Feedback Design in Targeted Exercise Digital Biofeedback Systems for Home Rehabilitation: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- Interventions: technology-based interventions where the user receives biofeedback. We will include only systems which have been evaluated on the target clinical population within its intended context of use.
- Exercise type: targeted exercises, i.e., specific rehabilitation movements which aim to improve strength, ROM or function in a specific joint, muscle or group of muscles. They are most often prescribed in terms of ‘repetitions’ and ‘sets’ and are intended to be repeated and progressed over time.
- Setting: the systems should be designed for use in an unsupervised home rehabilitation setting.
- Population: any clinical population receiving targeted exercises for rehabilitation.
- Studies written in the English language, from 2000 to July 2019.
2.2. Study Selection
2.3. Data Extraction
2.4. Data Analysis
3. Results
3.1. Search Results
3.2. System Characteristics
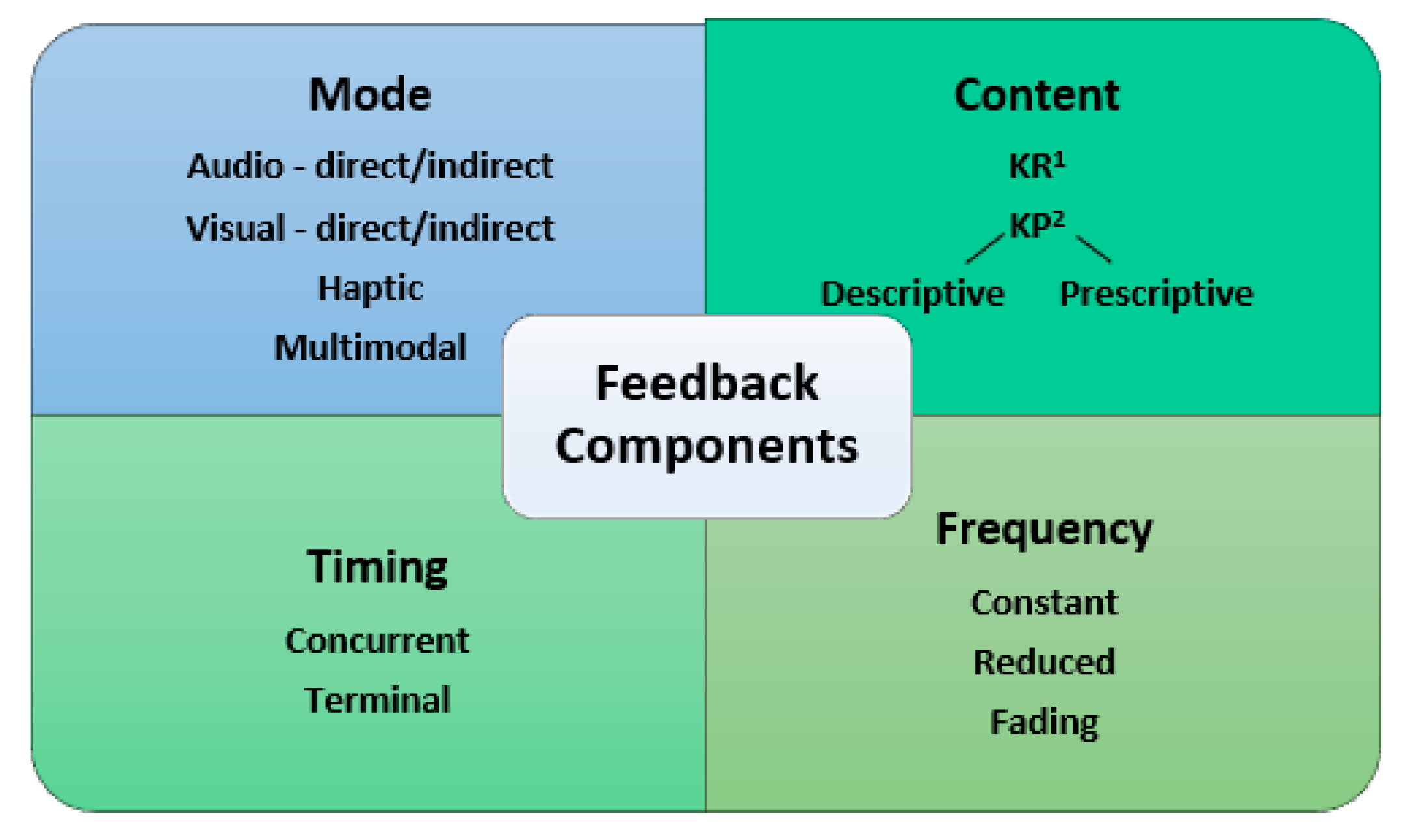
3.3. Feedback Components
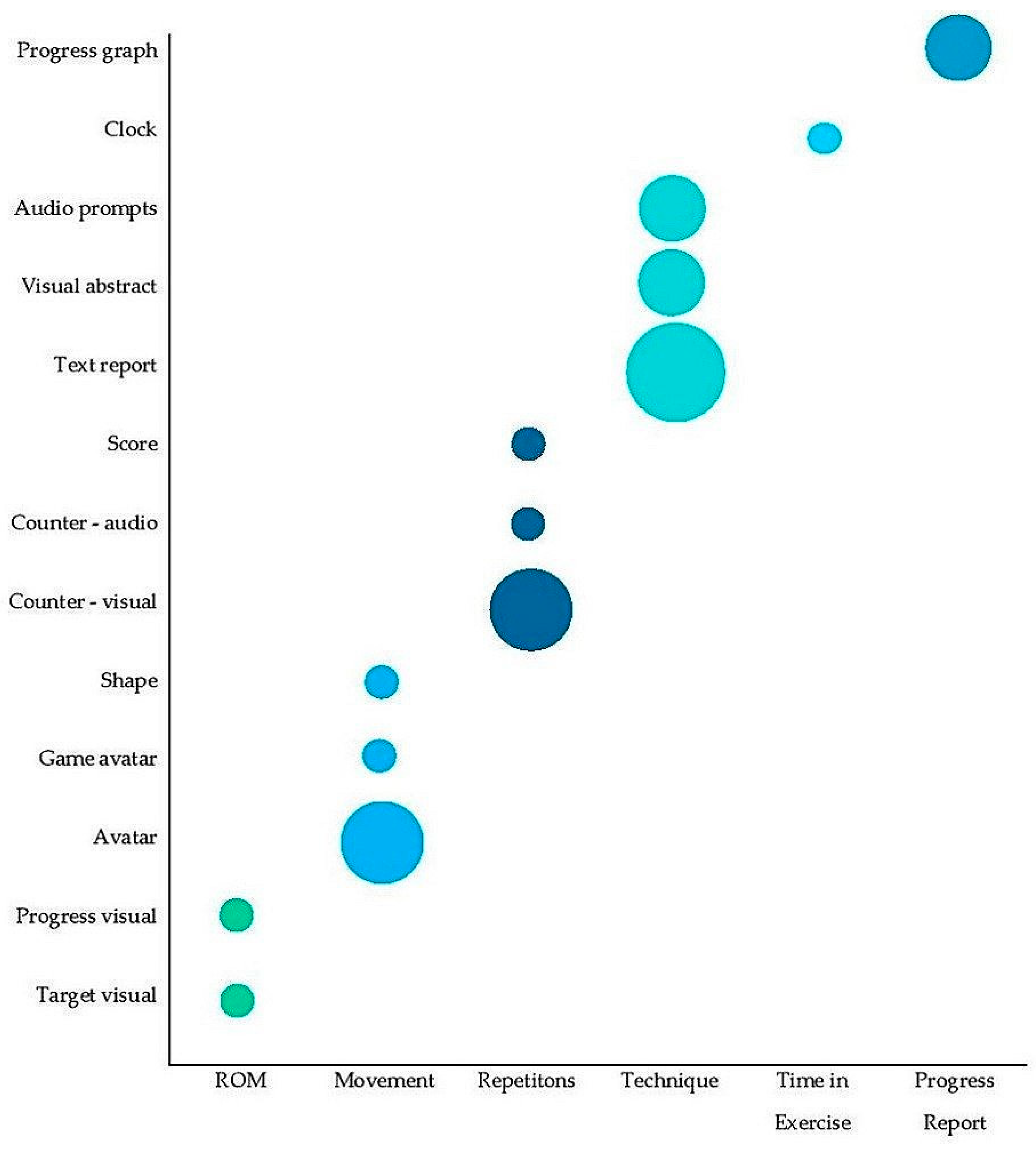
3.4. Feedback of Exercise Components
3.5. Evaluation of Feedback
4. Discussion
4.1. Systems Identified
4.2. Feedback Components
4.3. Rationale for Feedback
4.4. Feedback Evaluation
4.5. Review Limitations
4.6. Recommendations for Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fernando, C.K.; Basmajian, J.V. Biofeedback in Physical Medicine and Rehabilitation. Biofeedback Self. Regul. 1978, 3, 435–455. [Google Scholar] [CrossRef]
- Frank, D.L.; Khorshid, L.; Kiffer, J.F.; Moravec, C.S.; McKee, M.G. Biofeedback in Medicine: Who, When, Why and How? Ment. Health Fam. Med. 2010, 7, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wolf, S.L.; He, J. Recent Developments in Biofeedback for Neuromotor Rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Persson, U.; Caulfield, B. Biofeedback in Rehabilitation. J. Neuroeng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Storberget, M.; Grødahl, L.H.J.; Snodgrass, S.; van Vliet, P.; Heneghan, N. Verbal Augmented Feedback in the Rehabilitation of Lower Extremity Musculoskeletal Dysfunctions: A Systematic Review. BMJ Open Sport Exerc. Med. 2017, 3, e000256. [Google Scholar] [CrossRef] [PubMed]
- Fergus, P.; Kafiyat, K.; Merabti, M.; Taleb-Bendiab, A.; El Rhalibi, A. Remote Physiotherapy Treatments Using Wireless Body Sensor Networks. In Proceedings of the 2009 International Conference on Wireless Communications and Mobile Computing: Connecting the World Wirelessly, Leipzig, Germany, 21–24 June 2009; Volume 1191. [Google Scholar]
- Rawstorn, J.C.; Gant, N.; Meads, A.; Warren, I.; Maddison, R. Remotely Delivered Exercise-Based Cardiac Rehabilitation: Design and Content Development of a Novel MHealth Platform. JMIR Mhealth Uhealth 2016, 4, e57. [Google Scholar] [CrossRef]
- Sigrist, R.; Rauter, G.; Riener, R.; Wolf, P. Augmented Visual, Auditory, Haptic, and Multimodal Feedback in Motor Learning: A Review. Psychon. Bull. Rev. 2013, 20, 21–53. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Amir, H.; Scheidt, R.A. Computerized Biofeedback Knee Goniometer: Acceptance and Effect on Exercise Behavior in Post-Total Knee Arthroplasty Rehabilitation. Arch. Phys. Med. Rehabil. 2004, 85, 1026–1030. [Google Scholar] [CrossRef]
- Afzal, M.R.; Oh, M.-K.; Choi, H.Y.; Yoon, J. A Novel Balance Training System Using Multimodal Biofeedback. Biomed. Eng. Online 2016, 15, 42. [Google Scholar] [CrossRef][Green Version]
- Huang, M.C.; Lee, S.H.; Yeh, S.C.; Chan, R.C.; Rizzo, A.; Xu, W.; Wu, H.L.; Lin, S.H. Intelligent Frozen Shoulder Rehabilitation. IEEE Intell. Syst. 2014, 29, 22–28. [Google Scholar] [CrossRef]
- Sharma, D.A.; Chevidikunnan, M.F.; Khan, F.R.; Gaowgzeh, R.A. Effectiveness of Knowledge of Result and Knowledge of Performance in the Learning of a Skilled Motor Activity by Healthy Young Adults. J. Phys. Ther. Sci. 2016, 28, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Mountain, G.; Hammerton, J. A Review of the Evidence Underpinning the Use of Visual and Auditory Feedback for Computer Technology in Post-Stroke Upper-Limb Rehabilitation. Disabil. Rehabil. Assist. Technol. 2011, 6, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, A.A.A.; Seelen, H.A.M.; Willmann, R.D.; Kingma, H. Technology-Assisted Training of Arm-Hand Skills in Stroke: Concepts on Reacquisition of Motor Control and Therapist Guidelines for Rehabilitation Technology Design. J. Neuroeng. Rehabil. 2009, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alhasan, H.; Hood, V.; Mainwaring, F. The Effect of Visual Biofeedback on Balance in Elderly Population: A Systematic Review. Clin. Interv. Aging 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Lauber, B.; Keller, M. Improving Motor Performance: Selected Aspects of Augmented Feedback in Exercise and Health. Eur. J. Sport Sci. 2014, 14, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Hekler, E.B.; Professor, A.; Andersson, G.; Collins, L.M.; Doherty, A.; Hollis, C.; Rivera, D.E.; West, R.; Wyatt, J.C.; et al. Evaluating Digital Health Interventions: Key Questions and Approaches. Am. J. Prev. Med. 2016, 51, 843–851. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Orthopaedic Surgeons. Total Knee Replacement Exercise Guide. Available online: https://orthoinfo.aaos.org/en/recovery/total-knee-replacement-exercise-guide (accessed on 11 December 2019).
- American Academy of Orthopaedic Surgeons. Total Hip Replacement Exercise Guide. Available online: https://orthoinfo.aaos.org/en/recovery/total-hip-replacement-exercise-guide (accessed on 11 December 2019).
- Cancer Council Australia. Exercises after Surgery: A Guide for People Who have had Breast Cancer Surgery. Available online: https://www.cancer.org.au/content/about_cancer/factsheets/Breastexercisesaftersurgery_FactSheet_September 2014.pdf (accessed on 11 December 2019).
- Arthritis Research UK; Chartered Society of Physiotherapists. Carpal Tunnel Syndrome. Available online: https://www.csp.org.uk/system/files/2_carpal_tunnel.pdf (accessed on 11 December 2019).
- Arthritis Research UK; Chartered Society of Physiotherapists. Tennis Elbow. Available online: https://www.csp.org.uk/system/files/6_tennis_elbow.pdf (accessed on 11 December 2019).
- Martin, L.R.; Haskard, K.B.; Dimatteo, M.R. The Challenge of Patient Adherence. Ther. Clin. Risk Manag. 2005, 1, 189–199. [Google Scholar] [CrossRef]
- Bassett, S. Measuring Patient Adherence to Physiotherapy. J. Nov. Physiother. 2012, 2. [Google Scholar] [CrossRef]
- Husebø, A.M.L.; Dyrstad, S.M.; Søreide, J.A.; Bru, E. Predicting Exercise Adherence in Cancer Patients and Survivors: A Systematic Review and Meta-Analysis of Motivational and Behavioural Factor. J. Clin. Nurs. 2013, 22, 4–21. [Google Scholar] [CrossRef]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to Treatment Adherence in Physiotherapy Outpatient Clinics: A Systematic Review. Man. Ther. 2009, 15, 220–228. [Google Scholar] [CrossRef]
- Chamorro-Moriana, G.; Moreno, A.J.; Sevillano, J.L. Technology-Based Feedback and Its Efficacy in Improving Gait Parameters in Patients with Abnormal Gait: A Systematic Review. Sensors 2018, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.; van den Noort, J.C.; Dekker, J.; Harlaar, J. Gait Retraining With Real-Time Biofeedback to Reduce Knee Adduction Moment: Systematic Review of Effects and Methods Used. Arch. Phys. Med. Rehabil. 2017, 98, 137–150. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, L.M.A.; Barnes, A.; Wheat, J.S.; Heller, B.W. The Use of Biofeedback for Gait Retraining: A Mapping Review. Clin. Biomech. 2018, 59, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Z.H.; Wong, D.W.C.; Lam, W.K.; Wan, A.H.P.; Lee, W.C.C. Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review. Sensors 2016, 16, 442. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.F.C.; Sampaio, L.M.M.; Biasotto-Gonzalez, D.A.; dos Reis Nagano, R.C.; Lucareli, P.R.G.; Politti, F. Biofeedback for Pelvic Floor Muscle Training in Women with Stress Urinary Incontinence: A Systematic Review with Meta-Analysis. Physiotherapy 2019, 105, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Markopoulos, P.; Yu, B.; Chen, W.; Timmermans, A. Interactive Wearable Systems for Upper Body Rehabilitation: A Systematic Review. J. Neuroeng. Rehabil. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; Malley, L.O.; Arksey, H.; Malley, L.O. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2007, 8, 19–32. [Google Scholar] [CrossRef]
- Ananthanarayan, S.; Sheh, M.; Chien, A.; Profita, H.; Siek, K. Pt Viz: Towards a Wearable Device for Visualizing Knee Rehabilitation Exercises. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Paris, France, 27 April–2 May 2013; pp. 1247–1250. [Google Scholar]
- Argent, R.; Slevin, P.; Bevilacqua, A.; Neligan, M.; Daly, A. Wearable Sensor-Based Exercise Biofeedback for Orthopaedic Rehabilitation: A Mixed Methods User Evaluation of a Prototype System. Sensors 2019, 19, 432. [Google Scholar] [CrossRef]
- Blanquero, J.; Cortés-Vega, M.D.; García-Frasquet, M.Á.; Sánchez-Laulhé, P.R.; Nieto Díaz de los Bernardos, M.I.; Suero-Pineda, A. Exercises Using a Touchscreen Tablet Application Improved Functional Ability More than an Exercise Program Prescribed on Paper in People after Surgical Carpal Tunnel Release: A Randomised Trial. J. Physiother. 2019, 65, 81–87. [Google Scholar] [CrossRef]
- Doyle, J.; Bailey, C.; Dromey, B.; Scanaill, C.N. BASE—An Interactive Technology Solution to Deliver Balance and Strength Exercises to Older Adults. In Proceedings of the 2010 4th International Conference on Pervasive Computing Technologies for Healthcare, Munich, Germany, 22–25 March 2010. [Google Scholar]
- Lin, L.-F.; Lin, Y.-J.; Lin, Z.-H.; Chuang, L.-Y.; Hsu, W.-C.; Lin, Y.-H. Feasibility and Efficacy of Wearable Devices for Upper Limb Rehabilitation in Patients with Chronic Stroke: A Randomized Controlled Pilot Study. Eur. J. Phys. Rehabil. Med. 2018, 54, 388–396. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X.; Lu, Z.; Cao, S.; Wu, D.; Zhang, X. Development of an EMG-ACC-Based Upper Limb Rehabilitation Training System. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Ter Meer, L.P.; Yumak, Z.; Veltkamp, R.C. Usability Test of Exercise Games Designed for Rehabilitation of Elderly Patients After Hip Replacement Surgery: Pilot Study. JMIR Serious Games 2017, 5, e19. [Google Scholar] [CrossRef] [PubMed]
- Spina, G.; Huang, G.; Vaes, A.; Spruit, M.; Amft, O. COPDTrainer: A Smartphone-Based Motion Rehabilitation Training System with Real-Time Acoustic Feedback. In Proceedings of the ACM International Joint Conference on Pervasive and Ubiquitous Computing, Zurich, Switzerland, 8–12 September 2013; pp. 597–606. [Google Scholar]
- Ayoade, M.; Uzor, S.; Baillie, L. The Development and Evaluation of an Interactive System for Age Related Musculoskeletal Rehabilitation in the Home. In 14th International Conference on Human-Computer Interaction (INTERACT); Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–18. [Google Scholar]
- Ayoade, M.; Baillie, L. A Novel Knee Rehabilitation System for the Home. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Toronto, ON, Canada, 26 April–1 May 2014; pp. 2521–2530. [Google Scholar]
- Correia, F.D.; Nogueira, A.; Magalhães, I.; Guimarães, J.; Moreira, M.; Barradas, I.; Teixeira, L.; Tulha, J.; Seabra, R.; Lains, J.; et al. Home-Based Rehabilitation With A Novel Digital Biofeedback System versus Conventional In-Person Rehabilitation after Total Knee Replacement: A Feasibility Study. Sci. Rep. 2018, 8, 11299. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.D.; Nogueira, A.; Magalhães, I.; Guimarães, J.; Moreira, M.; Barradas, I.; Molinos, M.; Teixeira, L.; Tulha, J.; Seabra, R.; et al. Medium-Term Outcomes of Digital Versus Conventional Home-Based Rehabilitation After Total Knee Arthroplasty: Prospective, Parallel-Group Feasibility Study. JMIR Rehabil. Assist. Technol. 2019, 6, e13111. [Google Scholar] [CrossRef]
- Giorgino, T.; Tormene, P.; Maggioni, G.; Capozzi, D.; Quaglini, S.; Pistarini, C. Assessment of Sensorized Garments as a Flexible Support to Self-Administered Post-Stroke Physical Rehabilitation. Eur. J. Phys. Rehabil. Med. 2009, 45, 75–84. [Google Scholar]
- Giorgino, T.; Tormene, P.; Maggioni, G.; Pistarini, C.; Quaglini, S. Wireless Support to Poststroke Rehabilitation: MyHearts Neurological Rehabilitation Concept. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 1012–1018. [Google Scholar] [CrossRef]
- Mecklenburg, G.; Smittenaar, P.; Erhart-Hledik, J.C.; Perez, D.A.; Hunter, S. Effects of a 12-Week Digital Care Program for Chronic Knee Pain on Pain, Mobility, and Surgery Risk: Randomized Controlled Trial. J. Med. Internet Res. 2018, 20. [Google Scholar] [CrossRef]
- Smittenaar, P.; Erhart-hledik, J.C.; Kinsella, R.; Hunter, S.; Perez, D. Translating Comprehensive Conservative Care for Chronic Knee Pain Into a Digital Care Pathway: 12-Week and 6-Month Outcomes for the Hinge Health Program. JMIR Rehabil. Assist. Technol. 2017, 4, 7258. [Google Scholar] [CrossRef]
- Durfee, W.K.; Weinstein, S.A.; Bhatt, E.; Nagpal, A.; Carey, J.R. Design and Usability of a Home Telerehabilitation System to Train Hand Recovery Following Stroke. J. Med. Devices Trans. ASME 2009, 3, 1–8. [Google Scholar] [CrossRef]
- Durfee, W.; Carey, J.; Nuckley, D.; Deng, J. Design and Implementation of a Home Stroke Telerehabilitation System. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 2422–2425. [Google Scholar]
- Carey, J.R.; Durfee, W.K.; Bhatt, E.; Nagpal, A.; Weinstein, S.A.; Anderson, K.M.; Lewis, S.M. Comparison of Finger Tracking versus Simple Movement Training via Telerehabilitation to Alter Hand Function and Cortical Reorganization after Stroke. Neurorehabil. Neural Repair 2007, 21, 216–232. [Google Scholar] [CrossRef]
- Schmidt, R.A. Frequent Augmented Feedback Can Degrade Learning: Evidence and Interpretations. In Tutorials in Motor Neuroscience; Springer: Berlin/Heidelberg, Germany, 1991; pp. 59–75. [Google Scholar] [CrossRef]
- Verbrugghe, J.; Knippenberg, E.; Palmaers, S.; Matheve, T.; Smeets, W.; Feys, P.; Spooren, A.; Timmermans, A. Motion Detection Supported Exercise Therapy in Musculoskeletal Disorders: A Systematic Review. Eur. J. Phys. Rehabil. Med. 2018, 54, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.; Leung, N.; Hardisty, M.; Whyne, C.; Henry, P.; McLachlin, S. Shoulder Physiotherapy Exercise Recognition: Machine Learning the Inertial Signals from a Smartwatch. Physiol. Meas. 2018, 39, 075007. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.; Sweeney, K.T.; Caulfield, B. The Use of Inertial Sensors for the Classification of Rehabilitation Exercises. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 2965–2968. [Google Scholar]
- Bavan, L.; Surmacz, K.; Beard, D.; Mellon, S.; Rees, J. Adherence Monitoring of Rehabilitation Exercise with Inertial Sensors: A Clinical Validation Study. Gait Posture 2019, 70, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Brennan, L.; Argent, R.; Caulfield, B.; Kechadi, T. Segmenting Multivariate Time Series for Autonomous Rehabilitation Feedback Systems with ConvFSM. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 201.
- Kirnap, M.; Calis, M.; Turgut, A.O.; Halici, M.; Tuncel, M. The Efficacy of EMG-Biofeedback Training on Quadriceps Muscle Strength in Patients after Arthroscopic Meniscectomy. N. Z. Med. J. 2005, 118, 1–9. [Google Scholar]
- Huang, H.Y.; Lin, J.J.; Guo, Y.L.; Wang, W.T.J.; Chen, Y.J. EMG Biofeedback Effectiveness to Alter Muscle Activity Pattern and Scapular Kinematics in Subjects with and without Shoulder Impingement. J. Electromyogr. Kinesiol. 2013, 23, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.; Cairns, M.; Stokes, M. Use of Ultrasound Imaging by Physiotherapists: A Pilot Study to Survey Use, Skills and Training. Man. Ther. 2012, 17, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A Review of Wearable Sensors and Systems with Application in Rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 21. [Google Scholar] [CrossRef]
- Gómez-Espinosa, A.; Espinosa-Castillo, N.; Valdés-Aguirre, B. Foot-Mounted Inertial Measurement Units-Based Device for Ankle Rehabilitation. Appl. Sci. 2018, 8, 2032. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Bohensky, M.A.; Zomer, E.; Tacey, M.; Gorelik, A.; Brand, C.A.; De Steiger, R. The Projected Burden of Primary Total Knee and Hip Replacement for Osteoarthritis in Australia to the Year 2030. BMC Musculoskelet. Disord. 2019, 20, 90. [Google Scholar] [CrossRef]
- Ciou, S.H.; Hwang, Y.S.; Chen, C.C.; Luh, J.J.; Chen, S.C.; Chen, Y.L. Football APP Based on Smart Phone with FES in Drop Foot Rehabilitation. Technol. Heal. Care 2017, 25, 541–555. [Google Scholar] [CrossRef]
- Delbressine, F.; Timmermans, A.; Beursgens, L.; De Jong, M.; Van Dam, A.; Verweij, D.; Janssen, M.; Markopoulos, P. Motivating Arm-Hand Use for Stroke Patients by Serious Games. In Proceedings of the 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 8 August–1 September 2012; pp. 3564–3567. [Google Scholar]
- Schoenfeld, B.J.; Grgic, J.; Krieger, J. How Many Times per Week Should a Muscle Be Trained to Maximize Muscle Hypertrophy? A Systematic Review and Meta-Analysis of Studies Examining the Effects of Resistance Training Frequency. J. Sports Sci. 2019, 37, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.F.; Mollineda, A.; Casero, J.G.; Pla, F. A RGBD-Based Interactive System for Gaming-Driven Rehabilitation of Upper Limbs. Sensors 2019, 19, 3478. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, H.; Xu, W.; Wallis, R.I.; Sundaram, H.; Rikakis, T.; Ingalls, T.; Olson, L.; He, J. The Design of a Real-Time, Multimodal Biofeedback System for Stroke Patient Rehabilitation. In Proceedings of the 14th ACM International Conference on Multimedia, Santa Barbara, CA, USA, 23–27 October 2006; pp. 763–772. [Google Scholar]
- Blandin, Y.; Toussaint, L.; Shea, C.H. Specificity of Practice: Interaction Between Concurrent Sensory Information and Terminal Feedback. J. Exp. Psychol. Learn. Mem. Cogn. 2008, 34, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, R.; Newell, K. Influence of Augmented Feedback on Coordination Strategies. J. Mot. Behav. 2009, 41, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Magil, R. Motor Learning and Control, Concepts and Applications, 8th ed.; The McGraw-Hill Companies: New York, NY, USA, 2007. [Google Scholar]
- Aoyagi, Y.; Ohnishi, E.; Yamamoto, Y. Feedback Protocol of ‘Fading Knowledge of Results’ Is Effective for Prolonging Motor Learning Retention. J. Phys. Ther. Sci. 2019, 31, 687–691. [Google Scholar] [CrossRef]
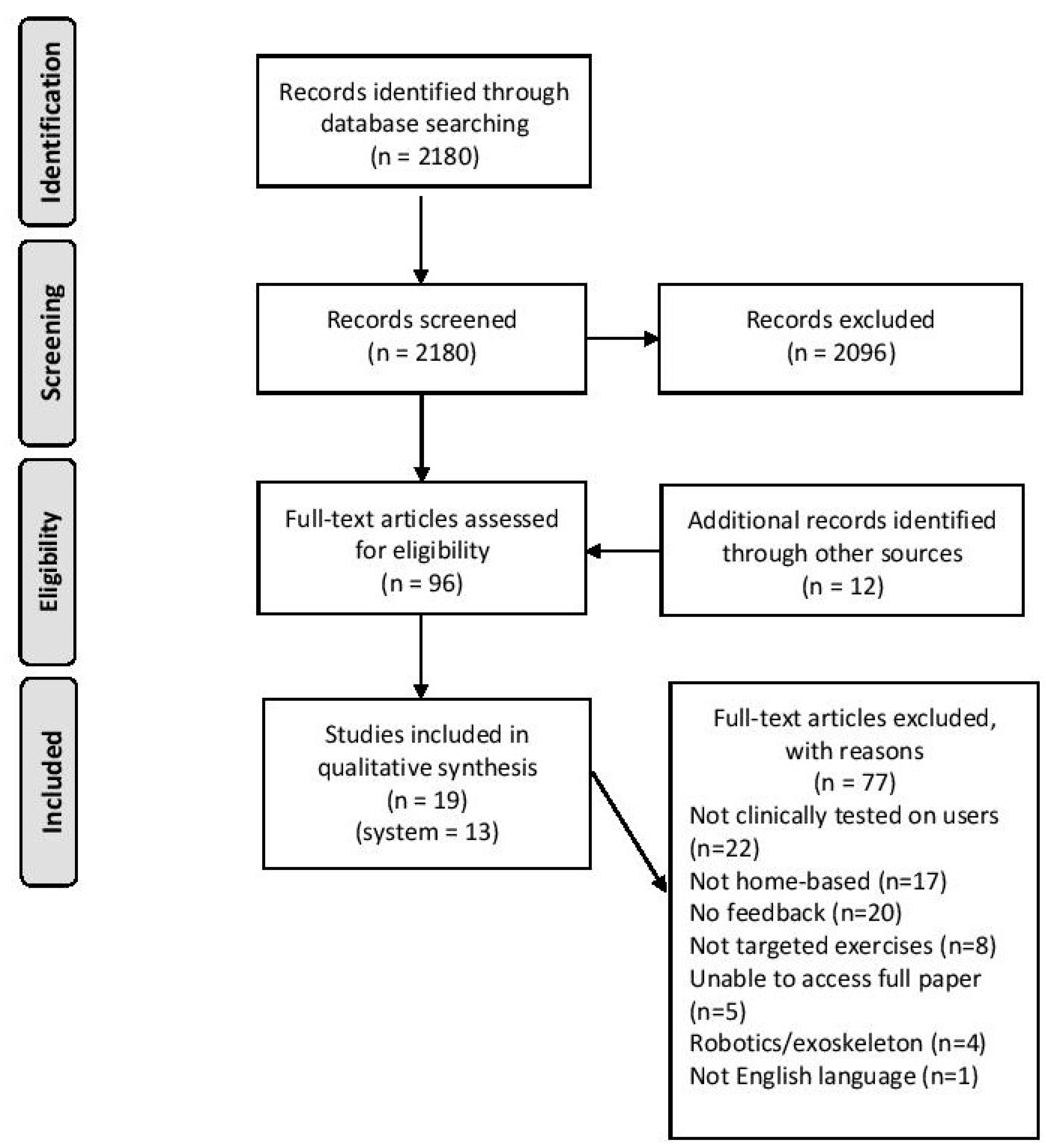
| Database | Search Strategy |
|---|---|
| PubMed | ((mobile app* OR mhealth OR mobile health OR ehealth OR smartphone OR acceleromet* OR wearable OR sensor system OR sensor-based system OR IMU OR inertial measurement unit* OR internet) AND (biofeedback OR bio-feedback OR feedback) AND (rehabilitation OR physiotherapy* OR physical therap*)) |
| ACM | (+ (web internet “mobile app*” mhealth “mobile health” ehealth smartphone acceleromet* wearable “sensor system” “sensor-based system” IMU “inertial measurement unit*”) + (biofeedback “bio-feedback” feedback) + (rehabilitation physiotherapy* “physical therapist” “home exercise”)) |
| PEDro | Biofeedback ‘Feedback technology’ Mhealth ‘Technology rehabilitation’ ‘Mobile rehabilitation’ ‘Mobile exercise’ ‘Wearable’ ‘Sensor exercise’ |
| Ref | Clinical Context | System Components | Feedback Design |
|---|---|---|---|
| Ananthanarayan et al., 2013 [34] ‘PT Viz’ | Condition: Chronic knee pain/post knee surgery Exercise: knee flexion and extension | Input sensor: neoprene bend sensor at back of knee, held in place by neoprene sleeves around thigh and calf Feedback device: electroluminescent wire lights in thigh sleeve | As user bends the knee, bars of electroluminescent wire light up; fully lit bars indicate full knee bend. |
| Argent et al., 2019 [35] | Condition: TKR 1 or UKR 2 Exercise: post-operative knee ROM and strengthening | Input sensor: IMU 3 in sleeve around calfFeedback device: tablet with application | Tablet application displays a 3D human avatar mirroring user’s lower limb movement. Repetitions are indicated with beeping noise and on-screen counter. A text report provides technique feedback. |
| Ayoade et al., 2013 [42], 2014 * [43] | Condition: TKR; falls Exercise: post-operative knee; falls rehabilitation | Input sensor: IMU (two for knee module, six for falls) Feedback device: computer with visualisation software | A stick-figure avatar simulates lower limb (knee) or body (falls) movements. The knee module contains a coloured fan graphic to indicate ROM progress, with corresponding colours indicating ROM per-repetition and a weekly progress chart. |
| Blanquero et al., 2019 [36] ‘ReHand’ | Condition: carpal tunnel release Exercise: fingers & wrist mobility dexterity, co-ordination | Input sensor: tablet touch screen Feedback device: tablet with Android application | The user performs exercises by touching the screen. Application displays exercise instructions and circles on which to place fingertips. Circles move with fingers, providing feedback on direction of movement and proximity to target. A countdown clock appears on screen. |
| Correia et al., 2018 * [44], 2019 [45] | Condition: TKR Exercise: post-operative knee ROM 4 and strengthening | Input sensor: IMU (3: calf, thigh, chest) Feedback device: tablet with application | The user aims to fill a ROM progress bar, earning stars by surpassing the target ROM. Movement or posture errors are communicated with audio and video feedback. A simple human avatar displaying user’s posture, a repetition counter and a timer also appear on screen. |
| Doyle et al., 2010 [37] ‘BASE’ | Condition: older adults at risk of falls Exercise: Otago programme for strength & balance | Input sensor: IMU (2), webcam/tracking markers (3) Feedback device: laptop with application | An avatar simulating user’s movements is superimposed with a ROM target line. Repetitions are counted on screen as the lower limbs passes this line. Walking exercises utilise audio prompts for feedback. Weekly progress record reports on compliance, target acquisition and repetition counts. |
| Durfee et al., 2009 * [50], Durfee et al. 2009 [51], Carey et al. 2007 [52] | Condition: CVA 5 Exercise: wrist & finger extension | Input sensor: electrogoniometer Microcontroller interface box Feedback device: laptop with application | Joint motions control the movement of a ball on screen. The user must trace a variety of waveform patterns with the ball. The resulting trace provides accuracy feedback, as does a text-based technique report and accuracy score. |
| Giorgino et al., 2009 * [46], 2009 [47] ‘NR System’ | Condition: CVA Exercise: upper limb simple movements; eating & combing | Input sensor: garment with kinesthetic strains sensors Feedback device: computer with application | Motion recognition software is trained by user performing exercises under supervision. User then exercises independently, and computer displays repetition counter and smiling/frowning faces indicating repetition classification. |
| Lin et al., 2018 [38] | Condition: CVA Exercise: upper limb simple movements | Input sensor: IMU (2: upper arm, forearm) Feedback device: smartphone with application | Smartphone application displays a human avatar simulating movement in front or side views. After six repetitions, system provides auditory and visual technique feedback and prompts. |
| Ling et al., 2017 [40] ‘Fietsgame’ | Condition: THR 6 Exercise: lower limb, e.g., steps, squats, lunges | Input sensor: Microsoft Kinect V2 Feedback device: television monitor | A human avatar simulating user’s movement performs a programme of games (selection of six for exercises, and six for balance training). Gamification feedback elements include scores, awards and sounds. Additional feedback on results and performance from game-specific features, e.g., background avatars dancing/clap if exercise performed correctly. |
| Liu et al., 2017 [39] | Condition: cerebral palsy Exercise: upper limb simple movements | Input sensor: surface EMG 7 circuit, accelerometer Feedback device: tablet with application | Upper limb joint motion & muscle activity signals control three different games. Gaming-style avatars (bird, cat, magician) complete tasks with gamified audio/visual elements, scores, performance grading, mean absolute value. |
| Smittenaar et al., 2017 * [49], Mecklenburg 2018 [48] | Condition: chronic knee pain Exercise: knee ROM and strengthening | Input sensor: motion sensors (2: thigh and calf) Feedback device: smartphone with application | Android platform delivers real-time technique feedback and progress screen. |
| Spina et al., 2013 [41] ‘COPD 8 Trainer’ | Condition: COPD Exercise: upper and lower limb variety | Input sensor: smartphone (IMU) in holster (relocated throughout exercising) Feedback device: smartphone with application | Application features real-time audio error correction (e.g., ‘move slower’) and repetition counting. A performance summary appears after exercising. |
| Name | Mode | Timing | Content | Quality | Rationale for Type of FB |
|---|---|---|---|---|---|
| Ananthanarayan et al., 2013 [34] | Visual | Concurrent | KR 1 | Descriptive | Not stated |
| Argent et al., 2019 [35] | Visual & audio | Concurrent & delayed | KR & KP 2 | Descriptive & prescriptive | Not stated |
| Ayoade et al., 2013, 201 [42,43] | Visual | Concurrent & delayed | KR & KP | Descriptive | Not stated |
| Blanquero et al., 2019 [36] | Visual | Concurrent | KR | Descriptive | Not stated |
| Correia et al., 2018 [44] | Visual & audio | Concurrent & delayed | KR & KP | Unclear | Not stated |
| Doyle et al., 2010 [37] | Multimodal | Concurrent | KR | Descriptive | Multimodal feedback to compensate for sensory impairments. Real-time feedback to assist exercise completion. User preference dictated choice of audio and visual feedback style. |
| Durfee et al., 2009 [50], Durfee et al. 2009 [51], Carey et al., 2007 [52] | Visual | Concurrent & delayed | KR & KP | Descriptive & prescriptive | Faded frequency KP used to prevent excessive extrinsic feedback interfering with user’s intrinsic error detection capability. Constant KR used to maintain motivation levels. State that tracking training emphasises motor learning principles outlined in Schmidt et al. [53] |
| Giorgino et al., 2009 [46], 2009 [47] | Visual | Concurrent | KR & KP | Descriptive | Visual feedback adapted for cognitively impaired users. |
| Lin et al., 2018 [38] | Visual &audio | Concurrent & delayed | KR & KP | Descriptive & prescriptive | Not stated |
| Ling et al., 2017 [40] | Visual & audio | Concurrent & delayed | KR | Descriptive | Not stated (Game) |
| Liu et al., 2017 [39] | Visual & audio | Concurrent | KR & KP | Descriptive | Not stated (Game) |
| Mecklenburg et al., 2018 [48], Smittenaar et al., 2017 [49] | Visual | Concurrent | KR | Unclear | Not stated |
| Spina et al., 2013 [41] | Audio & Visual | Concurrent & delayed | KR & KP | Prescriptive | Not stated |
| Name | Study Design | Participant Characteristics | Methodology | Outcome Measures |
|---|---|---|---|---|
| Ananthanarayan et al., 2013 [34] | Usability case series | N 1 = 6 Sex = four females, two males Age = 20–37 Country = USA Inclusion = history of knee surgery (n = 4) or chronic knee pain (n = 2) | Background questionnaire and usability session, followed by semi-structured interview. | Think aloud protocol & semi-structured interviews. |
| Argent et al., 2019 [35] | Usability case series | N = 15 Sex = nine females, six males Age = 63 ± 8.32 years Country = Ireland Inclusion = recent history of TKR 2 or UKR 3 | Participants used system at home for two weeks, then completed outcome measures. The first group (n = 5) were recruited at the end of their acute rehabilitation, the second group were recruited prior to surgery and used the system throughout their rehabilitation experience. | US 4, uMARS 5, and semi-structured interview. |
| Ayoade et al., 2013 [42] | Within-subjects systems comparison study | N = 11 (falls n = 5, TKR = 6) Sex = three females, eight males Age = 60 years and above Country = Scotland Inclusion = >60 years, history of falls or history of knee replacement | Evaluation of both the knee and falls systems consisted of two single-session assessments: a lab-based usability study (n = 5) and a home-based systems comparison study (n = 6). In the home-based study, participants first completed the exercises using booklets, then using the feedback system. | Observations, repetition pace, questionnaires, and semi-structured interviews. |
| Ayoade et al., 2014 [43] | Randomised controlled trial | N = 21 Sex = 11 females, 10 males Age = 47–85 years Country = Scotland Inclusion = undergoing TKR surgery | Participants randomised into rehabilitation visualisation system group, who used the feedback system at home, and control group, who received standard care of exercise DVD and booklet. Duration: 6 weeks. | Knee ROM 6, Oxford Knee Score, Intrinsic Motivation Inventory, adherence questionnaire, and SUS. |
| Doyle et al., 2010 [37] | Usability focus groups and case series | N = 12 Sex = not stated Age = older adults Country = Ireland Inclusion = older adults | First usability session: participants performed exercises with system using each of four different types of visual feedback, then completed walking exercises to evaluate two types of audio feedback. Second usability session: participants used system at home, completed system-navigation tasks. | Think Aloud protocol, observations, and interviews. |
| Carey et al., 2007 [52] | Randomised controlled trial | N = 20 Sex = five females, 15 males Age = 66.65 ± 9.6 years Country = USA Inclusion = chronic CVA 7, 30–80 years, visually able to use system, minimum ROM criteria applied | Intervention group (n = 10) used full system including tracking feedback at home, control group used system without tracking feedback function. Completed 180 trials per day for 10 days. | Battery of clinical hand assessments-Box and Block, Jebsen Taylor, finger ROM, and finger tracking activation paradigm using fMRI 8 |
| Durfee et al., 2009 [50] | Usability study | N = 20 Sex = five females, 15 males Age = 66.65 ± 9.6 years Country = USA Inclusion = chronic CVA, 30–80 years, visually able to use system, minimum ROM criteria applied | Participants completed RCT 9 as described in Carey et al., above. Then answered usability survey via telephone. | ix-question Likert scale questionnaire. |
| Giorgino et al. 2009 [47] | Usability study | N = 13 Sex = four females, nine males Age = 32–79 (mean 50) years Country = Italy Inclusion = hemiplegia & mild motor/cognitive impairment post CVA | Participants used system and completed evaluation questionnaire (limited details available). | User satisfaction survey. |
| Ling et al., 2017 [40] | Pilot usability study | N = 9 (two physiotherapists, seven patients) Sex = six females, three males Age= 74.5 ± 8.3 years (patients) Country = Netherlands Inclusion = post hip joint replacement of hip hemi-arthroplasty | Patient participants played six games under the guidance of a physiotherapist during a 60 min session. All participants completed outcome measures afterwards. | elf-report questionnaires, ‘general feedback’, objective data from software, e.g., knee angle and step width. |
| Liu et al., 2017 [39] | i. Usability testing. ii. Intervention case series. | N = 20 Sex = 12 females, eight males Age = 8.7 ± 2.8 years Country = China Inclusion = children with cerebral palsy diagnosis, voluntary movement and ‘normal cognitive capacity’ | i. ‘Game experience testing’: participants (n = 20) played each game in controlled environment ii. ‘Training effect test’: participants (n = 3) completed game training 2–3 times a week for one month, followed by once a week for 1.5 months. | i. Questionnaire, training time. ii. Fugl-Meyer Assessment & ADL 10 scale for upper extremity. SEMG 11 force and game accuracy. |
| Spina et al., 2013 [41] | Pilot case series study | N = 7 Sex = four females, three males Age = 60 ± 10 years Country = Netherlands Inclusion = COPD 12, undergoing pulmonary rehabilitation | In controlled environment, participants received instructions and systems was set up during ‘teach mode’. Participants then independently completed three sets of ten repetitions of each exercise. | ystem accuracy Impact of audio feedback on performance. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brennan, L.; Dorronzoro Zubiete, E.; Caulfield, B. Feedback Design in Targeted Exercise Digital Biofeedback Systems for Home Rehabilitation: A Scoping Review. Sensors 2020, 20, 181. https://doi.org/10.3390/s20010181
Brennan L, Dorronzoro Zubiete E, Caulfield B. Feedback Design in Targeted Exercise Digital Biofeedback Systems for Home Rehabilitation: A Scoping Review. Sensors. 2020; 20(1):181. https://doi.org/10.3390/s20010181
Chicago/Turabian StyleBrennan, Louise, Enrique Dorronzoro Zubiete, and Brian Caulfield. 2020. "Feedback Design in Targeted Exercise Digital Biofeedback Systems for Home Rehabilitation: A Scoping Review" Sensors 20, no. 1: 181. https://doi.org/10.3390/s20010181
APA StyleBrennan, L., Dorronzoro Zubiete, E., & Caulfield, B. (2020). Feedback Design in Targeted Exercise Digital Biofeedback Systems for Home Rehabilitation: A Scoping Review. Sensors, 20(1), 181. https://doi.org/10.3390/s20010181





