Identification of Fusarium Head Blight in Winter Wheat Ears Using Continuous Wavelet Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Areas
2.2. Data Acquisition
2.2.1. Wheat Ear Spectra Measurement
2.2.2. Determination of Disease Infestation Ratio (DIR)
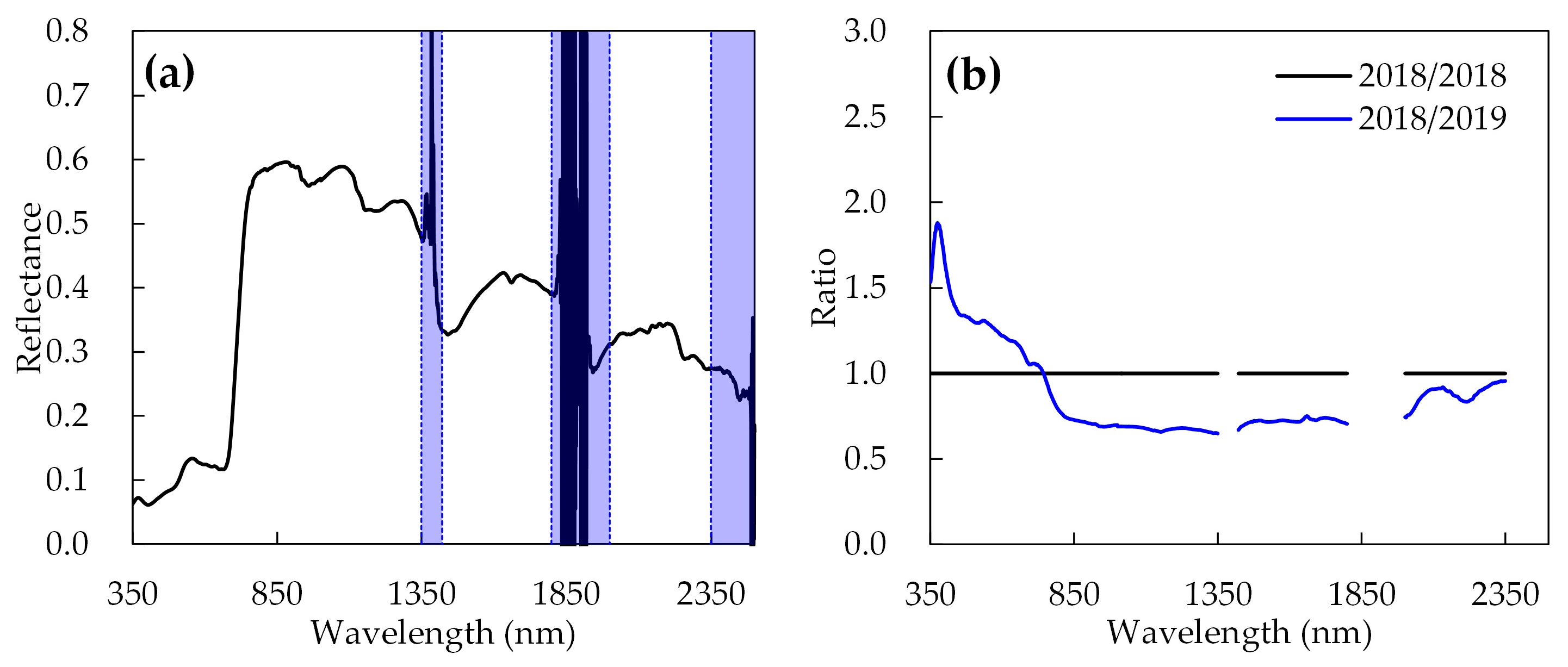
2.3. Preprocessing and Standardization of Spectral Reflectance Data
2.4. Wavelet Features Extraction for Fusarium Head Blight Using CWA
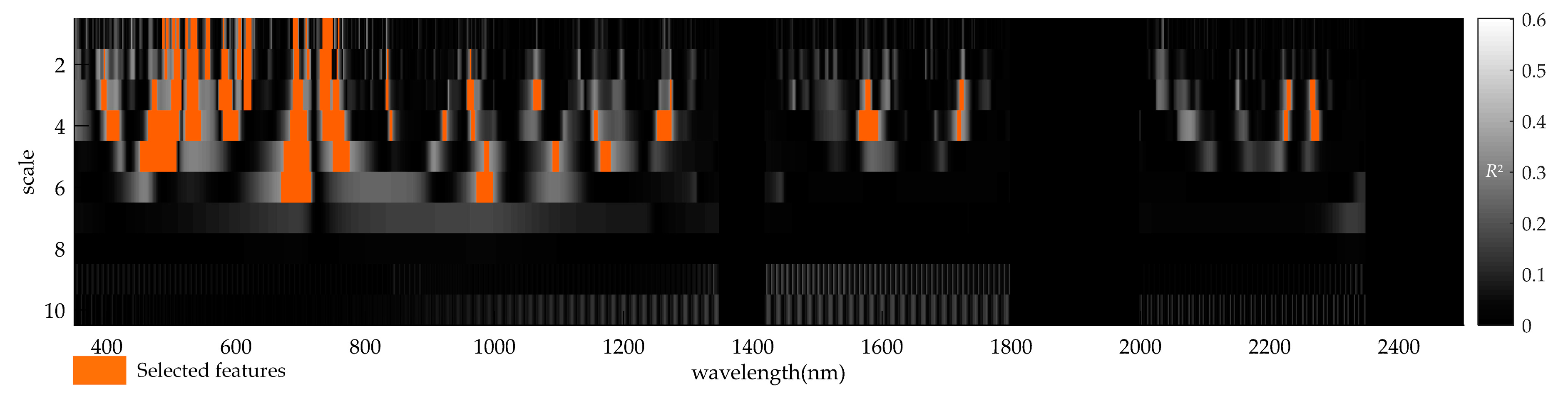
- Determine the sensitive wavelet regions with Fusarium head blight. A linear correlation analysis is first performed to determine the coefficient of determination (R2) between wavelet features and the DIR. The coefficients of determination (R2) between wavelet coefficients and DIR were generated to relate the scalogram with the disease infestation of wheat ears [14,39]. The top 5% ranking in descending order based on the R2 values of the correlation scalograms will be considered as the preliminary selection of wavelet regions with Fusarium head blight in this study. In addition, the p-value of independent t-test [45] can indicate the significance level of the difference between healthy and disease-infected eras. Thus, the statistically significant (p-value of t-test < 0.001) wavelet regions among the top 5% regions will be retained as the final sensitive wavelet feature regions.
- Determine the preliminary wavelet features. To reduce the computational load, only the features with the highest R2 within each wavelet region are retained as the preliminary wavelet features.
- Identify the optimal wavelet features for identifying Fusarium head blight. To reduce the redundancy among the wavelet features further, the coefficient of correlation R among the preliminary features will be calculated and summarized. The larger the absolute value of R, the greater is the correlation between the two wavelet features, i.e., the greater is the redundancy [46]. In this study, we assumed that only those preliminary wavelet features with an absolute R value lower than 0.8 are considered to have both strong correlation and relatively low redundancy. For the two mutually redundant preliminary wavelet features, the one with the higher correlation with the DIR will eventually be retained as the optimal wavelet features for the identification of Fusarium head blight.
2.5. Testing the Performance of the Wavelet Features for Fusarium Head Blight
3. Results and Discussion
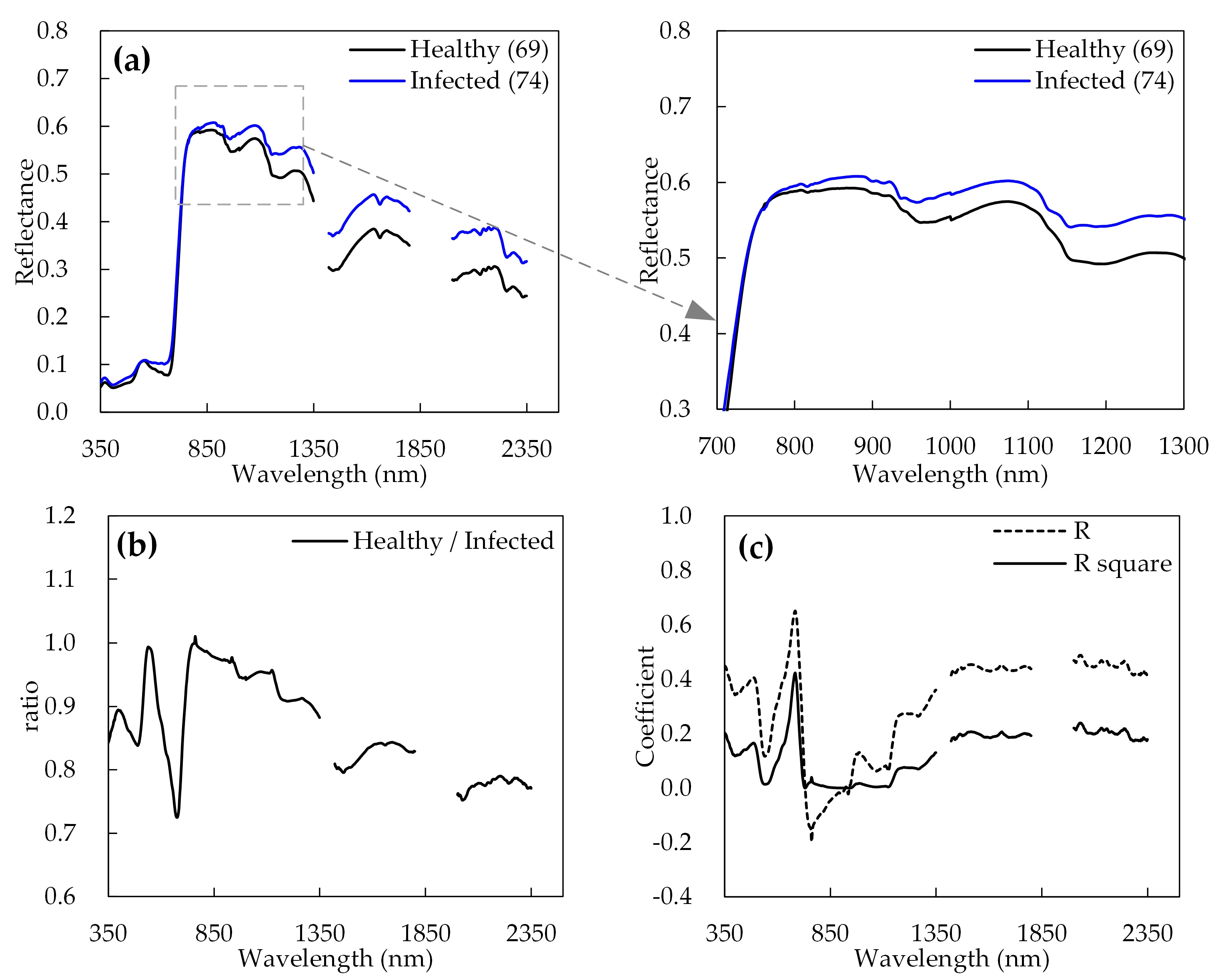
3.1. Changes in Reflectance Spectral Owing to Fusarium Head Blight
3.2. Wavelet Features and Their Sensitivities to Fusarium Head Blight
3.3. Capabilities of the Wavelet Features to Identify Fusarium Head Blight
3.4. Implications under Field Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, P.; Liu, W.; Yuan, J.; Wang, X.; Zhou, B.; Wang, S.; Zhang, S.; Feng, Y.; Yang, B.; Liu, G. Development and characterization of wheat-Leymus racemosus translocation lines with resistance to Fusarium Head Blight. Theo. Appl. Genet. 2005, 111, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Bauriegel, E.; Giebel, A.; Herppich, W.B. Hyperspectral and chlorophyll fluorescence imaging to analyse the impact of Fusarium culmorum on the photosynthetic integrity of infected wheat ears. Sensors 2011, 11, 3765–3779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miedaner, T.; Schneider, B.; Geiger, H.H. Deoxynivalenol (DON) content and Fusarium head blight resistance in segregating populations of winter rye and winter wheat. Crop Sci. 2003, 43, 519–526. [Google Scholar] [CrossRef]
- McCormick, S.P. The role of DON in pathogenicity. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2003; Chapter 7; pp. 165–183. [Google Scholar]
- Bauriegel, E.; Giebel, A.; Geyer, M.; Schmidt, U.; Herppich, W.B. Early detection of Fusarium infection in wheat using hyper-spectral imaging. Comput. Electron. Agr. 2011, 75, 304–312. [Google Scholar] [CrossRef]
- Whetton, R.L.; Waine, T.W.; Mouazen, A.M. Hyperspectral measurements of yellow rust and fusarium head blight in cereal crops: Part 2: On-line field measurement. Biosyst. Eng. 2018, 167, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Jie, L.; Wang, S.; Qi, H.; Li, S. Classifying wheat hyperspectral pixels of healthy heads and Fusarium head blight disease using a deep neural network in the wild field. Remote Sens. 2018, 10, 395. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Pan, Y.; Feng, H.; Zhao, X.; Yang, X.; Ding, C.; Yang, G. Development of Fusarium head blight classification index using hyperspectral microscopy images of winter wheat spikelets. Biosyst. Eng. 2019, 186, 83–99. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.; Huang, W.; Ma, H.; Zhao, J. Identification of Fusarium head blight in winter wheat ears based on Fisher’s linear discriminant analysis and a support vector machine. Appl. Sci. 2019, 9, 3894. [Google Scholar] [CrossRef] [Green Version]
- Mahlein, A.K.; Alisaac, E.; Al Masri, A.; Behmann, J.; Dehne, H.W.; Oerke, E.C. Comparison and combination of thermal, fluorescence, and hyperspectral imaging for monitoring Fusarium head blight of wheat on spikelet scale. Sensors 2019, 19, 2281. [Google Scholar] [CrossRef] [Green Version]
- Alisaac, E.; Behmann, J.; Kuska, M.; Dehne, H.W.; Mahlein, A.K. Hyperspectral quantification of wheat resistance to Fusarium head blight: Comparison of two Fusarium species. Eur. J. Plant Pathol. 2018, 152, 869–884. [Google Scholar] [CrossRef]
- Dammer, K.H.; Möller, B.; Rodemann, B.; Heppner, D. Detection of head blight (Fusarium ssp.) in winter wheat by color and multispectral image analyses. Crop. Prot. 2011, 30, 420–428. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yuan, L.; Wang, J.H.; Huang, W.J.; Chen, L.P.; Zhang, D.Y. Spectroscopic leaf level detection of powdery mildew for winter wheat using continuous wavelet analysis. J. Integr. Agric. 2012, 11, 1474–1484. [Google Scholar] [CrossRef]
- Jingcheng, Z.; Juhua, L.; Wenjiang, H.; Jihua, W. Continuous wavelet analysis based spectral feature selection for winter wheat yellow rust detection. Intell. Autom. Soft Co. 2011, 17, 531–540. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, W.; González-Moreno, P.; Luke, B.; Dong, Y.; Zheng, Q.; Ma, H.; Liu, L. Wavelet-based rust spectral feature set (WRSFs): A novel spectral feature set based on continuous wavelet transformation for tracking progressive host-pathogen interaction of yellow rust on wheat. Remote Sens. 2018, 10, 525. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Huang, W.; Yuan, L.; Zhao, C.; Du, S.; Zhang, J.; Zhao, J. Evaluation of spectral indices and continuous wavelet analysis to quantify aphid infestation in wheat. Precis. Agric. 2013, 14, 151–161. [Google Scholar] [CrossRef]
- Junjing, L.; Wenjiang, H.; Jinbao, J. Comparison of wavelet features and conventional spectral features on estimating severity of stripe rust in winter wheat. J. Triticeae Crops 2015, 35, 1456–1461. [Google Scholar]
- Zhang, J.; Pu, R.; Loraamm, R.W.; Yang, G.; Wang, J. Comparison between wavelet spectral features and conventional spectral features in detecting yellow rust for winter wheat. Comput. Electron. Agric. 2014, 100, 79–87. [Google Scholar] [CrossRef]
- Lu, J.; Huang, W.; Zhang, J.; Jiang, J. Quantitative identification of yellow rust and powdery mildew in winter wheat based on wavelet feature. Spectrosc. Spect. Anal. 2016, 36, 1854–1858. [Google Scholar]
- Shi, Y.; Huang, W.; Zhou, X. Evaluation of wavelet spectral features in pathological detection and discrimination of yellow rust and powdery mildew in winter wheat with hyperspectral reflectance data. J. Appl. Remote Sens. 2017, 11, 026025. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Yuan, L.; Chen, F.; Wu, K. Discrimination of winter wheat disease and insect stresses using continuous wavelet features extracted from foliar spectral measurements. Biosyst. Eng. 2017, 162, 20–29. [Google Scholar] [CrossRef]
- Huang, W.; Lu, J.; Ye, H.; Kong, W.; Mortimer, A.H.; Shi, Y. Quantitative identification of crop disease and nitrogen-water stress in winter wheat using continuous wavelet analysis. Int. J. Agric. Biol. Eng. 2018, 11, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Bao, Z.Y.; Tian, J.H.; Chen, X.; Zhang, H.B. Spectral differentiation among different diseases and pests in winter wheat using continuous wavelet analysis. Geogr. Geo-Inf. Sci. 2017, 33, 28–34. [Google Scholar]
- Menesatti, P.; Antonucci, F.; Pallottino, F.; Giorgi, S.; Matere, A.; Nocente, F.; Pasquini, M.; D’Egidio, M.G.; Costa, C. Laboratory vs. in-field spectral proximal sensing for early detection of Fusarium head blight infection in durum wheat. Biosyst. Eng. 2013, 114, 289–293. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Kim, M.S.; Dong, Y. Fusarium damage assessment in wheat kernels by Vis/NIR hyperspectral imaging. Sens. Instrum. Food Qual. Saf. 2011, 5, 63–71. [Google Scholar] [CrossRef]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 7, 179–188. [Google Scholar] [CrossRef]
- Rao, C.R. The utilization of multiple measurements in problems of biological classification. J. R. Stat. Soc. B 1948, 10, 159–203. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, H.; Ma, S. Improving kernel Fisher discriminant analysis for face recognition. IEEE Trans. Circuits Syst. Video Technol. 2004, 14, 42–49. [Google Scholar] [CrossRef]
- Witten, D.M.; Tibshirani, R. Penalized classification using Fisher’s linear discriminant. J. R. Stat. Soc. B 2011, 73, 753–772. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Plataniotis, K.N.; Venetsanopoulos, A.N. Regularization studies of linear discriminant analysis in small sample size scenarios with application to face recognition. Pattern Recognit. Lett. 2005, 26, 181–191. [Google Scholar] [CrossRef]
- Yang, J.; Frangi, A.F.; Yang, J.-Y.; Zhang, D.; Jin, Z. KPCA plus LDA: A complete kernel Fisher discriminant framework for feature extraction and recognition. IEEE Trans. Pattern Anal. 2005, 27, 230–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, L.; Bao, Z.; Zhang, H.; Zhang, Y.; Liang, X. Habitat monitoring to evaluate crop disease and pest distributions based on multi-source satellite remote sensing imagery. Optik 2017, 145, 66–73. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, C.; Xu, J.; Huang, L.; Zhang, D.; Liang, D. Forecasting the wheat powdery mildew (Blumeria graminis f. Sp. tritici) using a remote sensing-based decision-tree classification at a provincial scale. Australasian Plant Path. 2018, 47, 53–61. [Google Scholar] [CrossRef]
- Song, Y.; Linderholm, H.W.; Wang, C.; Tian, J.; Huo, Z.; Gao, P.; Song, Y.; Guo, A. The influence of excess precipitation on winter wheat under climate change in China from 1961 to 2017. Sci. Total Environ. 2019, 690, 189–196. [Google Scholar] [CrossRef]
- Xiao, J.; Huo, Z.; Li, N.; Xin, M.; Wang, L.; Lu, X. Progress in research on meteorological conditions of wheat scab. J. Nat. Disasters 2011, 20, 146–152. [Google Scholar]
- Wu, J.; Cheng, H.; Zhan, X. A study on variation characteristics of air humidity in Hefei area. J. Anhui Jianzhu Univ. 2016, 24, 74–78. [Google Scholar]
- Lin, C.; Tsogt, K.; Chang, C.-I. An empirical model-based method for signal restoration of SWIR in ASD Field Spectroradiometry. Photogramm. Eng. Remote Sens. 2012, 78, 119–127. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, G.A.; Feng, J.; Calvo-Polanco, M. Continuous wavelet analysis for the detection of green attack damage due to mountain pine beetle infestation. Remote Sens. Environ. 2010, 114, 899–910. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, A. Spectroscopic determination of leaf water content using continuous wavelet analysis. Remote Sens. Environ. 2010, 115, 659–670. [Google Scholar] [CrossRef]
- Blackburn, G.A.; Ferwerda, J.G. Retrieval of chlorophyll concentration from leaf reflectance spectra using wavelet analysis. Remote Sens. Environ. 2008, 112, 1614–1632. [Google Scholar] [CrossRef]
- Rivard, B.; Feng, J.; Gallie, A.; Sanchez-Azofeifa, A. Continuous wavelets for the improved use of spectral libraries and hyperspectral data. Remote Sens. Environ. 2008, 112, 2850–2862. [Google Scholar] [CrossRef]
- Mallat, S. Zero-crossings of a wavelet transform. IEEE Trans. Inf. Theory 1991, 37, 1019–1033. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A practical guide to wavelet analysis. B. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Heeren, T.; D’Agostino, R. Robustness of the two independent samples t-test when applied to ordinal scaled data. Stat. Med. 2010, 6, 79–90. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhao, Y.; Yang, Z. Combination feature selection based on relief. J. Fudan Univ. (Nat. Sci.) 2004, 43, 893–898. [Google Scholar]
- Bajwa, S.G.; Rupe, J.C.; Mason, J. Soybean disease monitoring with leaf reflectance. Remote Sens. 2017, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Mathre, D.E. Compendium of Barley Diseases. 1982. Available online: http://agris.fao.org/agris-search/search.do?recordID=US8263958 (accessed on 17 December 2019).
- Kadariya, M. Progress from Five Years of Selecting for Resistance to Fusarium Head Blight Severity in Spring Wheat; South Dakota State University: Brookings, SD, USA, 2006. [Google Scholar]
- Paarry, D.W.; Jenkinson, P.; Mcleod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Path. 2010, 44, 207–238. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef]
- Al Masri, A.; Hau, B.; Dehne, H.-W.; Mahlein, A.-K.; Oerke, E.-C. Impact of primary infection site of Fusarium species on head blight development in wheat ears evaluated by IR-thermography. Eur. J. Plant Pathol. 2017, 147, 855–868. [Google Scholar] [CrossRef]
- Kang, Z.; Buchenauer, H. A cytological and ultrastructural study on the infection process of Fusarium culmorum on wheat spikes. Mycol. Res. 2000, 104, 1083–1093. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Diaz-Varela, R.; Angileri, V.; Loudjani, P. Tree height quantification using very high resolution imagery acquired from an unmanned aerial vehicle (UAV) and automatic 3D photo-reconstruction methods. Eur. J. Agron. 2014, 55, 89–99. [Google Scholar] [CrossRef]
- Roosjen, P.P.; Brede, B.; Suomalainen, J.M.; Bartholomeus, H.M.; Kooistra, L.; Clevers, J.G. Improved estimation of leaf area index and leaf chlorophyll content of a potato crop using multi-angle spectral data–potential of unmanned aerial vehicle imagery. Int. J. Appl. Earth Obs. 2018, 66, 14–26. [Google Scholar] [CrossRef]

| Experiments | Number of Field Survey Ears | ||
|---|---|---|---|
| Healthy | Fusarium Head Blight Infected | Sum | |
| Exp. 1 (2018) | 34 | 53 | 87 |
| Exp. 2 (2019) | 71 | 56 | 127 |
| Wavelet Features | Correlation Coefficient among Different Wavelet Features | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WF01 | WF02 | WF03 | WF04 | WF05 | WF06 | WF07 | WF08 | WF09 | WF10 | WF11 | WF12 | WF13 | WF14 | WF15 | WF16 | WF17 | WF18 | WF19 | WF20 | WF21 | |
| WF01 | 1.000 | ||||||||||||||||||||
| WF02 | 0.648 | 1.000 | |||||||||||||||||||
| WF03 | 0.507 | 0.737 | 1.000 | ||||||||||||||||||
| WF04 | 0.589 | 0.811 | 0.797 | 1.000 | |||||||||||||||||
| WF05 | 0.534 | 0.853 | 0.824 | 0.841 | 1.000 | ||||||||||||||||
| WF06 | 0.715 | 0.736 | 0.734 | 0.866 | 0.824 | 1.000 | |||||||||||||||
| WF07 | 0.606 | 0.739 | 0.711 | 0.849 | 0.844 | 0.940 | 1.000 | ||||||||||||||
| WF08 | 0.530 | 0.694 | 0.844 | 0.714 | 0.779 | 0.704 | 0.706 | 1.000 | |||||||||||||
| WF09 | 0.435 | 0.635 | 0.773 | 0.643 | 0.703 | 0.578 | 0.553 | 0.913 | 1.000 | ||||||||||||
| WF10 | 0.389 | 0.763 | 0.766 | 0.729 | 0.812 | 0.650 | 0.659 | 0.807 | 0.702 | 1.000 | |||||||||||
| WF11 | 0.491 | 0.799 | 0.844 | 0.796 | 0.851 | 0.727 | 0.731 | 0.871 | 0.747 | 0.970 | 1.000 | ||||||||||
| WF12 | 0.432 | 0.768 | 0.744 | 0.711 | 0.813 | 0.636 | 0.644 | 0.777 | 0.657 | 0.931 | 0.919 | 1.000 | |||||||||
| WF13 | 0.467 | 0.669 | 0.700 | 0.679 | 0.680 | 0.674 | 0.699 | 0.680 | 0.547 | 0.714 | 0.786 | 0.700 | 1.000 | ||||||||
| WF14 | 0.364 | 0.734 | 0.738 | 0.695 | 0.755 | 0.562 | 0.589 | 0.753 | 0.616 | 0.943 | 0.938 | 0.913 | 0.723 | 1.000 | |||||||
| WF15 | 0.373 | 0.759 | 0.748 | 0.714 | 0.784 | 0.581 | 0.604 | 0.766 | 0.650 | 0.960 | 0.947 | 0.910 | 0.709 | 0.987 | 1.000 | ||||||
| WF16 | 0.386 | 0.760 | 0.754 | 0.720 | 0.783 | 0.594 | 0.624 | 0.762 | 0.625 | 0.943 | 0.946 | 0.909 | 0.738 | 0.997 | 0.990 | 1.000 | |||||
| WF17 | 0.589 | 0.774 | 0.731 | 0.688 | 0.777 | 0.741 | 0.681 | 0.751 | 0.640 | 0.862 | 0.880 | 0.852 | 0.756 | 0.847 | 0.835 | 0.855 | 1.000 | ||||
| WF18 | 0.654 | 0.900 | 0.695 | 0.766 | 0.820 | 0.814 | 0.806 | 0.658 | 0.579 | 0.680 | 0.727 | 0.700 | 0.689 | 0.639 | 0.667 | 0.677 | 0.788 | 1.000 | |||
| WF19 | 0.536 | 0.827 | 0.689 | 0.700 | 0.710 | 0.554 | 0.548 | 0.665 | 0.636 | 0.710 | 0.750 | 0.695 | 0.646 | 0.733 | 0.757 | 0.751 | 0.693 | 0.757 | 1.000 | ||
| WF20 | 0.796 | 0.704 | 0.572 | 0.511 | 0.600 | 0.612 | 0.519 | 0.645 | 0.615 | 0.549 | 0.607 | 0.587 | 0.491 | 0.514 | 0.547 | 0.531 | 0.676 | 0.722 | 0.656 | 1.000 | |
| WF21 | 0.831 | 0.750 | 0.698 | 0.640 | 0.675 | 0.712 | 0.627 | 0.724 | 0.663 | 0.600 | 0.685 | 0.644 | 0.599 | 0.579 | 0.594 | 0.597 | 0.725 | 0.762 | 0.686 | 0.938 | 1.000 |
| Wavelet Features | Wavelength/nm | Scale | R2 | Significance of t-Test |
|---|---|---|---|---|
| WF02 | 471 | 4 | 0.539 | *** |
| WF06 | 696 | 1 | 0.602 | *** |
| WF09 | 841 | 4 | 0.441 | *** |
| WF11 | 963 | 3 | 0.548 | *** |
| WF13 | 1069 | 3 | 0.422 | *** |
| WF21 | 2272 | 4 | 0.544 | *** |
| Validation | Field Truth | ||||||
|---|---|---|---|---|---|---|---|
| Wavelet Features | Healthy | Fusarium Head Blight | Sum | UA/% | OA/% | Kappa Coefficient | |
| Six wavelet features in the whole spectral wavelength range | Healthy | 31 | 3 | 34 | 91.2 | 88.7 | 0.775 |
| Fusarium head blight | 5 | 32 | 37 | 86.5 | |||
| Sum | 36 | 35 | 71 | ||||
| PA/% | 86.1 | 91.4 | |||||
| Four wavelet features concentrated in the range of 400–1000 nm | Healthy | 29 | 3 | 32 | 90.6 | 85.9 | 0.719 |
| Fusarium head blight | 7 | 32 | 39 | 82.1 | |||
| Sum | 36 | 35 | 71 | ||||
| PA/% | 80.6 | 91.4 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Huang, W.; Jing, Y.; Pignatti, S.; Laneve, G.; Dong, Y.; Ye, H.; Liu, L.; Guo, A.; Jiang, J. Identification of Fusarium Head Blight in Winter Wheat Ears Using Continuous Wavelet Analysis. Sensors 2020, 20, 20. https://doi.org/10.3390/s20010020
Ma H, Huang W, Jing Y, Pignatti S, Laneve G, Dong Y, Ye H, Liu L, Guo A, Jiang J. Identification of Fusarium Head Blight in Winter Wheat Ears Using Continuous Wavelet Analysis. Sensors. 2020; 20(1):20. https://doi.org/10.3390/s20010020
Chicago/Turabian StyleMa, Huiqin, Wenjiang Huang, Yuanshu Jing, Stefano Pignatti, Giovanni Laneve, Yingying Dong, Huichun Ye, Linyi Liu, Anting Guo, and Jing Jiang. 2020. "Identification of Fusarium Head Blight in Winter Wheat Ears Using Continuous Wavelet Analysis" Sensors 20, no. 1: 20. https://doi.org/10.3390/s20010020
APA StyleMa, H., Huang, W., Jing, Y., Pignatti, S., Laneve, G., Dong, Y., Ye, H., Liu, L., Guo, A., & Jiang, J. (2020). Identification of Fusarium Head Blight in Winter Wheat Ears Using Continuous Wavelet Analysis. Sensors, 20(1), 20. https://doi.org/10.3390/s20010020







