Detection of the Crystallization Process of Paracetamol with a Multi-Mode Optical Fiber in a Reflective Configuration
Abstract
1. Introduction
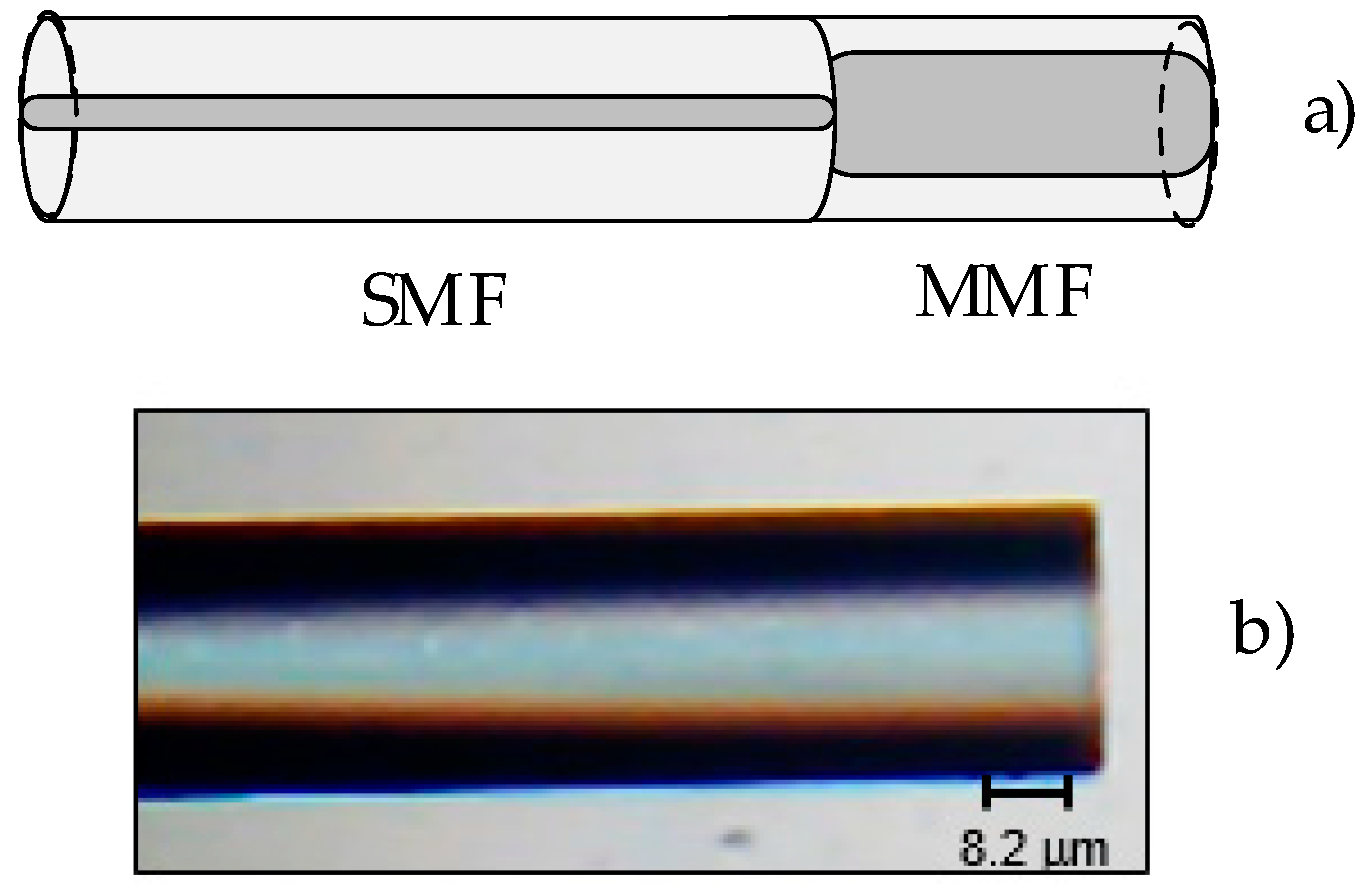
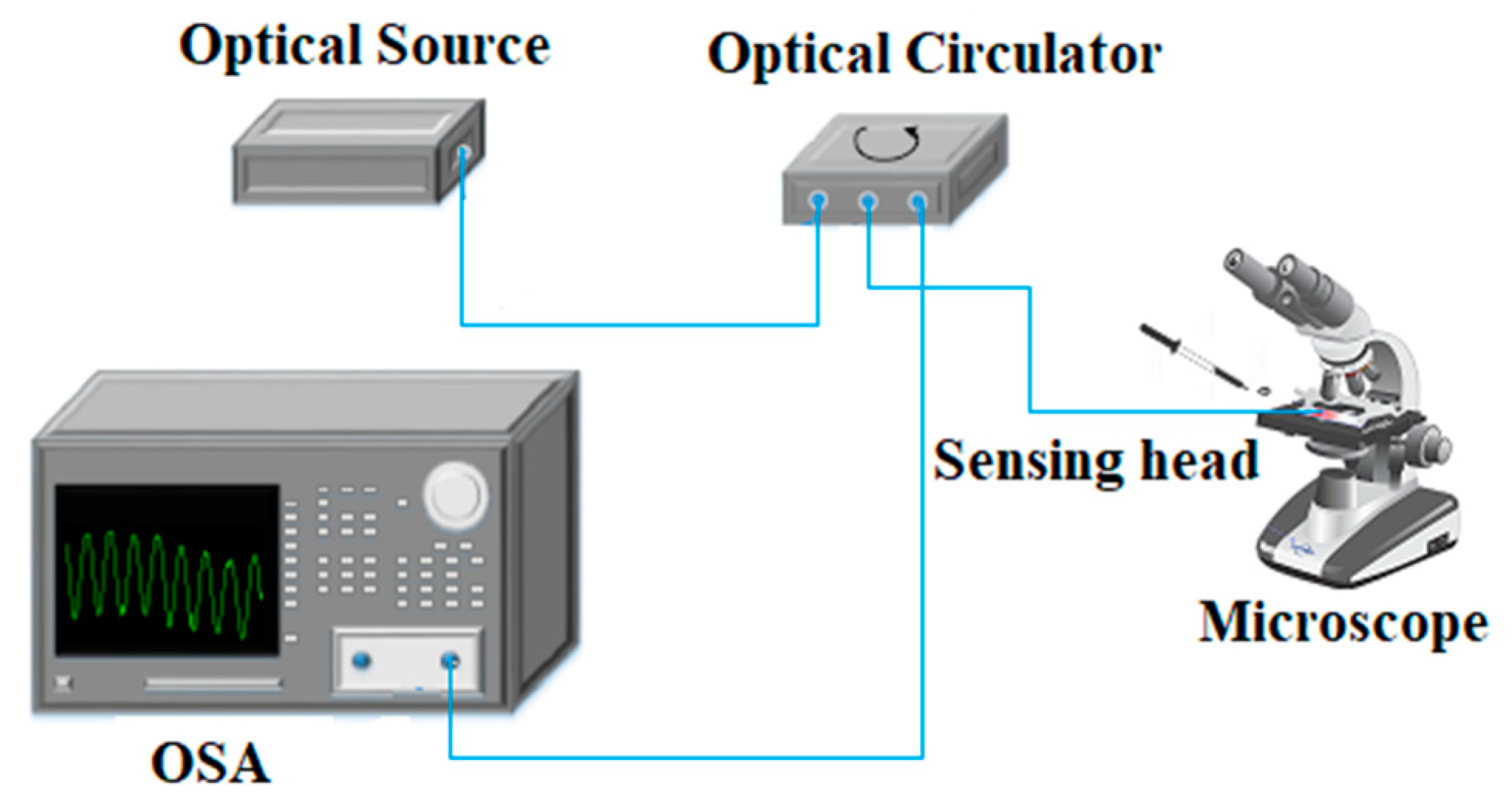
2. Multi-mode Fiber-Based Tip Sensor Characterization
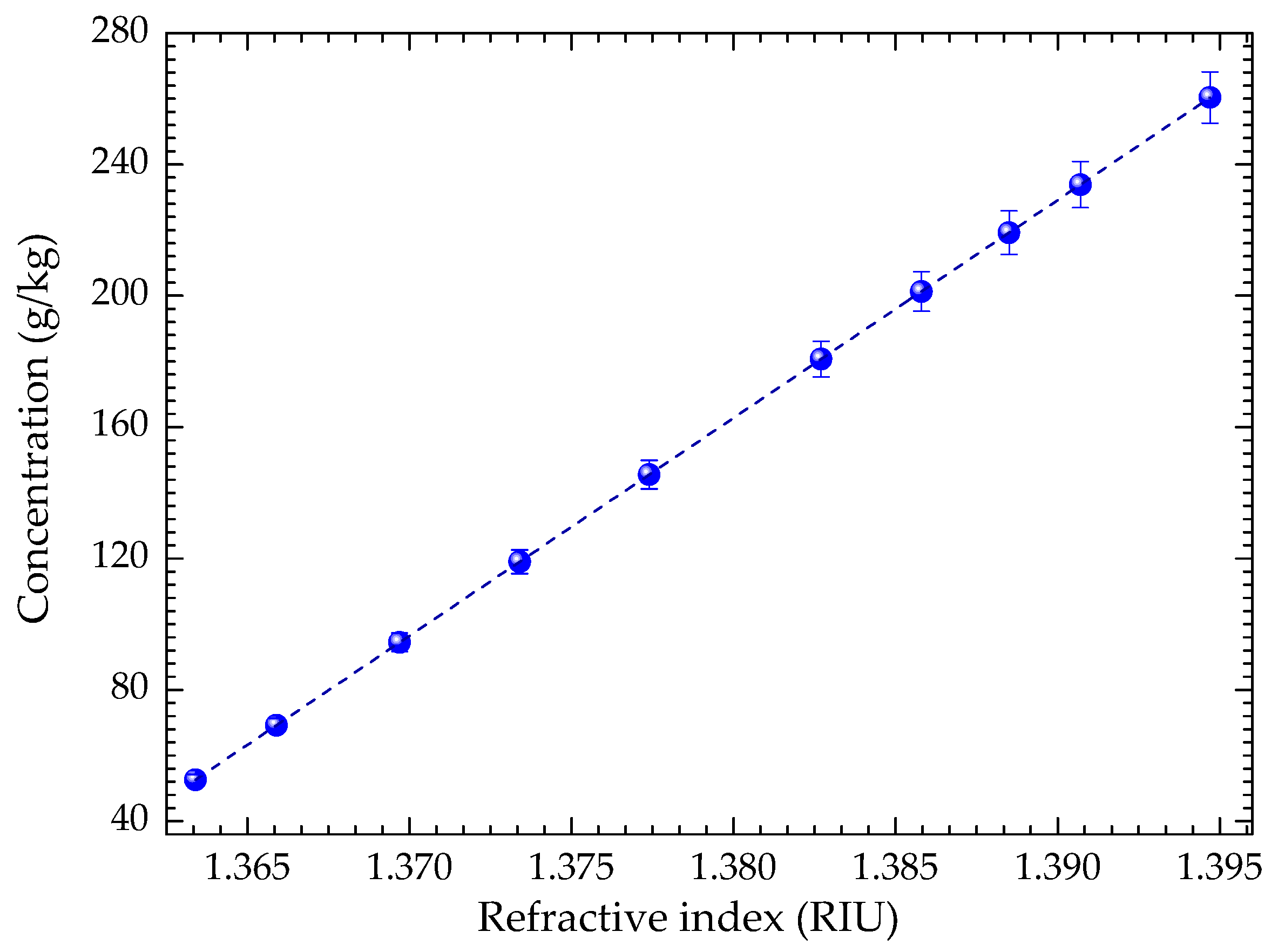
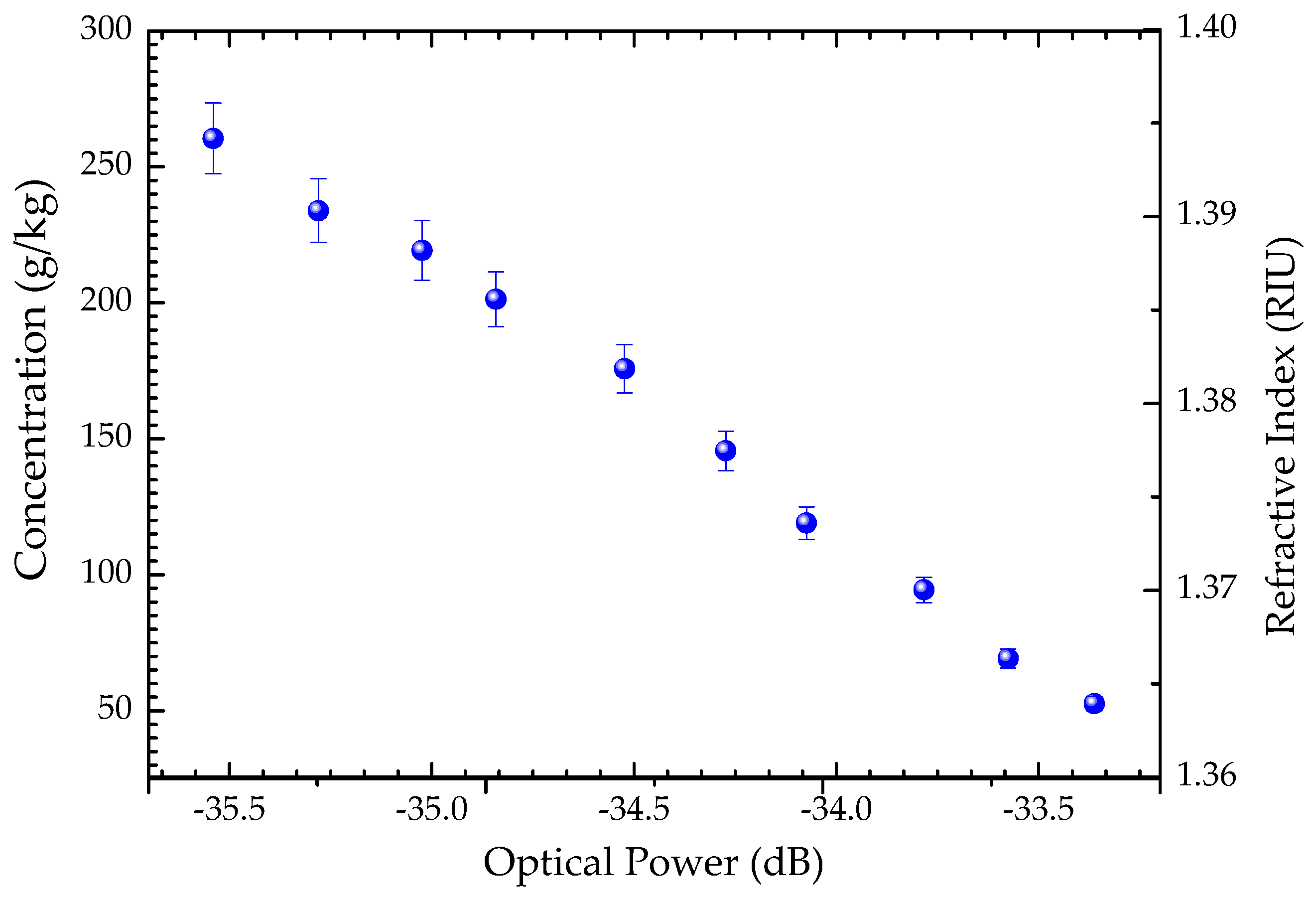
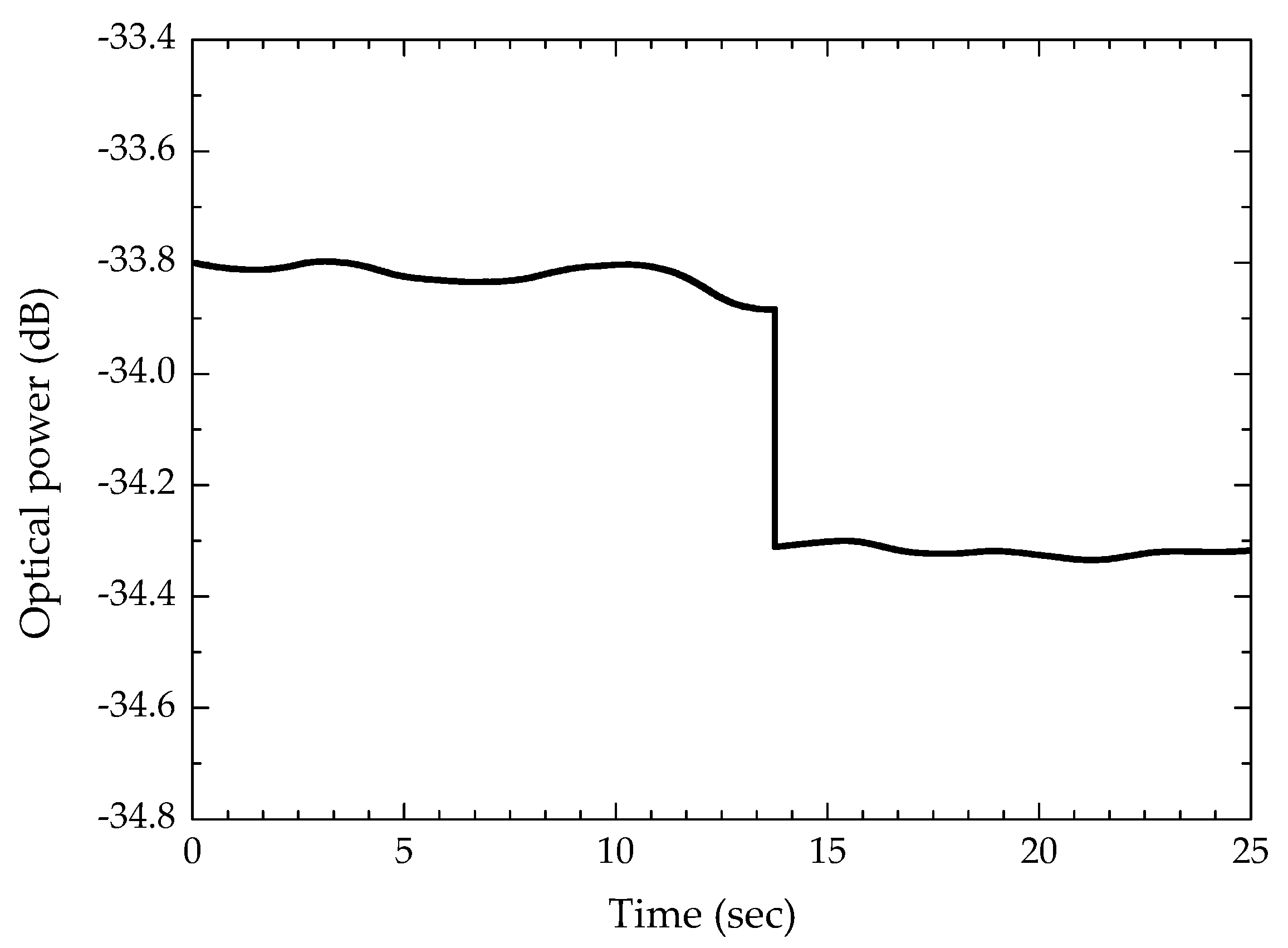
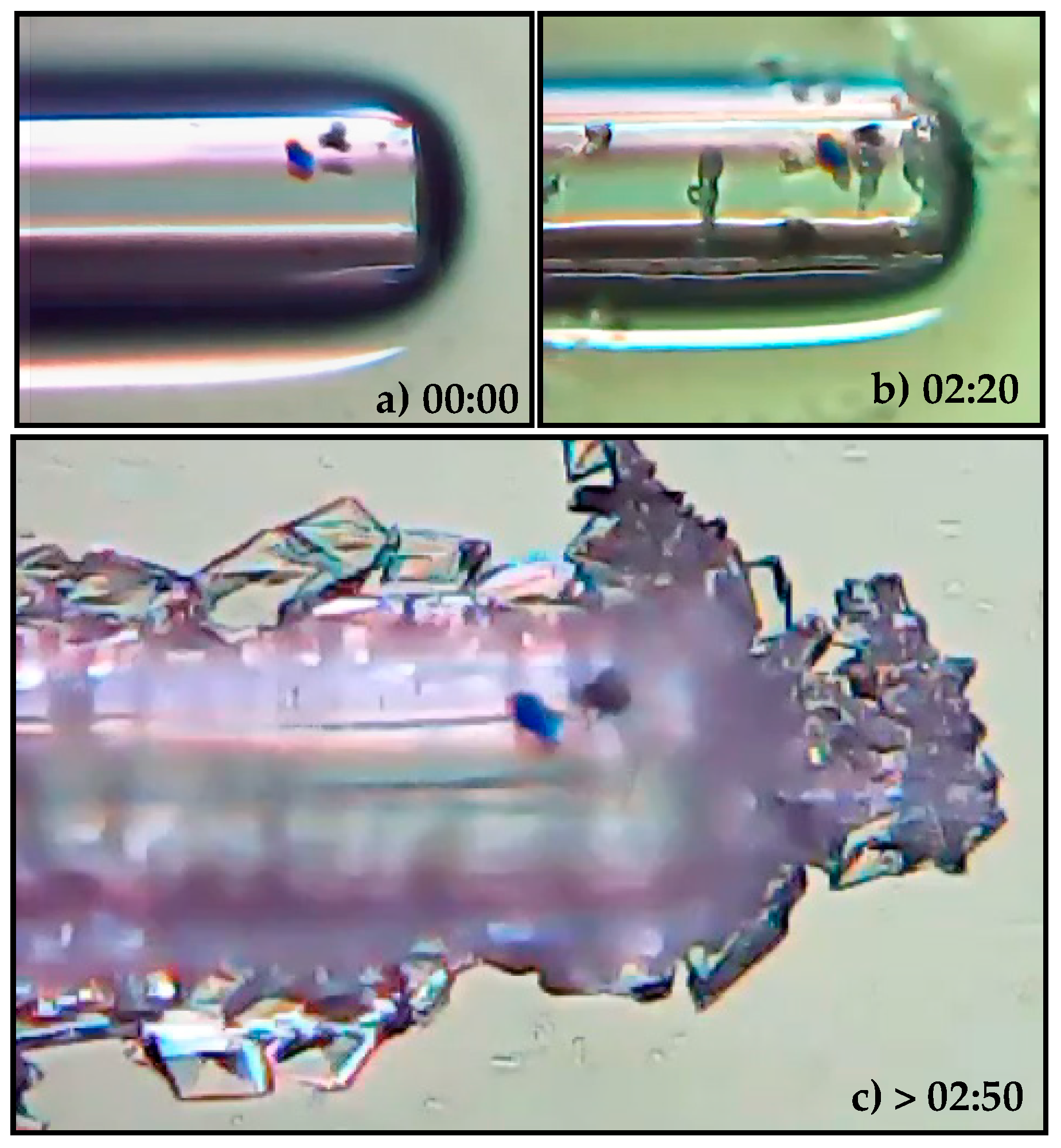
3. Monitoring the Crystallization Process of Paracetamol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nagy, Z.K.; Fevotte, G.; Kramer, H.; Simon, L.L. Recent advances in the monitoring, modeling and control of crystallization systems. Chem. Eng. Res. Des. 2013, 91, 1903–1922. [Google Scholar] [CrossRef]
- Yu, L.X.; Lionberguer, R.A.; Raw, A.S.; D’Costa, R.; Wu, H.; Hussain, A.S. Applications of process analytical technology to crystallization processes. Adv. Drug Deliv. Rev. 2004, 56, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; McGlone, T.; Yerdelen, S.; Srirambhatla, V.; Mabbott, F.; Gurung, R.; Briuglia, M.L.; Ahmed, B.; Polyzois, H.; McGinty, J.; et al. Enabling precision manufacturing of active pharmaceutical ingredients: workflow for seeded cooling continuous crystallizations. Mol. Syst. Des. Eng. 2018, 3, 518–549. [Google Scholar] [CrossRef]
- Erdemir, D.; Lee, A.Y.; Myerson, A.S. Polymorph selection: the role of nucleation, crystal growth and molecular modeling. Curr. Opin. Drug Discov. Dev. 2007, 10, 746–755. [Google Scholar]
- Erdemir, D.; Lee, A.Y.; Myerson, A.S. Nucleation of crystals from solution: classical and two step models. Acc. Chem. Res. 2009, 42, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Towler, C.S.; Davey, R.J.; Lancaster, R.W.; Price, C.J. Impact of molecular speciation on crystal nucleation in polymorphic systems: the conundrum of glycine and molecular ‘self poisoning’. J. Am. Chem. Soc. 2004, 126, 13347–13353. [Google Scholar] [CrossRef]
- He, G.; Tjahjono, M.; Chow, P.S.; Tan, R.B.H.; Garland, M. In situ determination of metastable zone width using dielectric constant measurement. Org. Proc. Res. Dev. 2010, 14, 1469–1472. [Google Scholar] [CrossRef]
- He, G.; Hermanto, M.W.; Tjahjono, M.; Chow, P.S.; Tan, R.B.H.; Garland, M. Comparison of dielectric constant meter with turbidity meter and focused beam reflectance measurement for metastable zone width determination. Chem. Eng. Res. Des. 2012, 90, 259–265. [Google Scholar] [CrossRef]
- Pertig, D.; Buchfink, R.; Petersen, S.; Stelzer, T.; Ulrich, J. Inline analyzing of industrial crystallization processes by an innovative ultrasonic probe technique. Chem. Eng. Tech. 2011, 34, 639–646. [Google Scholar] [CrossRef]
- Stelzer, T.; Pertig, D.; Ulrich, J. Ultrasonic crystallization monitoring technique for simultaneous in-line measurement of liquid and polid Phase. J. Cryst. Growth 2013, 362, 71–76. [Google Scholar] [CrossRef]
- Simon, L.L.; Merz, T.; Dubuis, S.; Lieb, A.; Hungerbuhler, K. In-situ monitoring of pharmaceutical and specialty chemicals crystallization processes using endoscopy-stroboscopy and multivariate image analysis. Chem. Eng. Res. Des. 2012, 90, 1847–1855. [Google Scholar] [CrossRef]
- Boerkamp, M.; Lamb, D.W.; Lye, P.G. An intrinsic exposed core optical fibre sensor as a quantitative surface crystallization monitoring sensor. Sens. Actuators B 2003, 177, 964–969. [Google Scholar] [CrossRef]
- Simon, L.L.; Nagy, Z.K.; Hungerbuhler, K. Comparison of external bulk video imaging with focused beam reflectance measurement and ultra-violet visible spectroscopy for crystallization nucleation detection and metastable zone identification in food and pharmaceutical crystallization processes. Chem. Eng. Sci. 2009, 64, 3344–3351. [Google Scholar] [CrossRef]
- Gherras, N.; Serris, E.; Fevotte, G. Monitoring industrial pharmaceutical crystallization processes using acoustic emission in pure and impure media. Int. J. Pharm. 2012, 439, 109–119. [Google Scholar] [CrossRef]
- Di Martino, P.; Conflant, P.; Drache, M.; Huvenne, J.P.; Hermann, A.M. Preparation and physical characterization of form II and III of paracetamol. J. Therm. Anal. 1997, 48, 447–458. [Google Scholar] [CrossRef]
- Sibik, J.; Sargent, M.J.; Franklin, M.; Zeitler, J.A. Crystallization and phase changes in paracetamol from the amorphous solid to the liquid phase. Mol. Pharm. 2014, 11, 1326–1334. [Google Scholar] [CrossRef]
- Di Martino, P.; Palmieri, G.F.; Martelli, S. Molecular mobility of the paracetamol amorphous form. Chem. Pharm. Bull. 2000, 48, 1105–1108. [Google Scholar] [CrossRef][Green Version]
- Johari, G.P.; Kim, S.; Shanker, R.M. Dielectric studies of molecular motions in amorphous solid and ultraviscous acetaminophen. J. Pharm. Sci. 2005, 94, 2207–2223. [Google Scholar] [CrossRef]
- Méndez del Rio, J.R.; Rousseau, R.W. Batch and tubular-batch crystallization of paracetamol: crystal size distribution and polymorph formation. Cryst. Growth Des. 2006, 6, 1407–1414. [Google Scholar]
- Kachrimanis, K.; Braun, D.E.; Griesser, U.J. Quantitative analysis of paracetamol polymorphs in powder mixtures by FT-Raman spectroscopy and PLS regression. J. Pharm. Biomed. Anal. 2007, 43, 407–412. [Google Scholar] [CrossRef]
- Qi, S.; Avalle, P.; Saklatvala, R.; Craig, D.Q.M. An investigation into the effects of thermal history on the crystallisation behaviour of amorphous paracetamol. Eur. J. Pharm. Biopharm. 2008, 69, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Nanubolu, J.B.; Burley, J.C. Investigating the recrystallization behavior of amorphous paracetamol by variable temperature Raman studies and surface Raman mapping. Mol. Pharm. 2012, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.C.; Lee, M.J.; Seo, D.Y.; Lee, H.E.; Choi, Y.; Kim, W.S.; Kim, C.S.; Jeong, M.Y.; Choi, G.J. Polymorph transformation in paracetamol monitored by in-line NIR spectroscopy during a cooling crystallization process. AAPS Pharm. Sci. Tech. 2011, 12, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoubi, N.; Koundourellis, J.E.; Malamataris, S. FT-IR and Raman spectroscopic methods for identification and quantitation of orthorhombic and monoclinic paracetamol in powder mixes. J. Pharm. Biomed. Anal. 2002, 29, 459–467. [Google Scholar] [CrossRef]
- Kauffmana, J.F.; Batykeferb, L.M.; Tuschelb, D.D. Raman detected differential scanning calorimetry of polymorphic transformations in acetaminophen. J. Pharm. Biomed. Anal. 2008, 48, 1310–1315. [Google Scholar] [CrossRef]
- Novais, S.; Ferreira, M.S.; Pinto, J.L. Relative humidity fiber sensor based on multi-mode interferometer coated with agarose-gel. Coatings 2018, 8, 453. [Google Scholar] [CrossRef]
- Gomes, A.D.; Kobelke, J.; Bierlich, J.; Schuster, K.; Bartelt, H.; Frazão, O. Optical fiber prove viscometer based on hollow capillary tube. J. Light. Technol. 2019, 37, 4456–4461. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, L.; Novais, S.; Ferreira, A.; Frazão, O.; Silva, S. Detection of the Crystallization Process of Paracetamol with a Multi-Mode Optical Fiber in a Reflective Configuration. Sensors 2020, 20, 87. https://doi.org/10.3390/s20010087
Soares L, Novais S, Ferreira A, Frazão O, Silva S. Detection of the Crystallization Process of Paracetamol with a Multi-Mode Optical Fiber in a Reflective Configuration. Sensors. 2020; 20(1):87. https://doi.org/10.3390/s20010087
Chicago/Turabian StyleSoares, Liliana, Susana Novais, António Ferreira, Orlando Frazão, and Susana Silva. 2020. "Detection of the Crystallization Process of Paracetamol with a Multi-Mode Optical Fiber in a Reflective Configuration" Sensors 20, no. 1: 87. https://doi.org/10.3390/s20010087
APA StyleSoares, L., Novais, S., Ferreira, A., Frazão, O., & Silva, S. (2020). Detection of the Crystallization Process of Paracetamol with a Multi-Mode Optical Fiber in a Reflective Configuration. Sensors, 20(1), 87. https://doi.org/10.3390/s20010087





