A Highly Sensitive Amperometric Glutamate Oxidase Microbiosensor Based on a Reduced Graphene Oxide/Prussian Blue Nanocube/Gold Nanoparticle Composite Film-Modified Pt Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
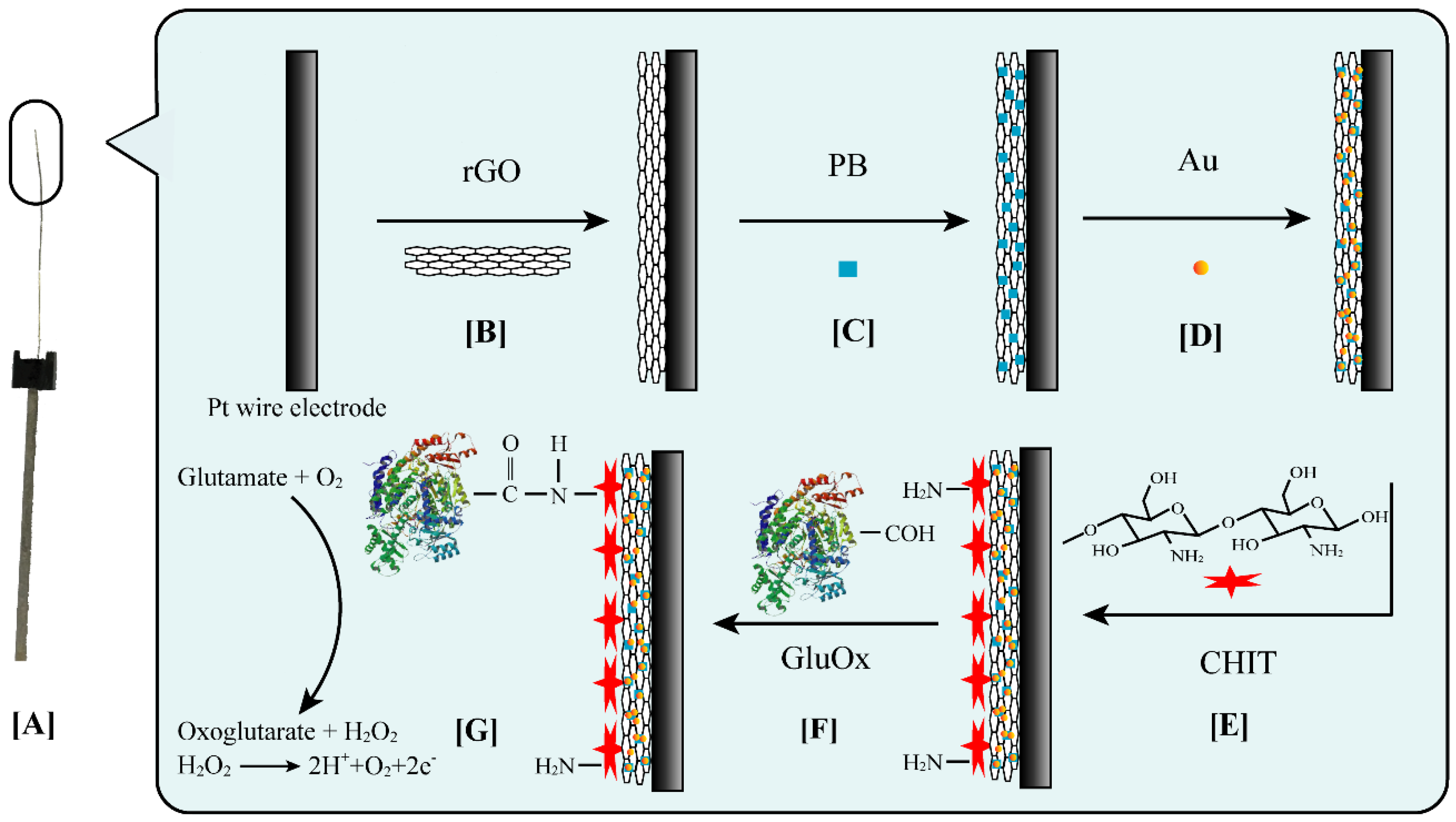
2.3. Fabrication of the Modified Electrode
2.4. Analytical Procedure
3. Results and Discussion
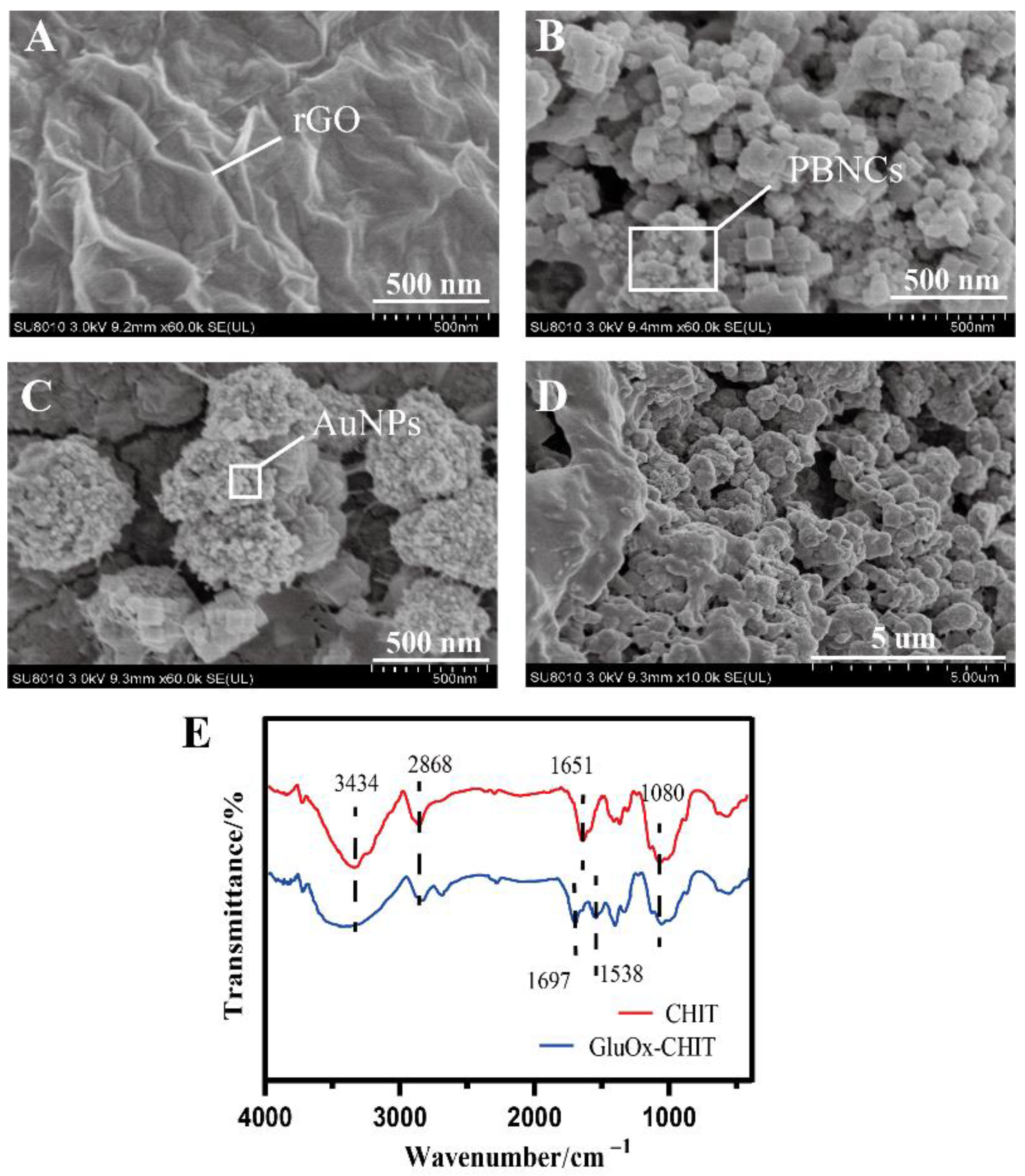
3.1. Morphology and Characterization
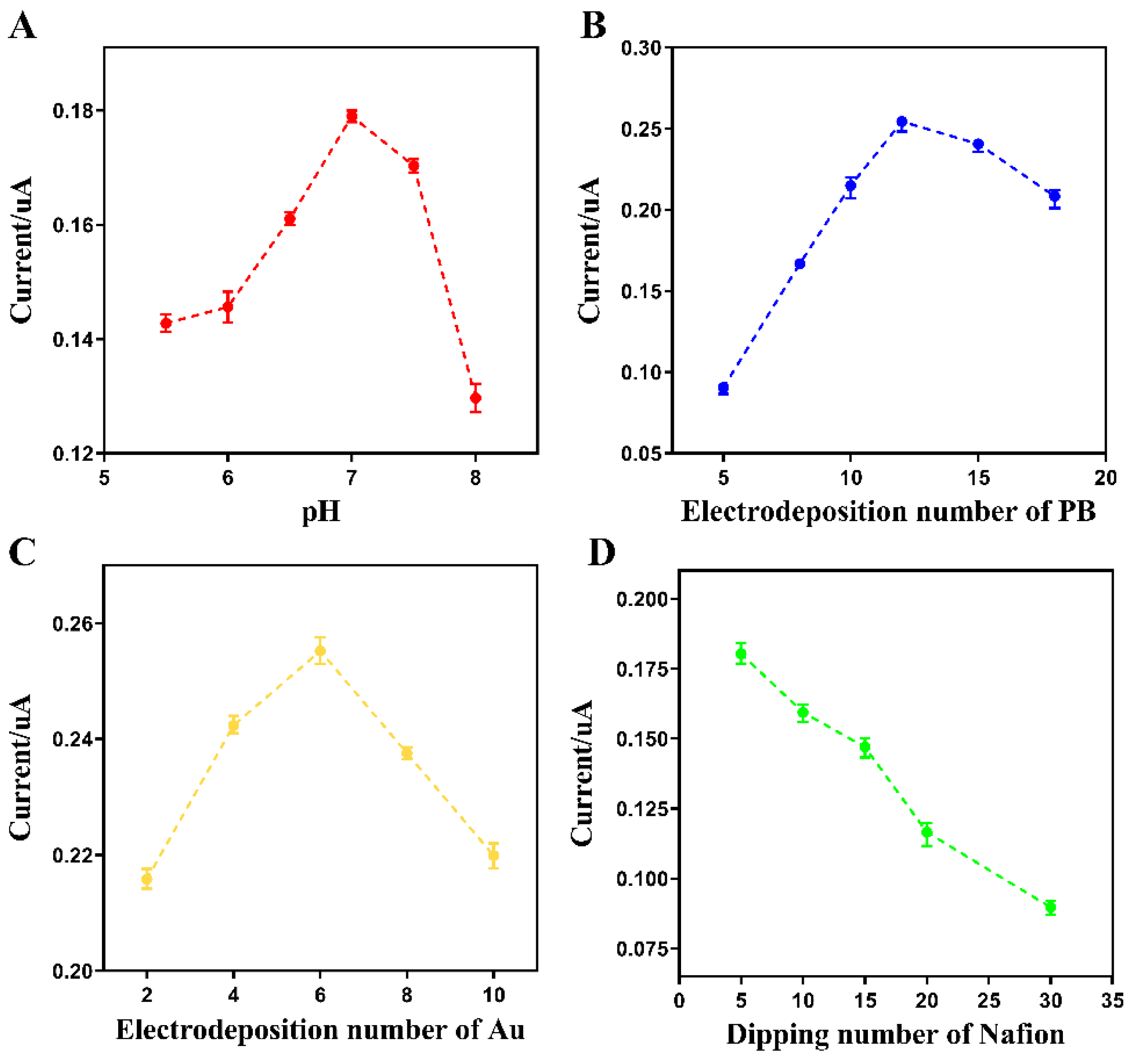
3.2. Optimization of Experimental Parameters
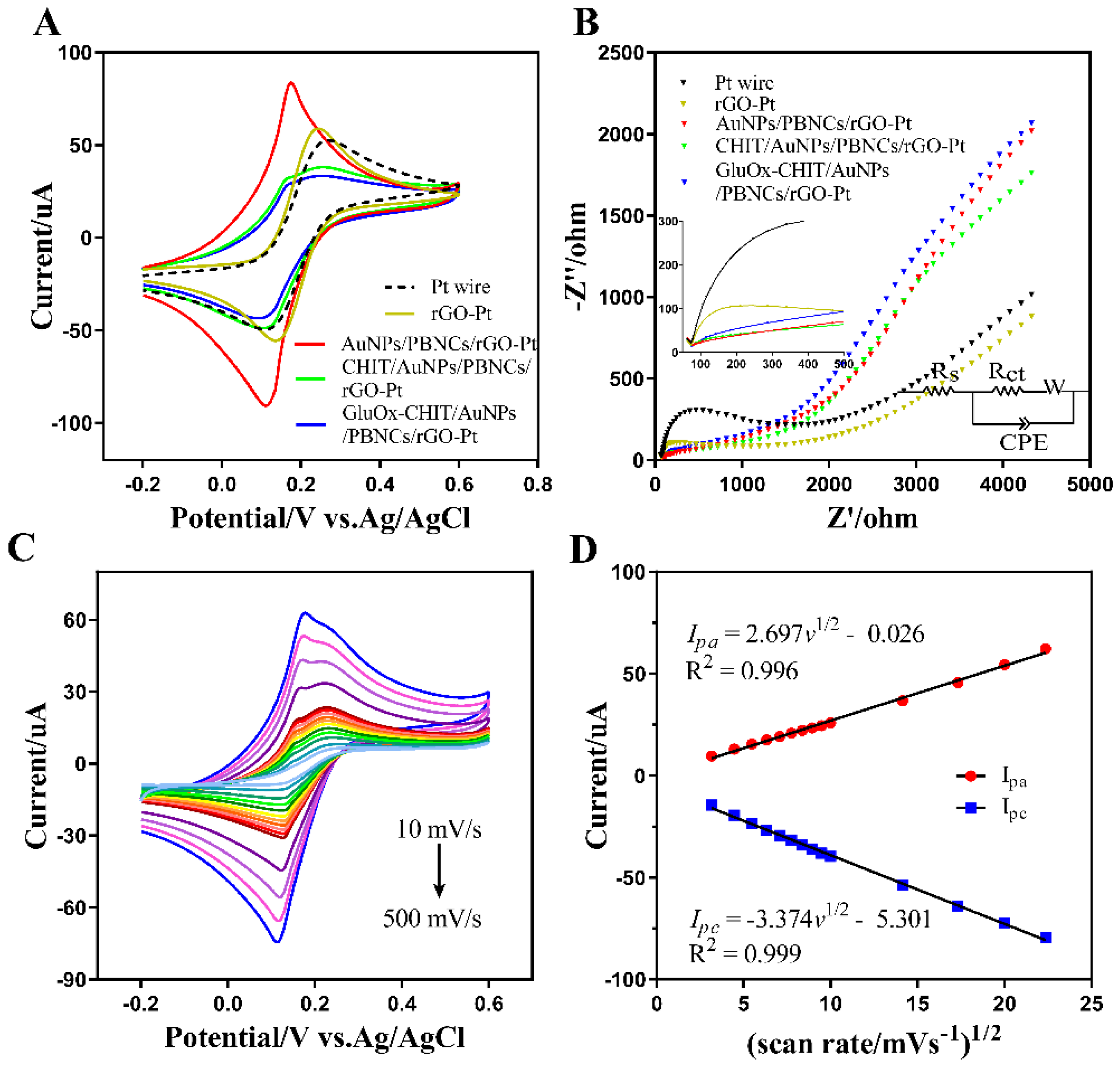
3.3. Electrochemical Activities
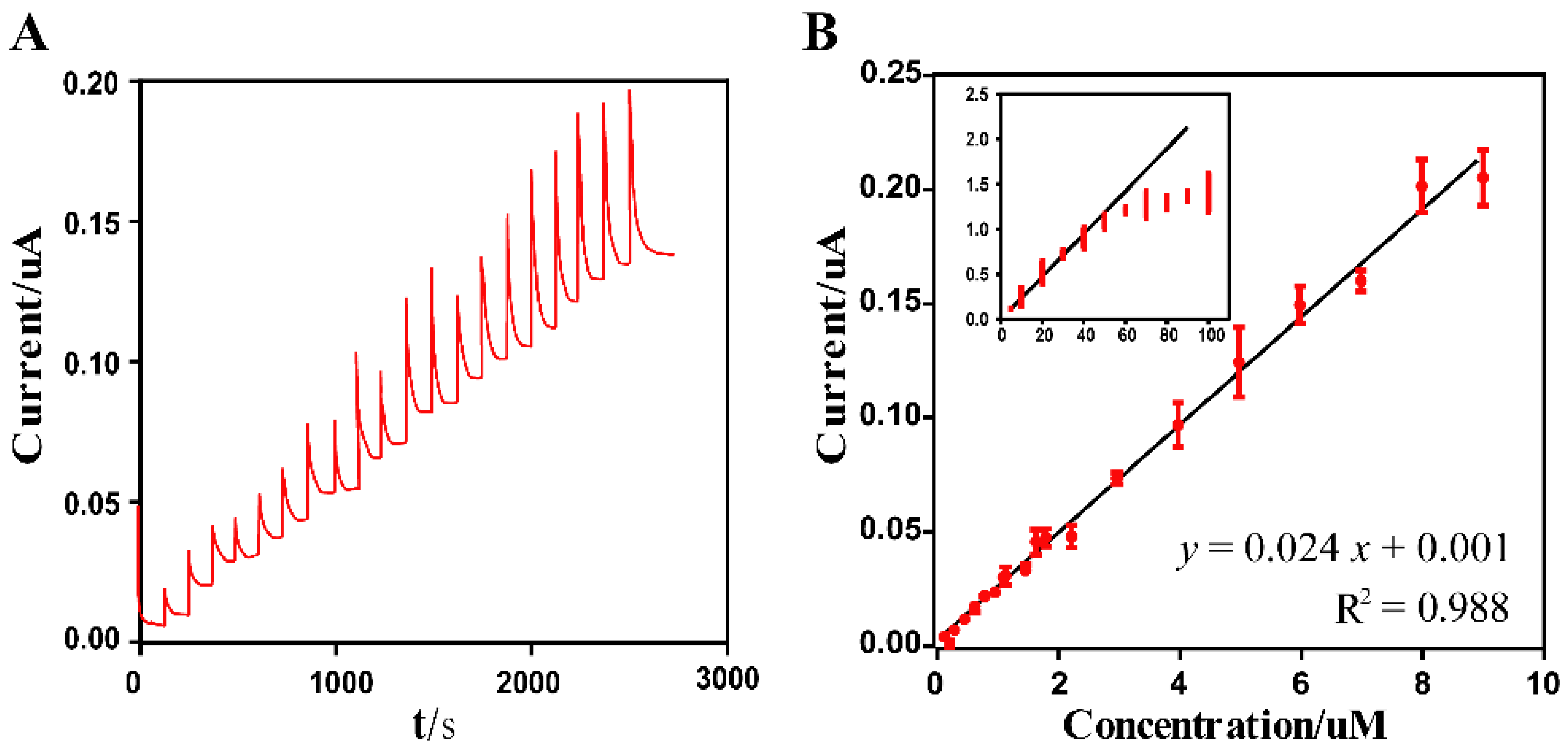
3.4. Electrochemical Response of Glutamate
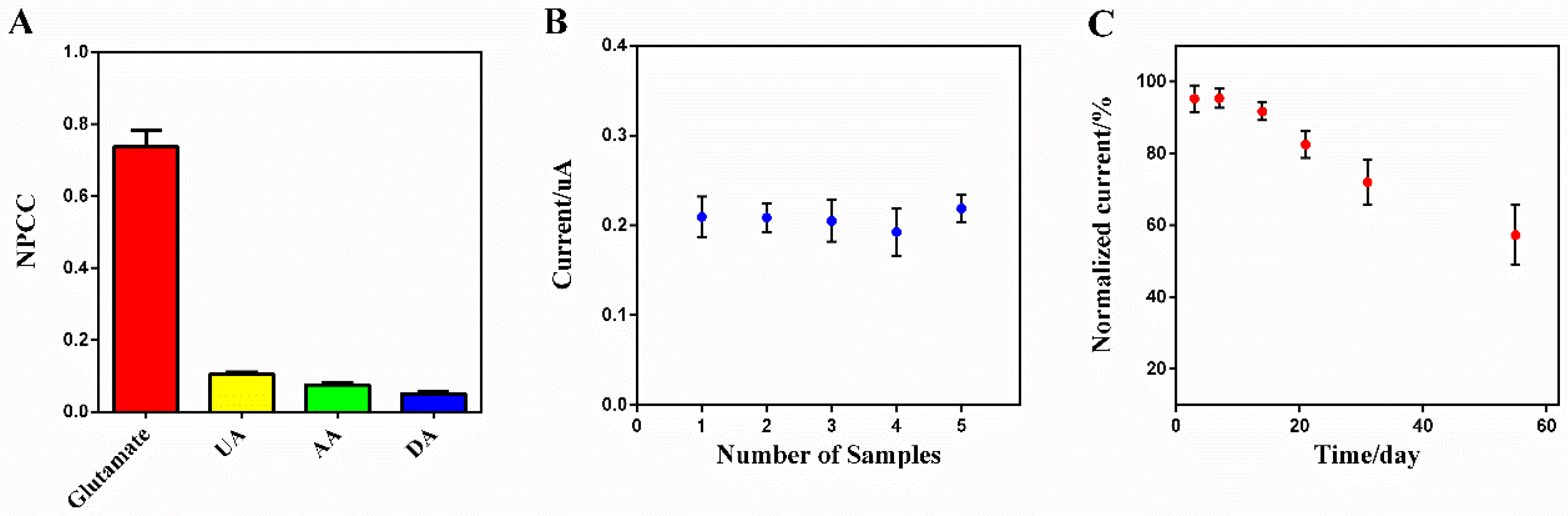
3.5. Specificity, Reproducibility, and Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012, 13, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry 2004, 9, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Waters, N.; Holm-Waters, S.; Tedroff, J.; Nilsson, M.; Carlsson, M.L. Interactions Between Monoamines, Glutamate, and GABA in Schizophrenia: New Evidence. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 237–260. [Google Scholar] [CrossRef] [PubMed]
- DosSantos, M.F.; Ferreira, N.; Toback, R.L.; Carvalho, A.C.; DaSilva, A.F. Potential Mechanisms Supporting the Value of Motor Cortex Stimulation to Treat Chronic Pain Syndromes. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Griffith, H.R.; Okonkwo, O.C.; O’Brien, T.; den Hollander, J.A. Reduced brain glutamate in patients with Parkinson’s disease. NMR Biomed. 2008, 21, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.A.; Sombati, S.; DeLorenzo, R.J. Glutamate Injury—Induced Epileptogenesis in Hippocampal Neurons. Stroke 2001, 32, 2344–2350. [Google Scholar] [CrossRef]
- Paul, I.A.; Skolnick, P. Glutamate and depression: Clinical and preclinical studies. Ann. N. Y. Acad. Sci. 2003, 1003, 250–272. [Google Scholar] [CrossRef]
- Swanson, C.J.; Bures, M.; Johnson, M.P.; Linden, A.-M.; Monn, J.A.; Schoepp, D.D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005, 4, 131–144. [Google Scholar] [CrossRef]
- Galvan, A.; Smith, Y.; Wichmann, T. Continuous monitoring of intracerebral glutamate levels in awake monkeys using microdialysis and enzyme fluorometric detection. J. Neurosci. Methods 2003, 126, 175–185. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, Y.; Xu, L.; Yang, X.; Li, C. Amperometric glutamate biosensor based on self-assembling glutamate dehydrogenase and dendrimer-encapsulated platinum nanoparticles onto carbon nanotubes. Talanta 2007, 73, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; de la Flor, R.; Atkins, A.; Slone-Murphy, J.; Dawson, L.A. Development and application of a liquid chromatography/tandem mass spectrometric assay for measurement of N-acetylaspartate, N-acetylaspartylglutamate and glutamate in brain slice superfusates and tissue extracts. J. Chromatogr. B 2008, 876, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, A.; Mondal, J.; Chandra, R.; Patra, G.K. Exploitation of a simple Schiff base as a ratiometric and colorimetric chemosensor for glutamic acid. Anal. Methods 2015, 7, 8146–8151. [Google Scholar] [CrossRef]
- Okon, S.L.; Ronkainen, N.J. Enzyme-based electrochemical glutamate biosensors. Electrochem. Sens. Technol. 2017, 13. [Google Scholar] [CrossRef]
- Hamdan, S.K. In vivo electrochemical biosensor for brain glutamate detection: A mini review. Malays. J. Med. Sci. MJMS 2014, 21, 12. [Google Scholar] [PubMed]
- Hughes, G.; Pemberton, R.M.; Fielden, P.R.; Hart, J.P. The design, development and application of electrochemical glutamate biosensors. TrAC Trends Anal. Chem. 2016, 79, 106–113. [Google Scholar] [CrossRef]
- Chang, K.-S.; Hsu, W.-L.; Chen, H.-Y.; Chang, C.-K.; Chen, C.-Y. Determination of glutamate pyruvate transaminase activity in clinical specimens using a biosensor composed of immobilized L-glutamate oxidase in a photo-crosslinkable polymer membrane on a palladium-deposited screen-printed carbon electrode. Anal. Chim. Acta 2003, 481, 199–208. [Google Scholar] [CrossRef]
- Kwong, A.W.K.; Gründig, B.; Hu, J.; Renneberg, R. Comparative study of hydrogel-immobilized L-glutamate oxidases for a novel thick-film biosensor and its application in food samples. Biotechnol. Lett. 2000, 22, 267–272. [Google Scholar] [CrossRef]
- Alvarez-Crespo, S.L.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Amperometric glutamate biosensor based on poly(o-phenylenediamine) film electrogenerated onto modified carbon paste electrodes. Biosens. Bioelectron. 1997, 12, 739–747. [Google Scholar] [CrossRef]
- Hughes, G.; Pemberton, R.; Fielden, P.; Hart, J.P. Development of a disposable screen-printed amperometric biosensor based on glutamate dehydrogenase, for the determination of glutamate in clinical and food applications. Anal. Bioanal. Electrochem. 2014, 6, 435–449. [Google Scholar]
- Qin, S.; van der Zeyden, M.; Oldenziel, W.H.; Cremers, T.I.; Westerink, B.H. Microsensors for in vivo Measurement of Glutamate in Brain Tissue. Sensors 2008, 8, 6860–6884. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.K.; Chattopadhyay, P.; Roychudhuri, U.; Chakraborty, R. A biosensor based on co-immobilized L-glutamate oxidase and L-glutamate dehydrogenase for analysis of monosodium glutamate in food. Biosens. Bioelectron. 2006, 21, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Im, H.-S.; Lee, D.-H.; Kim, H.-S.; Choi, Y.-K. Ferrocene functionalized single-walled carbon nanotube bundles. Hybrid interdigitated construction film for L-glutamate detection. J. Phys. Chem. C 2007, 111, 1200–1206. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; O’Neill, R.D.; González-Mora, J.L. Glutamate microbiosensors based on Prussian Blue modified carbon fiber electrodes for neuroscience applications: In-vitro characterization. Sens. Actuators B Chem. 2016, 235, 117–125. [Google Scholar] [CrossRef]
- O’Neill, R.D.; Rocchitta, G.; McMahon, C.P.; Serra, P.A.; Lowry, J.P. Designing sensitive and selective polymer/enzyme composite biosensors for brain monitoring in vivo. TrAC Trends Anal. Chem. 2008, 27, 78–88. [Google Scholar] [CrossRef]
- D’Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69. [Google Scholar] [CrossRef]
- Kirwan, S.M.; Rocchitta, G.; McMahon, C.P.; Craig, J.D.; Killoran, S.J.; O’Brien, K.B.; Serra, P.A.; Lowry, J.P.; O’Neill, R.D. Modifications of poly (o-phenylenediamine) permselective layer on Pt-Ir for biosensor application in neurochemical monitoring. Sensors 2007, 7, 420–437. [Google Scholar] [CrossRef]
- Matos-Peralta, Y.; Antuch, M. Review—Prussian Blue and Its Analogs as Appealing Materials for Electrochemical Sensing and Biosensing. J. Electrochem. Soc. 2019, 167. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Y.; Zhang, B.; Lu, L. In situ Controllable Growth of Prussian Blue Nanocubes on Reduced Graphene Oxide: Facile Synthesis and Their Application as Enhanced Nanoelectrocatalyst for H2O2 Reduction. ACS Appl. Mater. Interfaces 2010, 2, 2339–2346. [Google Scholar] [CrossRef]
- Li, S.-J.; Du, J.-M.; Shi, Y.-F.; Li, W.-J.; Liu, S.-R. Functionalization of graphene with Prussian blue and its application for amperometric sensing of H2O2. J. Solid State Electrochem. 2012, 16, 2235–2241. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors. Biosens. Bioelectron. 2017, 89, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zheng, L.; Bao, S.; Gao, H.; Zhu, F.; Wu, Q. Graphene-induced tiny flowers of organometallic polymers with ultrathin petals for hydrogen peroxide sensing. Carbon 2015, 93, 719–730. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J.; Xiao, S.; Yang, Z.; Pang, P.; Bai, W.; Gao, Y. Facile and controllable synthesis of Prussian blue nanocubes on TiO2–graphene composite nanosheets for nonenzymatic detection of hydrogen peroxide. Anal. Methods 2014, 6, 9761–9768. [Google Scholar] [CrossRef]
- Hu, J.; Wisetsuwannaphum, S.; Foord, J.S. Glutamate biosensors based on diamond and graphene platforms. Faraday Discuss. 2014, 172, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Myoung, N.; Hong, H.-G. Facile and controllable synthesis of Prussian blue on chitosan-functionalized graphene nanosheets for the electrochemical detection of hydrogen peroxide. Electrochim. Acta 2012, 81, 37–43. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Li, X.-R.; Xu, M.-C.; Chen, H.-Y.; Xu, J.-J. Bimetallic Au@ Pt@ Au core–shell nanoparticles on graphene oxide nanosheets for high-performance H2O2 bi-directional sensing. J. Mater. Chem. B 2015, 3, 4355–4362. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of Polyaniline and Polypyrrole Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. Polymers 2019, 11, 377. [Google Scholar] [CrossRef]
- Zotti, G.; Vercelli, B.; Berlin, A. Gold Nanoparticle Linking to Polypyrrole and Polythiophene: Monolayers and Multilayers. Chem. Mater. 2008, 20, 6509–6516. [Google Scholar] [CrossRef]
- Bai, X.; Shiu, K.-K. Investigation of the optimal weight contents of reduced graphene oxide–gold nanoparticles composites and theirs application in electrochemical biosensors. J. Electroanal. Chem. 2014, 720, 84–91. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Dong, H.; Yu, Q.; Zhang, S.; Chen, H. Sensitive detection of dopamine using a platinum microelectrode modified by reduced graphene oxide and gold nanoparticles. J. Electroanal. Chem. 2019, 848, 113244. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G. Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens. Bioelectron. 2005, 21, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Dalkıran, B.; Erden, P.E.; Kılıç, E. Graphene and tricobalt tetraoxide nanoparticles based biosensor for electrochemical glutamate sensing. Artif. Cells Nanomed. Biotechnol. 2016, 45, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Pundir, C.S. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Gregorio-Jauregui, K.M.; Pineda, M.G.; Rivera-Salinas, J.E.; Hurtado, G.; Saade, H.; Martinez, J.L.; Ilyina, A.; López, R.G. One-Step Method for Preparation of Magnetic Nanoparticles Coated with Chitosan. J. Nanomater. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, G.-Y.; Tian, Y. Simultaneous Determination of Glutamate and Calcium Ion in Rat Brain during Spreading Depression and Ischemia Processes. Chin. J. Anal. Chem. 2019, 47, 347–354. [Google Scholar] [CrossRef]
- Clay, M.; Monbouquette, H.G. A Detailed Model of Electroenzymatic Glutamate Biosensors To Aid in Sensor Optimization and in Applications in Vivo. ACS Chem. Neurosci. 2018, 9, 241–251. [Google Scholar] [CrossRef]
- Valiūnienė, A.; Rekertaitė, A.I.; Ramanavičienė, A.; Mikoliūnaitė, L.; Ramanavičius, A. Fast Fourier transformation electrochemical impedance spectroscopy for the investigation of inactivation of glucose biosensor based on graphite electrode modified by Prussian blue, polypyrrole and glucose oxidase. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 165–171. [Google Scholar]
- Wei, W.; Song, Y.; Wang, L.; Zhang, S.; Luo, J.; Xu, S.; Cai, X. An implantable microelectrode array for simultaneous L-glutamate and electrophysiological recordings in vivo. Microsyst. Nanoeng. 2015, 1. [Google Scholar] [CrossRef]
- Barman, S.C.; Hossain, M.F.; Yoon, H.; Park, J.Y. Carboxyl Terminated Reduced Graphene Oxide (Crbxl-RGO) and Pt Nanoparticles Based Ultra-Sensitive and Selective Electrochemical Biosensor for Glutamate Detection. J. Electrochem. Soc. 2018, 165, B296–B301. [Google Scholar] [CrossRef]
- Devi, R.; Gogoi, S.; Barua, S.; Sankar Dutta, H.; Bordoloi, M.; Khan, R. Electrochemical detection of monosodium glutamate in foodstuffs based on Au@MoS2/chitosan modified glassy carbon electrode. Food Chem. 2019, 276, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Jagadale, A.; Zhou, X.; Blaisdell, D.; Yang, S. Carbon nanofibers (CNFs) supported cobalt-nickel sulfide (CoNi 2 S 4) nanoparticles hybrid anode for high performance lithium ion capacitor. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Özel, R.E.; Ispas, C.; Ganesana, M.; Leiter, J.C.; Andreescu, S. Glutamate oxidase biosensor based on mixed ceria and titania nanoparticles for the detection of glutamate in hypoxic environments. Biosens. Bioelectron. 2014, 52, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Xu, J.; Razeeb, K.M. Disposable biosensor based on immobilisation of glutamate oxidase on Pt nanoparticles modified Au nanowire array electrode. Biosens. Bioelectron. 2010, 26, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Gourine, A.V.; Huckstepp, R.T.R.; Dale, N. A microelectrode biosensor for real time monitoring of l-glutamate release. Anal. Chim. Acta 2009, 645, 86–91. [Google Scholar] [CrossRef] [PubMed]
| Biosensor Configuration | Electrode Type | Eap (V) (Ag/AgCl) | Size | LOD (µM) | Linearity (µM) | Storage Stability | Reproducibility | References |
|---|---|---|---|---|---|---|---|---|
| PB; PoPD/ PEI/GluOx | Carbon fiber | 0.05 V | d: 10 µm, l: 250 µm | <2.00 | 0–150 | 30 days: 90% | 4.20% | [24] |
| CeO2/TiO2/AsOx/BSA/GluOx/Chit | Pt wire | 0.60 V | d: 125 µm, l: 2 mm | 0.49 | 0–50 | 10 days: 80% | <5.00% | [54] |
| Crbxl-RGO/PtNPs/Gldh/CHIT | Au plate | 0.57 V (DPV) | (2 × 3.75) mm2 | 0.10 | 4–900 | 7 days: 91% | 5.86% | [50] |
| PtNPs/NAEs | -- | 0.65 V | -- | 14.00 | 0–800 | 14 days: 98% | 6.65% | [55] |
| Gel layer | Pt wire | 0.60 V | d: 50 µm, l: 0.5 mm | 0.05 | 0.5–100 | 150 days: 95% | -- | [56] |
| cMWCNT/AuNPs/CHIT/GluOx | Au | 0.20 V | 0.36 cm2 | 1.60 | 5–500 | 7 days: 97.8% | -- | [44] |
| rGO/PBNCs/AuNPs/CHIT/GluOx | Pt wire | 0.50 V | d: 100 µm l: 6 mm | 0.04 | 0.05–40 | 15 days: 92.14% | 4.44% | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yu, Q.; Fu, W.; Chen, X.; Zhang, Q.; Dong, S.; Chen, H.; Zhang, S. A Highly Sensitive Amperometric Glutamate Oxidase Microbiosensor Based on a Reduced Graphene Oxide/Prussian Blue Nanocube/Gold Nanoparticle Composite Film-Modified Pt Electrode. Sensors 2020, 20, 2924. https://doi.org/10.3390/s20102924
Chen J, Yu Q, Fu W, Chen X, Zhang Q, Dong S, Chen H, Zhang S. A Highly Sensitive Amperometric Glutamate Oxidase Microbiosensor Based on a Reduced Graphene Oxide/Prussian Blue Nanocube/Gold Nanoparticle Composite Film-Modified Pt Electrode. Sensors. 2020; 20(10):2924. https://doi.org/10.3390/s20102924
Chicago/Turabian StyleChen, Jing, Qiwen Yu, Wei Fu, Xing Chen, Quan Zhang, Shurong Dong, Hang Chen, and Shaomin Zhang. 2020. "A Highly Sensitive Amperometric Glutamate Oxidase Microbiosensor Based on a Reduced Graphene Oxide/Prussian Blue Nanocube/Gold Nanoparticle Composite Film-Modified Pt Electrode" Sensors 20, no. 10: 2924. https://doi.org/10.3390/s20102924
APA StyleChen, J., Yu, Q., Fu, W., Chen, X., Zhang, Q., Dong, S., Chen, H., & Zhang, S. (2020). A Highly Sensitive Amperometric Glutamate Oxidase Microbiosensor Based on a Reduced Graphene Oxide/Prussian Blue Nanocube/Gold Nanoparticle Composite Film-Modified Pt Electrode. Sensors, 20(10), 2924. https://doi.org/10.3390/s20102924







