Development of an In Situ Analyzer Based on Sequential Injection Analysis and Liquid Waveguide Capillary Flow Cell for the Determination of Dissolved Reactive Phosphorus in Natural Waters
Abstract
1. Introduction
2. Materials and Methods
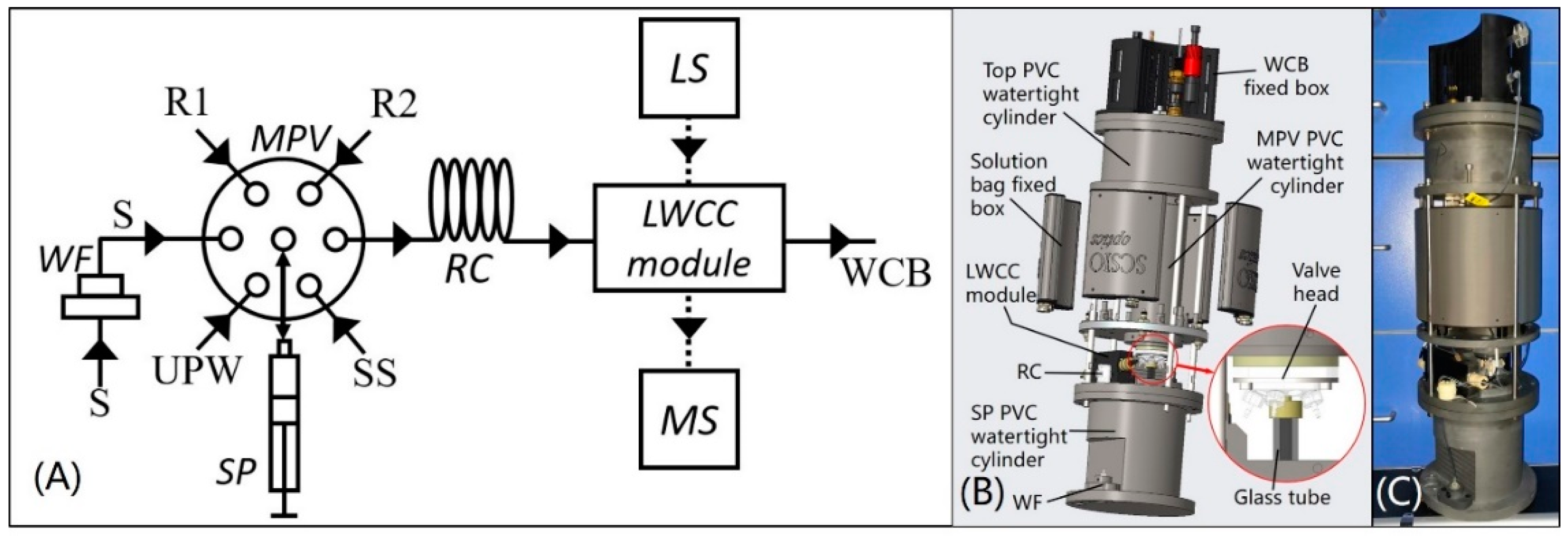
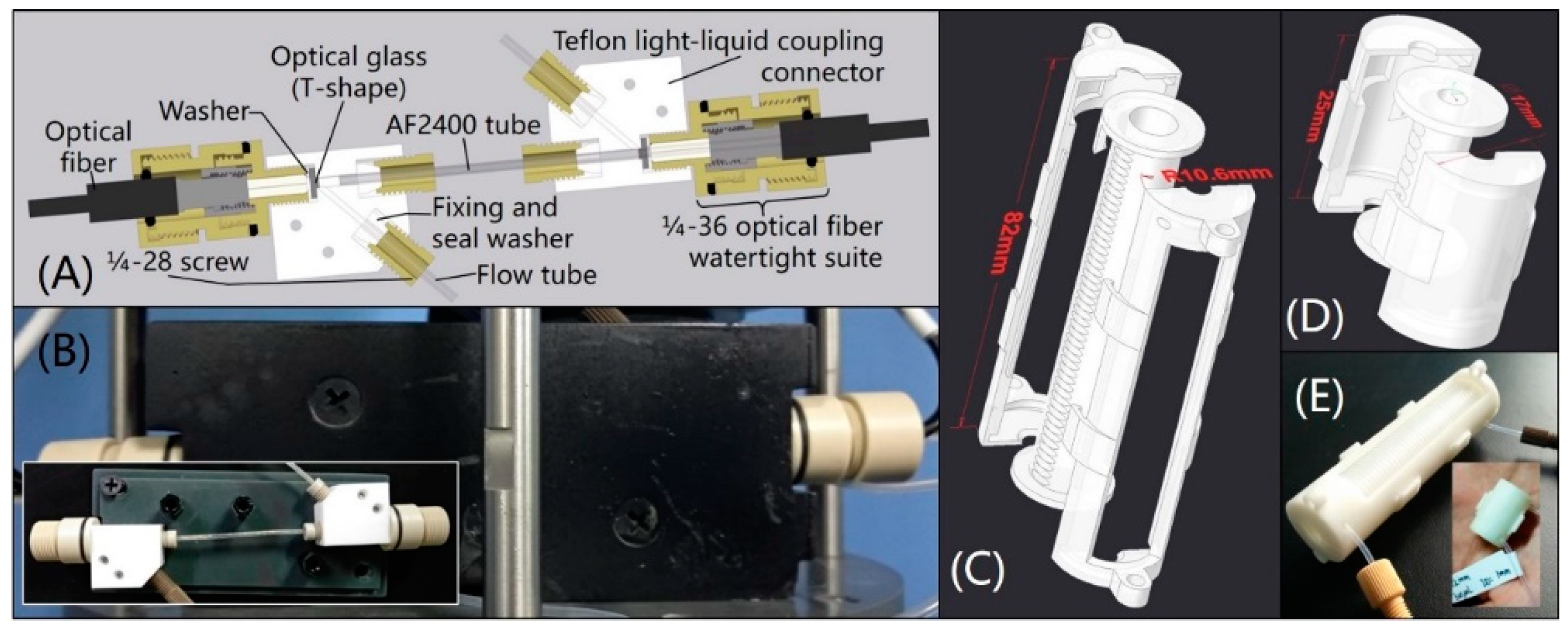
2.1. Apparatus
2.2. Reagents
2.3. Method
3. Results and Discussion
3.1. SIA System Optimization
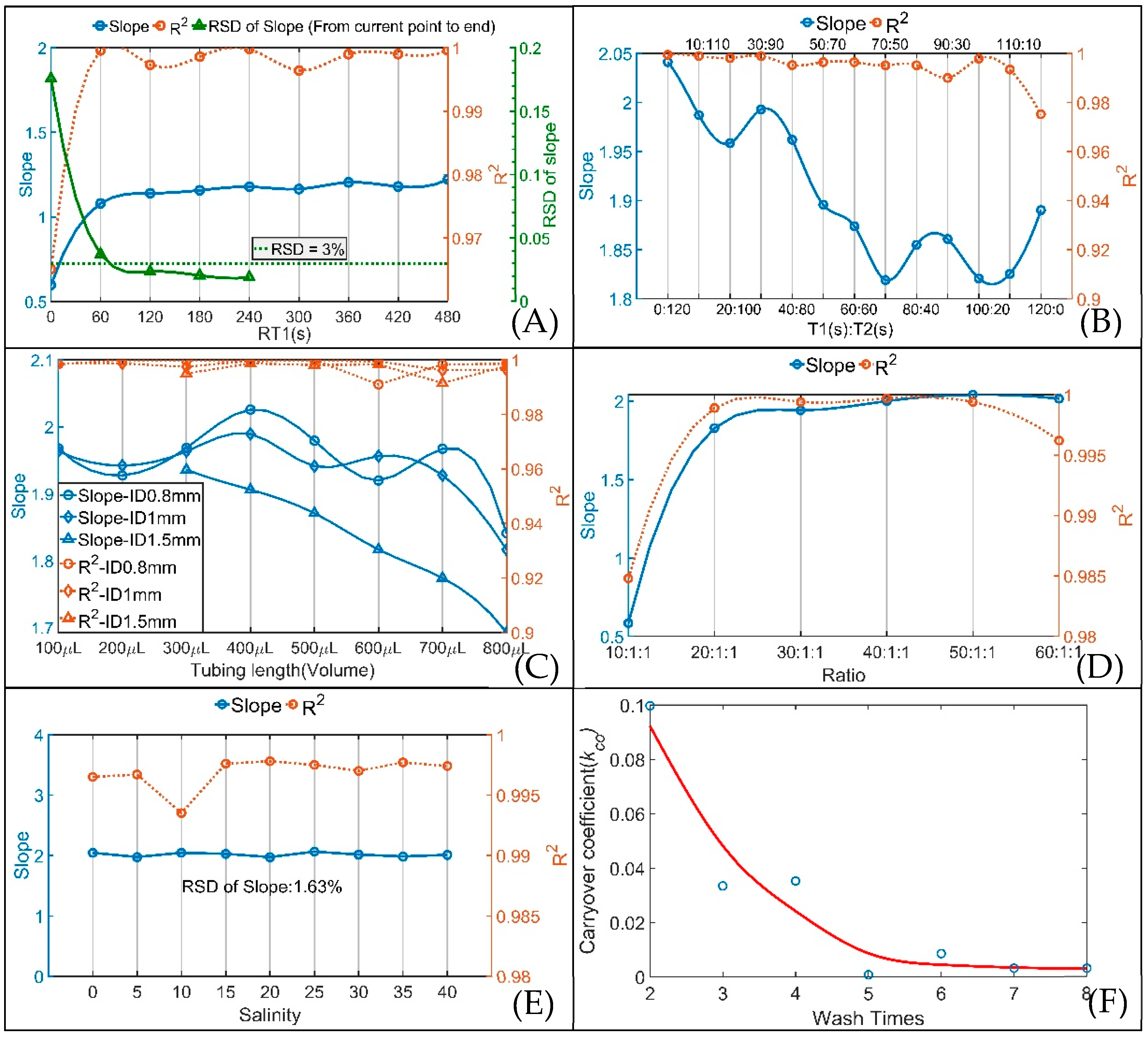
3.1.1. The Effect of SIA System Parameters
3.1.2. The Effect of Sample Salinity
3.1.3. Carryover Effect
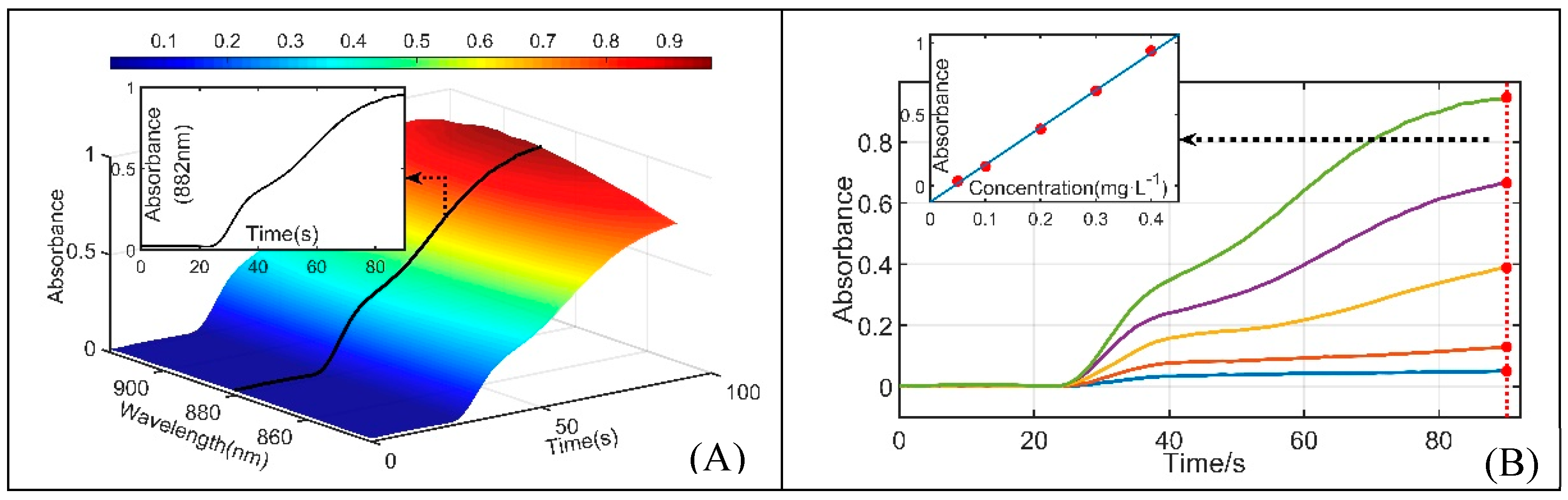
3.2. Performances
3.3. Method Comparison
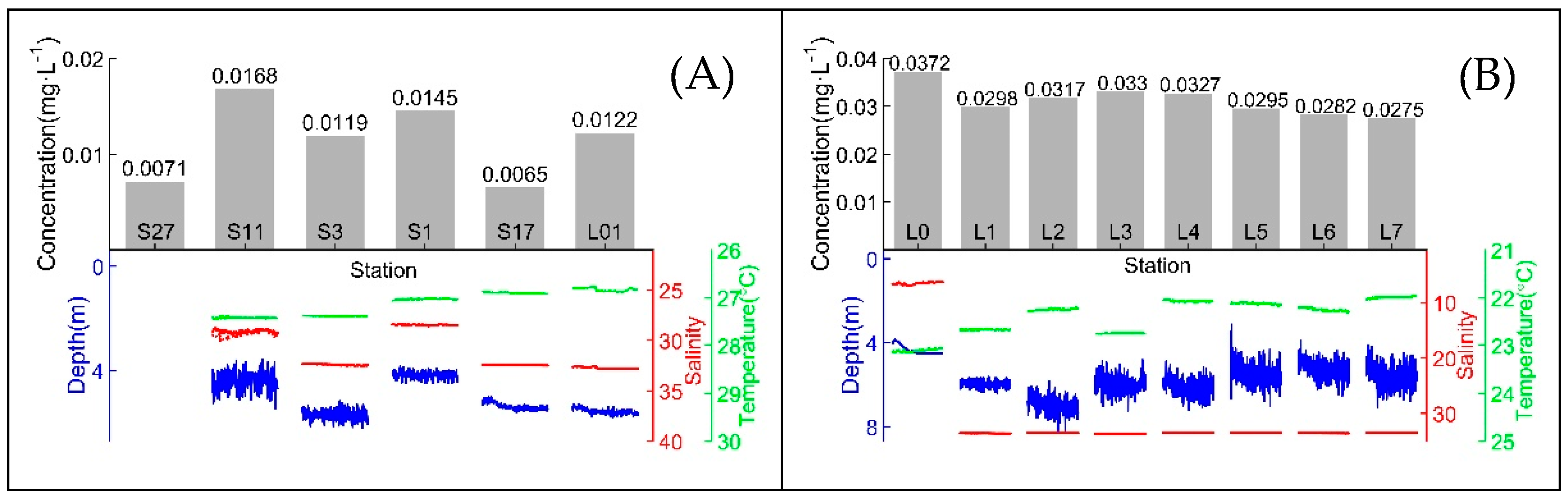
3.4. In situ and Online Measurements and Data Analysis
4. Conclusions
5. Patents
- Liu, S.Y. Detecting analyte in solvent stream for micro-chemical analysis utilising flow through detectors-using light absorption and fluorescence as measure of chemical properties of small amounts of flowing fluid analyte, esp. in conjunction with liq. chromatography and capillary electrophoresis. US5444807-A, 1995-08-22.
- Li, C.; Yang, Z.; Xu, C.; Zhang, Z.; Gou, M.; Lu, G.; Cao, W.; Yang, Y. An in situ analysis device for the detection of nutrients in seawater. ZL201810904083.5, 2019-10-08.
- Li, C.; Yang, Z.; Gou, M.; Xu, C.; Cao, W. A multi-adaptive tube looper for wet chemical measurement. ZL201710764520.3, 2019-10-15.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Desmit, X.; Thieu, V.; Billen, G.; Campuzano, F.; Duliere, V.; Garnier, J.; Lassaletta, L.; Menesguen, A.; Neves, R.; Pinto, L.; et al. Reducing marine eutrophication may require a paradigmatic change. Sci. Total Environ. 2018, 635, 1444–1466. [Google Scholar] [CrossRef] [PubMed]
- Doney, S.C. The Growing Human Footprint on Coastal and Open-Ocean Biogeochemistry. Science 2010, 328, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Borja, A.; Carstensen, J.; Elliott, M.; Krause-Jensen, D.; Marba, N. Paradigms in the Recovery of Estuarine and Coastal Ecosystems. Estuaries Coasts 2015, 38, 1202–1212. [Google Scholar] [CrossRef]
- McCrackin, M.L.; Jones, H.P.; Jones, P.C.; Moreno-Mateos, D. Recovery of lakes and coastal marine ecosystems from eutrophication: A global meta-analysis. Limnol. Oceanogr. 2017, 62, 507–518. [Google Scholar] [CrossRef]
- Reinhard, C.T.; Planavsky, N.J.; Gill, B.C.; Ozaki, K.; Robbins, L.J.; Lyons, T.W.; Fischer, W.W.; Wang, C.J.; Cole, D.B.; Konhauser, K.O. Evolution of the global phosphorus cycle. Nature 2017, 541, 386–389. [Google Scholar] [CrossRef]
- Burson, A.; Stomp, M.; Akil, L.; Brussaard, C.P.D.; Huisman, J. Unbalanced reduction of nutrient loads has created an offshore gradient from phosphorus to nitrogen limitation in the North Sea. Limnol. Oceanogr. 2016, 61, 869–888. [Google Scholar] [CrossRef]
- D’Angelo, C.; Wiedenmann, J. Impacts of nutrient enrichment on coral reefs: New perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 2014, 7, 82–93. [Google Scholar] [CrossRef]
- Adornato, L.R.; Kaltenbacher, E.A.; Greenhow, D.R.; Byrne, R.H. High-resolution in situ analysis of nitrate and phosphate in the oligotrophic ocean. Environ. Sci. Technol. 2007, 41, 4045–4052. [Google Scholar] [CrossRef]
- Khongpet, W.; Pencharee, S.; Puangpila, C.; Hartwell, S.K.; Lapanantnoppakhun, S.; Jakmunee, J. A compact hydrodynamic sequential injection system for consecutive online determination of phosphate and ammonium. Microchem. J. 2019, 147, 403–410. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Lu, G.; Cao, W. A Study of Fast Detection of Nitrite in Seawater Based on Sequential Injection Analysis. Spectrosc. Spectr. Anal. 2019, 39, 589–595. [Google Scholar]
- Nightingale, A.M.; Beaton, A.D.; Mowlem, M.C. Trends in microfluidic systems for in situ chemical analysis of natural waters. Sens. Actuators B Chem. 2015, 221, 1398–1405. [Google Scholar] [CrossRef]
- Jońca, J.; Comtat, M.; Garçon, V. In Situ Phosphate Monitoring in Seawater: Today and Tomorrow. In Smart Sensors for Real-Time Water Quality Monitoring; Mukhopadhyay, S.C., Mason, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–44. [Google Scholar]
- Worsfold, P.J.; Clough, R.; Lohan, M.C.; Monbet, P.; Ellis, P.S.; Quetel, C.R.; Floor, G.H.; McKelvie, I.D. Flow injection analysis as a tool for enhancing oceanographic nutrient measurements—A review. Anal. Chim. Acta 2013, 803, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.; Marshall, G.D. Sequential injection: A new concept for chemical sensors, process analysis and laboratory assays. Anal. Chim. Acta 1990, 237, 329–343. [Google Scholar] [CrossRef]
- Galhardo, C.X.; Masini, J.C. Spectrophotometric determination of phosphate and silicate by sequential injection using molybdenum blue chemistry. Anal. Chim. Acta 2000, 417, 191–200. [Google Scholar] [CrossRef]
- Mesquita, R.B.R.; Ferreira, M.T.S.O.B.; Toth, I.V.; Bordalo, A.A.; McKelvie, I.D.; Rangel, A.O.S.S. Development of a flow method for the determination of phosphate in estuarine and freshwaters-Comparison of flow cells in spectrophotometric sequential injection analysis. Anal. Chim. Acta 2011, 701, 15–22. [Google Scholar] [CrossRef]
- Worsfold, P.; McKelvie, I.; Monbet, P. Determination of phosphorus in natural waters: A historical review. Anal. Chim. Acta 2016, 918, 8–20. [Google Scholar] [CrossRef]
- Clinton-Bailey, G.S.; Grand, M.M.; Beaton, A.D.; Nightingale, A.M.; Owsianka, D.R.; Slavikt, G.J.; Connelly, D.P.; Cardwell, C.L.; Mowlem, M.C. A Lab-on-Chip Analyzer for In Situ Measurement of Soluble Reactive Phosphate: Improved Phosphate Blue Assay and Application to Fluvial Monitoring. Environ. Sci. Technol. 2017, 51, 9989–9995. [Google Scholar] [CrossRef]
- Grand, M.M.; Clinton-Bailey, G.S.; Beaton, A.D.; Schaap, A.M.; Johengen, T.H.; Tamburri, M.N.; Connelly, D.P.; Mowlem, M.C.; Achterberg, E.P. A Lab-On-Chip Phosphate Analyzer for Long-Term In Situ Monitoring at Fixed Observatories: Optimization and Performance Evaluation in Estuarine and Oligotrophic Coastal Waters. Front. Mar. Sci. 2017, 4, 255. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Dallas, T.; Dasgupta, P.K. Light at the end of the tunnel: Recent analytical applications of liquid-core waveguides. TrAC Trends Anal. Chem. 2004, 23, 385–392. [Google Scholar] [CrossRef]
- Pascoa, R.N.; Toth, I.V.; Rangel, A.O. Review on recent applications of the liquid waveguide capillary cell in flow based analysis techniques to enhance the sensitivity of spectroscopic detection methods. Anal. Chim Acta 2012, 739, 1–13. [Google Scholar] [CrossRef]
- Gimbert, L.J.; Worsfold, P.J. Environmental applications of liquid-waveguide-capillary cells coupled with spectroscopic detection. TrAC Trends Anal. Chem. 2007, 26, 914–930. [Google Scholar] [CrossRef]
- Amornthammarong, N.; Zhang, J.Z. Liquid-waveguide spectrophotometric measurement of low silicate in natural waters. Talanta 2009, 79, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, M.; Huang, Y.; Yuan, D.; Zhu, Y. Simultaneous determination of nanomolar nitrite and nitrate in seawater using reverse flow injection analysis coupled with a long path length liquid waveguide capillary cell. Talanta 2013, 117, 456–462. [Google Scholar] [CrossRef]
- Ma, J.; Adornato, L.; Byrne, R.H.; Yuan, D. Determination of nanomolar levels of nutrients in seawater. TrAC Trends Anal. Chem. 2014, 60, 1–15. [Google Scholar] [CrossRef]
- Zimmer, L.A.; Cutter, G.A. High resolution determination of nanomolar concentrations of dissolved reactive phosphate in ocean surface waters using long path liquid waveguide capillary cells (LWCC) and spectrometric detection. Limnol. Oceanogr. Methods 2012, 10, 568–580. [Google Scholar] [CrossRef]
- Lenehan, C.E.; Barnett, N.W.; Lewis, S.W. Sequential injection analysis. Analyst 2002, 127, 997–1020. [Google Scholar] [CrossRef] [PubMed]
- Broughton, P.M.G.; Buttolph, M.A.; Gowenlock, A.H.; Neill, D.W.; Skentelbery, R.G. Recommended scheme for the evaluation of instruments for automatic analysis in the clinical biochemistry laboratory. J. Clin. Pathol. 1969, 22, 278–284. [Google Scholar]
- Zhang, J.Z. Distinction and quantification of carry-over and sample interaction in gas segmented continuous flow analysis. J. Autom. Chem. 1997, 19, 205–212. [Google Scholar] [CrossRef]
- Thiers, R.E.; Meyn, J.; Wildermann, R.F. Use of a Computer Program to Correct for Sample Interaction: A Significant Adjunct to Continuous-Flow Analysis. Clin. Chem. 1970, 16, 832–839. [Google Scholar] [CrossRef]
- Jiang, Q.; He, J.; Wu, J.; He, M.; Bartley, E.; Ye, G.; Christakos, G. Space-Time Characterization and Risk Assessment of Nutrient Pollutant Concentrations in China’s Near Seas. J. Geophys. Res. Ocean. 2019, 124, 4449–4463. [Google Scholar] [CrossRef]
- Liu, H.; Huang, L.; Tan, Y.; Ke, Z.; Liu, J.; Zhao, C.; Wang, J. Seasonal variations of chlorophyll a and primary production and their influencing factors in the Pearl River Estuary. J. Trop. Oceanogr. 2017, 36, 81–91. [Google Scholar]

| Sample | Salinity | Concentration (mg·L−1) | Added (mg·L−1) | Found (mg·L−1) | Recovery (%) |
|---|---|---|---|---|---|
| Standard solution (prepared with pure water) | 0 | 0.05 | / | 0.0532 | 106.4 |
| 0.15 | 0.1463 | 97.5 | |||
| 0.25 | 0.2481 | 99.2 | |||
| 0.35 | 0.3547 | 101.3 | |||
| 0.4 | 0.4036 | 100.9 | |||
| Standard solution (prepared with artificial seawater) | 32 | 0.05 | / | 0.0482 | 96.4 |
| 0.1 | 0.0933 | 93.3 | |||
| 0.2 | 0.1961 | 98.1 | |||
| 0.3 | 0.2933 | 97.8 | |||
| 0.4 | 0.4014 | 100.3 | |||
| River water (Pearl River) | 0 | 0.1464 | 0.1 | 0.2428 | 96.4 |
| 0.2 | 0.3491 | 101.3 | |||
| 0.3 | 0.4593 | 104.3 | |||
| Seawater 1 | 33.7 | 0.0534 | 0.025 | 0.0771 | 94.8 |
| 0.05 | 0.1068 | 106.8 | |||
| 0.1 | 0.1529 | 99.5 | |||
| Seawater 2 | 33.5 | 0.0566 | 0.025 | 0.0805 | 95.6 |
| 0.05 | 0.1088 | 104.4 | |||
| 0.1 | 0.1584 | 101.8 | |||
| Seawater 3 | 33.6 | 0.0547 | 0.025 | 0.0795 | 99.2 |
| 0.05 | 0.1063 | 103.2 | |||
| 0.1 | 0.1554 | 100.7 |
| Cruise | Station | DRP Concentration (mg·L−1) | Salinity | Temperature (°C) |
|---|---|---|---|---|
| Healthy Ocean | S0 | 0.0117 | 2.0 | 26.5 |
| S12 | 0.0247 | 30.33 | 25.8 | |
| S13 | 0.0056 | 30.29 | 25.82 | |
| S14 | 0.0228 | 28.75 | 25.15 | |
| S16 | 0.0172 | 27.53 | 24.92 | |
| S18 | 0.0196 | 30.37 | 25.88 | |
| S25 | 0.0118 | 30.2 | 25.6 | |
| S31 | 0.0217 | 31.4 | 26.4 | |
| Instrument Acceptance | L0 | 0.0278 | 7.28 | 24.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Li, C.; Zhang, Z.; Lu, G.; Cai, Z.; Cao, W. Development of an In Situ Analyzer Based on Sequential Injection Analysis and Liquid Waveguide Capillary Flow Cell for the Determination of Dissolved Reactive Phosphorus in Natural Waters. Sensors 2020, 20, 2967. https://doi.org/10.3390/s20102967
Yang Z, Li C, Zhang Z, Lu G, Cai Z, Cao W. Development of an In Situ Analyzer Based on Sequential Injection Analysis and Liquid Waveguide Capillary Flow Cell for the Determination of Dissolved Reactive Phosphorus in Natural Waters. Sensors. 2020; 20(10):2967. https://doi.org/10.3390/s20102967
Chicago/Turabian StyleYang, Zeming, Cai Li, Zhenzhao Zhang, Guixin Lu, Zifeng Cai, and Wenxi Cao. 2020. "Development of an In Situ Analyzer Based on Sequential Injection Analysis and Liquid Waveguide Capillary Flow Cell for the Determination of Dissolved Reactive Phosphorus in Natural Waters" Sensors 20, no. 10: 2967. https://doi.org/10.3390/s20102967
APA StyleYang, Z., Li, C., Zhang, Z., Lu, G., Cai, Z., & Cao, W. (2020). Development of an In Situ Analyzer Based on Sequential Injection Analysis and Liquid Waveguide Capillary Flow Cell for the Determination of Dissolved Reactive Phosphorus in Natural Waters. Sensors, 20(10), 2967. https://doi.org/10.3390/s20102967





