Preparation and Investigation of Silver Nanoparticle–Antibody Bioconjugates for Electrochemical Immunoassay of Tick-Borne Encephalitis
Abstract
1. Introduction
2. Materials and Methods
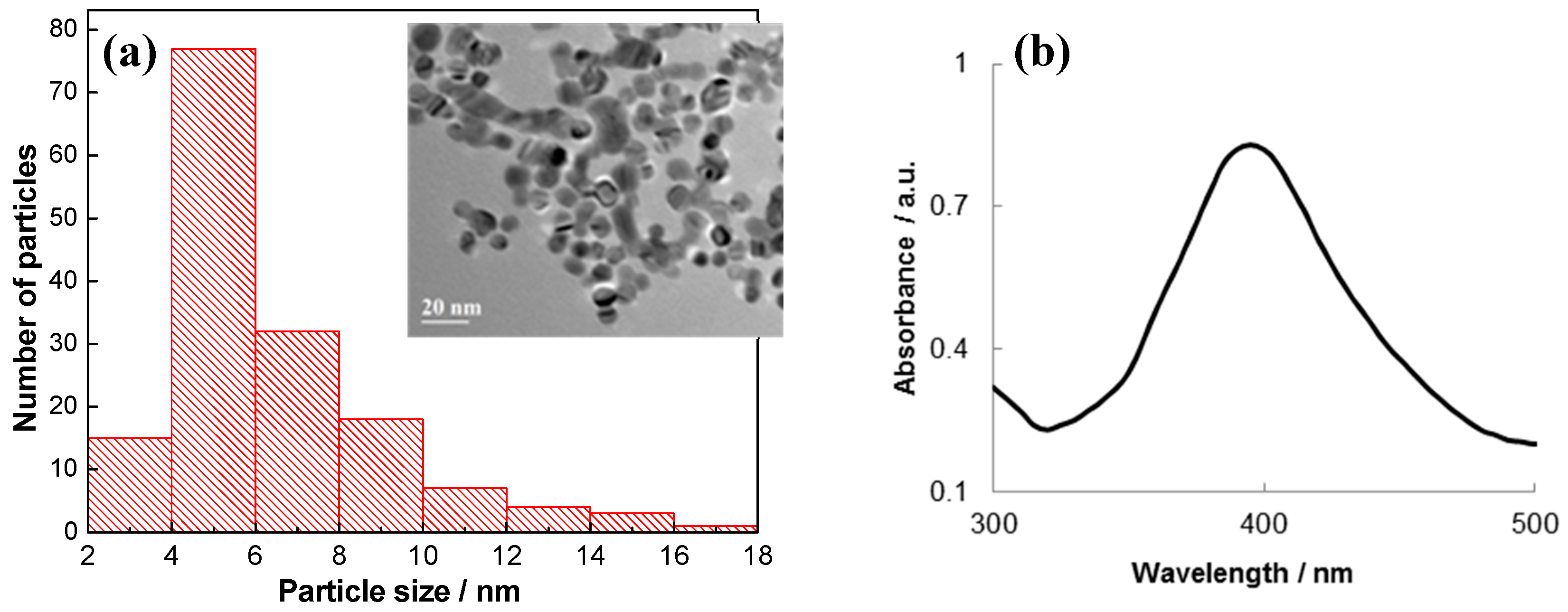
2.1. The Synthesis and Characterization of Ag NPs
2.2. Stabilizing Ag NPs with BSA
2.3. Flocculation Test
2.4. Preparation of Ab@AgNP
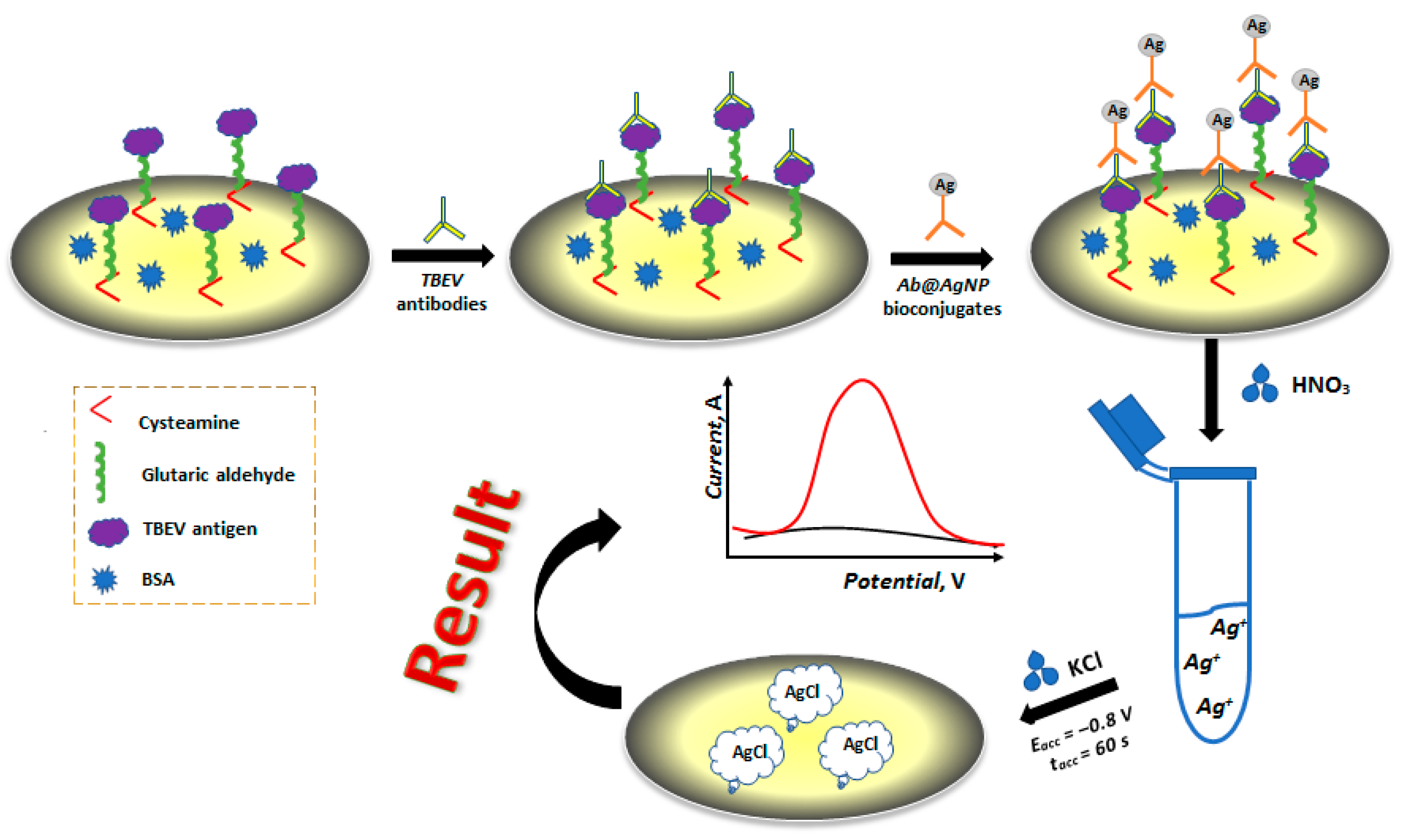
2.5. Production of an Electrochemical Immunosensor Based on Ab@AgNP Bioconjugates
2.6. Electrochemical Detection of Ag in Ab@AgNP Bioconjugates
2.7. Application of Electrochemical Immunosensor to the Analysis of Immunological Products
3. Results
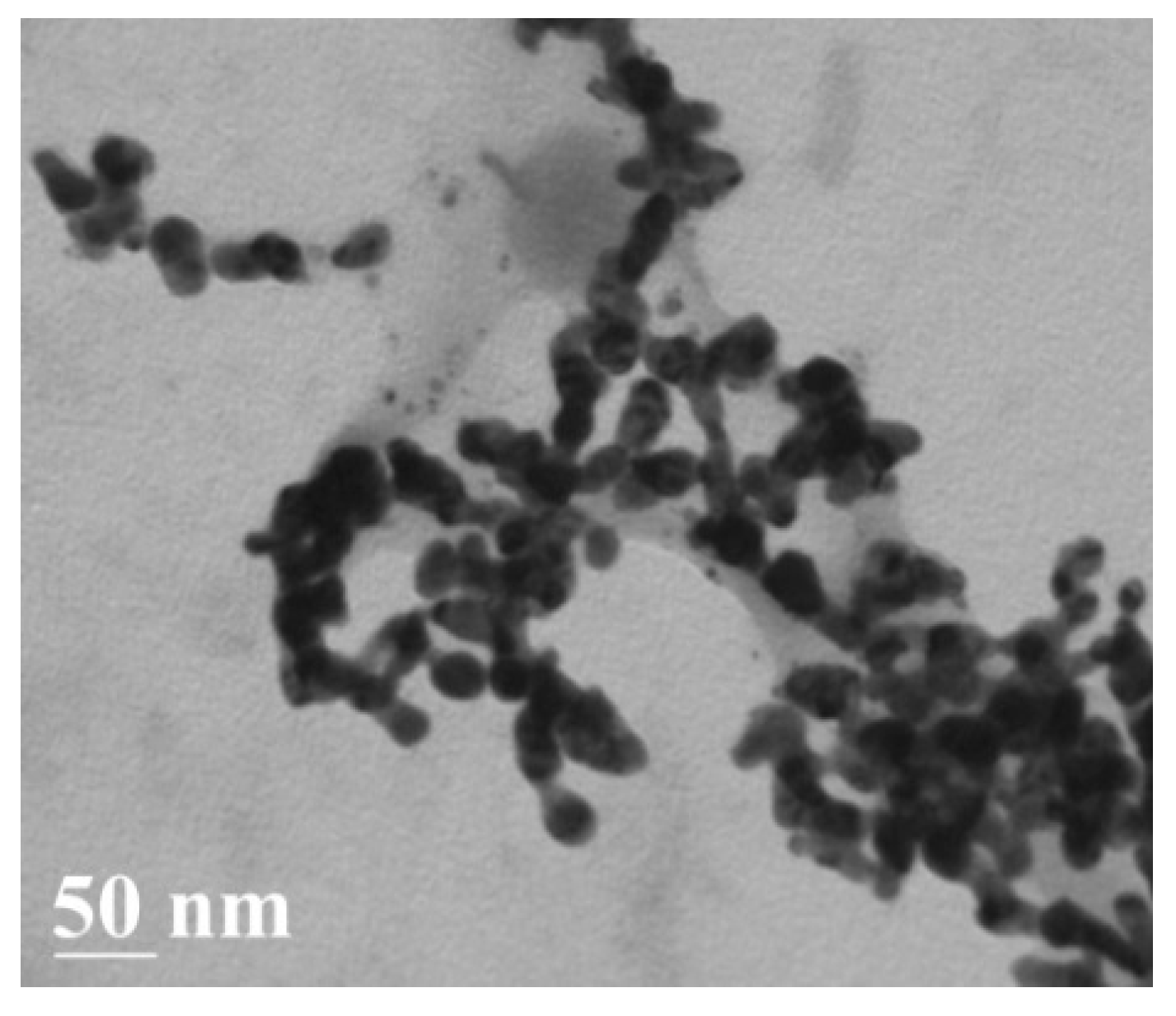
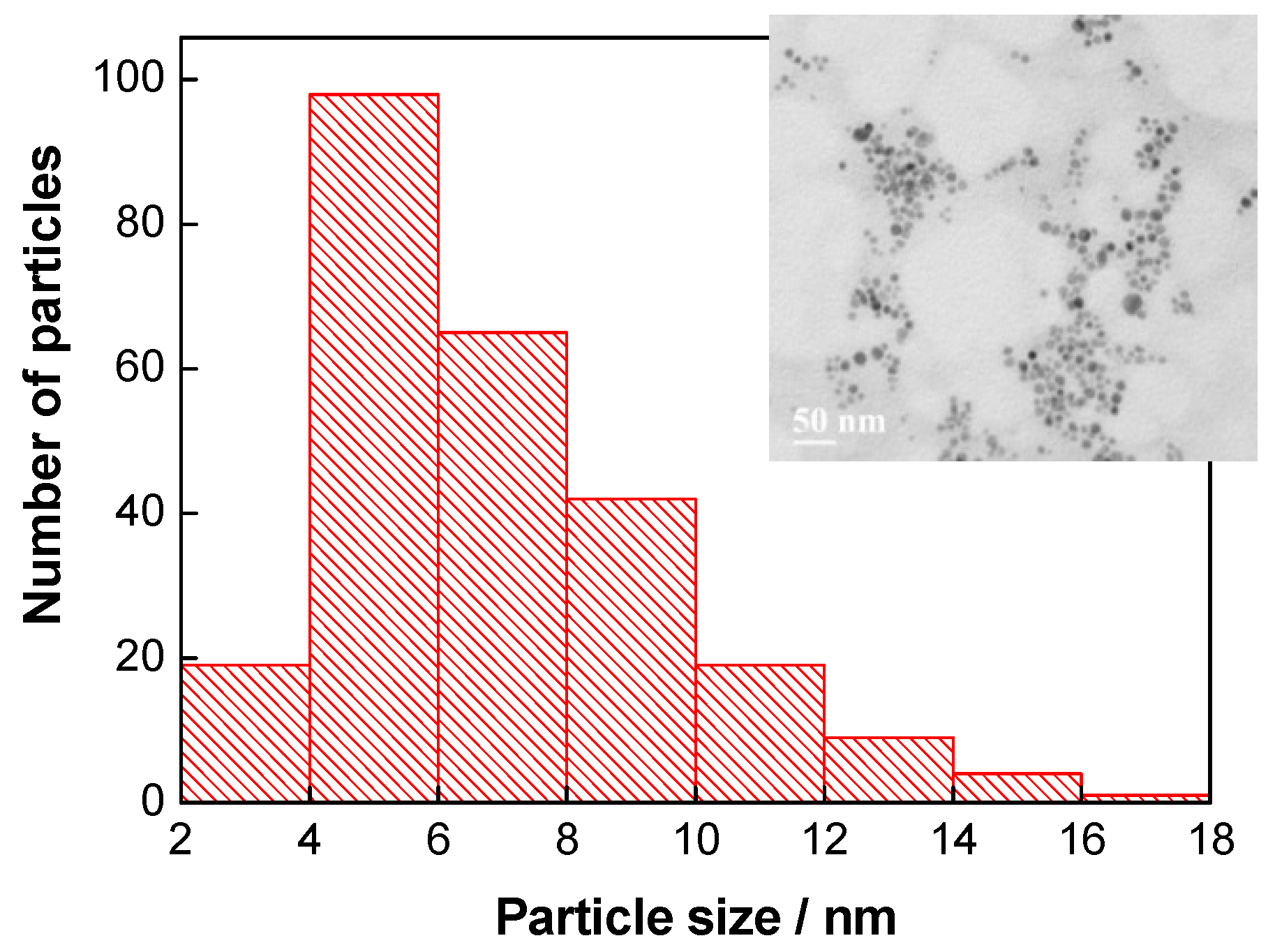
3.1. Characterization of Ab@AgNP Bioconjugates
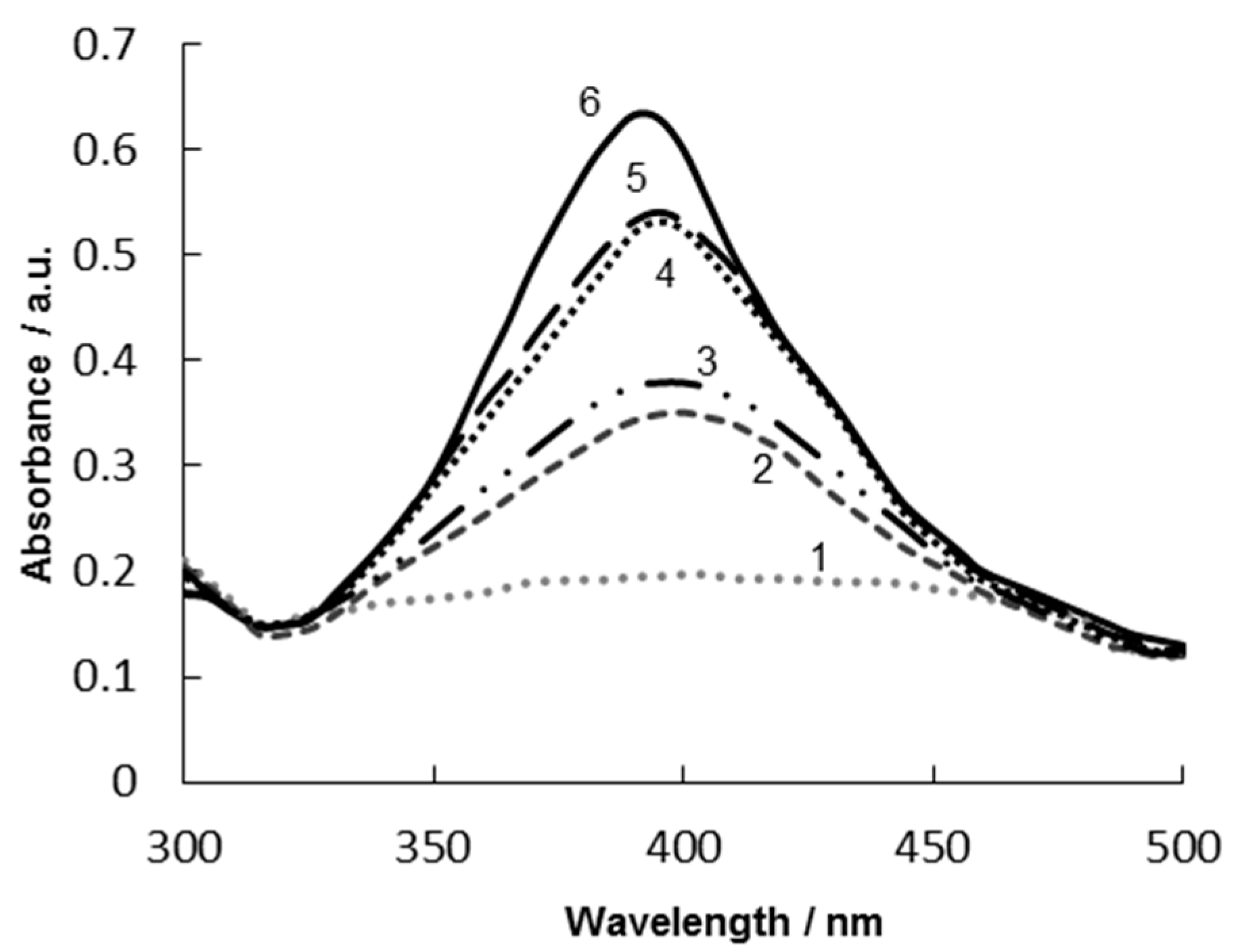
3.2. Optimization of BSA Concentration for Stabilization of Ag NPs
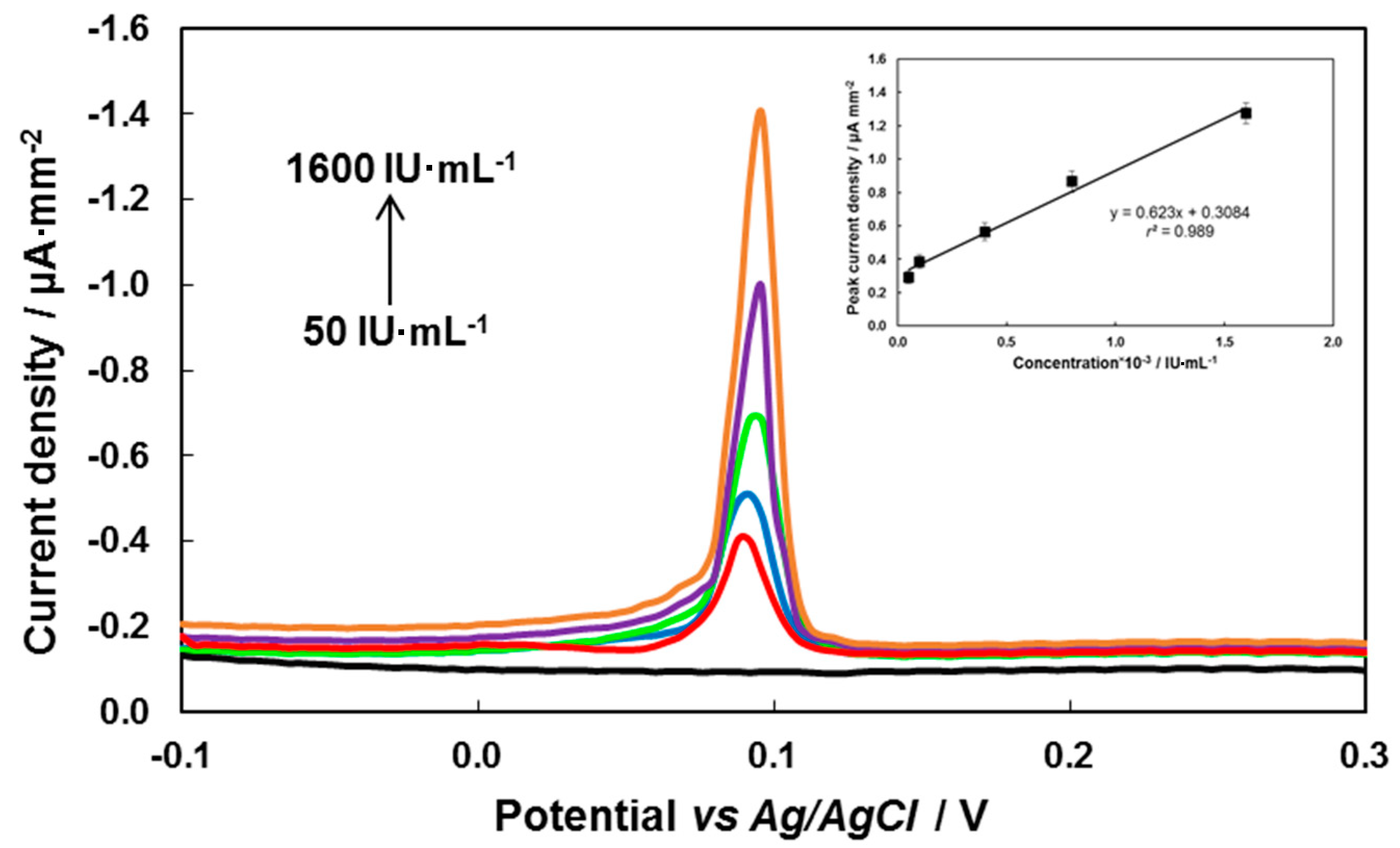
3.3. Electrochemical Determination of Antibodies to TBEV
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khasnatinov, M.; Danchinova, G.; Liapunov, A.; Manzarova, E.; Petrova, I.; Liapunova, N.; Solovarov, I. Prevalence of tick-borne pathogens in hard ticks that attacked human hosts in Eastern Siberia. Int. J. Biomed. 2017, 7, 307–309. [Google Scholar] [CrossRef]
- Holzmann, H. Diagnosis of tick-borne encephalitis. Vaccine 2003, 21, 36–40. [Google Scholar] [CrossRef]
- Bogovic, P.; Strle, F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases. 2015, 3, 430–441. [Google Scholar] [CrossRef]
- Bayry, J.; Lacroix-Desmazes, S.; Kazatchkine, M.D. Intravenous immunoglobulin for infectious diseases: Tailor-made or universal? J. Infect. Dis. 2003, 188, 1610–1611. [Google Scholar] [CrossRef]
- Veje, M.; Studah, M.; Johansson, M.; Johansson, P.; Nolskog, P.; Bergstrom, T. Diagnosing tick-borne encephalitis: A re-evaluation of notified cases. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 339–344. [Google Scholar] [CrossRef]
- Espina, V.; Woodhouse, E.C.; Wulfkuhle, J.; Asmussen, H.D.; Petricoin, E.F., III; Liotta, L.A. Protein microarray detection strategies: Focus on direct detection technologies. J. Immunol. Meth. 2004, 290, 121–133. [Google Scholar] [CrossRef]
- Brainina, K.; Kozitsina, A.; Beikin, J. Electrochemical immunosensor for Forest-Spring encephalitis based on protein A labeled with colloidal gold. Anal. Bioanal. Chem. 2003, 376, 481–485. [Google Scholar] [CrossRef]
- Brainina, K.; Kozitsina, A.; Rubtsova, M.; Sergeev, B.; Saraeva, S. Electrochemical immunosensor for diagnosis of the forest-spring encephalitis. In Comprehensive Analytical Chemistry, 2nd ed.; Alegret, S., Merkoci, A., Eds.; Elsevier: Oxford, UK, 2007; Volume 49, pp. 265–269. ISBN 978-0-444-53053-0. [Google Scholar]
- Zhang, L.; Yuan, R.; Chai, Y.; Cao, S.; Lib, Q. A label-free amperometric immunosenor based on multi-layer assembly of polymerized o-phenylenediamine and gold nanoparticles for determination of Japanese B encephalitis vaccine. Anal. Chim. Acta 2005, 531, 1–5. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, R.; Chai, Y.; Chen, S.; Wang, N.; Zhu, Q. Layer-by-layer self-assembly of films of nano-Au and Co(bpy)33+ for the determination of Japanese B encephalitis vaccine. Biochem. Eng. J. 2006, 28, 231–236. [Google Scholar] [CrossRef]
- Hien, H.T.; Giang, H.T.; Trung, T.; Tuan, C.V. Enhancement of biosensing performance using a polyaniline/multiwalled carbon nanotubes nanocomposite. J. Mater. Sci. 2017, 52, 1694–1703. [Google Scholar] [CrossRef]
- Lai, H.C.; Chin, S.F.; Pang, S.C.; Sum, M.S.H.; Perera, D. Carbon nanoparticles based electrochemical biosensor strip for detection of Japanese Encephalitis Virus. J. Nanomater. 2017, 2017, 3615707. [Google Scholar] [CrossRef]
- Huy, T.Q.; Hanh, N.T.H.; Thuy, N.T.; Chung, P.V.; Nga, P.T.; Tuan, M.A. A novel biosensor based on serum antibody immobilization for rapid detection of viral antigens. Talanta 2011, 86, 271–277. [Google Scholar] [CrossRef]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Thomas, M.B.; Thomas, R.; Raichur, A.M.; Chakravortty, D. Interaction of silver nanoparticles with serum proteins affects their antimicrobial activity in vivo. Antimicrob. Agents Chemother. 2013, 57, 4945–4955. [Google Scholar] [CrossRef]
- You, C.C.; Chompoosor, A.; Rotello, V.M. The biomacromolecule-nanoparticle interface. Nano Today 2007, 2, 34–43. [Google Scholar] [CrossRef]
- Noskova, G.N.; Zakharova, E.A.; Chernov, V.I.; Zaichko, A.V.; Elesova, E.E.; Kabakaev, A.S. Fabrication and application gold microelectrode ensemble based on carbon black-polyethylene composite electrode. Anal. Methods 2011, 3, 1130–1135. [Google Scholar] [CrossRef]
- Transatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Hoare, J.P. A cyclic voltammetric study of the gold-oxygen system. J. Electrochem. Soc. 1984, 13, 1808–1815. [Google Scholar] [CrossRef]
- Barhoumi, H.; Maaref, A.; Jaffrezic-Renault, N. Impedimetric urea biosensor based on modified gold electrode with urease immobilized on glutathione layer. Sens. Transducers 2014, 27, 47–53. [Google Scholar]
- Zewde, B.; Ambaye, A.; Stubbs, J.; Raghavan, D. A review of stabilized silver nanoparticles—Synthesis, biological properties, characterization, and potential areas of applications. JSM Nanotechnol. Nanomed. 2016, 4, 1043–1056. [Google Scholar]
- Perevezentseva, D.; Gorchakov, E.; Petrushin, M. Influence of silver nanoparticles conditions synthesis on their electrochemical properties. AIP Conf. Proc. 2016, 1772, 020005-1–020005-5. [Google Scholar] [CrossRef]
- Eksperiandova, L.P.; Belikov, K.N.; Khimchenko, S.V.; Blank, T.A. Once again about determination and detection limits. J. Anal. Chem. 2010, 65, 229–234. [Google Scholar] [CrossRef]
| Electrode | Modifier | Method/Label | Target | Linearity Range (mg·mL−1) | Limit of Detection (ng·mL−1) | Ref. |
|---|---|---|---|---|---|---|
| Thick-film graphite electrode | Glutaric aldehyde, Nafion, or nitrocellulose | Anodic stripping voltammetry/protein A with Au NPs | Antibodies | 10−7 − 10−2 | 0.1 | [7] |
| Screen-printed electrode | - | Linear sweep voltammetry/protein A with Ag NPs | Antibodies | 10−7 − 10−2 | 0.5 | [8] |
| Platinum electrode | Nano-Au/o-phenylenediamine polymer film with deposited Prussian blue | Amperometry/label-free | Antigen | a 1.1∙10−8 – 1.9∙10−6 | a 6∙10−9 | [9] |
| Gold disc electrode | l-cysteine + nano-Au and [Co(bpy)3]3+ | Potentiometry/label-free | Antigen | a 8.1∙10−8 − 3.0∙10−6 | a 3.5∙10−8 | [10] |
| Platinum microelectrode | Polyaniline/multiwalled carbon nanotubes | Electrochemical impedance spectroscopy/label-free | Antigen | 2.0∙10−6 − 2.5∙10−4 | - | [11] |
| Screen-printed electrode | Carbon nanoparticles modified with chitosan | Electrochemical impedance spectroscopy/label-free | Antigen | 1.0∙10−6 − 2.0·10−5 | 0.36 | [12] |
| Screen-printed electrode | Silanized surface with protein A/glutaric aldehyde | Electrochemical impedance spectroscopy/label-free | Antigen | 10−3 − 10−2 | 750 | [13] |
| Immunological Product | Declared by Producer (IU·mL−1) | Found by ELISA (IU·mL−1) (C1) | Found by Electrochemical Method (IU·mL−1) (C2) | C2/C1 (%) |
|---|---|---|---|---|
| Human immunoglobulin against TBEV (FSUC SIC “Microgen”, Russia) | Not less than 80 | 86 ± 4 | 87 ± 4 | 101 |
| Human immunoglobulin against TBEV (FSUC SIC “Microgen”, Russia) | Not less than 160 | 172 ± 8 | 165 ± 4 | 96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khristunova, Y.; Korotkova, E.; Kratochvil, B.; Barek, J.; Dorozhko, E.; Vyskocil, V.; Plotnikov, E.; Voronova, O.; Sidelnikov, V. Preparation and Investigation of Silver Nanoparticle–Antibody Bioconjugates for Electrochemical Immunoassay of Tick-Borne Encephalitis. Sensors 2019, 19, 2103. https://doi.org/10.3390/s19092103
Khristunova Y, Korotkova E, Kratochvil B, Barek J, Dorozhko E, Vyskocil V, Plotnikov E, Voronova O, Sidelnikov V. Preparation and Investigation of Silver Nanoparticle–Antibody Bioconjugates for Electrochemical Immunoassay of Tick-Borne Encephalitis. Sensors. 2019; 19(9):2103. https://doi.org/10.3390/s19092103
Chicago/Turabian StyleKhristunova, Yekaterina, Elena Korotkova, Bohumil Kratochvil, Jiri Barek, Elena Dorozhko, Vlastimil Vyskocil, Evgenii Plotnikov, Olesya Voronova, and Vladimir Sidelnikov. 2019. "Preparation and Investigation of Silver Nanoparticle–Antibody Bioconjugates for Electrochemical Immunoassay of Tick-Borne Encephalitis" Sensors 19, no. 9: 2103. https://doi.org/10.3390/s19092103
APA StyleKhristunova, Y., Korotkova, E., Kratochvil, B., Barek, J., Dorozhko, E., Vyskocil, V., Plotnikov, E., Voronova, O., & Sidelnikov, V. (2019). Preparation and Investigation of Silver Nanoparticle–Antibody Bioconjugates for Electrochemical Immunoassay of Tick-Borne Encephalitis. Sensors, 19(9), 2103. https://doi.org/10.3390/s19092103







