Electrical Modeling of the Growth and Differentiation of Skeletal Myoblasts Cell Cultures for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
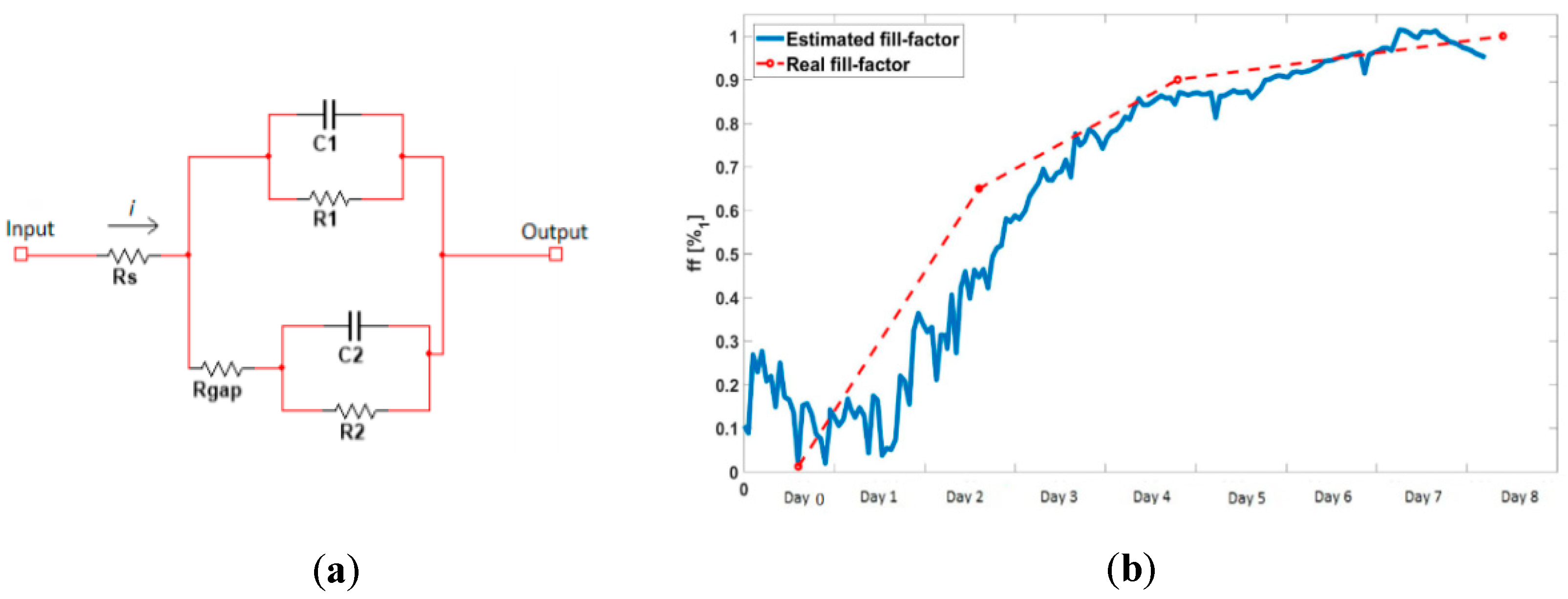
2.1. Oscillation-Based Circuit
2.2. Protocol of Isolation and Cultivation of Muscle Stem Cells
2.3. Experimental Growth and Differentiation of Muscle Stem Cells in the 8W10E PET Cultureware
3. Results
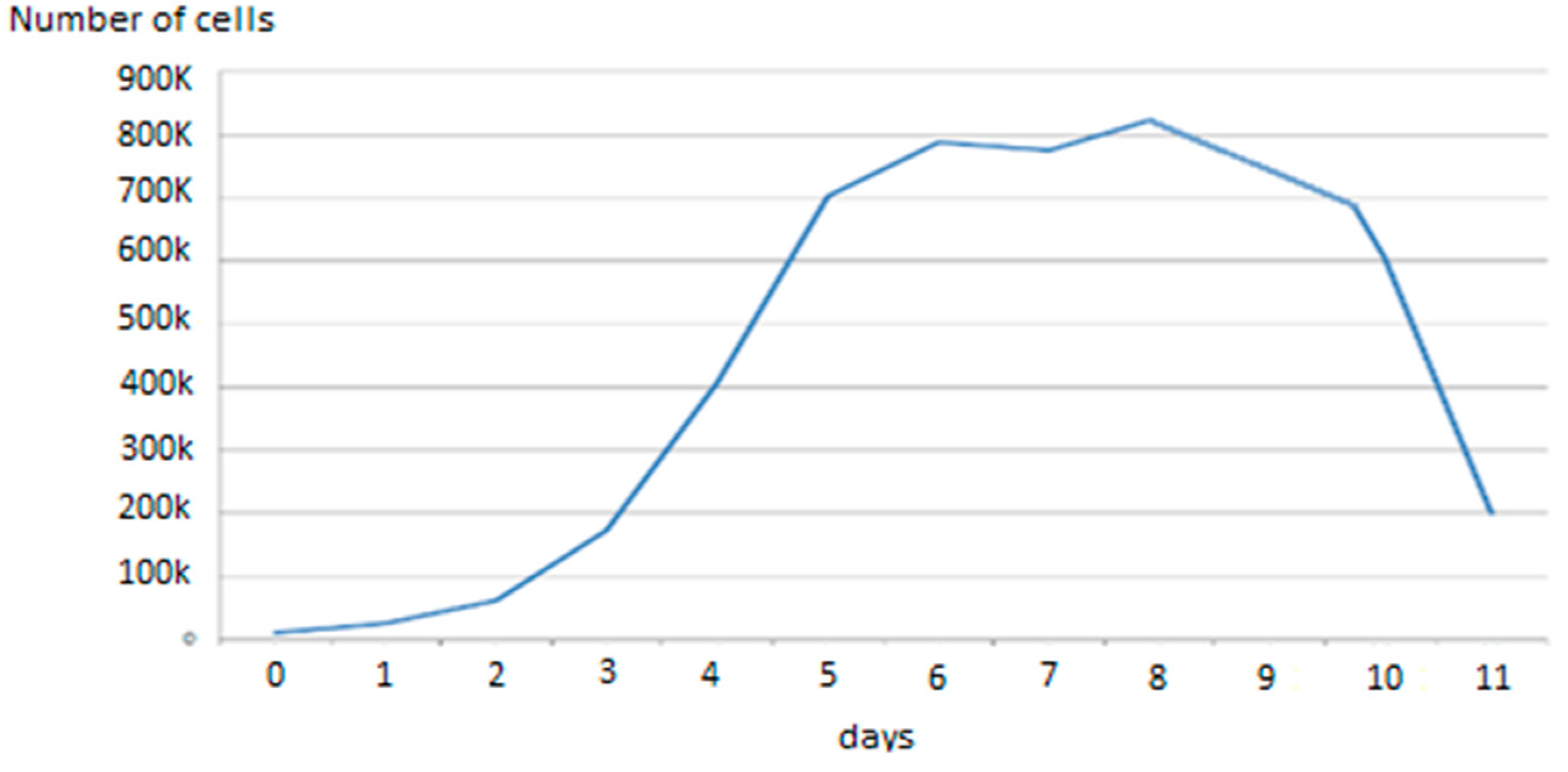
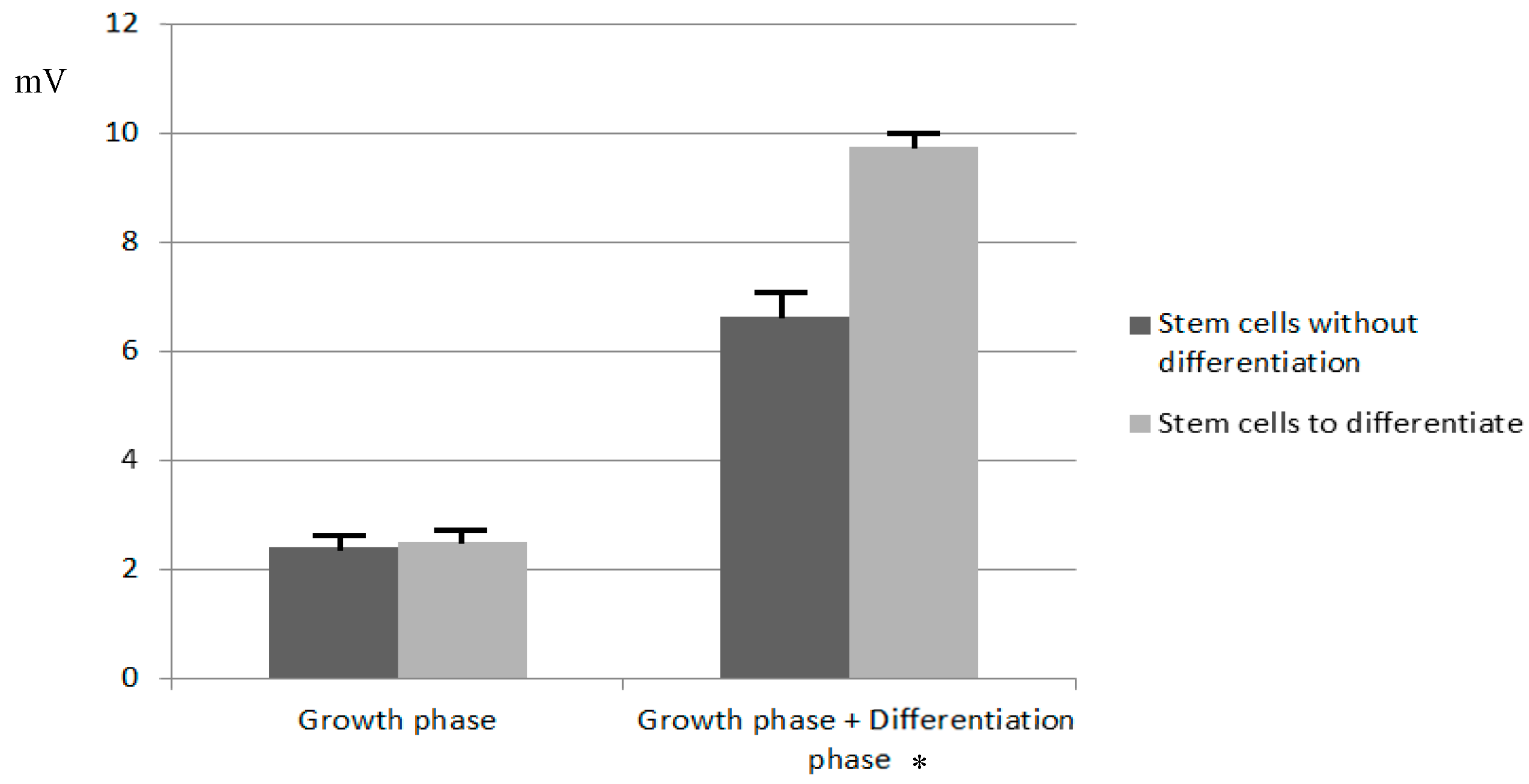
3.1. Study of the Effect of Current on Cell Growth and Differentiation
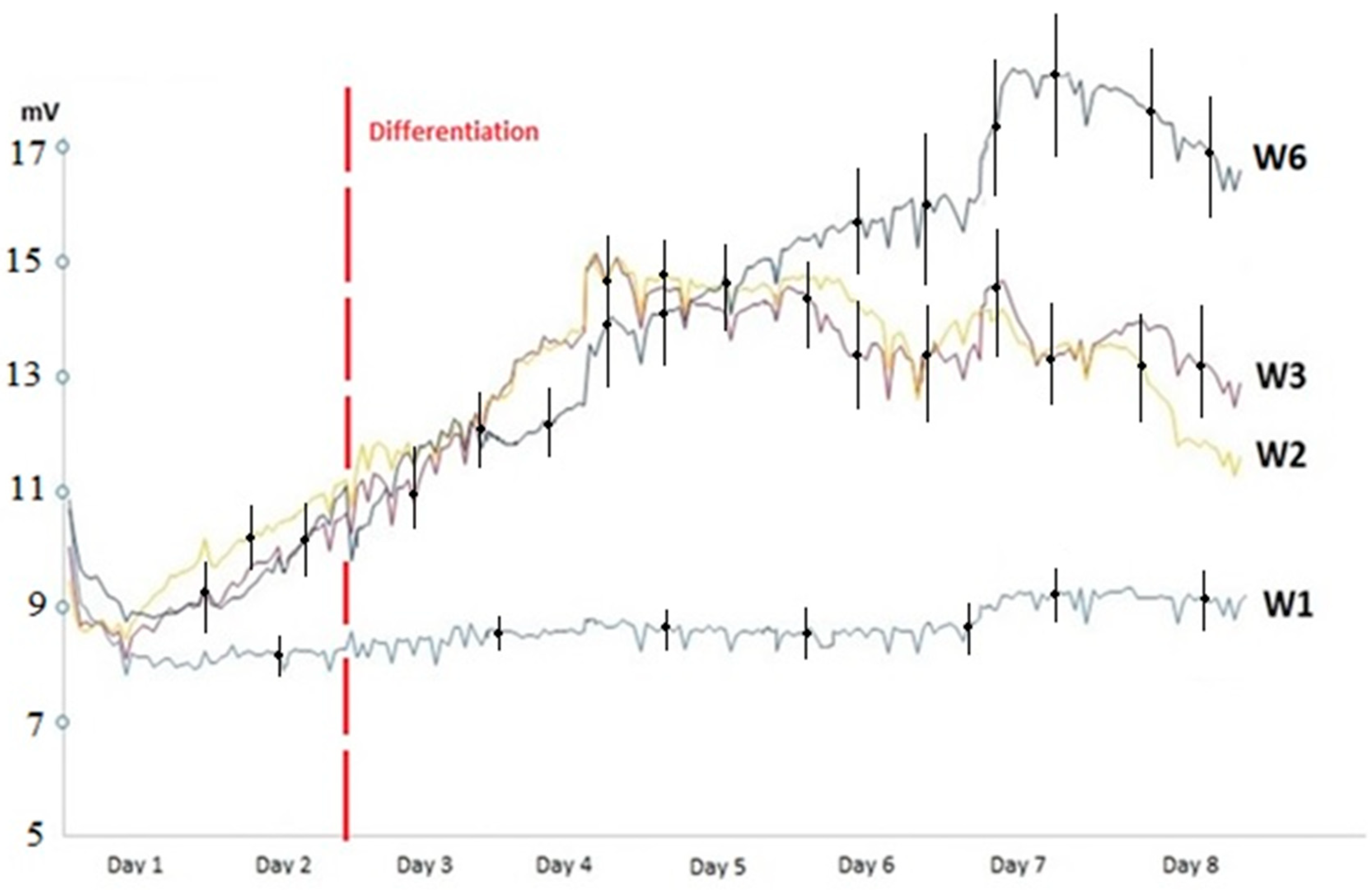
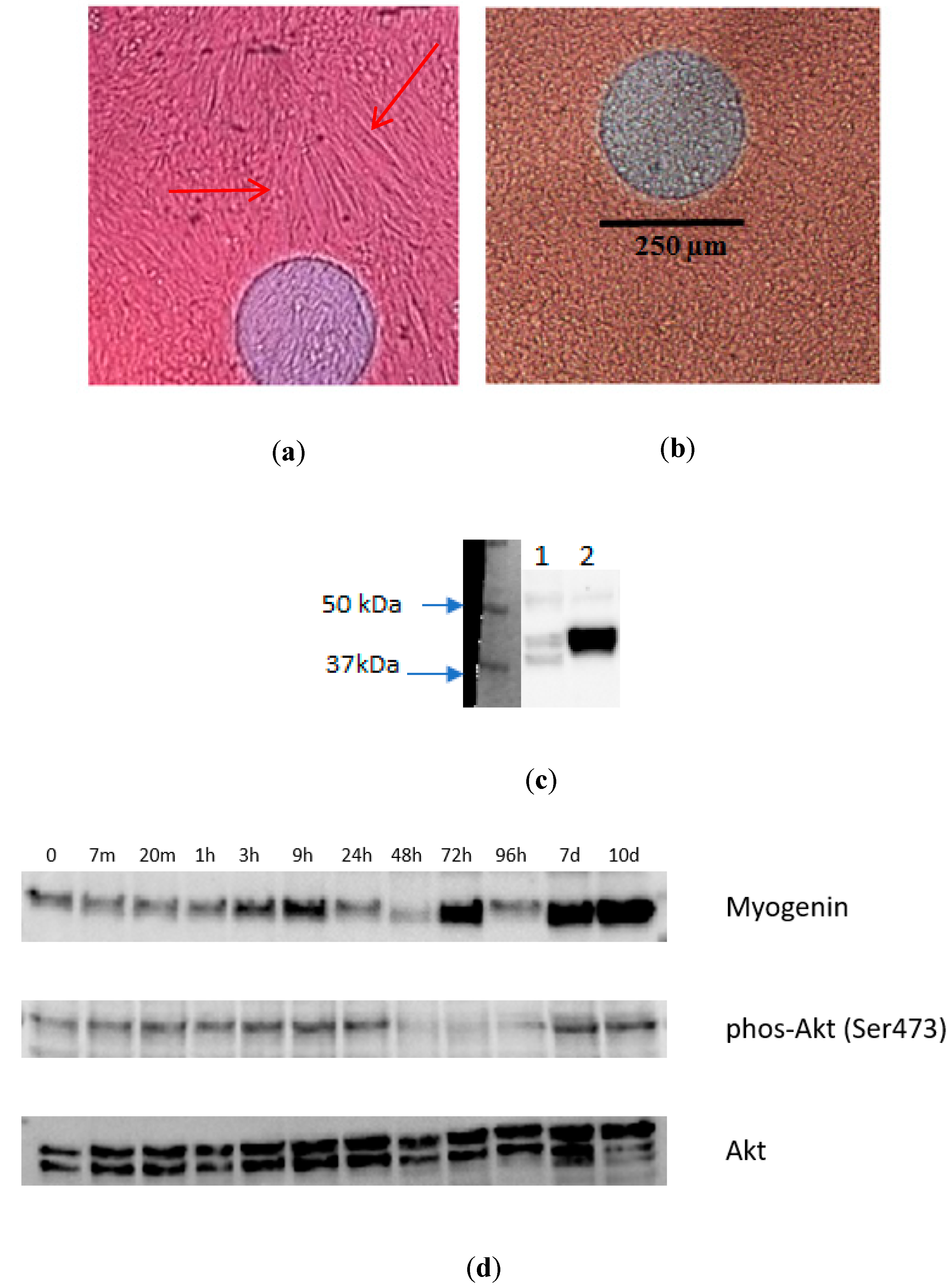
3.2. Monitoring of the Growth and Differentiation of Myoblasts in the 8W10E PET Cultureware
4. Discussion
4.1. Monitoring of the Growth and Differentiation of Myoblasts
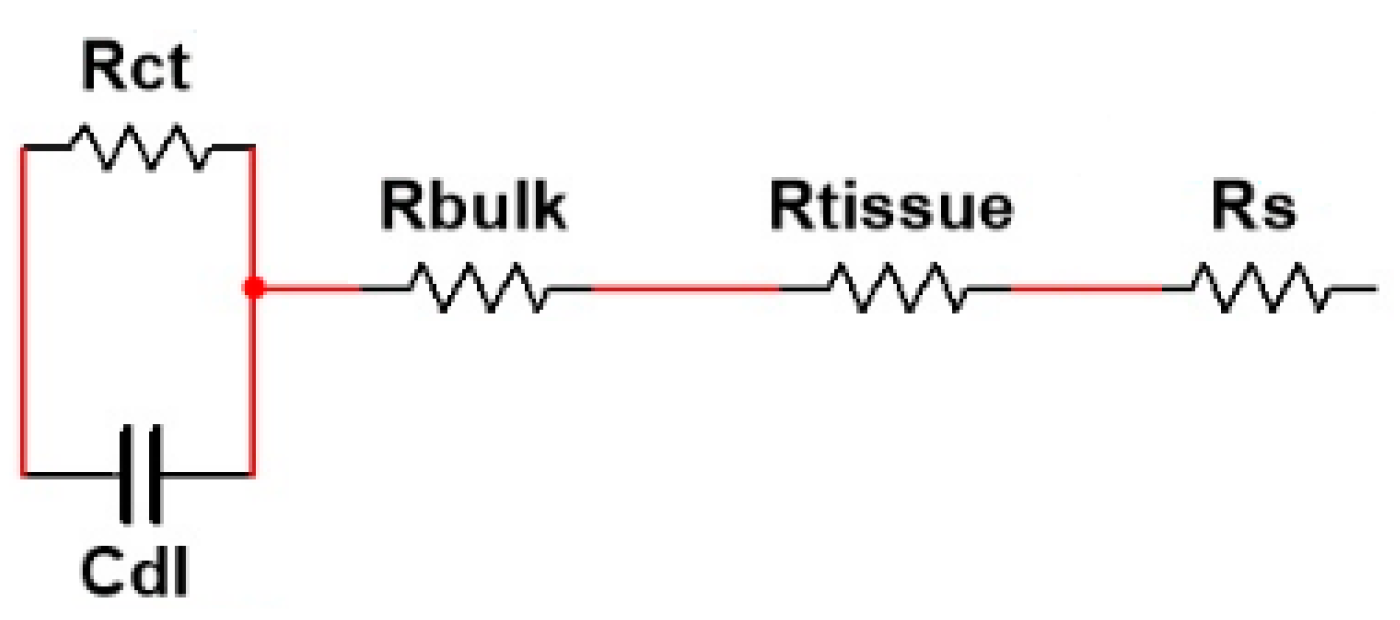
4.2. Electrical Modeling of the Cellular Growth and Differentiation
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Giaever, I.; Keese, C.R. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef]
- Daza Navarro, P.; Olmo Fernández, A.; Cañete, D.; Yufera Garcia, A. Monitoring living cell assays with bio-impedance sensors. Sens. Actuators B Chem. 2013, 176, 605–610. [Google Scholar] [CrossRef]
- Pérez, P.; Maldonado, A.; Yufera, A.; Huertas, G.; Rueda, A. Towards Bio-impedance Based Labs: A Review. J. Electr. Eng. 2016, 4, 116–127. [Google Scholar]
- Onnela, N.; Savolainen, V.; Juuti-Uusitalo, K.; Vaajasaari, H.; Skottman, H.; Hyttinen, J. Electric impedance of human embryonic stem cell-derived retinal pigment epithelium. Med. Biol. Eng. Comput. 2012, 50, 107–116. [Google Scholar] [CrossRef]
- Park, H.E.; Kim, N.; Koh, H.S.; Cho, S.; Sung, J.-S.; Kim, J.Y. Real-time monitoring of neural differentiation of human mesenchymal stem cells by electric cell-substrate impedance sensing. J. Biomed. Biotechnol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Büth, H.; Cho, S.; Impidjati; Thielecke, H. Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J. Biotechnol. 2010, 148, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bagnaninchi, P.; Drummond, N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. 2011. Proc. Natl. Acad. Sci. USA 2011, 108, 6462–6467. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, R.C.; Zhang, J.; Griffith, E.H.; Frank, M.W.; Starly, B.; Loboa, E.G. Electrical Cell-Substrate Impedance Spectroscopy can monitor age-grouped human adipose stem cell variability during osteogenic differentiation. STEM CELLS Transl. Med. 2017, 6, 502–511. [Google Scholar] [CrossRef]
- Liao, H.; Zhou, G.-Q. Development and progress of engineering of skeletal muscle tissue. Tissue Eng. Part B Rev. 2009, 15, 319–331. [Google Scholar] [CrossRef]
- Jun, S.B.; Hynd, M.R.; Smith, K.L.; Song, J.K.; Turner, J.N.; Shain, W.; Kim, S.J. Electrical stimulation-induced cell clustering in cultured neural networks. Med. Boil. Eng. 2007, 45, 1015–1021. [Google Scholar] [CrossRef]
- Cheema, U.; Yang, S.-Y.; Mudera, V.; Goldspink, G.; Brown, R.A. 3-D in vitro model of early skeletal muscle development. Cell Motil. Cytoskelet. 2003, 54, 226–236. [Google Scholar] [CrossRef]
- Lei, K.F.; Wu, M.-H.; Hsu, C.-W.; Chen, Y.-D. Real-time and non-invasive impedimetric monitoring of cell proliferation and chemosensitivity in a perfusion 3D cell culture microfluidic chip. Biosens. Bioelectron. 2014, 51, 16–21. [Google Scholar] [CrossRef]
- Khan, M.A.; Cantù, E.; Tonello, S.; Serpelloni, M.; Lopomo, N.; Sardini, E.A. Review on Biomaterials for 3D Conductive Scaffolds for Stimulating and Monitoring Cellular Activities. Appl. Sci. 2019, 9, 961. [Google Scholar] [CrossRef]
- Whulanza, Y.; Ucciferri, N.; Domenici, C.; Vozzi, G.; Ahluwalia, A. Sensing scaffolds to monitor cellular activity using impedance measurements. Biosens. Bioelectron. 2011, 26, 3303–3308. [Google Scholar] [CrossRef]
- Del Agua, I.; Mantione, D.; Marina, S.; Pitsalidis, C.; Ferro, M.; Sanchez-Sanchez, A.; Owens, R.M.; Malliaras, G.G.; Mecerreyes, D. Conducting Polymer Scaffolds based on PEDOT and Xanthan Gum for live-cell Monitoring. ACS Omega 2018, 3, 7424–7431. [Google Scholar] [CrossRef]
- Tonello, S.; Lopomo, N.; Serpelloni, M.; Serzanti, M.; Dell’Era, P.; Sardini, E. Preliminary Study of Inkjet Printed Sensors for Monitoring Cell Cultures. Procedia Eng. 2016, 168, 578–581. [Google Scholar] [CrossRef]
- Villanueva, P.; Pereira, S.; Olmo, A.; Pérez, P.; Yuste, Y.; Yúfera, A.; De La Portilla, F. Electrical pulse stimulation of skeletal myoblasts cell cultures with simulated action potentials. J. Tissue Eng. Regen. Med. 2019, 13, 1265–1269. [Google Scholar] [CrossRef]
- Huertas, G.; Maldonado, A.; Yúfera, A.; Rueda, A.; Huertas, J.L. The Bio-Oscillator: A Circuit for Cell-Culture Assays. IEEE Trans. Circuits Syst. Part 2 Express Briefs 2015, 62, 164–168. [Google Scholar] [CrossRef]
- Pérez, P.; Maldonado-Jacobi, A.; López-Angulo, A.; Martínez, C.; Olmo, A.; Huertas, G.; Yúfera, A. Remote Sensing of Cell Culture Assays. In Cell Culture; InTech Europe: Rijeka, Croatia, 2017; pp. 135–155. ISBN 978-953-51-4875-3. [Google Scholar]
- Pérez, P.; Huertas, G.; Maldonado-Jacobi, A.; Martín, M.; Serrano, J.A.; Olmo, A.; Daza, P.; Yúfera, A. Sensing Cell-Culture Assays with Low-Cost Circuitry. Sci. Rep. 2018, 8, 8841. [Google Scholar] [CrossRef]
- Applied Biophysics. Available online: http://www.biophysics.com/ (accessed on 1 June 2020).
- Sarker, K.P.; Lee, K.-Y. L6 myoblast differentiation is modulated by Cdk5 via the PI3K–AKT–p70S6K signaling pathway. Oncogene 2004, 23, 6064–6070. [Google Scholar] [CrossRef]
- Serrano, J.A.; Huertas, G.; Maldonado-Jacobi, A.; Olmo, A.; Pérez, P.; Martin, M.E.; Daza, P.; Yúfera, A. An Empirical-Mathematical Approach for Calibration and Fitting Cell-Electrode Electrical Models in Bioimpedance Tests. Sensors 2018, 18, 2354. [Google Scholar] [CrossRef] [PubMed]
- Mercanzini, A.; Bensadoun, J.-C.; Bertsch, A.; Renaud, P.; Colin, P. In Vivo Electrical Impedance Spectroscopy of Tissue Reaction to Microelectrode Arrays. IEEE Trans. Biomed. Eng. 2009, 56, 1909–1918. [Google Scholar] [CrossRef]

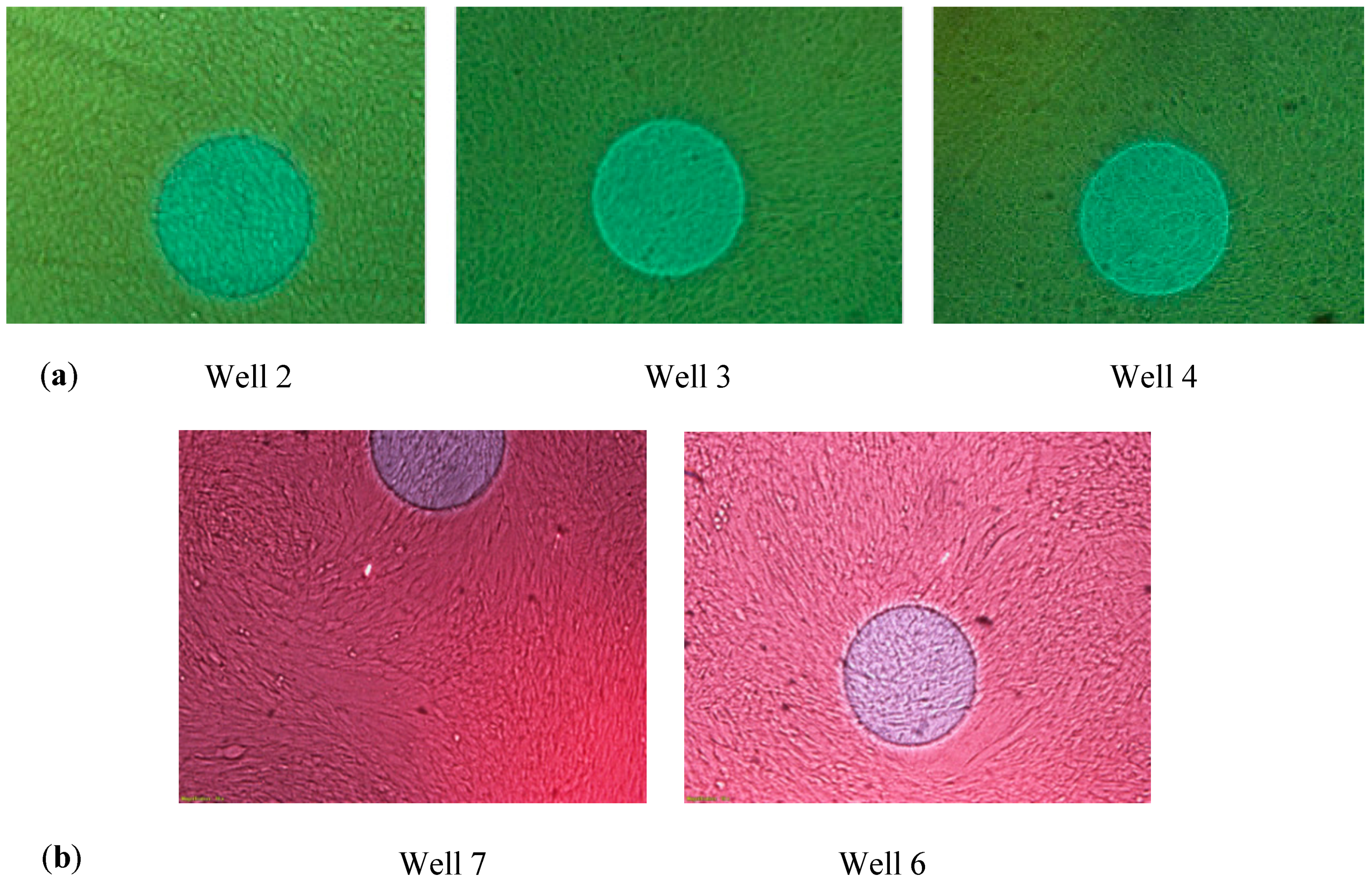
| # Well | Culture | Impedance Measurement |
|---|---|---|
| 1 | Growth medium | Yes |
| 2 | Stem cells without differentiation | Yes |
| 3 | Stem cells without differentiation | Yes |
| 4 | Stem cells without differentiation | No |
| 5 | Differentiation medium | Yes |
| 6 | Stem cells for differentiation | Yes |
| 7 | Stem cells for differentiation | No |
| #Well | W2 | W3 | W6 | W2 | W3 | W6 |
|---|---|---|---|---|---|---|
| # Experiment | 1 | 1 | 1 | 2 | 2 | 2 |
| Differentiation | No | No | Yes | No | No | Yes |
| Rgap [Ω] | 1017.6 | 1009.2 | - | 845.3 | 846 | - |
| Rbulk [Ω] | - | - | 1013.4 | - | - | 845.65 |
| Rtissue [Ω] | - | - | 158.92 | - | - | 114.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmo, A.; Yuste, Y.; Serrano, J.A.; Maldonado-Jacobi, A.; Pérez, P.; Huertas, G.; Pereira, S.; Yufera, A.; de la Portilla, F. Electrical Modeling of the Growth and Differentiation of Skeletal Myoblasts Cell Cultures for Tissue Engineering. Sensors 2020, 20, 3152. https://doi.org/10.3390/s20113152
Olmo A, Yuste Y, Serrano JA, Maldonado-Jacobi A, Pérez P, Huertas G, Pereira S, Yufera A, de la Portilla F. Electrical Modeling of the Growth and Differentiation of Skeletal Myoblasts Cell Cultures for Tissue Engineering. Sensors. 2020; 20(11):3152. https://doi.org/10.3390/s20113152
Chicago/Turabian StyleOlmo, Alberto, Yaiza Yuste, Juan Alfonso Serrano, Andres Maldonado-Jacobi, Pablo Pérez, Gloria Huertas, Sheila Pereira, Alberto Yufera, and Fernando de la Portilla. 2020. "Electrical Modeling of the Growth and Differentiation of Skeletal Myoblasts Cell Cultures for Tissue Engineering" Sensors 20, no. 11: 3152. https://doi.org/10.3390/s20113152
APA StyleOlmo, A., Yuste, Y., Serrano, J. A., Maldonado-Jacobi, A., Pérez, P., Huertas, G., Pereira, S., Yufera, A., & de la Portilla, F. (2020). Electrical Modeling of the Growth and Differentiation of Skeletal Myoblasts Cell Cultures for Tissue Engineering. Sensors, 20(11), 3152. https://doi.org/10.3390/s20113152







