MOFs-Derived Nano-CuO Modified Electrode as a Sensor for Determination of Hydrazine Hydrate in Aqueous Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
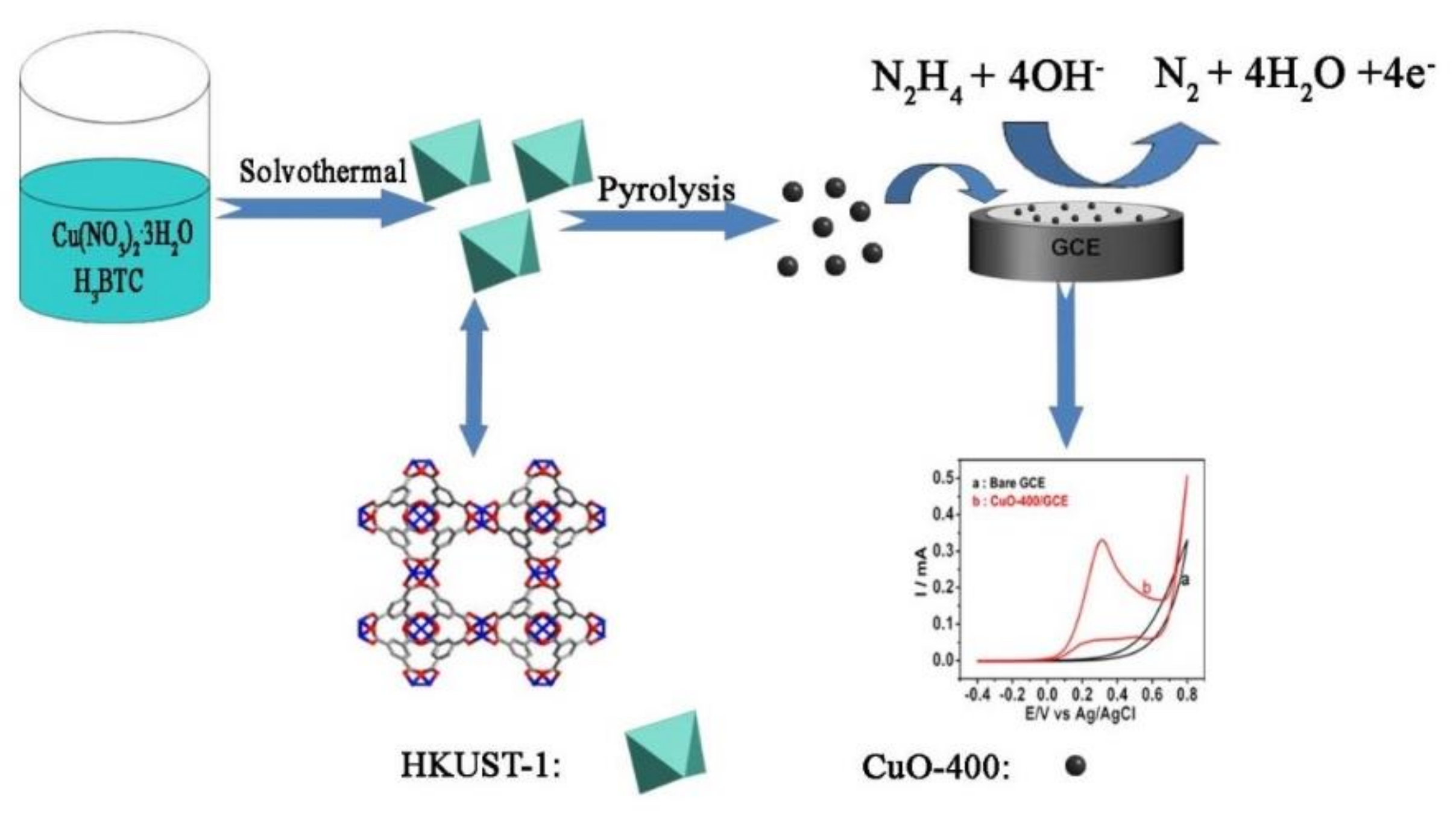
2.2. Preparation of Nano-CuO
2.3. Fabrication of Nano-CuO Sensor
2.4. Electrochemical Detection
3. Results and Discussion
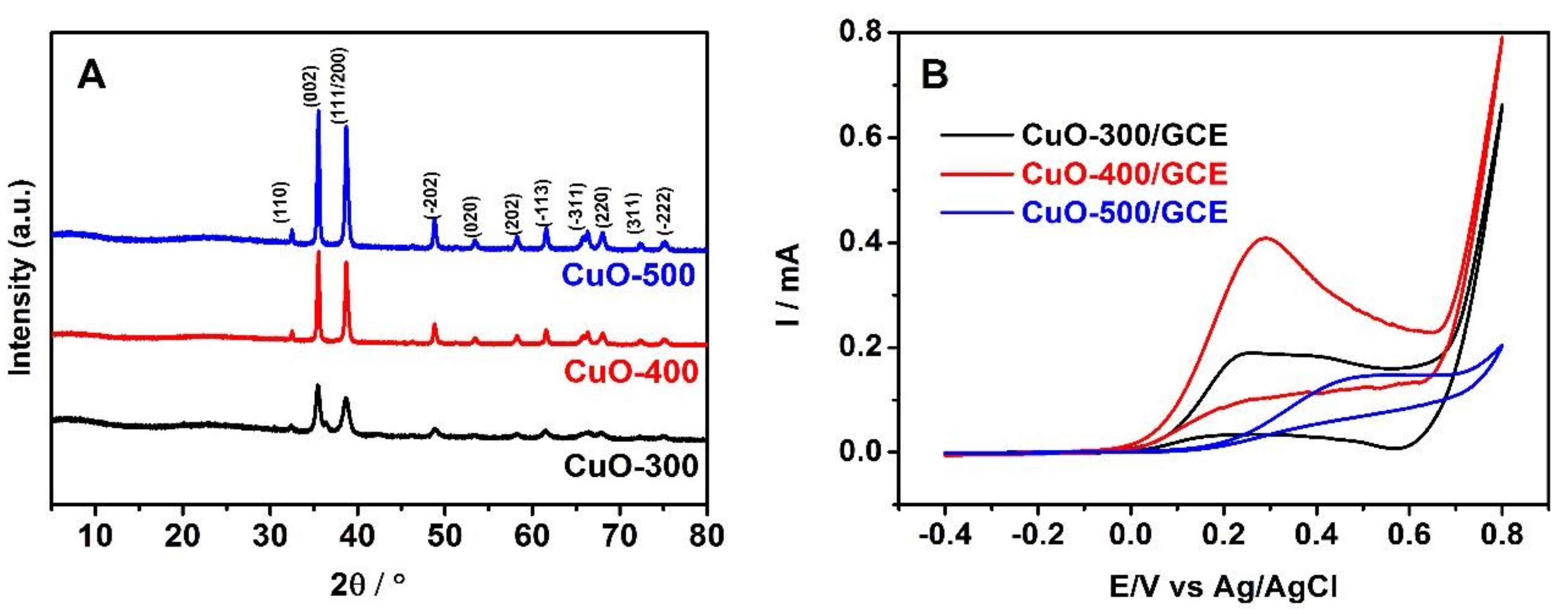
3.1. Characterization of HKUST-1
3.2. Analysis of Nano-CuO
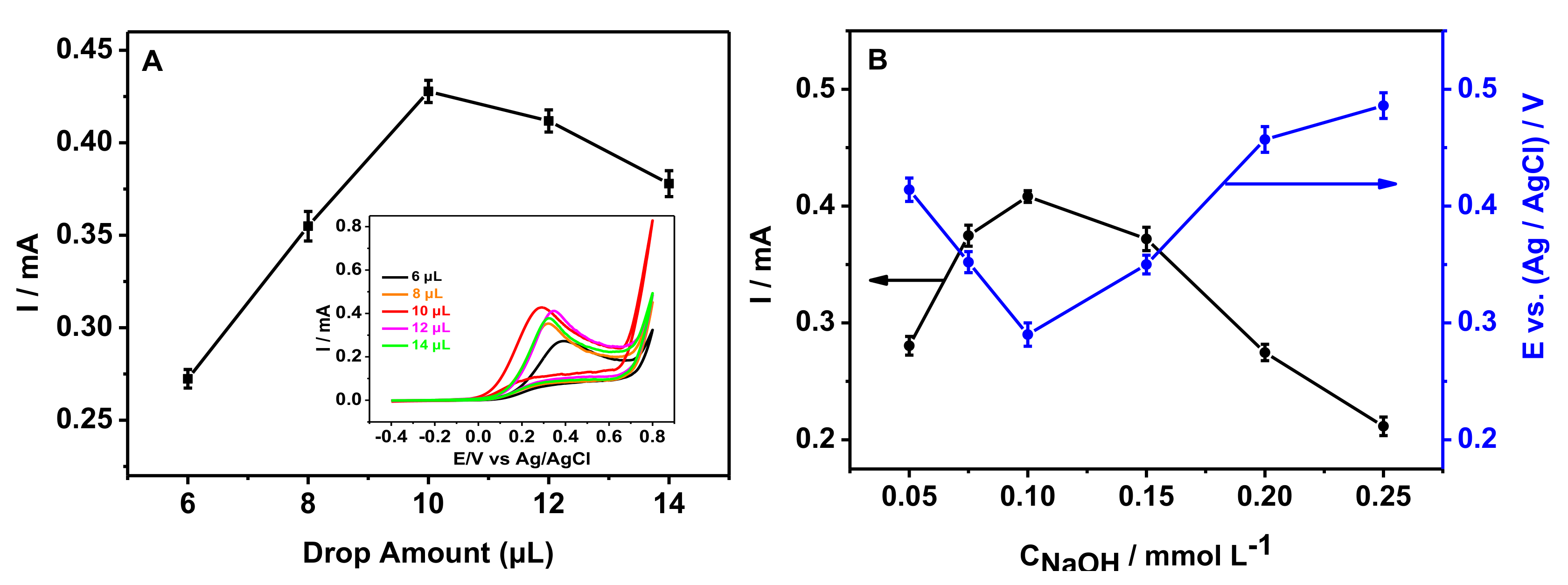
3.3. Optimization of the Experimental Conditions
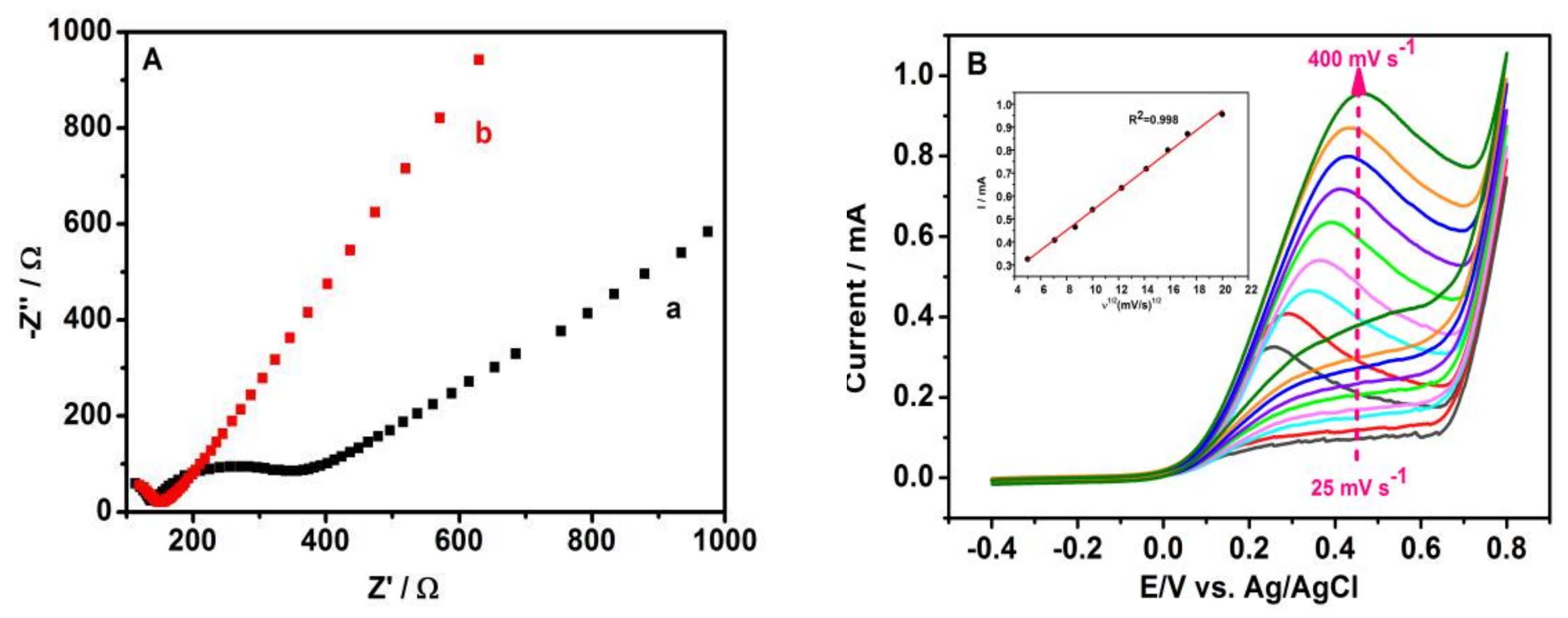
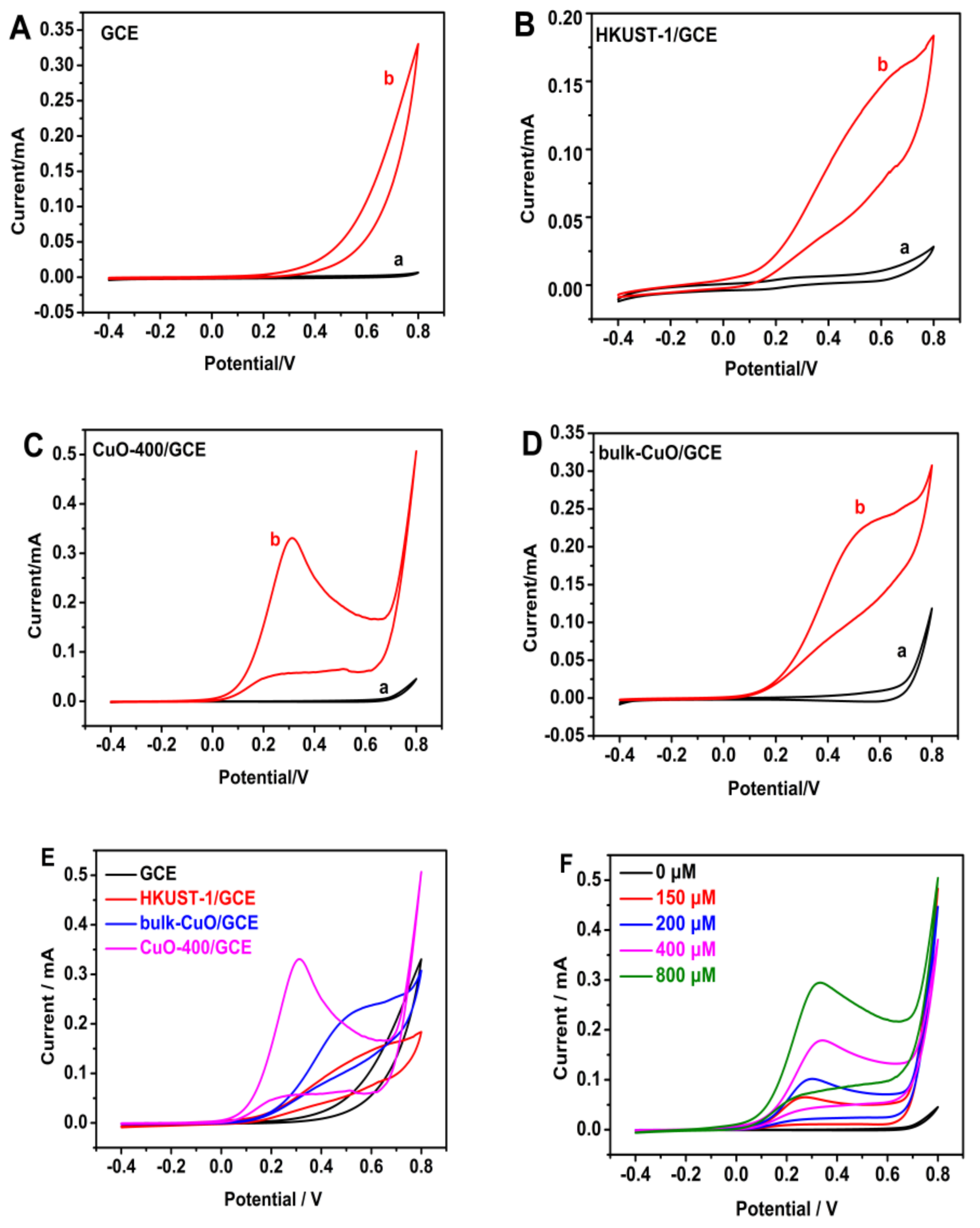
3.4. Electrochemical Properties of Nano-CuO-400/GCE Sensor
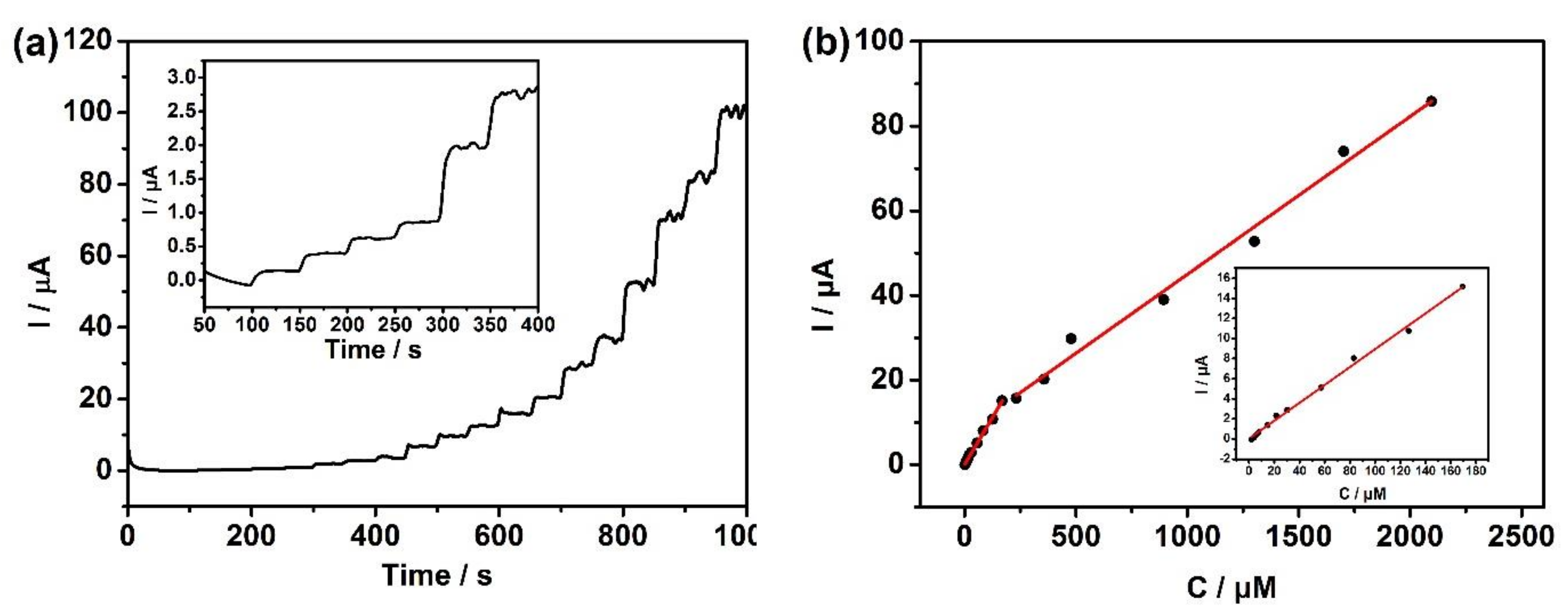
3.5. The Detection Limit of the Sensor for Hydrazine Hydrate
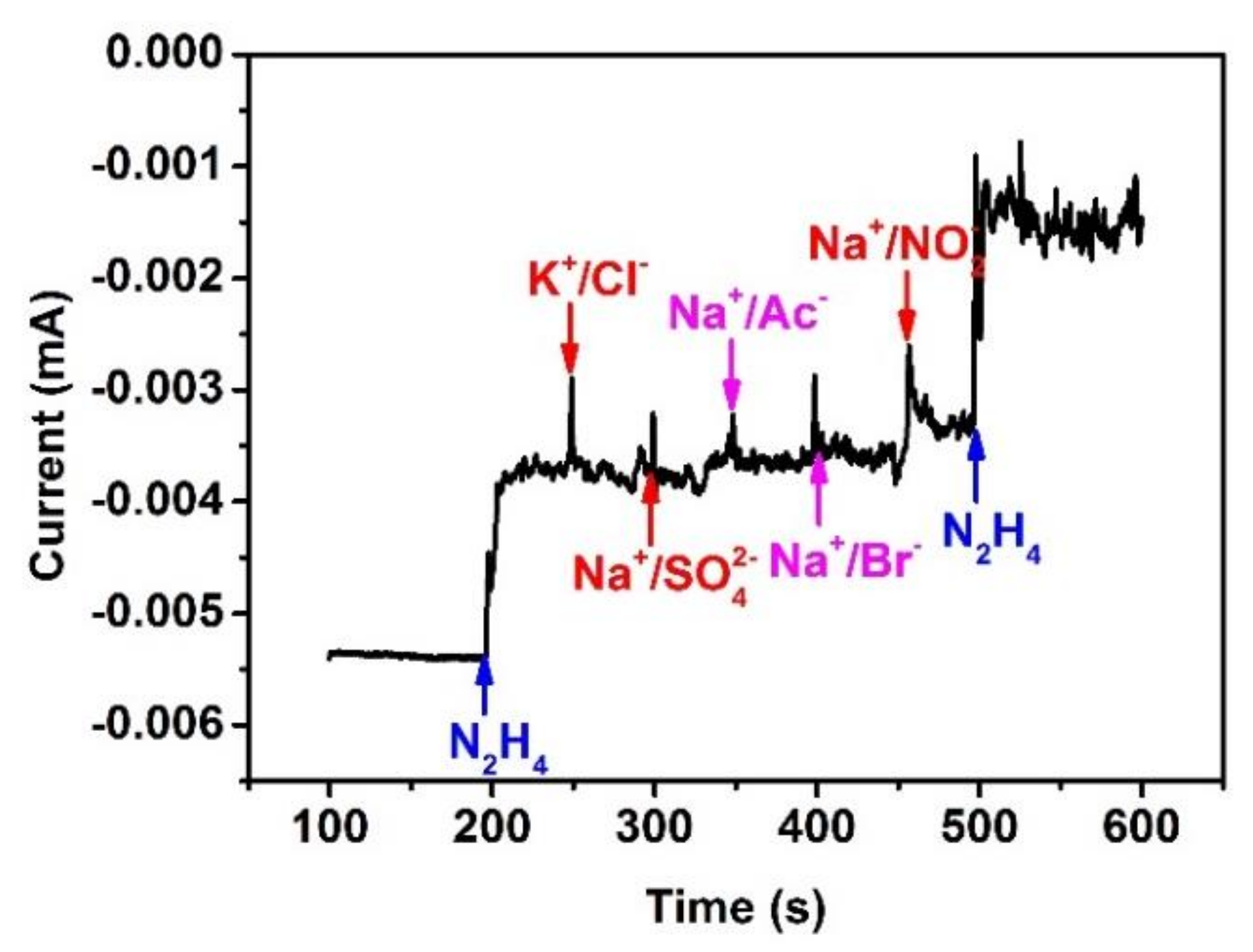
3.6. Anti-Interference Capability, Reproducibility, Recovery and Stability of the Sensor
3.7. Applications to the Water Samples
4. Conclusions
5. Data Availability
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Prathap, M.A.; Anuraj, V.; Satpati, B.; Srivastava, R. Facile preparation of Ni(OH)2–MnO2 hybrid material and its application in the electrocatalytic oxidation of hydrazine. J. Hazard. Mater. 2013, 262, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Zelnick, S.D.; Mattie, D.R.; Stepaniak, P.C. Occupational exposure to hydrazines: Treatment of acute central nervous system toxicity. Aviat. Spaceenviron. Med. 2003, 74, 1285–1291. [Google Scholar]
- Sun, M.; Guo, J.; Yang, Q.; Xiao, N.; Li, Y. A new fluorescent and colorimetric sensor for hydrazine and its application in biological systems. J. Mater. Chem. B 2014, 2, 1846–1851. [Google Scholar] [CrossRef]
- Afsharas, A.; Tsyrulneva, I.; Zaporozhets, O. Spectroscopic, visual test techniques and optical sensors for determination of hydrazine and its derivatives. Methods Objects Chem. Anal. 2015, 10, 97–107. [Google Scholar] [CrossRef]
- Liu, F.; Li, W.; Li, F.; Sun, S. Determination of hydrazine hydrate based on electrochemiluminescence of Ru(bpy)32+. Environ. Monit. Assess. 2013, 185, 4153–4158. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Narayanasastri, S.; Reddy, A.R.K. Single step derivatization with CF3 enone of thiophene at ambient temperature to determine propellant grade hydrazines: A study by GC and GC-MS. Analyst 2015, 140, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.A.; Shin, H.S. Simple and sensitive determination of hydrazine in drinking water by ultra-high-performance liquid chromatography-tandem mass spectrometry after derivatization with naphthalene-2, 3-dialdehyde. J. Chromatogr. A 2015, 1395, 73–78. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Sun, Z.; Xia, L.; Chen, G.; You, J. A simple and sensitive HPLC method based on pre-column fluorescence labelling for multiple classes of plant growth regulator determination in food samples. Food Chem. 2015, 170, 123–130. [Google Scholar] [CrossRef]
- Mani, V.; Huang, S.T.; Devasenathipathy, R.; Yang, T.C.K. Electropolymerization of cobalt tetraamino-phthalocyanine at reduced graphene oxide for electrochemical determination of cysteine and hydrazine. RSC Adv. 2016, 6, 38463–38469. [Google Scholar] [CrossRef]
- Helal, A.; Qamaruddin, M.; Aziz, M.A.; Shaikh, M.N.; Yamani, Z.H. MB-UiO-66-NH2 Metal-Organic Framework as Chromogenic and Fluorogenic Sensor for Hydrazine Hydrate in Aqueous Solution. ChemistrySelect 2017, 2, 7630–7636. [Google Scholar] [CrossRef]
- Panchompoo, J.; Aldous, L.; Downing, C.; Crossley, A.; Compton, R.G. Facile synthesis of Pd nanoparticle modified carbon black for electroanalysis: Application to the detection of hydrazine. Electroanalysis 2011, 23, 1568–1578. [Google Scholar] [CrossRef]
- Haghighi, B.; Hamidi, H.; Bozorgzadeh, S. Sensitive and selective determination of hydrazine using glassy carbon electrode modified with Pd nanoparticles decorated multiwalled carbon nanotubes. Anal. Bioanal. Chem. 2010, 398, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Luo, Y.; Lu, W.; Hu, J.; Liao, F.; Sun, X. Immobilization of Au nanoparticles on Au electrode for hydrazine detection: Using thiolated single-stranded DNA as a linker. Thin Solid Film 2011, 519, 6130–6134. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Zhang, L.; Zhang, H.; Li, C.M.; Lei, Y. Preparation of TiO2-Pt hybrid nanofibers and their application for sensitive hydrazine detection. Nanoscale 2011, 3, 1149–1157. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Dilbaghi, N.; Umar, A. Zinc oxide nanocones as potential scaffold for the fabrication of ultra-high sensitive hydrazine chemical sensor. Ceram. Int. 2015, 41, 3101–3108. [Google Scholar] [CrossRef]
- Umar, A.; Akhtar, M.S.; Al-Hajry, A.; Al-Assiri, M.S.; Dar, G.N.; Islam, M.S. Enhanced photocatalytic degradation of harmful dye and phenyl hydrazine chemical sensing using ZnO nanourchins. Chem. Eng. J. 2015, 262, 588–596. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, G.; He, P.; Lei, W.; Dong, F.; Yang, D.; Suo, Z. Novel one-pot hydrothermal fabrication of cuprous oxide-attapulgite/grapheme for non-enzyme glucose sensing. Anal. Methods 2015, 7, 2747–2753. [Google Scholar] [CrossRef]
- Gu, W.; Wang, M.; Mao, X.; Wang, Y.; Li, L.; Xia, W. A facile sensitive l-tyrosine electrochemical sensor based on a coupled CuO/Cu2O nanoparticles and multi-walled carbon nanotubes nanocomposite film. Anal. Methods 2014, 7, 1313–1320. [Google Scholar] [CrossRef]
- Luo, B.; Li, X.; Yang, J.; Li, X.; Xue, L.; Li, X.; Gu, J.; Wang, M.; Jiang, L. Non-enzymatic electrochemical sensors for the detection of hydrogen peroxide based on Cu2O/Cu nanocomposites. Anal. Methods 2014, 6, 1114–1120. [Google Scholar] [CrossRef]
- Le, W.Z.; Liu, Y.Q. Preparation of nano-copper oxide modified glassy carbon electrode by a novel film plating/potential cycling method and its characterization. Sens. Actuators B Chem. 2009, 141, 147–153. [Google Scholar] [CrossRef]
- Rosca, V.; Koper, M.T.M. Electrocatalytic oxidation of hydrazine on platinum electrodes in alkaline solutions. Electrochim. Acta 2008, 53, 5199–5205. [Google Scholar] [CrossRef]
- Khan, S.B.; Faisal, M.; Rahman, M.M. Highly sensitive and stable phenyl hydrazine chemical sensors based on CuO flower shapes and hollow spheres. New J. Chem. 2013, 37, 1098–1104. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Li, P.; Sang, S.; Zhang, W.; Hu, J.; Lian, K. A highly sensitive electrochemical sensor based on Cu/Cu2O@carbon nanocomposite structures for hydrazine detection. Anal. Methods 2015, 7, 9040–9046. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, L.; Yang, Z. An amperometric sensor for hydrazine based on nano-copper oxide modified electrode. J. Solid State Electrochem. 2011, 15, 821–827. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zhang, Y.B.; Lin, J.B.; Chen, X.M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Y.; Yao, Z.; Guo, J.; Lin, Q.; Zhang, J.; Zhang, Z.; Wei, F.; Xiang, S. An antiferromagnetic metalloring pyrazolate (Pz) framework with [Cu12(μ2-OH)12(Pz)12] nodes for separation of C2H2/CH4 mixture. J. Mater. Chem. A 2018, 6, 19681–19688. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, S.; Chen, L.; Huang, J.; Ma, Z.; Li, Z.; Yao, Z.; Zhang, J.; Zhang, Z.; Xiang, S. Additive-induced supramolecular isomerism and enhancement of robustness in Co(II)-based MOFs for efficiently trapping acetylene from acetylene-containing mixtures. ACS Appl. Mater. Interfaces 2018, 10, 30912–30918. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, S.; Chen, L.; Huang, J.; Ma, Z.; Li, Z.; Yao, Z.; Zhang, J.; Zhang, Z.; Xiang, S. Enhanced intrinsic proton conductivity of metal–organic frameworks by tuning the degree of interpenetration. Cryst. Growth Des. 2018, 18, 3724–3728. [Google Scholar]
- Wang, X.; Chen, Z.; Zhao, X.; Yao, T.; Chen, W.; You, R.; Zhao, C.; Wu, G.; Wang, J.; Huang, W.; et al. Regulation of coordination number over single Co sites: Triggering the efficient electroreduction of CO2. Angew. Chem. Int. Ed. 2018, 57, 1944–1948. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, C.; Wu, Z.Y.; Xiong, Y.; Xu, Q.; Yu, S.H.; Jiang, H.L. From bimetallic metal-organic framework to porous carbon: High surface area and multicomponent active dopants for excellent electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, D.; Ye, Y.; Wu, L.; Yao, Z.; Ma, X.; Wang, L.; Zhang, Z.; Xiang, S. Thermal conversion of MOF@MOF: Synthesis of an N-doped carbon material with excellent ORR performance. ChemPlusChem 2018, 83, 1044–1051. [Google Scholar] [CrossRef]
- Sun, X.; Lu, L.; Zhu, Q.; Wu, C.; Yang, D.; Chen, C.; Han, B. MoP nanoparticles supported on indium-doped porous carbon: Outstanding catalysts for highly efficient CO2 electroreduction. Angew. Chem. Int. Ed. 2018, 57, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Nair, S.V.; Rai, A.K. Low temperature synthesis of carbon-wrapped CuO synthesized without using a conventional carbon source for Li ion Battery Application. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 94, 113–117. [Google Scholar] [CrossRef]
- Choi, K.J.; Jang, H.W. One-dimensional oxide nanostructures as gas-sensing materials: Review and issues. Sensors 2010, 10, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ahammad, A.J.; Jin, J.H.; Ahn, S.J.; Lee, J.J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Prathap, M.A.; Kaur, B.; Srivastava, R. Hydrothermal synthesis of CuO micro-/nanostructures and their applications in the oxidative degradation of methylene blue and non-enzymatic sensing of glucose/H2O2. J. Colloid Interface Sci. 2012, 370, 144–154. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W.; Wu, X. Copper sulfide reduced graphene oxide nanocomposite for detection of hydrazine and hydrogen peroxide at low potential in neutral medium. Electrochim. Acta 2014, 123, 260–267. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Wu, D.; Li, Z.; Lin, Q.; Ma, X.; Zhang, Z.; Xiang, S. MOFs-Derived Nano-CuO Modified Electrode as a Sensor for Determination of Hydrazine Hydrate in Aqueous Medium. Sensors 2020, 20, 140. https://doi.org/10.3390/s20010140
Lu Y, Wu D, Li Z, Lin Q, Ma X, Zhang Z, Xiang S. MOFs-Derived Nano-CuO Modified Electrode as a Sensor for Determination of Hydrazine Hydrate in Aqueous Medium. Sensors. 2020; 20(1):140. https://doi.org/10.3390/s20010140
Chicago/Turabian StyleLu, Yaqi, Dan Wu, Ziyin Li, Quanjie Lin, Xiuling Ma, Zhangjing Zhang, and Shengchang Xiang. 2020. "MOFs-Derived Nano-CuO Modified Electrode as a Sensor for Determination of Hydrazine Hydrate in Aqueous Medium" Sensors 20, no. 1: 140. https://doi.org/10.3390/s20010140
APA StyleLu, Y., Wu, D., Li, Z., Lin, Q., Ma, X., Zhang, Z., & Xiang, S. (2020). MOFs-Derived Nano-CuO Modified Electrode as a Sensor for Determination of Hydrazine Hydrate in Aqueous Medium. Sensors, 20(1), 140. https://doi.org/10.3390/s20010140




