Feasibility of a Sensor-Based Technological Platform in Assessing Gait and Sleep of In-Hospital Stroke and Incomplete Spinal Cord Injury (iSCI) Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
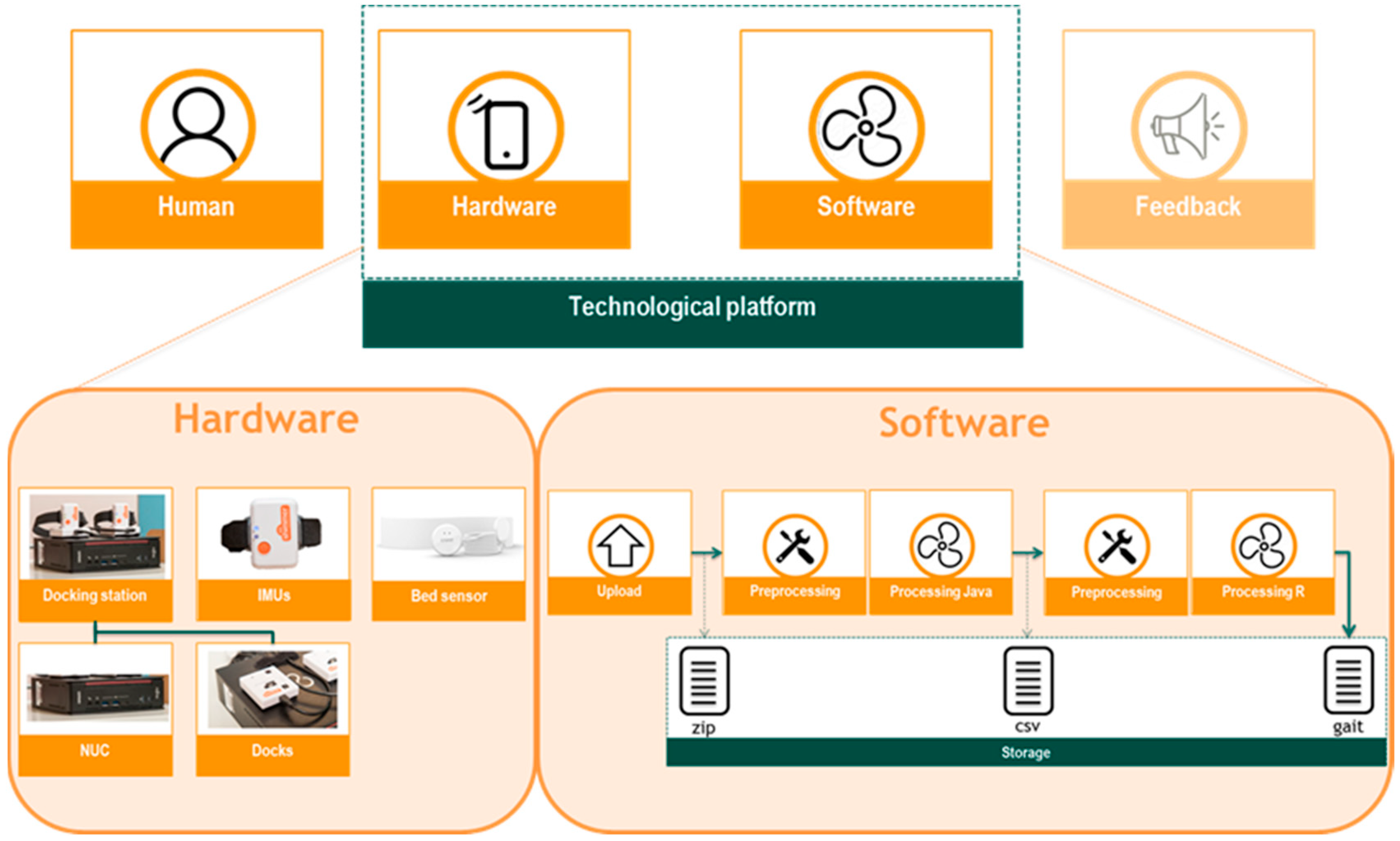
2.3. Technological Platform Architecture
2.3.1. Hardware
2.3.2. Software
Data Upload
Data Pre-processing
Data Processing
Data Storage
2.4. Experimental Protocol
2.5. Analysis and Outcome Measures
3. Results
3.1. Participants
3.2. Measurement Days and Nights
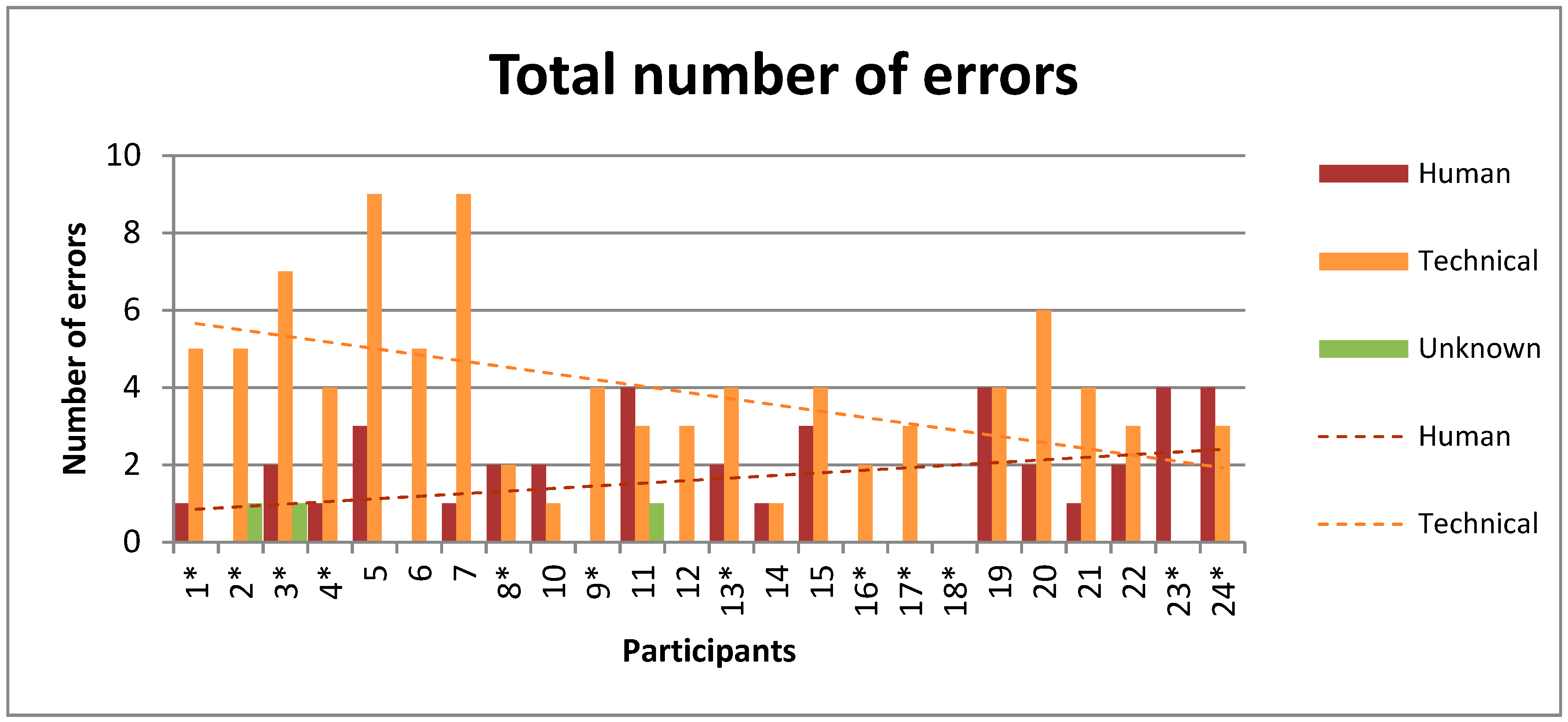
3.3. Error Causes
3.4. User Experience
4. Discussion
4.1. Feasibility
4.2. Error Causes
4.3. User Experience
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frazer, S.W.T.; Hellebrand, W.E.H.; Keijsers, N.W. Variation and achievement of ambulatory activity among pa tients with chronic stroke. J. Rehabil. Med. 2013, 45, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Gebruers, N.; Vanroy, C.; Truijen, S.; Engelborghs, S.; De Deyn, P.P. Monitoring of Physical Activity After Stroke: A Systematic Review of Accelerometry-Based Measures. Arch. Phys. Med. Rehabil. 2010, 91, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Teeter, L.; Gassaway, J.; Taylor, S.; LaBarbera, J.; McDowell, S.; Backus, D.; Zanca, J.M.; Natale, A.; Cabrera, J.; Smout, R.J.; et al. Relationship of physical therapy inpatient rehabilitation interventions and patient characteristics to outcomes following spinal cord injury: The SCIRehab project. J. Spinal Cord Med. 2012, 35, 503–526. [Google Scholar] [CrossRef] [PubMed]
- Kijima, Y.; Kiyama, R.; Sekine, M.; Tamura, T.; Fujimoto, T.; Maeda, T.; Ohshige, T. Estimation of Gait Independence Using a Tri-Axial Accelerometer in Stroke Patients. J. Aging Phys. Act. 2018, 26, 61–67. [Google Scholar] [CrossRef]
- Mansfield, A.; Wong, J.S.; Bryce, J.; Brunton, K.; Inness, E.L.; Knorr, S.; Jones, S.; Taati, B.; McIlroy, W.E. Use of Accelerometer-Based Feedback of Walking Activity for Appraising Progress with Walking-Related Goals in Inpatient Stroke Rehabilitation. Neurorehabil. Neural Repair 2015, 29, 847–857. [Google Scholar] [CrossRef]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; Mackay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef]
- Mahendran, N.; Kuys, S.S.; Brauer, S.G. Recovery of ambulation activity across the first six months post-stroke. Gait Posture 2016, 49, 271–276. [Google Scholar] [CrossRef]
- Scivoletto, G.; Morganti, B.; Molinari, M. Early versus delayed inpatient spinal cord injury rehabilitation: An Italian study. Arch. Phys. Med. Rehabil. 2005, 86, 512–516. [Google Scholar] [CrossRef]
- Sumida, M.; Fujimoto, M.; Tokuhiro, A.; Tominaga, T.; Magara, A.; Uchida, R. Early rehabilitation effect for traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 2001, 82, 391–395. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. A Novel Approach to Ambulatory Monitoring. Neurorehabil. Neural Repair 2011, 25, 6–14. [Google Scholar] [CrossRef]
- Zbogar, D.; Eng, J.J.; Miller, W.C.; Krassioukov, A.V.; Verrier, M.C. Physical activity outside of structured therapy during inpatient spinal cord injury rehabilitation. J. Neuroeng. Rehabil. 2016, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Bakken, L.N.; Lee, K.A.; Kim, H.S.; Finset, A.; Lerdal, A. Sleep-Wake Patterns during the Acute Phase after First-Ever Stroke. Stroke Res. Treat. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bakken, L.N.; Kim, H.S.; Finset, A.; Lerdal, A. Subjective sleep quality in relation to objective sleep estimates: Comparison, gender differences and changes between the acute phase and the six-month follow-up after stroke. J. Adv. Nurs. 2014, 70, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Hall, M.L.; Strollo, P.J.; Kamarck, T.W.; Owens, J.; Lee, L.; Reis, S.E.; Matthews, K.A. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical polysomnographic measures in a community sample. J. Clin. Sleep Med. 2008, 4, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Lester, J.; Choudhury, T.; Borriello, G. A Practical Approach to Recognizing Physical Activities; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–16. [Google Scholar]
- Van Den Berg-Emons, R.J.; L’Ortye, A.A.; Buffart, L.M.; Nieuwenhuijsen, C.; Nooijen, C.F.; Bergen, M.P.; Stam, H.J.; Bussmann, J.B. Validation of the physical activity scale for individuals with physical disabilities. Arch. Phys. Med. Rehabil. 2011, 92, 923–928. [Google Scholar] [CrossRef]
- Klassen, T.D.; Simpson, L.A.; Lim, S.B.; Louie, D.R.; Parappilly, B.; Sakakibara, B.M.; Zbogar, D.; Eng, J.J. “Stepping Up” Activity Poststroke: Ankle-Positioned Accelerometer Can Accurately Record Steps During Slow Walking. Phys. Ther. 2016, 96, 355–360. [Google Scholar] [CrossRef]
- Mansfield, A.; Wong, J.S.; Bayley, M.; Biasin, L.; Brooks, D.; Brunton, K.; Howe, J.-A.; Inness, E.L.; Jones, S.; Lymburner, J.; et al. Using wireless technology in clinical practice: does feedback of daily walking activity improve walking outcomes of individuals receiving rehabilitation post-stroke? Study protocol for a randomized controlled trial. BMC Neurol. 2013, 13, 93. [Google Scholar] [CrossRef]
- Norvang, O.P.; Hokstad, A.; Taraldsen, K.; Tan, X.; Lydersen, S.; Indredavik, B.; Askim, T. Time spent lying, sitting, and upright during hospitalization after stroke: a prospective observation study. BMC Neurol. 2018, 18, 138. [Google Scholar] [CrossRef]
- Capela, N.A.; Lemaire, E.D.; Baddour, N.; Rudolf, M.; Goljar, N.; Burger, H. Evaluation of a smartphone human activity recognition application with able-bodied and stroke participants. J. Neuroeng. Rehabil. 2016, 13, 5. [Google Scholar] [CrossRef]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef]
- Shull, P.B.; Jirattigalachote, W.; Hunt, M.A.; Cutkosky, M.R.; Delp, S.L. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.O.; Matteucci, M.; Cerutti, S.; Bianchi, A.M.; Kortelainen, J.M. Automatic detection of sleep macrostructure based on bed sensors. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2009, 5555–5558. [Google Scholar] [PubMed]
- Roebuck, A.; Monasterio, V.; Gederi, E.; Osipov, M.; Behar, J.; Malhotra, A.; Penzel, T.; Clifford, G.D. A review of signals used in sleep analysis. Physiol. Meas. 2014, 35, R1–R57. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Mora, G.; Elvia, P.; Bianchi, A.M.; Kortelainen, J.; Tenhunen, M.; Himanen, S.L.; Mendez, M.O.; Arce-Santana, E.; Gutierrez-Navarro, O. Sleep-wake detection based on respiratory signal acquired through a Pressure Bed Sensor. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 3452–3455. [Google Scholar]
- Woodward, R.B.; Shefelbine, S.J.; Vaidyanathan, R. Pervasive Monitoring of Motion and Muscle Activation: Inertial and Mechanomyography Fusion. IEEE/ASME Trans. Mechatronics 2017, 22, 2022–2033. [Google Scholar] [CrossRef]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PM R 2018, 10, S220–S232. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef]
- Godfrey, A.; Hetherington, V.; Shum, H.; Bonato, P.; Lovell, N.H.; Stuart, S. From A to Z: Wearable technology explained. Maturitas 2018, 113, 40–47. [Google Scholar] [CrossRef]
- Bonato, P. Advances in wearable technology and applications in physical medicine and rehabilitation. J. Neuroeng. Rehabil. 2005, 2, 2–5. [Google Scholar] [CrossRef]
- Albert, M.V.; Azeze, Y.; Courtois, M.; Jayaraman, A. In-lab versus at-home activity recognition in ambulatory subjects with incomplete spinal cord injury. J. Neuroeng. Rehabil. 2017, 14, 10. [Google Scholar] [CrossRef]
- Cresswell, K.M.; Bates, D.W.; Sheikh, A. Ten key considerations for the successful implementation and adoption of large-scale health information technology. J. Am. Med. Informatics Assoc. 2013, 20, 9–13. [Google Scholar] [CrossRef]
- Paré, G.; Sicotte, C.; Jaana, M.; Girouard, D. Prioritizing the risk factors influencing the success of clinical information system projects: A Delphi study in Canada. Methods Inf. Med. 2008, 47, 251–259. [Google Scholar] [PubMed]
- Johansson, D.; Malmgren, K.; Alt Murphy, M. Wearable sensors for clinical applications in epilepsy, Parkinson’s disease, and stroke: a mixed-methods systematic review. J. Neurol. 2018, 265, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications in Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-baker, L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Weeks, D.L.; Sprint, G.L.; Stilwill, V.; Meisen-Vehrs, A.L.; Cook, D.J. Implementing Wearable Sensors for Continuous Assessment of Daytime Heart Rate Response in Inpatient Rehabilitation. Telemed. e-Health 2018, 24, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Weenk, M.; van Goor, H.; Frietman, B.; Engelen, L.J.; van Laarhoven, C.J.; Smit, J.; Bredie, S.J.; van de Belt, T.H. Continuous Monitoring of Vital Signs Using Wearable Devices on the General Ward: Pilot Study. JMIR mHealth uHealth 2017, 5, e91. [Google Scholar] [CrossRef]
- Kleiman, E.; Millner, A.J.; Joyce, V.W.; Nash, C.C.; Buonopane, R.J.; Nock, M.K. Using wearable physiological monitors with suicidal adolescent inpatients: Feasibility and acceptability study. J. Med. Internet Res. 2019, 7, e13725. [Google Scholar]
- Kroll, R.R.; McKenzie, E.D.; Boyd, J.G.; Sheth, P.; Howes, D.; Wood, M.; Maslove, D.M. Use of wearable devices for post-discharge monitoring of ICU patients: A feasibility study. J. Intensive Care 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Levitt, D.L.; Spanakis, E.K.; Ryan, K.A.; Silver, K.D. Insulin Pump and Continuous Glucose Monitor Initiation in Hospitalized Patients with Type 2 Diabetes Mellitus. Diabetes Technol. Ther. 2018, 20, 32–38. [Google Scholar] [CrossRef]
- Cameron, F.M.; Ly, T.T.; Buckingham, B.A.; Maahs, D.M.; Forlenza, G.P.; Levy, C.J.; Lam, D.; Clinton, P.; Messer, L.H.; Westfall, E.; et al. Closed-Loop Control Without Meal Announcement in Type 1 Diabetes. Diabetes Technol. Ther. 2017, 19, 527–532. [Google Scholar] [CrossRef]
- Ishikawa, S.; Stevens, S.L.; Ka ng, M.; Morgan, D.W. Reliability of daily step activity monitoring in adults with incomplete spinal cord injury. J. Rehabil. Res. Dev. 2011, 48, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Mudge, S.; Stott, N.S. Test—retest reliability of the StepWatch Activity Monitor outputs in individuals with chronic stroke. Clin. Rehabil. 2008, 22, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.A.; Pal, J.; Becker, I. Measuring Free-Living Physical Activity in Adults with and Without Neurologic Dysfunction With a Triaxial Accelerometer. Arch. Phys. Med. Rehabil. 2008, 89, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Ainsworth, B.E.; Thompson, R.W.; Bassett, D.R. Sources of variance in daily physical activity levels as measured by an accelerometer. Med. Sci. Sport. Exerc. 2002, 34, 1376–1381. [Google Scholar] [CrossRef]
- Beniczky, S.; Polster, T.; Kjaer, T.W.; Hjalgrim, H. Detection of generalized tonic-clonic seizures by a wireless wrist accelerometer: A prospective, multicenter study. Epilepsia 2013, 54, e58–e61. [Google Scholar] [CrossRef]
- Bayly, J.; Carino, J.; Petrovski, S.; Smit, M.; Fernando, D.A.; Vinton, A.; Yan, B.; Gubbi, J.R.; Palaniswami, M.S.; O’Brien, T.J. Time-frequency mapping of the rhythmic limb movements distinguishes convulsive epileptic from psychogenic nonepileptic seizures. Epilepsia 2013, 54, 1402–1408. [Google Scholar] [CrossRef]
- Weiss, A.; Herman, T.; Giladi, N.; Hausdorff, J.M. Objective Assessment of Fall Risk in Parkinson’s Disease Using a Body-Fixed Sensor Worn for 3 Days. PLoS One 2014, 9, e96675. [Google Scholar] [CrossRef]
- Askim, T.; Bernhardt, J.; Churilov, L.; Fredriksen, K.; Indredavik, B. Changes in physical activity and related functional and disability levels in the first six months after stroke: A longitudinal follow-up study. J. Rehabil. Med. 2013, 45, 423–428. [Google Scholar] [CrossRef]
- Uswatte, G.; Giuliani, C.; Winstein, C.; Zeringue, A.; Hobbs, L.; Wolf, S.L. Validity of Accelerometry for Monitoring Real-World Arm Activity in Patients With Subacute Stroke: Evidence From the Extremity Constraint-Induced Therapy Evaluation Trial. Arch. Phys. Med. Rehabil. 2006, 87, 1340–1345. [Google Scholar] [CrossRef]
- Velez, M.; Fisher, R.S.; Bartlett, V.; Le, S. Tracking generalized tonic-clonic seizures with a wrist accelerometer linked to an online database. Seizure 2016, 39, 13–18. [Google Scholar] [CrossRef]
- Sanchez, M.; Bussmann, J.; Janssen, W.; Horemans, H.; Chastin, S.; Heijenbrok, M.; Stam, H. Accelerometric assessment of different dimensions of natural walking during the first year after stroke: Recovery of amount, distribution, quality and speed of walking. J. Rehabil. Med. 2015, 47, 714–721. [Google Scholar] [CrossRef]
- Gebruers, N.; Truijen, S.; Engelborghs, S.; Nagels, G.; Brouns, R.; De Deyn, P.P. Actigraphic Measurement of Motor Deficits in Acute Ischemic Stroke. Cerebrovasc. Dis. 2008, 26, 533–540. [Google Scholar] [CrossRef]
- Urbin, M.A.; Waddell, K.J.; Lang, C.E. Acceleration Metrics Are Responsive to Change in Upper Extremity Function of Stroke Survivors. Arch. Phys. Med. Rehabil. 2015, 96, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.R.; Hammond, F.; Hart, T.; Bickett, A.K.; Temkin, N.R.; Dikmen, S. Participant recruitment and retention in rehabilitation research. Am. J. Phys. Med. Rehabil. 2008, 87, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable sensors for remote health monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Schniepp, R.; Möhwald, K.; Wuehr, M. Clinical and automated gait analysis in patients with vestibular, cerebellar, and functional gait disorders: perspectives and limitations. J. Neurol. 2019, 266, 118–122. [Google Scholar] [CrossRef]

| Expected | Automatically Obtained | After Reprocessing | |||
|---|---|---|---|---|---|
| Upload | (pre)Processed | Lost | Lost | ||
| Days | 147 (100%) | 114/147 (78%) | 67/147 (46%) | 80/147 (54%) | 21/147 (14%) |
| Nights | 130 (100%) | 125/130 (96%) | n.a. | 5/130 (4%) | n.a. |
| Target Component | Error Cause | # Reported | # Lost (Root Cause) |
|---|---|---|---|
| Human/Hardware | Data not transmitted from dock to NUC | 11 | 1 |
| Hardware | Loss of Wi-Fi connectivity | 4 | 0 |
| Hardware | NUC booting to the bios screen instead of Linux | 18 | 0 |
| Preprocessing | Repository not ready (R or Java) | 12 | 0 |
| Preprocessing | Azure batch jobs maximum reached | 2 | 0 |
| Processing | No left and/or right IMU data available | 16 | 14 |
| Processing | Data left and right IMU not matched properly by NUC | 28 | 0 |
| Technical | 91 | 15 | |
| Human | 39 | 3 | |
| Unknown | 3 | 3 | |
| Total | 133 | 21 |
| iSCI | Stroke | Total | P-value | |
|---|---|---|---|---|
| Put IMUs on independently? (yes/no) | 10/2 | 9/3 | 19/5 | P = 0 1 |
| Easiness of use (0–10) | 9 (7–10) | 8 (6–10) | 8 (6–10) | P = 0.132 |
| Impeded by the IMUs? (yes/no) | 0/12 | 0/12 | 0/24 | - |
| Bothered by wearing IMUs? (0–10) | 0 (0–2) | 0 (0–3) | 0 (0–3) | P = 0.208 |
| Whole clinical admission? (yes/no) | 10/2 | 10/2 | 20/4 | P = 1 |
| Interested in activity? (yes/no) | 9/3 | 9/3 | 18/6 | P = 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendriks, M.M.S.; Vos-van der Hulst, M.; Keijsers, N.L.W. Feasibility of a Sensor-Based Technological Platform in Assessing Gait and Sleep of In-Hospital Stroke and Incomplete Spinal Cord Injury (iSCI) Patients. Sensors 2020, 20, 2748. https://doi.org/10.3390/s20102748
Hendriks MMS, Vos-van der Hulst M, Keijsers NLW. Feasibility of a Sensor-Based Technological Platform in Assessing Gait and Sleep of In-Hospital Stroke and Incomplete Spinal Cord Injury (iSCI) Patients. Sensors. 2020; 20(10):2748. https://doi.org/10.3390/s20102748
Chicago/Turabian StyleHendriks, Maartje M. S., Marije Vos-van der Hulst, and Noel L. W. Keijsers. 2020. "Feasibility of a Sensor-Based Technological Platform in Assessing Gait and Sleep of In-Hospital Stroke and Incomplete Spinal Cord Injury (iSCI) Patients" Sensors 20, no. 10: 2748. https://doi.org/10.3390/s20102748
APA StyleHendriks, M. M. S., Vos-van der Hulst, M., & Keijsers, N. L. W. (2020). Feasibility of a Sensor-Based Technological Platform in Assessing Gait and Sleep of In-Hospital Stroke and Incomplete Spinal Cord Injury (iSCI) Patients. Sensors, 20(10), 2748. https://doi.org/10.3390/s20102748





