, O.; Faraji, F.; Janowich, J.; Kong, E.; Larraburo, Y.; Rolle, C.; Johnston, E.; et al. Video game training enhances cognitive control in older adults. Nature 2013, 501, 97–101. [Google Scholar] [CrossRef]
- Oei, A.C.; Patterson, M.D. Enhancing Cognition with Video Games: A Multiple Game Training Study. PLoS ONE 2013, 8, e58546. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; Stock, A.K.; Beste, C.; Colzato, L.S. Action video gaming and cognitive control: Playing first person shooter games is associated with improved action cascading but not inhibition. PLoS ONE 2015, 10, e0144364. [Google Scholar] [CrossRef] [PubMed]
- Boot, W.R.; Kramer, A.F.; Simons, D.J.; Fabiani, M.; Gratton, G. The effects of video game playing on attention, memory, and executive control. Acta Psychol. 2008, 2, 226. [Google Scholar] [CrossRef] [PubMed]
- Green, C.S.; Bavelier, D. Learning, attentional control, and action video games. Curr. Biol. 2012, 22, R197–R206. [Google Scholar] [CrossRef] [PubMed]
- Ninaus, M.; Pereira, G.; Stefitz, R.; Prada, R.; Paiva, A.; Neuper, C.; Wood, G. Game elements improve performance in a working memory training task. Int. J. Serious Games 2015, 2, 3–16. [Google Scholar] [CrossRef]
- Chopin, A.; Bediou, B.; Bavelier, D. Altering perception: The case of action video gaming. Curr. Opin. Psychol. 2019, 29, 168–173. [Google Scholar] [CrossRef]
- Mack, D.J.; Ilg, U.J. The effects of video game play on the characteristics of saccadic eye movements. Vision Res. 2014, 102, 26–32. [Google Scholar] [CrossRef]
- Mack, D.J.; Wiesmann, H.; Ilg, U.J. Video game players show higher performance but no difference in speed of attention shifts. Acta Psychol. 2016, 169, 11–19. [Google Scholar] [CrossRef]
- Dye, M.W.G.; Green, C.S.; Bavelier, D. Increasing Speed of Processing With Action Video Games. Curr. Dir. Psychol. Sci. 2009, 18, 321–326. [Google Scholar] [CrossRef]
- Dye, M.W.G.; Green, C.S.; Bavelier, D. The development of attention skills in action video game players. Neuropsychologia 2009, 47, 1780–1789. [Google Scholar] [CrossRef]
- Chisholm, J.D.; Kingstone, A. Action video game players’ visual search advantage extends to biologically relevant stimuli. Acta Psychol. 2015, 159, 93–99. [Google Scholar] [CrossRef]
- Bediou, B.; Adams, D.M.; Mayer, R.E.; Tipton, E.; Green, C.S.; Bavelier, D. Meta-analysis of action video game impact on perceptual, attentional, and cognitive skills. Psychol. Bull. 2018, 144, 77–110. [Google Scholar] [CrossRef] [PubMed]
- West, G.L.; Al-Aidroos, N.; Pratt, J. Action video game experience affects oculomotor performance. Acta Psychol. 2013, 142, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.S.; Prinzmetal, W.; Shimamura, A.P.; Landau, A.N. Improved control of exogenous attention in action video game players. Front. Psychol. 2014, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, J.D.; Kingstone, A. Improved top-down control reduces oculomotor capture: The case of action video game players. Atten. Percept. Psychophys. 2012, 74, 257–262. [Google Scholar] [CrossRef]
- Littel, M.; Van Den Berg, I.; Luijten, M.; Van Rooij, A.J.; Keemink, L.; Franken, I.H.A. Error processing and response inhibition in excessive computer game players: An event-related potential study. Addict. Biol. 2012, 17, 934–947. [Google Scholar] [CrossRef]
- Azizi, E.; Stainer, M.J.; Abel, L.A. Is experience in multi-genre video game playing accompanied by impulsivity? Acta Psychol. 2018, 190, 47–84. [Google Scholar] [CrossRef]
- Munoz, D.P.; Everling, S. Look away: The anti-saccade task and the voluntary control of eye movement. Nat. Rev. Neurosci. 2004, 5, 218–228. [Google Scholar] [CrossRef]
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef]
- Antoniades, C.; Ettinger, U.; Gaymard, B.; Gilchrist, I.; Kristjánsson, A.; Kennard, C.; John Leigh, R.; Noorani, I.; Pouget, P.; Smyrnis, N.; et al. An internationally standardised antisaccade protocol. Vis. Res. 2013, 84, 1–5. [Google Scholar] [CrossRef]
- Saslow, M.G. Latency for saccadic eye movement. J. Opt. Soc. Am. 1967, 57, 1030–1033. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Oonk, H.M.; Barnes, L.L.; Hughes, H.C. Effects of warning signals and fixation point offsets on the latencies of pro- versus antisaccades: Implications for an interpretation of the gap effect. Exp. Brain Res. 1995, 103, 287–293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stampe, D.M. Heuristic filtering and reliable calibration methods for video-based pupil-tracking systems. Behav. Res. Methods Instrum. Comput. 1993, 25, 137–142. [Google Scholar] [CrossRef]
- Behrens, F.; MacKeben, M.; Schröder-Preikschat, W. An improved algorithm for automatic detection of saccades in eye movement data and for calculating saccade parameters. Behav. Res. Methods 2010, 42, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hallett, P.E. Primary and secondary saccades to goals defined by instructions. Vis. Res. 1978, 18, 1279–1296. [Google Scholar] [CrossRef]
- Tangney, J.P.; Baumeister, R.F.; Boone, A.L. High Self-Control Predicts Good Adjustment, Less Pathology, Better Grades, and Interpersonal Success. J. Pers. 2004, 72, 271–324. [Google Scholar] [CrossRef]
- Carver, C.S.; White, T.L. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Lee, S.-R.; Lee, W.-H.; Park, J.-S.; Kim, S.-M.; Kim, J.-W.; Shim, J.-H. The Study on Reliability and Validity of Korean Version of the Barratt Impulsiveness Scale-11-Revised in Nonclinical Adult Subjects. J. Korean Neuropsychiatr. Assoc. 2014, 51, 378–386. [Google Scholar] [CrossRef]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An Inventory for Measuring Clinical Anxiety: Psychometric Properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Kapci, E.G.; Uslu, R.; Turkcapar, H.; Karaoglan, A. Beck depression inventory II: Evaluation of the psychometric properties and cut-off points in a Turkish adult population. Depress. Anxiety 2008, 25, E104–E110. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.J. Measures of Anxiety. Arthritis Care 2011, 63, S467–S472. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.; Fillmore, M.T.; Milich, R. Separating automatic and intentional inhibitory mechanisms of attention in adults with attention-deficit/hyperactivity disorder. J. Abnorm. Psychol. 2011, 120, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.A.; Swing, E.L.; Lim, C.G.; Khoo, A. Video game playing, attention problems, and impulsiveness: Evidence of bidirectional causality. Psychol. Pop. Media Cult. 2012, 1, 62–70. [Google Scholar] [CrossRef]
- Deleuze, J.; Christiaens, M.; Nuyens, F.; Billieux, J. Shoot at first sight! First person shooter players display reduced reaction time and compromised inhibitory control in comparison to other video game players. Comput. Hum. Behav. 2017, 72, 570–576. [Google Scholar] [CrossRef]
- Dong, G.; Potenza, M.N. A cognitive-behavioral model of Internet gaming disorder: Theoretical underpinnings and clinical implications. J. Psychiatr. Res. 2014, 58, 7–11. [Google Scholar] [CrossRef]
- Fauth-Bühler, M.; Mann, K. Neurobiological correlates of internet gaming disorder: Similarities to pathological gambling. Addict. Behav. 2017, 64, 349–356. [Google Scholar] [CrossRef]
- Saunders, J.B.; Degenhardt, L.; Farrell, M. Excessive gambling and gaming: Addictive disorders? Lancet Psychiatry 2017, 4, 433–435. [Google Scholar] [CrossRef]
- Chisholm, J.D.; Hickey, C.; Theeuwes, J.; Kingstone, A. Reduced attentional capture in action video game players. Atten. Percept. Psychophys. 2010, 72, 667–671. [Google Scholar] [CrossRef]
- Leigh, R.J.; Zee, D.S. The Neurology of Eye Movements; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Munoz, D.P.; Armstrong, I.T.; Hampton, K.A.; Moore, K.D. Altered Control of Visual Fixation and Saccadic Eye Movements in Attention-Deficit Hyperactivity Disorder. J. Neurophysiol. 2003, 90, 503–514. [Google Scholar] [CrossRef]
- Yep, R.; Soncin, S.; Brien, D.C.; Coe, B.C.; Marin, A.; Munoz, D.P. Using an emotional saccade task to characterize executive functioning and emotion processing in attention-deficit hyperactivity disorder and bipolar disorder. Brain Cogn. 2018, 124, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, R.C.; Knopik, V.S.; Sweet, L.H.; Fischer, M.; Seidenberg, M.; Rao, S.M. Neural correlates of inhibitory control in adult attention deficit/hyperactivity disorder: Evidence from the Milwaukee longitudinal sample. Psychiatry Res. Neuroimaging 2011, 194, 119–129. [Google Scholar] [CrossRef]
- Liu, G.C.; Yen, J.Y.; Chen, C.Y.; Yen, C.F.; Chen, C.S.; Lin, W.C.; Ko, C.H. Brain activation for response inhibition under gaming cue distraction in internet gaming disorder. Kaohsiung J. Med. Sci. 2014, 30, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Quay, H.-C. The Behavioral Reward and Inhibition System In childhood Behavior Disorder. In Attention Deficit Disorder, New Research in Attention, Treatment, and Psychopharmacology; Bloomingdale, L.M., Ed.; Pergamon Press: Oxford, UK, 1988; pp. 176–186. [Google Scholar]
- Quay, H.C. The psychobiology of undersocialized aggressive conduct disorder: A theoretical perspective. Dev. Psychopathol. 1993, 5, 165–180. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wu, Y.-C.; Su, C.-H.; Lin, P.-C.; Ko, C.-H.; Yen, J.-Y. Association between Internet gaming disorder and generalized anxiety disorder. J. Behav. Addict. 2017, 6, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.A.; Coles, M.E.; Gibb, B.E. Retrospective reports of behavioral inhibition and young adults’ current symptoms of social anxiety, depression, and anxious arousal. J. Anxiety Disord. 2009, 23, 884–890. [Google Scholar] [CrossRef]
- Morgan, B.E.; Van Honk, J.; Hermans, E.J.; Scholten, M.R.M.; Stein, D.J.; Kahn, R.S. Gray’s BIS/BAS dimensions in non-comorbid, non-medicated social anxiety disorder. World J. Biol. Psychiatry 2009, 10, 925–928. [Google Scholar] [CrossRef]
- Yen, J.Y.; Ko, C.H.; Yen, C.F.; Chen, C.S.; Chen, C.C. The association between harmful alcohol use and Internet addiction among college students: Comparison of personality. Psychiatry Clin. Neurosci. 2009, 63, 218–224. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.S.; Kim, B.-N.; Cheong, J.H.; Han, D.H. The Factors for the Aggression in Patients with On-Line Game Addiction: Behavioral Inhibition/Activation System and Comorbid Disease. J. Korean Neuropsychiatr. Assoc. 2014, 52, 84–90. [Google Scholar] [CrossRef]
- Zvyagintsev, M.; Klasen, M.; Weber, R.; Sarkheil, P.; Esposito, F.; Schwenzer, M.; Mathiak, K. Violence-related content in video game may lead to functional connectivity changes in brain networks as revealed by fMRI-ICA in young men. Neuroscience 2016, 320, 247–258. [Google Scholar] [CrossRef]
- Lim, J.A.; Lee, J.Y.; Jung, H.Y.; Sohn, B.K.; Choi, S.W.; Kim, Y.J.; Kim, D.J.; Choi, J.S. Changes of quality of life and cognitive function in individuals with Internet gaming disorder: A 6-month follow-up. Medicine 2016, 95, e5695. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D. Pathological video-game use among youth ages 8 to 18: A national study: Research article. Psychol. Sci. 2009, 20, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Rehbein, F.; Psych, G.; Kleimann, M.; Mediasci, G.; Mößle, T. Prevalence and risk factors of video game dependency in adolescence: Results of a German nationwide survey. Cyberpsychology Behav. Soc. Netw. 2010, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Pontes, H.M.; Király, O.; Demetrovics, Z.; Griffiths, M.D. The conceptualisation and measurement of DSM-5 internet gaming disorder: The development of the IGD-20 test. PLoS ONE 2014, 9, e110137. [Google Scholar] [CrossRef]
- Spinella, M. Neurobehavioral correlates of impulsivity: Evidence of prefrontal involvement. Int. J. Neurosci. 2004, 114, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Jacob, G.A.; Gutz, L.; Bader, K.; Lieb, K.; Tüscher, O.; Stahl, C. Impulsivity in borderline personality disorder: Impairment in self-report measures, but not behavioral inhibition. Psychopathology 2010, 43, 180–188. [Google Scholar] [CrossRef]
- Taylor, A.J.G. Trait impulsivity and oculomotor inhibition. Stud. Psychol. 2016, 58, 134–144. [Google Scholar] [CrossRef]
- Preciado, D.; Theeuwes, J. To look or not to look? Reward, selection history, and oculomotor guidance. J. Neurophysiol. 2018, 120, 1740–1752. [Google Scholar] [CrossRef]
- Brewer, G.A.; Spillers, G.J.; McMillan, B.; Unsworth, N. Extensive performance on the antisaccade task does not lead to negative transfer. Psychon. Bull. Rev. 2011, 18, 923–929. [Google Scholar] [CrossRef]
- García-Blanco, A.C.; Perea, M.; Salmerón, L. Attention orienting and inhibitory control across the different mood states in bipolar disorder: An emotional antisaccade task. Biol. Psychol. 2013, 94, 556–561. [Google Scholar] [CrossRef]
- Ogletree, S.M.; Drake, R. College students’ video game participation and perceptions: Gender differences and implications. Sex Roles 2007, 56, 537–542. [Google Scholar] [CrossRef]
- Boot, W.R.; Blakely, D.P.; Simons, D.J. Do action video games improve perception and cognition? Front. Psychol. 2011, 2, 226. [Google Scholar] [CrossRef] [PubMed]
- Heponiemi, T.; Keltikangas-Järvinen, L.; Kettunen, J.; Puttonen, S.; Ravaja, N. BIS-BAS sensitivity and cardiac autonomic stress profiles. Psychophysiology 2004, 41, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, L.; Vandeweghe, L.; Vandewalle, J.; Van Durme, K.; Vandevivere, E.; Wante, L.; McIntosh, K.; Verbeken, S.; Moens, E.; Goossens, L.; et al. Measuring Punishment and Reward Sensitivity in children and adolescents with a parent-report version of the Bis/Bas-scales. Pers. Individ. Differ. 2015, 87, 272–277. [Google Scholar] [CrossRef]
- Wilson, S.J.; Glue, P.; Ball, D.; Nutt, D.J. Saccadic eye movement parameters in normal subjects. Electroencephalogr. Clin. Neurophysiol. 1993, 86, 69–74. [Google Scholar] [CrossRef]
- Cross, C.P.; Copping, L.T.; Campbell, A. Sex Differences in Impulsivity: A Meta-Analysis. Psychol. Bull. 2011, 137, 97–130. [Google Scholar] [CrossRef]
- Jorm, A.F.; Christensen, H.; Henderson, A.S.; Jacomb, P.A.; Körten, A.E.; Rodgers, B. Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: Factor structure, validity and norms in a large community sample. Pers. Individ. Differ. 1998, 26, 49–58. [Google Scholar] [CrossRef]
- Cherney, I.D. Mom, let me play more computer games: They improve my mental rotation skills. Sex Roles 2008, 59, 776–786. [Google Scholar] [CrossRef]
- Hoeft, F.; Watson, C.L.; Kesler, S.R.; Bettinger, K.E.; Reiss, A.L. Gender differences in the mesocorticolimbic system during computer game-play. J. Psychiatr. Res. 2008, 42, 253–258. [Google Scholar] [CrossRef]
- Shibuya, A.; Sakamoto, A.; Ihori, N.; Yukawa, S. The effects of the presence and contexts of video game violence on children: A longitudinal study in Japan. Simul. Gaming 2008, 39, 528–539. [Google Scholar] [CrossRef]
- Connell, C.J.W.; Thompson, B.; Kuhn, G.; Gant, N. Exercise-induced fatigue and caffeine supplementation affect psychomotor performance but not covert visuo-spatial attention. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Doettl, S.M.; Easterday, M.K.; Plyler, P.N.; Behn, L.L.; Poget, A.S. Mental tasking and rotary Chair-Induced vestibular nystagmus utilizing Video-Oculography. Int. J. Audiol. 2020, 59, 360–366. [Google Scholar] [CrossRef] [PubMed]
- McNerney, K.M.; Coad, M.L.; Burkard, R. The influence of caffeine on rotary chair and oculomotor testing. J. Am. Acad. Audiol. 2018, 29, 587–595. [Google Scholar] [CrossRef] [PubMed]


| Scale | VGP (n = 18) | NVGP (n = 12) | p-Value | Effect Size (Cohen’s d) |
|---|---|---|---|---|
| Prosaccade RT (ms) | 166.6 ± 28.5 | 183.9 ± 29.9 | 0.122 (t) | 0.5943 |
| Antisaccade RT (ms) | 228.3 ± 35.5 | 244 ± 31.6 | 0.226 (t) | 0.4614 |
| Prosaccade Error Rate (%) | 3.4 ± 2.42 | 1.78 ± 1.76 | 0.085 (W) | 0.7440 |
| Antisaccade Error Rate (%) | 17.4 ± 12.3 | 7.4 ± 4.7 | 0.005 ** (t) | 1 |
| BSCS | 37.7 ± 4.6 | 36.9 ± 5.4 | 0.686 (t) | 0.1523 |
| BIS/BAS | 15.5 ± 3.2/26.2 ± 5.1 | 18 ± 2.1/26.3 ± 5.2 | 0.023 */0.954 (t) | 0.8949/0.0216 |
| BIS-11-R | 61.3 ± 10.8 | 59.7 ± 11.1 | 0.701 (t) | 0.1447 |
| BDI-II | 10.4 ± 7.1 | 8.8 ± 5.9 | 0.534 (t) | 0.2349 |
| BAI | 5.8 ± 6.1 | 5.4 ± 3.6 | 0.671 (W) | 0.0791 |
| Group All Participant | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pro RT | Anti RT | Anti Error | BSCS | BIS-11-R | BDI-II | BAI | BIS | BAS | |
| Pro RT | - | 0.74 *** | −0.50 ** | 0.11 | 0.22 | 0.06 | −0.20 | 0.03 | −0.03 |
| Anti RT | - | - | −0.34 | 0.07 | 0.29 | 0.07 | −0.09 | −0.05 | −0.18 |
| Anti Error | - | - | - | 0.24 | 0.16 | 0.26 | 0.19 | −0.16 | 0.03 |
| BSCS | - | - | - | - | 0.49 ** | 0.22 | 0.06 | −0.48 ** | −0.12 |
| BIS-11-R | - | - | - | - | - | 0.40 * | 0.23 | −0.42 * | −0.43 * |
| BDI-II | - | - | - | - | - | - | 0.43 * | −0.26 | −0.24 |
| BAI | - | - | - | - | - | - | - | −0.10 | −0.20 |
| BIS | - | - | - | - | - | - | - | - | 0.46 * |
| BAS | - | - | - | - | - | - | - | - | - |
| Group VGP. Above Main Diagonal | |||||||||
| Group NVGP. Below Main Diagonal | |||||||||
| Pro RT | Anti RT | Anti Error | BSCS | BIS-11-R | BDI-II | BAI | BIS | BAS | |
| Pro RT | - | 0.72 *** | −0.55 * | 0.05 | 0.04 | 0.04 | −0.29 | −0.07 | −0.05 |
| Anti RT | 0.73 ** | - | −0.40 | −0.01 | 0.33 | 0.10 | −0.10 | −0.12 | −0.29 |
| Anti Error | −0.15 | 0.21 | - | 0.18 | 0.12 | 0.22 | 0.19 | 0.03 | 0.10 |
| BSCS | 0.25 | 0.25 | 0.53 | - | 0.34 | 0.02 | −0.03 | −0.50 * | 0.15 |
| BIS−11-R | 0.57 | 0.28 | 0.30 | 0.67 * | - | 0.40 | 0.18 | −0.52 * | −0.42 |
| BDI-II | 0.23 | 0.11 | 0.31 | 0.52 | 0.38 | - | 0.40 | −0.28 | −0.37 |
| BAI | 0.04 | −0.02 | 0.25 | 0.27 | 0.36 | 0.53 | - | −0.11 | −0.41 |
| BIS | −0.19 | −0.29 | 0.08 | −0.54 | −0.27 | −0.12 | −0.02 | - | 0.48 * |
| BAS | −0.01 | −0.01 | −0.19 | −0.47 | −0.45 | 0.00 | 0.34 | 0.58 * | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.H.; Park, H.-J.; Yeo, S.-H.; Kim, H. A Multimodal Analysis Combining Behavioral Experiments and Survey-Based Methods to Assess the Cognitive Effect of Video Game Playing: Good or Evil? Sensors 2020, 20, 3219. https://doi.org/10.3390/s20113219
Jeong JH, Park H-J, Yeo S-H, Kim H. A Multimodal Analysis Combining Behavioral Experiments and Survey-Based Methods to Assess the Cognitive Effect of Video Game Playing: Good or Evil? Sensors. 2020; 20(11):3219. https://doi.org/10.3390/s20113219
Chicago/Turabian StyleJeong, Ji Hyeok, Hyun-Jung Park, Sang-Hoon Yeo, and Hyungmin Kim. 2020. "A Multimodal Analysis Combining Behavioral Experiments and Survey-Based Methods to Assess the Cognitive Effect of Video Game Playing: Good or Evil?" Sensors 20, no. 11: 3219. https://doi.org/10.3390/s20113219
APA StyleJeong, J. H., Park, H.-J., Yeo, S.-H., & Kim, H. (2020). A Multimodal Analysis Combining Behavioral Experiments and Survey-Based Methods to Assess the Cognitive Effect of Video Game Playing: Good or Evil? Sensors, 20(11), 3219. https://doi.org/10.3390/s20113219








