Focal Vibration Training (Equistasi®) to Improve Posture Stability. A Retrospective Study in Parkinson’s Disease
Abstract
1. Introduction
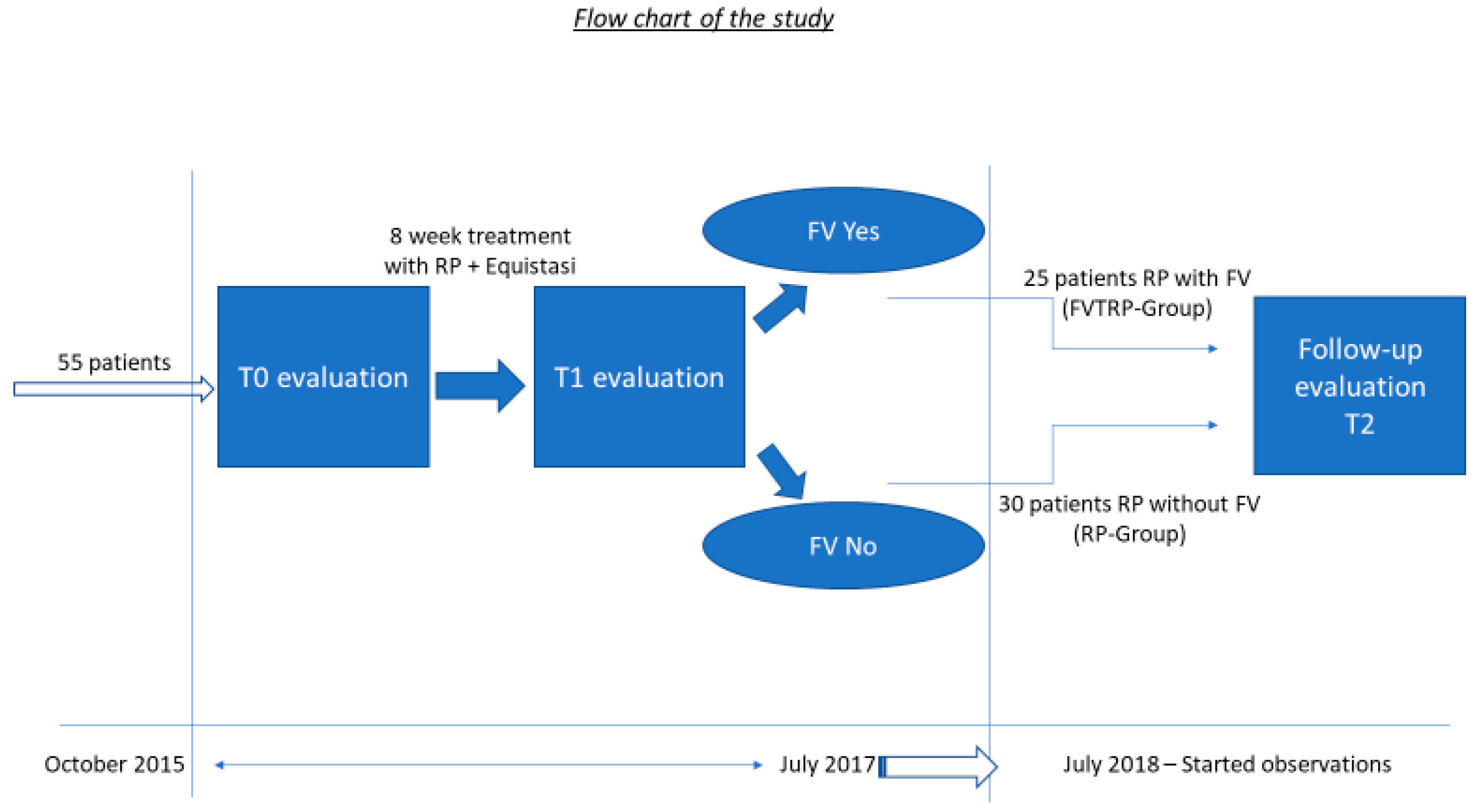
2. Materials and Methods
2.1. Study Procedure, Observed Measures and Requirements for Obtaining the FV Equistasi®
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Albani, G.; Veneziano, G.; Lunardon, C.; Vinci, C.; Daniele, A.; Cossa, F.; Mauro, A. Feasibility of home exercises to enhance the benefits of tango dancing in people with Parkinson’s disease. Complement. Ther. Med. 2019, 42, 233–239. [Google Scholar] [CrossRef]
- Mak, M.K.; Wong-Yu, I.S.; Shen, X.; Chung, C.L. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat. Rev. Neurol. 2017, 13, 689–703. [Google Scholar] [CrossRef]
- Shen, X.; Wong-Yu, I.S.; Mak, M.K. Effects of Exercise on Falls, Balance, and Gait Ability in Parkinson’s Disease: A Meta-analysis. Neurorehabil. Neural. Repair. 2016, 30, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, B.; Jansen, B.; Omelina, L.; Van Sint Jan, S. The use of commercial video games in rehabilitation: A systematic review. Int. J. Rehabil. Res. 2016, 39, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Palamara, G.; Gotti, F.; Maestri, R.; Bera, R.; Gargantini, R.; Bossio, F.; Zivi, I.; Volpe, D.; Ferrazzoli, D.; Frazzitta, G. Aquatic Therapy Versus Land-Based Rehabilitation Alone for the Treatment of Balance Dysfunction in Parkinson Disease: A Randomized Controlled Study With 6-Month Follow-Up. Arch. Phys. Med. Rehabil. 2017, 98, 1077–1085. [Google Scholar] [CrossRef]
- Shen, X.; Mak, M.K. Balance and Gait Training With Augmented Feedback Improves Balance Confidence in People With Parkinson’s Disease: A Randomized Controlled Trial. Neurorehabil. Neural. Repair. 2014, 28, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, D.; DeAngelis, T.R.; Hendron, K.; Thomas, C.A.; Saint-Hilaire, M.; Ellis, T. Highly Challenging Balance Program Reduces Fall Rate in Parkinson Disease. J. Neurol. Phys. Ther. 2016, 40, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.J.; Phillips, J.R.; Jahanshahi, M.; Moustafa, A.A. Postural instability and falls in Parkinson’s disease. Rev. Neurosci. 2016, 27, 549–555. [Google Scholar] [CrossRef]
- Grimbergen, Y.A.; Munneke, M.; Bloem, B.R. Falls in Parkinson’s disease. Curr. Opin. Neurol. 2004, 17, 405–415. [Google Scholar] [CrossRef]
- Bekkers, E.M.J.; Dijkstra, B.W.; Dockx, K.; Heremans, E.; Verschueren, S.M.P.; Nieuwboer, A. Clinical balance scales indicate worse postural control in people with Parkinson’s disease who exhibit freezing of gait compared to those who do not: A meta-analysis. Gait Posture. 2017, 56, 134–140. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People with Parkinson’s Disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Smulders, K.; Dale, M.L.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B. Pharmacological treatment in Parkinson’s disease: Effects on gait. Parkinsonism Relat. Disord. 2016, 31, 3–13. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Gruendlinger, L.; Scollins, L.; O’Herron, S.; Tarsy, D. Deep brain stimulation effects on gait variability in Parkinson’s disease. Mov. Disord. 2009, 24, 1688–1692. [Google Scholar] [CrossRef]
- Volpe, D.; Giantin, M.G.; Fasano, A. A wearable proprioceptive stabilizer (Equistasi®) for rehabilitation of postural instability in Parkinson’s disease: A phase II randomized double-blind, double-dummy, controlled study. PLoS ONE 2014, 9, e112065. [Google Scholar] [CrossRef]
- Spina, E.; Carotenuto, A.; Aceto, M.G.; Cerillo, I.; Silvestre, F.; Arace, F.; Paone, P.; Orefice, G.; Iodice, R. The effects of mechanical focal vibration on walking impairment in multiple sclerosis patients: A randomized, double-blinded vs placebo study. Restor. Neurol. Neurosci. 2016, 34, 869–876. [Google Scholar] [CrossRef]
- Leonardi, L.; Aceto, M.G.; Marcotulli, C.; Arcuria, G.; Serrao, M.; Pierelli, F.; Paone, P.; Filla, A.; Roca, A.; Casali, C. A wearable proprioceptive stabilizer for rehabilitation of limb and gait ataxia in hereditary cerebellar ataxias: a pilot open-labeled study. Neurol. Sci. 2017, 38, 459–463. [Google Scholar] [CrossRef]
- Alfonsi, E.; Paone, P.; Tassorelli, C.; De Icco, R.; Moglia, A.; Alvisi, E.; Marchetta, L.; Fresia, M.; Montini, A.; Calabrese, M.; et al. Acute effects of high-frequency microfocal vibratory stimulation on the H reflex of the soleus muscle. A double-blind study in healthy subjects. Funct. Neurol. 2015, 30, 269–274. [Google Scholar]
- Kossev, A.; Siggelkow, S.; Kapels, H.; Dengler, R.; Rollnik, J.D. Crossed effects of muscle vibration on motor-evoked potentials. Clin. Neurophysiol. 2001, 112, 453–456. [Google Scholar] [CrossRef]
- Equistasi. Available online: www.equistasi.com (accessed on 30 April 2019).
- Fahn, S.; Marsden, C.; Calne, D.; Goldstein, M. Recent Developments in Parkinson’s Disease; Macmillan Health Care Information: Florham Park, NJ, USA, 1987; pp. 153–163, 293–304. [Google Scholar]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patient. JAGS 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The timed ‘‘Up & Go’’: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Catelan, D.; Biggeri, A.; Barbone, F. Power and study size (what’s wrong in post hoc power evaluation). Epidemiol. Prev. 2011, 35, 236–240. [Google Scholar]
- Colin, P.; Eleveld, D.J.; Jonckheere, S.; Van Bocxlaer, J.; De Waele, J.; Vermeulen, A. What about confidence intervals? A word of caution when interpreting PTA simulations. J. Antimicrob. Chemother. 2016, 71, 2502–2508. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Grasso, R.; Lacquaniti, F. Influence of leg muscle vibration on human walking. J. Neurophysiol. 2000, 84, 1737–1747. [Google Scholar] [CrossRef]
- Courtine, G.; De Nunzio, A.M.; Schmid, M.; Beretta, M.V.; Schieppati, M. Stance- and locomotion-dependent processing of vibration-induced proprioceptive inflow from multiple muscles in humans. J. Neurophysiol. 2007, 97, 772–779. [Google Scholar] [CrossRef]
- Nanhoe-Mahabier, W.; Allum, J.H.; Pasman, E.P.; Overeem, S.; Bloem, B.R. The effects of vibrotactile biofeedback training on trunk sway in Parkinson’s disease patients. Parkinsonism Relat. Disord. 2012, 18, 1017–1021. [Google Scholar] [CrossRef]
- De Nunzio, A.M.; Grasso, M.; Nardone, A.; Godi, M.; Schieppati, M. Alternate rhythmic vibratory stimulation of trunk muscles affects walking cadence and velocity in Parkinson’s disease. Clin. Neurophysiol. 2010, 121, 240–247. [Google Scholar] [CrossRef]
- Spolaor, F.; Guiotto, A.; Pavan, D.; Arab Yaghoubi, L.; Peppe, A.; Paone, P.; Sawacha, Z.; Volpe, D. The neurorehabilitation device Equistasi® impacts positively on the gait of Parkinson’s disease subjects. Gait Posture 2018, 66, S37–S38. [Google Scholar] [CrossRef]

| Characteristics of Patients | Mean (± sd) |
|---|---|
| Male/Female | 37/18 |
| Age (y) | 69.33 (8.21) |
| Disease Duration (y) | 8.09 (5.00) |
| LLD (mmg/die) | 490.3 (222.4) |
| H/Y | 2.58 (0.86) |
| UPDRS III | 39.2 (17.9) |
| TINETTI SCALE | 18.33 (6.2) |
| BBS SCALE | 46.7 (6.2) |
| T.U.G. (s) | 14.82 (5.1) |
| FVTRP (± sd) n = 24 | RP (± sd) n = 29 | p Value | |
|---|---|---|---|
| Age (y) | 68.6 (8.6) | 69.4 (7.8) | 0.773 |
| Disease Duration (Y) | 7.88 (5.6) | 7.16 (4.4) | 0.581 |
| H/Y | 2.73 (0.7) | 2.55 (0.9) | 0.761 |
| LLD (mmg/die) | 485 (176) | 527 (228) | 0.544 |
| UPDRS III | 37.3 (19) | 39.4 (18) | 0.671 |
| Tinetti | 17.3 (5.3) | 19.4 (6.9) | 0.222 |
| BBS | 41.04 (9.3) | 43.45 (10.6) | 0.378 |
| TUG | 15.9 (5.4) | 14.41 (5.7) | 0.267 |
| FVTRP (± sd) n = 24 | RP (± sd) n = 29 | |||||
|---|---|---|---|---|---|---|
| T0 | T2 | p Value | T0 | T2 | p Value | |
| FALLS | 2.1 (0.7) | 1.25 (0.6) | 0.036° | 1.9 (0.6) | 1.94 (0.8) | 0.420 |
| LEDD (mmg/die) | 505.2 (158) | 465.7 (169.1) | 0.163 | 531.2 (227) | 582.2 (239) | 0.040 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serio, F.; Minosa, C.; De Luca, M.; Conte, P.; Albani, G.; Peppe, A. Focal Vibration Training (Equistasi®) to Improve Posture Stability. A Retrospective Study in Parkinson’s Disease. Sensors 2019, 19, 2101. https://doi.org/10.3390/s19092101
Serio F, Minosa C, De Luca M, Conte P, Albani G, Peppe A. Focal Vibration Training (Equistasi®) to Improve Posture Stability. A Retrospective Study in Parkinson’s Disease. Sensors. 2019; 19(9):2101. https://doi.org/10.3390/s19092101
Chicago/Turabian StyleSerio, Francesco, Cosimo Minosa, Matteo De Luca, Pierguido Conte, Giovanni Albani, and Antonella Peppe. 2019. "Focal Vibration Training (Equistasi®) to Improve Posture Stability. A Retrospective Study in Parkinson’s Disease" Sensors 19, no. 9: 2101. https://doi.org/10.3390/s19092101
APA StyleSerio, F., Minosa, C., De Luca, M., Conte, P., Albani, G., & Peppe, A. (2019). Focal Vibration Training (Equistasi®) to Improve Posture Stability. A Retrospective Study in Parkinson’s Disease. Sensors, 19(9), 2101. https://doi.org/10.3390/s19092101





