Intelligent Occlusion Stabilization Splint with Stress-Sensor System for Bruxism Diagnosis and Treatment
Abstract
1. Introduction
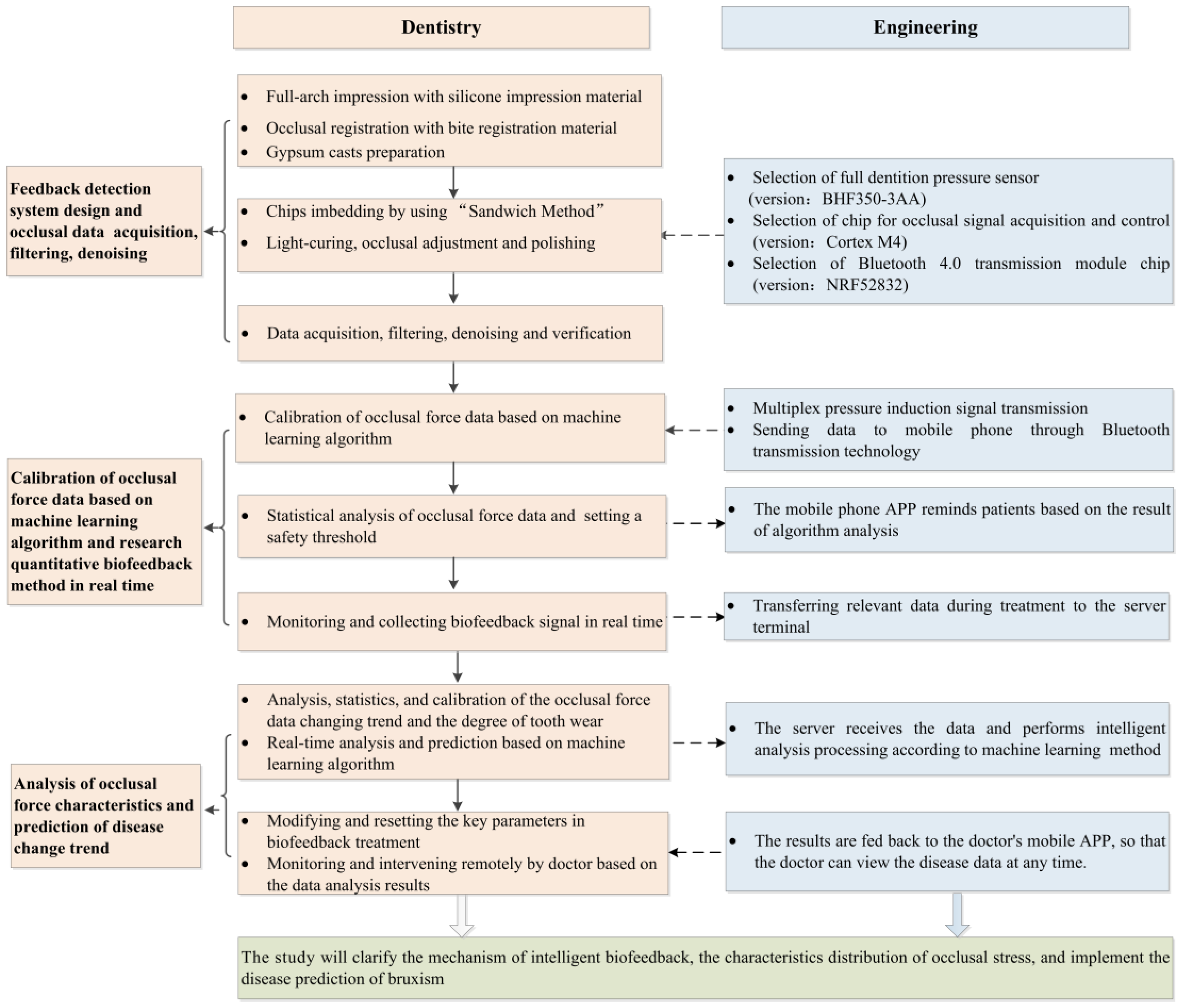
2. Materials and Methods
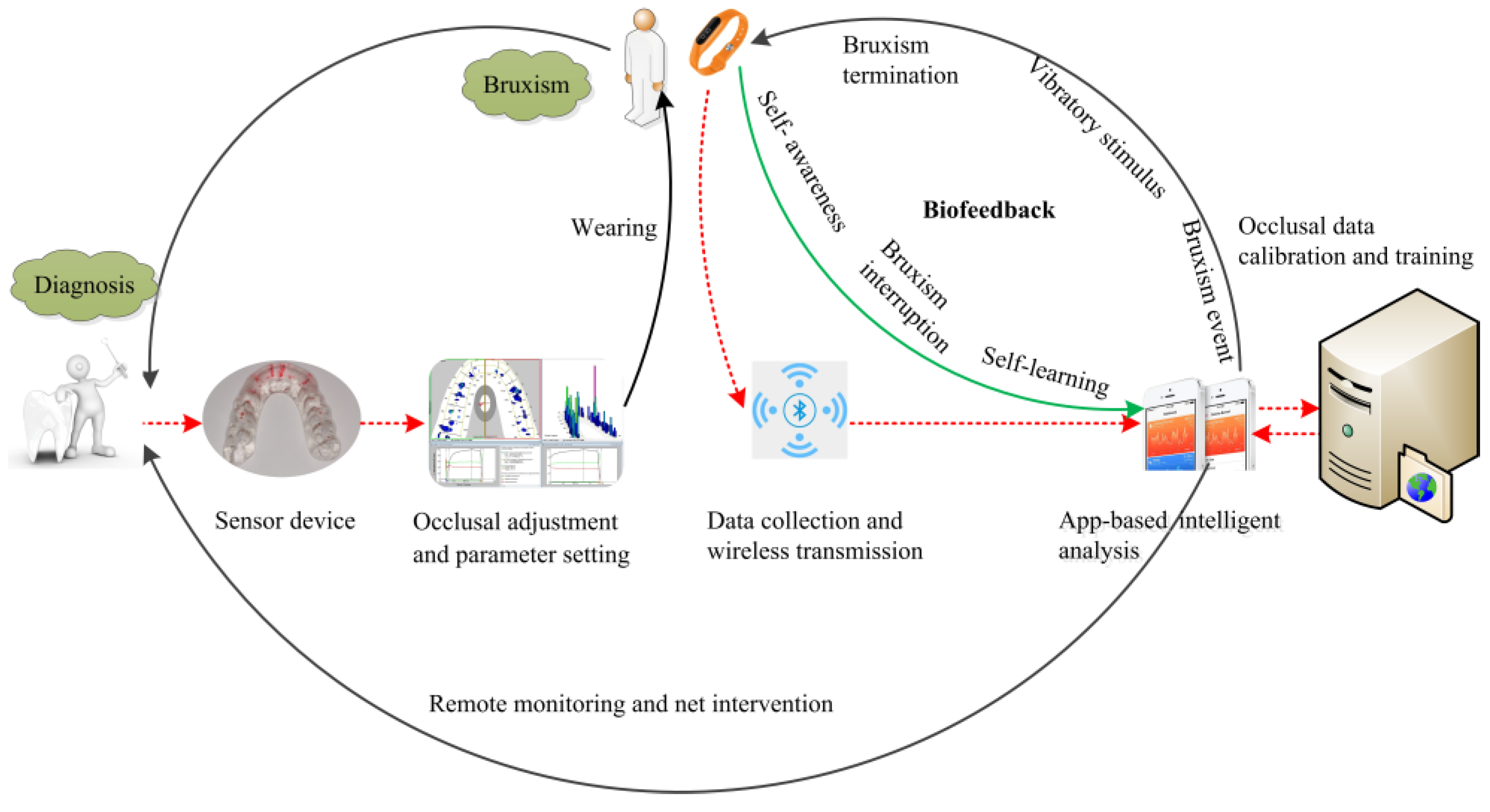
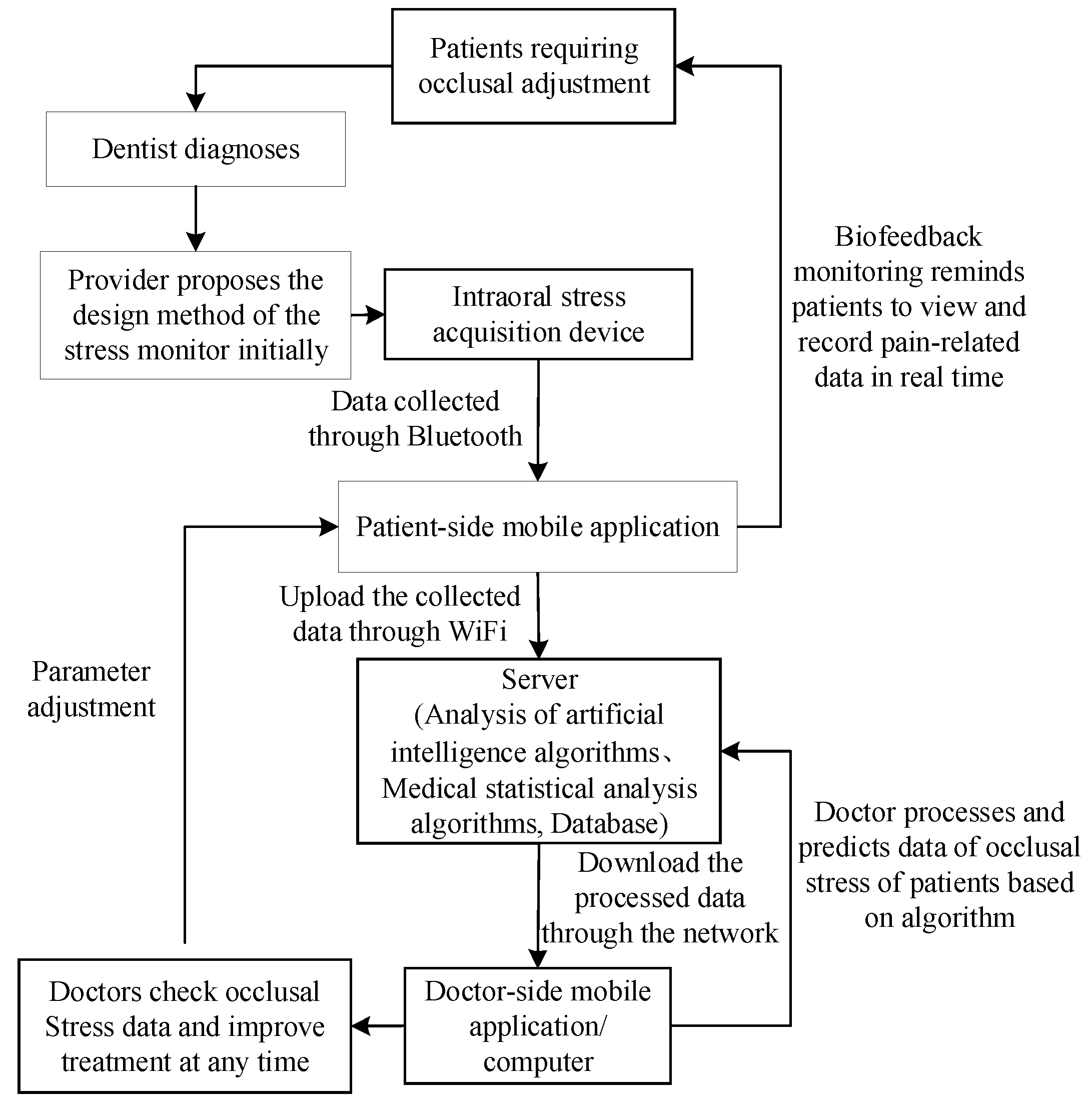
2.1. Overall Treatment Scheme: Biofeedback System Scheme
2.2. Full-Dentition Stress Sensors Integration and Imbedding Methodology—"Sandwich Method”
2.2.1. Early-Stage Preparation
2.2.2. Sandwich Method
2.3. Sensors Components and System Design
- The pressure signal acquisition module included a set of sensitive piezoresistive chips (HuaLanHai, BHF350-3AA, GuangZhou, China). The sensor chip can output corresponding voltage changes with dental pressure variation.
- The main control module was mainly used for receiving occlusal force signals collected by a stress sensor, while also processing, storing and packing the collected data. In this system, a ultralow-power MCU (Nordic Semiconductor, NRF52832, Oslo, Norway) was used as the main controller to control the acquisition of multiple stress signals, convert analog signals into digital signals through a multichannel analog-to-digital converter (ADC) (Nordic Semiconductor, SAADC, Oslo, Norway) conversion module and then send the processed data to a server for further analysis. The power supply of the control module was accomplished by using a button battery (Panasonic Semiconductor, Panasonic CR2032, Celebes, Indonesia), which can work continuously for at least six months. The MCU should turn off automatically, whenever the required operating voltage could not be supplied by the battery.
- The wireless transceiver module (ST Microelectronics, STM32, Geneva, Switzerland) was mainly used to send the occlusal force data processed by the MCU to a server terminal. Bluetooth wireless transmission has the advantage of low power consumption and significant reduction of power consumption of a sensor system, which can be used to update occlusal data in real time. The server terminal parsed the data package according to the Bluetooth protocol and stored the parsed data in the server for further data analyses.
- The server terminal module utilized a common server (Hewlett-Packard Development Company, L.P, HP Z840, Beijing, China) with a Graphics Processing Unit (GPU) coprocessor (NVIDIA, TiTan XP, Santa Clara, CA, USA) for machine learning algorithm training. The server platform ran application software to receive data from the sensor system and showed the curves generated based on sensor-captured pressure.
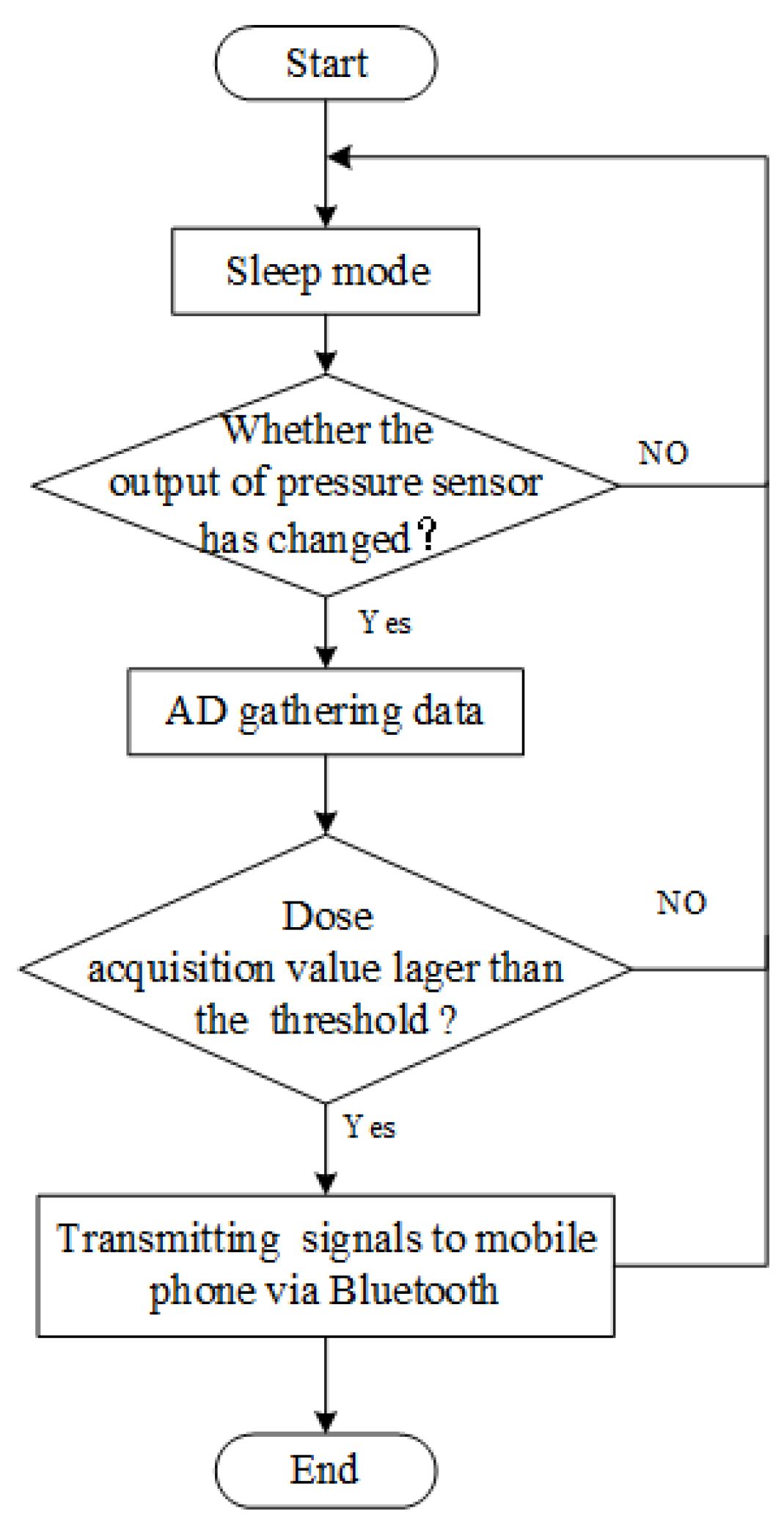
2.3.1. Sensor System Design Consideration
2.3.2. Workflow of an Intraoral Sensor Pressure Detection System
2.4. Data Collection and Analysis
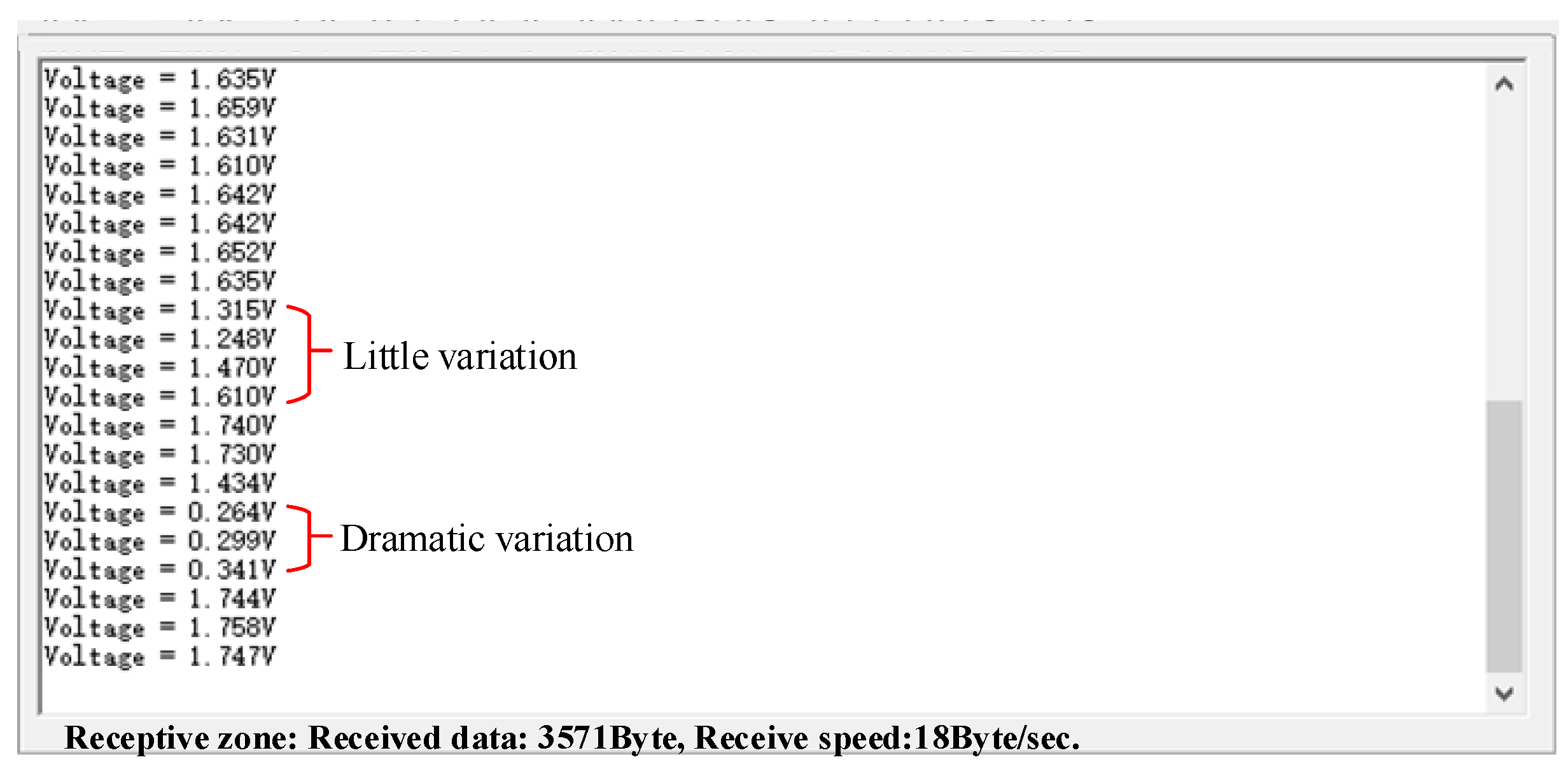
2.4.1. Data Denoising
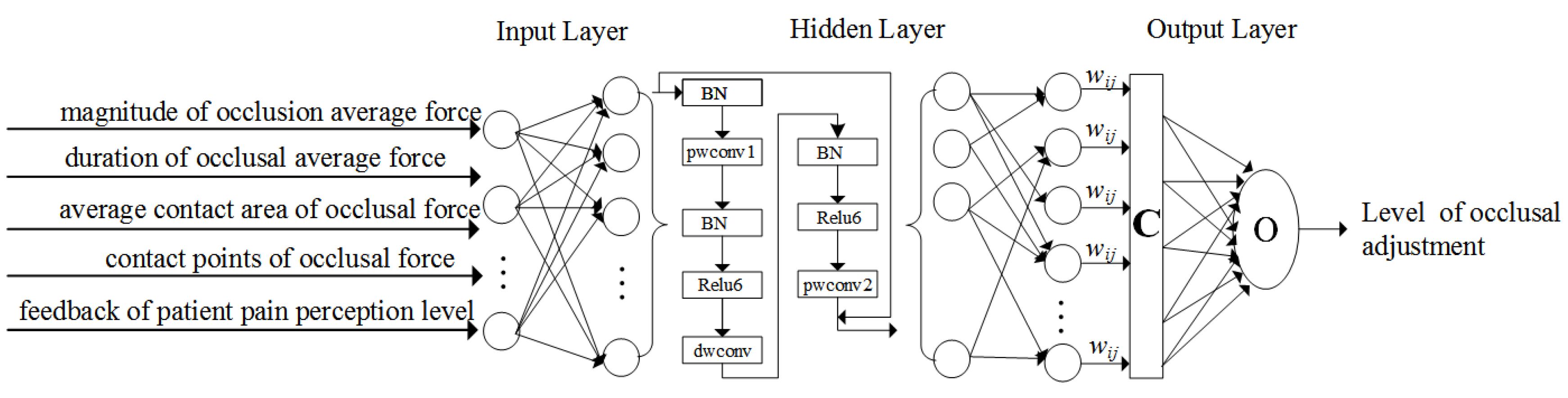
2.4.2. Data Analysis with Intelligent Machine Learning Algorithms
2.5. Diagnosis and Treatment Scheme
3. Results
3.1. Sandwich Method Verification
3.2. Sensor Prototype System Building
3.3. Experimental Data from the Sensor Prototype System
3.4. Experiment of the Configuration of a Machine Learning Algorithm
4. Discussion
4.1. Main Results
4.2. Occlusal Adjustment
4.3. Chip Selection
4.4. Embedding Challenges
4.5. Experiments and Future Work
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lobbezoo, F.; Jacobs, R.; De Laat, A.; Aarab, G.; Wetselaar, P.; Manfredini, D. Chewing on bruxism. Diagnosis, imaging, epidemiology and aetiology. Ned. Tijdschr. Tandheelkd. 2017, 124, 309–316. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Glaros, A.G.; Kato, T.; Koyano, K.; Lavigne, G.J.; de Leeuw, R.; Manfredini, D.; Svensson, P.; Winocur, E.; et al. Bruxism defined and graded: An international consensus. J. Oral. Rehabil. 2013, 40, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Martynowicz, H.; Gac, P.; Brzecka, A.; Poreba, R.; Wojakowska, A.; Mazur, G.; Smardz, J.; Wieckiewicz, M. The relationship between sleep bruxism and obstructive sleep apnea based on polysomnographic findings. J. Clin. Med. 2019, 8, 1653. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Restrepo, C.; Diaz-Serrano, K.; Winocur, E.; Lobbezoo, F. Prevalence of sleep bruxism in children: A systematic review of the literature. J. Oral. Rehabil. 2013, 40, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, J.; Luo, L.; Wang, Y. Does bruxism contribute to dental implant failure? A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2016, 18, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral. Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Castroflorio, T.; Deregibus, A.; Bargellini, A.; Debernardi, C.; Manfredini, D. Detection of sleep bruxism: Comparison between an electromyographic and electrocardiographic portable holter and polysomnography. J. Oral. Rehabil. 2014, 41, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Jokubauskas, L.; Baltrušaitytė, A. Baltrusaityte, Efficacy of biofeedback therapy on sleep bruxism: A systematic review and meta-analysis. J. Oral. Rehabil. 2018, 45, 485–495. [Google Scholar] [CrossRef]
- Wang, L.F.; Long, H.; Deng, M.; Xu, H.; Fang, J.; Fan, Y.; Bai, D.; Han, X.L. Biofeedback treatment for sleep bruxism: A systematic review. Sleep Breath. 2014, 18, 235–242. [Google Scholar] [CrossRef]
- Baba, K.; Clark, G.T.; Watanabe, T.; Ohyama, T. Bruxism force detection by a piezoelectric film-based recording device in sleeping humans. J. Orofac. Pain 2003, 17, 58–64. [Google Scholar]
- Nakamura, H.; Takaba, M.; Abe, Y.; Yoshizawa, S.; Suganuma, T.; Yoshida, Y.; Nakazato, Y.; Ono, Y.; Clark, G.T.; Baba, K.; et al. Effects of a contingent vibratory stimulus delivered by an intra-oral device on sleep bruxism: A pilot study. Sleep Breath. 2019, 23, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yang, J.; Zhang, F.; Yin, X.; Wei, X.; Wang, C. Efficacy of biofeedback therapy via a mini wireless device on sleep bruxism contrasted with occlusal splint: A pilot study. J. Biomed. Res. 2015, 29, 160–168. [Google Scholar]
- Macedo, C.R.; Silva, A.B.; Machado, M.A.; Saconato, H.; Prado, G.F. Occlusal splints for treating sleep bruxism (tooth grinding). Cochrane Database Syst. Rev. 2007, 17, 5514. [Google Scholar] [CrossRef] [PubMed]
- Karakis, D.; Dogan, A.; Bek, B. Evaluation of the effect of two different occlusal splints on maximum occlusal force in patients with sleep bruxism: A pilot study. J. Adv. Prosthodont. 2014, 6, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Tsukiyama, Y.; Kuwatsuru, R.; Koyano, K. The effect of intermittent use of occlusal splint devices on sleep bruxism: A 4-week observation with a portable electromyographic recording device. J. Oral. Rehabil. 2015, 42, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, S.; Huschto, T.; Adamov, A.; Bohm, L.; Busser, A.; Flother, F.F.; Hinzmann, R.; Konig, H.; McAhren, S.M.; Robertson, D.H.; et al. Predicting the early risk of chronic kidney disease in patients with diabetes using real-world data. Nat. Med. 2019, 25, 57–59. [Google Scholar] [CrossRef]
- Ilovar, S.; Zolger, D.; Castrillon, E.; Car, J.; Huckvale, K. Biofeedback for treatment of awake and sleep bruxism in adults: Systematic review protocol. Syst. Rev. 2014, 3, 42. [Google Scholar] [CrossRef]
- Sumonsiri, P.; Thongudomporn, U.; Paphangkorakit, J.; Premprabha, T. Assessment of the relationship between masticatory performance, occlusal contact area, chewing time and cycles, and gastric emptying scintigraphy in dentate subjects. J. Oral. Rehabil. 2019, 46, 787–791. [Google Scholar] [CrossRef]
- Beddis, H.; Pemberton, M.; Davies, S. Sleep bruxism: An overview for clinicians. Br. Dent. J. 2018, 225, 497–501. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Davies, S.; van der Zaag, J.; van Selms, M.K.; Hamburger, H.L.; Naeije, M. Principles for the management of bruxism. J. Oral. Rehabil. 2008, 35, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Shulman, J. Teaching patients how to stop bruxing habits. J. Am. Dent. Assoc. 2001, 132, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
- Majithia, I.P.; Arora, V.; Anil Kumar, S.; Saxena, V.; Mittal, M. Comparison of articulating paper markings and T Scan III recordings to evaluate occlusal force in normal and rehabilitated maxillofacial trauma patients. Med. J. Armed. Forces. India 2015, 71, S382–S388. [Google Scholar] [CrossRef] [PubMed]
- Candirli, C.; Korkmaz, Y.T.; Celikoglu, M.; Altintas, S.H.; Coskun, U.; Memis, S. Dentists’ knowledge of occlusal splint therapy for bruxism and temporomandibular joint disorders. Niger. J. Clin. Pract. 2016, 19, 496–501. [Google Scholar] [PubMed]
- Nota, A.; Tecco, S.; Cioffi, C.; Beraldi, A.; Padulo, J.; Baldini, A. Occlusion time analysis in military pilots affected by bruxism. Sci. Rep. 2019, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Huettig, F.; Kustermann, A.; Kuscu, E.; Geis-Gerstorfer, J.; Spintzyk, S. Polishability and wear resistance of splint material for oral appliances produced with conventional, subtractive, and additive manufacturing. J. Mech. Behav. Biomed. Mater. 2017, 75, 175–179. [Google Scholar] [CrossRef]
- Berntsen, C.; Kleven, M.; Heian, M.; Hjortsjö, C. Clinical comparison of conventional and additive manufactured stabilization splints. Acta Biomater. Odontol. Scand. 2018, 4, 81–89. [Google Scholar] [CrossRef]
- Dedem, P.; Türp, J.C. Digital Michigan splint—from intraoral scanning to plasterless manufacturing. Int. J. Comput. Dent. 2016, 19, 63–76. [Google Scholar]
- Sfondrini, M.F.; Gandini, P.; Malfatto, M.; Di Corato, F.; Trovati, F.; Scribante, A. Computerized Casts for Orthodontic Purpose Using Powder-Free Intraoral Scanners: Accuracy, Execution Time, and Patient Feedback. Biomed. Res. Int. 2018, 23, 4103232. [Google Scholar] [CrossRef]
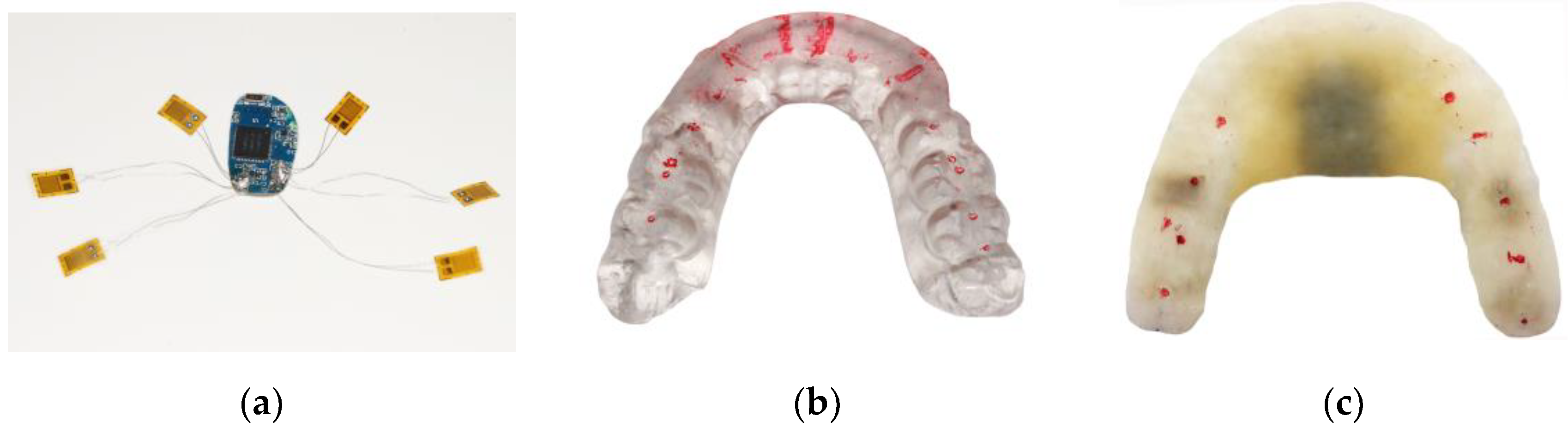
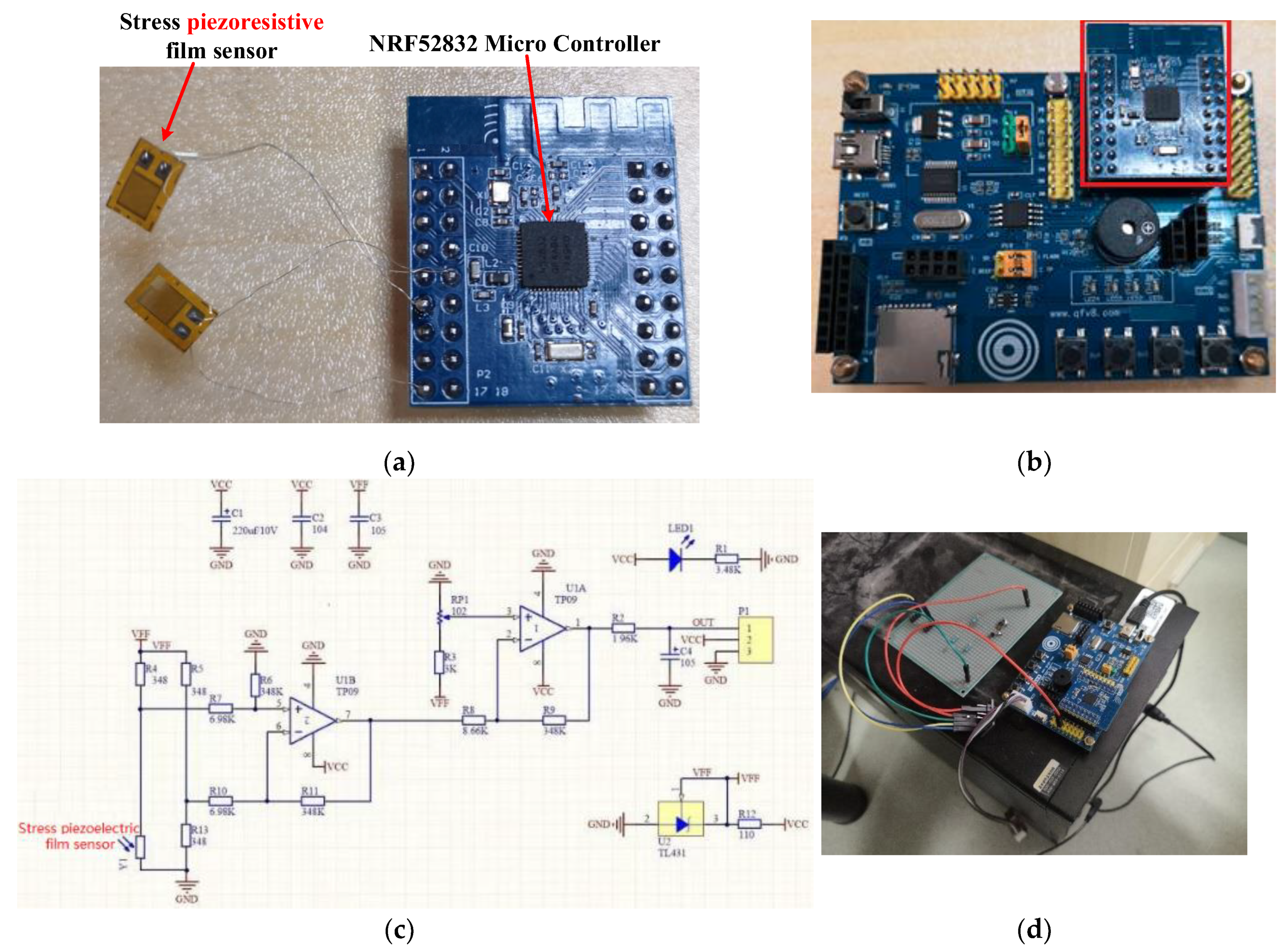
| Item | Parameter |
|---|---|
| Each layer of the light-cured resin | 1 mm |
| Thickness of the piezoresistive-film sensor | 0.3 mm |
| Light-curing time | 5 min |
| Thickness of the OSS | 2 mm |
| Operator | Input of the Neural Network | Output of the Neural Network | Recursion Time |
|---|---|---|---|
| Conv1d | 16 | 32 | 1 |
| Maxpooling | 32 | 32 | 1 |
| Bottleneck1d | 32 | 32 | 6 |
| Conv1d | 32 | 64 | 1 |
| Maxpooling | 64 | 64 | 1 |
| Bottleneck1d | 64 | 64 | 6 |
| Conv1d | 64 | 128 | 1 |
| Maxpooling | 128 | 128 | 1 |
| Bottleneck1d | 128 | 128 | 6 |
| Conv1d | 128 | 256 | 1 |
| Maxpooling | 256 | 256 | 1 |
| GAP | 256 | 256 | 1 |
| Fully Connected | 256 | 64 | 1 |
| Fully Connected | 64 | 3 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Liu, L.; Gao, P.; Zheng, Y.; Hou, W.; Wang, J. Intelligent Occlusion Stabilization Splint with Stress-Sensor System for Bruxism Diagnosis and Treatment. Sensors 2020, 20, 89. https://doi.org/10.3390/s20010089
Gao J, Liu L, Gao P, Zheng Y, Hou W, Wang J. Intelligent Occlusion Stabilization Splint with Stress-Sensor System for Bruxism Diagnosis and Treatment. Sensors. 2020; 20(1):89. https://doi.org/10.3390/s20010089
Chicago/Turabian StyleGao, Jinxia, Longjun Liu, Peng Gao, Yihuan Zheng, Wenxuan Hou, and Junhui Wang. 2020. "Intelligent Occlusion Stabilization Splint with Stress-Sensor System for Bruxism Diagnosis and Treatment" Sensors 20, no. 1: 89. https://doi.org/10.3390/s20010089
APA StyleGao, J., Liu, L., Gao, P., Zheng, Y., Hou, W., & Wang, J. (2020). Intelligent Occlusion Stabilization Splint with Stress-Sensor System for Bruxism Diagnosis and Treatment. Sensors, 20(1), 89. https://doi.org/10.3390/s20010089




