Latest Trends in Electrochemical Sensors for Neurotransmitters: A Review
Abstract
1. Introduction
2. Electrochemical Sensors
3. Non-Enzymatic Electrochemical Sensors
3.1. Amino Acid Neurotransmitters
Glutamate
3.2. Biogenic Amines Neurotransmitters
3.2.1. Dopamine
3.2.2. Epinephrine
3.2.3. Norepinephrine
3.2.4. Oxytocin
3.2.5. Serotonin
4. Enzyme Sensors
4.1. Amino Acid Neurotransmitters
Glutamate
4.2. Biogenic Amine Neurotransmitters
4.2.1. Dopamine
4.2.2. Acetylcholine
5. Biosensors for In Vitro, In Vivo and Ex Vivo Measurements
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Costantino, G. Elenco delle Cocciniglie osservate in Sicilia. Bolletino di Zool. 1950, 17, 1–24. [Google Scholar] [CrossRef][Green Version]
- Aoki, I.; Shirane, K.; Tokimoto, T.; Nakagawa, K. Separation of fine particles using rotating tube with alternate flow. Rev. Sci. Instrum. 1986, 57, 2859–2861. [Google Scholar] [CrossRef]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Chandra, P. Clinical implications and electrochemical biosensing of monoamine neurotransmitters in body fluids, in vitro, in vivo, and ex vivo models. Biosens. Bioelectron. 2018, 121, 137–152. [Google Scholar] [CrossRef]
- Bucher, E.S.; Wightman, R.M. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nanoparticles, 2D material and conducting polymer modified electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef]
- Selvolini, G.; Băjan, I.; Hosu, O.; Cristea, C.; Săndulescu, R.; Marrazza, G. DNA-based sensor for the detection of an organophosphorus pesticide: Profenofos. Sensors 2018, 18, 2035. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, A.; Rossi, C.; Marrazza, G. Bio-inspired fish robot based on chemical sensors. Sens. Actuators B Chem. 2017, 239, 325–329. [Google Scholar] [CrossRef]
- Rapini, R.; Cincinelli, A.; Marrazza, G. Acetamiprid multidetection by disposable electrochemical DNA aptasensor. Talanta 2016, 161, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Souza, L.M.; Franco, D.L.; Castro, A.C.H.; Oliveira, A.A.; Boodts, J.F.C.; Brito-Madurro, A.G.; Madurro, J.M. Formation of novel polymeric films derived from 4-hydroxybenzoic acid. Mater. Chem. Phys. 2011, 129, 46–52. [Google Scholar] [CrossRef]
- Ferreira, D.C.; MacHado, A.E.D.H.; Tiago, F.D.S.; Madurro, J.M.; Madurro, A.G.B.; Abrahão, O. Molecular modeling study on the possible polymers formed during the electropolymerization of 3-hydroxyphenylacetic acid. J. Mol. Graph. Model. 2012, 34, 18–27. [Google Scholar] [CrossRef]
- Silva, F.D.A.D.S.; Lopes, C.B.; Kubota, L.T.; Lima, P.R.; Goulart, M.O.F. Poly-xanthurenic acid modified electrodes: An amperometric sensor for the simultaneous determination of ascorbic and uric acids. Sens. Actuators B Chem. 2012, 168, 289–296. [Google Scholar] [CrossRef]
- Song, W.; Chen, Y.; Xu, J.; Tian, D.B. A selective voltammetric detection for dopamine using poly (gallic acid) film modified electrode. Chin. Chem. Lett. 2010, 21, 349–352. [Google Scholar] [CrossRef]
- Herzog, G.; Gorgy, K.; Gulon, T.; Cosnier, S. Electrogeneration and characterization of photoactivable films and their application for enzyme grafting. Electrochem. Commun. 2005, 7, 808–814. [Google Scholar] [CrossRef]
- Hosu, O.; Elouarzaki, K.; Gorgy, K.; Cristea, C.; Sandulescu, R.; Marks, R.S.; Cosnier, S. Nanostructured photoactivatable electrode surface based on pyrene diazirine. Electrochem. Commun. 2017, 80, 5–8. [Google Scholar] [CrossRef]
- Hosu, O.; Bârsan, M.M.; Cristea, C.; Săndulescu, R.; Brett, C.M.A. Nanostructured electropolymerized poly(methylene blue) films from deep eutectic solvents. Optimization and characterization. Electrochim. Acta 2017, 232, 285–295. [Google Scholar] [CrossRef]
- Peña, R.C.; Bertotti, M.; Brett, C.M.A. Methylene Blue/Multiwall Carbon Nanotube Modified Electrode for the Amperometric Determination of Hydrogen Peroxide. Electroanalysis 2011, 23, 2290–2296. [Google Scholar] [CrossRef]
- Hosu, O.; Barsan, M.M.; Cristea, C.; Săndulescu, R.; Brett, C.M.A. Nanocomposites based on carbon nanotubes and redox-active polymers synthesized in a deep eutectic solvent as a new electrochemical sensing platform. Microchim. Acta 2017, 184, 3919–3927. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef]
- Cui, X.; Lee, V.A.; Raphael, Y.; Wiler, J.A.; Hetke, J.F.; Anderson, D.J.; Martin, D.C. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 2001, 56, 261–272. [Google Scholar] [CrossRef]
- Ku, S.; Palanisamy, S.; Chen, S.M. Highly selective dopamine electrochemical sensor based on electrochemically pretreated graphite and nafion composite modified screen printed carbon electrode. J. Colloid Interface Sci. 2013, 411, 182–186. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Y.; Weng, B.; Zhang, W.; Harris, A.T.; Minett, A.I.; Yue, Z.; Huang, X.F.; Chen, J. Sensitive and selective dopamine determination in human serum with inkjet printed Nafion/MWCNT chips. Electrochem. Commun. 2013, 37, 32–35. [Google Scholar] [CrossRef]
- Noroozifar, M.; Khorasani-Motlagh, M.; Hassani Nadiki, H.; Saeed Hadavi, M.; Mehdi Foroughi, M. Modified fluorine-doped tin oxide electrode with inorganic ruthenium red dye-multiwalled carbon nanotubes for simultaneous determination of a dopamine, uric acid, and tryptophan. Sens. Actuators B Chem. 2014, 204, 333–341. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Rocha-Santos, T.A.P.; Cardoso, S.; Duarte, A.C.; Cardosa, S. Strategies for enhancing the analytical performance of nanomaterial-based sensors. TrAC Trends Anal. Chem. 2013, 47, 27–36. [Google Scholar] [CrossRef]
- Tertiş, M.; Hosu, O.; Fritea, L.; Farcau, C.; Cernat, A.; Săndulescu, R.; Cristea, C. A Novel Label-Free Immunosensor Based on Activated Graphene Oxide for Acetaminophen Detection. Electroanalysis 2015, 27, 638–647. [Google Scholar] [CrossRef]
- Cernat, A.; Tertiş, M.; Săndulescu, R.; Bedioui, F.; Cristea, A.; Cristea, C. Electrochemical sensors based on carbon nanomaterials for acetaminophen detection: A review. Anal. Chim. Acta 2015, 886, 16–28. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Ads, E.H.; Ahmed, Y.M.; Galal, A. Determination of some neurotransmitters at cyclodextrin/ionic liquid crystal/graphene composite electrode. Electrochim. Acta 2016, 199, 319–331. [Google Scholar] [CrossRef]
- Albishri, H.M.; Abd El-Hady, D. Hyphenation of enzyme/graphene oxide-ionic liquid/glassy carbon biosensors with anodic differential pulse stripping voltammetry for reliable determination of choline and acetylcholine in human serum. Talanta 2019, 200, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ying, Y.; Li, L.; Xu, T.; Wu, Y.; Guo, X.; Wang, F.; Shen, H.; Wen, Y.; Yang, H. Stretched graphene tented by polycaprolactone and polypyrrole net–bracket for neurotransmitter detection. Appl. Surf. Sci. 2017, 396, 832–840. [Google Scholar] [CrossRef]
- Zhang, S.J.; Kang, K.; Niu, L.M.; Kang, W.J. Electroanalysis of neurotransmitters via 3D gold nanoparticles and a graphene composite coupled with a microdialysis device. J. Electroanal. Chem. 2019, 834, 249–257. [Google Scholar] [CrossRef]
- Ragavan, K.V.; Egan, P.; Neethirajan, S. Multi mimetic Graphene Palladium nanocomposite based colorimetric paper sensor for the detection of neurotransmitters. Sens. Actuators B Chem. 2018, 273, 1385–1394. [Google Scholar] [CrossRef]
- Tonel, M.Z.; González-Durruthy, M.; Zanella, I.; Fagan, S.B. Interactions of graphene derivatives with glutamate-neurotransmitter: A parallel first principles—Docking investigation. J. Mol. Graph. Model. 2019, 88, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Chen, Y.; Liu, Y.; Zhang, Q.; Xiong, L.; Huang, H.; Li, L.; Yu, X.; Wei, L. Facile synthesis of nitrogen-doped graphene aerogels for electrochemical detection of dopamine. Solid State Sci. 2018, 86, 6–11. [Google Scholar] [CrossRef]
- Raphey, V.R.; Henna, T.K.; Nivitha, K.P.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Ku, S.; Chen, S.M. Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim. Acta 2013, 180, 1037–1042. [Google Scholar] [CrossRef]
- Niu, L.M.; Lian, K.Q.; Shi, H.M.; Wu, Y.B.; Kang, W.J.; Bi, S.Y. Characterization of an ultrasensitive biosensor based on a nano-Au/DNA/nano-Au/poly(SFR) composite and its application in the simultaneous determination of dopamine, uric acid, guanine, and adenine. Sens. Actuators B Chem. 2013, 178, 10–18. [Google Scholar] [CrossRef]
- Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N. Fast and selective plasmonic serotonin detection with Aptamer-gold nanoparticle conjugates. Sensors 2017, 17, 681. [Google Scholar] [CrossRef]
- Govindaraju, S.; Ankireddy, S.R.; Viswanath, B.; Kim, J.; Yun, K. Fluorescent Gold Nanoclusters for Selective Detection of Dopamine in Cerebrospinal fluid. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Kaur, B.; Srivastava, R. A polyaniline-zeolite nanocomposite material based acetylcholinesterase biosensor for the sensitive detection of acetylcholine and organophosphates. New J. Chem. 2015, 39, 6899–6906. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X.; Xia, Y. Electrochemical detection of serotonin based on a poly(bromocresol green) film and Fe3O4nanoparticles in a chitosan matrix. RSC Adv. 2017, 7, 1847–1851. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Simon, D.T.; Pham, M.C.; Noël, V.; Berggren, M. Detection of glutamate and acetylcholine with organic electrochemical transistors based on conducting polymer/platinum nanoparticle composites. Adv. Mater. 2014, 26, 5658–5664. [Google Scholar] [CrossRef]
- Tsierkezos, N.G.; Ritter, U.; Nugraha Thaha, Y.; Knauer, A.; Fernandes, D.; Kelarakis, A.; McCarthy, E.K. Boron-doped multi-walled carbon nanotubes as sensing material for analysis of dopamine and epinephrine in presence of uric acid. Chem. Phys. Lett. 2018, 710, 157–167. [Google Scholar] [CrossRef]
- Si, B.; Song, E. Recent Advances in the Detection of Neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Wilson, D.; Gonçalves, D.; Oliveira, O.N. Low-cost screen-printed electrodes based on electrochemically reduced graphene oxide-carbon black nanocomposites for dopamine, epinephrine and paracetamol detection. J. Colloid Interface Sci. 2018, 515, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, B.; He, X.; Li, F.; Ding, Y.; Fei, J. Carbon nanomaterial based electrochemical sensors for biogenic amines. Microchim. Acta 2013, 180, 935–956. [Google Scholar] [CrossRef]
- Tąta, A.; Gralec, B.; Proniewicz, E. Unsupported platinum nanoparticles as effective sensors of neurotransmitters and possible drug curriers. Appl. Surf. Sci. 2018, 435, 256–264. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, R.; Chai, Y.; Zhang, Y.; Hu, F.; Zhang, M. Au-nanoclusters incorporated 3-amino-5-mercapto-1,2,4-triazole film modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2011, 30, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Hanko, M.; Švorc, Ľ.; Planková, A.; Mikuš, P. Overview and recent advances in electrochemical sensing of glutathione—A review. Anal. Chim. Acta 2019, 1062, 1–27. [Google Scholar] [CrossRef]
- Shadlaghani, A.; Farzaneh, M.; Kinser, D.; Reid, R.C. Direct Electrochemical Detection of Glutamate, Acetylcholine, Choline, and Adenosine Using Non-Enzymatic Electrodes. Sensors 2019, 19, 447. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Yang, X. A sensitive choline biosensor using Fe3O4 magnetic nanoparticles as peroxidase mimics. Analyst 2011, 136, 4960–4965. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, G.; Armelin, E.; Alemán, C. Selective detection of dopamine combining multilayers of conducting polymers with gold nanoparticles. J. Phys. Chem. B 2014, 118, 4669–4682. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Owens, R.M.; Malliaras, G.G. The rise of organic bioelectronics. Chem. Mater. 2014, 26, 679–685. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Patrascu, D.; David, I.; David, V.; Mihailciuc, C.; Stamatin, I.; Ciurea, J.; Nagy, L.; Nagy, G.; Ciucu, A.A. Selective voltammetric determination of electroactive neuromodulating species in biological samples using iron(II) phthalocyanine modified multi-wall carbon nanotubes paste electrode. Sens. Actuators B Chem. 2011, 156, 731–736. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Cai, Z.; Oyama, M.; Chen, X. Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim. Acta 2014, 181, 689–705. [Google Scholar] [CrossRef]
- Narayanan, T.N.; Vusa, C.S.R.; Alwarappan, S. Selective and efficient electrochemical biosensing of ultrathin molybdenum disulfide sheets. Nanotechnology 2014, 25. [Google Scholar] [CrossRef]
- Meldrum, B.S. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr. 2018, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.R. The role of glutamate in central nervous system health and disease—A review. Vet. J. 2007, 173, 278–286. [Google Scholar] [CrossRef]
- Nedergaard, M.; Takano, T.; Hansen, A.J. Beyond the role of glutamate as a neurotransmitter. Nat. Rev. Neurosci. 2002, 3, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Jessen, R.; Wood, P.M.; Wanner, I.N.A.B.; Mahoney, J.; An, K.; Bates, M.; Bunge, M.B. Invariant Mantling of Growth Cones by Schwann Cell Precursors Characterize Growing Peripheral Nerve Fronts. Glia 2006, 438, 424–438. [Google Scholar] [CrossRef]
- Baurmash, H.D. Transplantation of Alloplastic Submandibular Glands as Not Clinically Applicable Treatment for Xerostomia. J. Oral Maxillofac. Surg. 2007, 65, 1267–1269. [Google Scholar] [CrossRef]
- Hinzman, J.M.; Thomas, T.C.; Quintero, J.E.; Gerhardt, G.A.; Lifshitz, J. Disruptions in the Regulation of Extracellular Glutamate by Neurons and Glia in the Rat Striatum Two Days after Diffuse Brain Injury. J. Neurotrauma 2012, 29, 1197–1208. [Google Scholar] [CrossRef]
- Chang, Y.; Lin, T.Y.; Lu, C.W.; Huang, S.K.; Wang, Y.C.; Wang, S.J. Xanthohumol-induced presynaptic reduction of glutamate release in the rat hippocampus. Food Funct. 2016, 7, 212–226. [Google Scholar] [CrossRef]
- Wilson, C.L.; Natarajan, V.; Hayward, S.L.; Khalimonchuk, O.; Kidambi, S. Mitochondrial dysfunction and loss of glutamate uptake in primary astrocytes exposed to titanium dioxide nanoparticles. Nanoscale 2015, 7, 18477–18488. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Hasan, M.; Mathewson, A.; Razeeb, K.M. Disposable sensor based on enzyme-free Ni nanowire array electrode to detect glutamate. Biosens. Bioelectron. 2013, 40, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Diestra, D.; Thapa, B.; Beltran-Huarac, J.; Weiner, B.R.; Morell, G. l-cysteine capped ZnS:Mn quantum dots for room-temperature detection of dopamine with high sensitivity and selectivity. Biosens. Bioelectron. 2017, 87, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, Y.; Li, C.; Zheng, B.; Li, Y.; Zhang, T.; Zhang, Z.; Yang, M. Morphology-controlled synthesis of Bi2S3 nanorods-reduced graphene oxide composites with high-performance for electrochemical detection of dopamine. Sens. Actuators B Chem. 2018, 257, 936–943. [Google Scholar] [CrossRef]
- Yan, X.; Lu, N.; Gu, Y.; Li, C.; Zhang, T.; Liu, H.; Zhang, Z.; Zhai, S. Catalytic activity of biomimetic model of cytochrome P450 in oxidation of dopamine. Talanta 2018, 179, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.T.E.; An, S.; Choe, S.R.; Kwak, C.H.; Huh, Y.S.; Lee, J.; Han, Y.K. Fabrication of 3D honeycomb-like porous polyurethane-functionalized reduced graphene oxide for detection of dopamine. Biosens. Bioelectron. 2016, 86, 122–128. [Google Scholar] [CrossRef]
- Ramachandran, A.; Panda, S.; Karunakaran Yesodha, S. Physiological level and selective electrochemical sensing of dopamine by a solution processable graphene and its enhanced sensing property in general. Sens. Actuators B Chem. 2018, 256, 488–497. [Google Scholar] [CrossRef]
- Sivasubramanian, R.; Biji, P. Preparation of copper (I) oxide nanohexagon decorated reduced graphene oxide nanocomposite and its application in electrochemical sensing of dopamine. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2016, 210, 10–18. [Google Scholar] [CrossRef]
- Yu, G.; Xia, J.; Zhang, F.; Wang, Z. Hierarchical and hybrid RGO/ZIF-8 nanocomposite as electrochemical sensor for ultrasensitive determination of dopamine. J. Electroanal. Chem. 2017, 801, 496–502. [Google Scholar] [CrossRef]
- Hou, J.; Xu, C.; Zhao, D.; Zhou, J. Facile fabrication of hierarchical nanoporous AuAg alloy and its highly sensitive detection towards dopamine and uric acid. Sens. Actuators B Chem. 2016, 225, 241–248. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, J.; Yan, L.; Zhu, L.; Liu, B. An electrochemical sensor for selective detection of dopamine based on nickel tetrasulfonated phthalocyanine functionalized nitrogen-doped graphene nanocomposites. J. Electroanal. Chem. 2016, 779, 92–98. [Google Scholar] [CrossRef]
- da Silva, L.V.; Lopes, C.B.; da Silva, W.C.; de Paiva, Y.G.; Silva, F.; de Assis dos Santos Silva, F.; Lima, P.R.; Kubota, L.T.; Goulart, M.O.F. Electropolymerization of ferulic acid on multi-walled carbon nanotubes modified glassy carbon electrode as a versatile platform for NADH, dopamine and epinephrine separate detection. Microchem. J. 2017, 133, 460–467. [Google Scholar] [CrossRef]
- Figueiredo-Filho, L.C.S.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Simultaneous voltammetric determination of dopamine and epinephrine in human body fluid samples using a glassy carbon electrode modified with nickel oxide nanoparticles and carbon nanotubes within a dihexadecylphosphate film. Analyst 2014, 139, 2842–2849. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Mortazavi, M.; Khoobi, A. Fabrication of a graphene oxide nano-sheet modified electrode for determination of dopamine in the presence of tyrosine: A multivariate optimization strategy. J. Mol. Liq. 2016, 215, 31–38. [Google Scholar] [CrossRef]
- Sun, L.; Li, H.; Li, M.; Li, C.; Li, P.; Yang, B. Simultaneous determination of ascorbic acid, dopamine, uric acid, tryptophan, and nitrite on a novel carbon electrode. J. Electroanal. Chem. 2016, 783, 167–175. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, X.; Ding, Y.; Luo, L.; Xu, D.; Ning, Y. Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan. Talanta 2016, 146, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.; Gupta, P.; Goyal, R.N.; Shim, Y.B. Graphene/conducting polymer nano-composite loaded screen printed carbon sensor for simultaneous determination of dopamine and 5-hydroxytryptamine. Sens. Actuators B Chem. 2017, 239, 993–1002. [Google Scholar] [CrossRef]
- Majidi, M.R.; Pournaghi-Azar, M.H.; Fadakar Bajeh Baj, R. Graphene nanoplatelets like structures formed on ionic liquid modified carbon-ceramic electrode: As a sensing platform for simultaneous determination of dopamine and acetaminophen. J. Mol. Liq. 2016, 220, 778–787. [Google Scholar] [CrossRef]
- Dinesh, B.; Saraswathi, R.; Senthil Kumar, A. Water based homogenous carbon ink modified electrode as an efficient sensor system for simultaneous detection of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2017, 233, 92–104. [Google Scholar] [CrossRef]
- Moraes, F.C.; Golinelli, D.L.C.; Mascaro, L.H.; MacHado, S.A.S. Determination of epinephrine in urine using multi-walled carbon nanotube modified with cobalt phthalocyanine in a paraffin composite electrode. Sens. Actuators B Chem. 2010, 148, 492–497. [Google Scholar] [CrossRef]
- Tezerjani, M.D.; Benvidi, A.; Dehghani Firouzabadi, A.; Mazloum-Ardakani, M.; Akbari, A. Epinephrine electrochemical sensor based on a carbon paste electrode modified with hydroquinone derivative and graphene oxide nano-sheets: Simultaneous determination of epinephrine, acetaminophen and dopamine. Meas. J. Int. Meas. Confed. 2017, 101, 183–189. [Google Scholar] [CrossRef]
- Goyal, R.N.; Aziz, M.A.; Oyama, M.; Chatterjee, S.; Rana, A.R.S. Nanogold based electrochemical sensor for determination of norepinephrine in biological fluids. Sens. Actuators B Chem. 2011, 153, 232–238. [Google Scholar] [CrossRef]
- Asai, K.; Ivandini, T.A.; Einaga, Y. Continuous and selective measurement of oxytocin and vasopressin using boron-doped diamond electrodes. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Sadanandhan, N.K.; Cheriyathuchenaaramvalli, M.; Devaki, S.J.; Ravindranatha Menon, A.R. PEDOT-reduced graphene oxide-silver hybrid nanocomposite modified transducer for the detection of serotonin. J. Electroanal. Chem. 2017, 794, 244–253. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Van Hien, H.; Kim, N.H.; Lee, J.H. A novel sensitive sensor for serotonin based on high-quality of AuAg nanoalloy encapsulated graphene electrocatalyst. Biosens. Bioelectron. 2017, 96, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.J.E.; Martínez, A.M.; Ribeiro, W.F.; Bichinho, K.M.; Di Nezio, M.S.; Pistonesi, M.F.; Araujo, M.C.U. Determination of tryptamine in foods using square wave adsorptive stripping voltammetry. Talanta 2016, 154, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tertiş, M.; Florea, A.; Adumitrăchioaie, A.; Cernat, A.; Bogdan, D.; Barbu-Tudoran, L.; Jaffrezic Renault, N.; Săndulescu, R.; Cristea, C. Detection of Dopamine by a Biomimetic Electrochemical Sensor Based on Polythioaniline-Bridged Gold Nanoparticles. Chempluschem 2017, 82, 561–569. [Google Scholar] [CrossRef]
- Immanuel, S.; Aparna, T.K.; Sivasubramanian, R. A facile preparation of Au—SiO2 nanocomposite for simultaneous electrochemical detection of dopamine and uric acid. Surf. Interfaces 2019, 14, 82–91. [Google Scholar] [CrossRef]
- Qiu, Z.; Yang, T.; Gao, R.; Jie, G.; Hou, W. An electrochemical ratiometric sensor based on 2D MOF nanosheet/Au/polyxanthurenic acid composite for detection of dopamine. J. Electroanal. Chem. 2019, 835, 123–129. [Google Scholar] [CrossRef]
- Yang, C.; Liu, M.M.; Bai, F.Q.; Guo, Z.Z.; Liu, H.; Zhong, G.X.; Peng, H.P.; Chen, W.; Lin, X.H.; Lei, Y.; et al. An electrochemical biosensor for sensitive detection of nicotine-induced dopamine secreted by PC12 cells. J. Electroanal. Chem. 2019, 832, 217–224. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Beitollahi, H.; Mohseni, M.A.S.; Benvidi, A.; Naeimi, H.; Nejati-Barzoki, M.; Taghavinia, N. Simultaneous determination of epinephrine and acetaminophen concentrations using a novel carbon paste electrode prepared with 2,2′-[1,2 butanediylbis(nitriloethylidyne)]-bis-hydroquinone and TiO2 nanoparticles. Colloids Surf. B Biointerfaces 2010, 76, 82–87. [Google Scholar] [CrossRef]
- Michael, D.J.; Wightman, R.M. Electrochemical monitoring of biogenic amine neurotransmission in real time. J. Pharm. Biomed. Anal. 1999, 19, 33–46. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Beitollahi, H.; Amini, M.K.; Mirkhalaf, F.; Mirjalili, B.F. A highly sensitive nanostructure-based electrochemical sensor for electrocatalytic determination of norepinephrine in the presence of acetaminophen and tryptophan. Biosens. Bioelectron. 2011, 26, 2102–2106. [Google Scholar] [CrossRef]
- Emran, M.Y.; Shenashen, M.A.; Mekawy, M.; Azzam, A.M.; Akhtar, N.; Gomaa, H.; Selim, M.M.; Faheem, A.; El-Safty, S.A. Ultrasensitive in-vitro monitoring of monoamine neurotransmitters from dopaminergic cells. Sens. Actuators B Chem. 2018, 259, 114–124. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.A.; Boccia, M.L. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress 2002, 5, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Bales, K.L.; van Westerhuyzen, J.A.; Lewis-Reese, A.D.; Grotte, N.D.; Lanter, J.A.; Carter, C.S. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm. Behav. 2007, 52, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Tertiș, M.; Cernat, A.; Lacatiș, D.; Florea, A.; Bogdan, D.; Suciu, M.; Săndulescu, R.; Cristea, C. Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture. Electrochem. Commun. 2017, 75, 43–47. [Google Scholar] [CrossRef]
- Gug, I.T.; Tertis, M.; Hosu, O.; Cristea, C. Salivary biomarkers detection: Analytical and immunological methods overview. TrAC Trends Anal. Chem. 2019, 113, 301–316. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
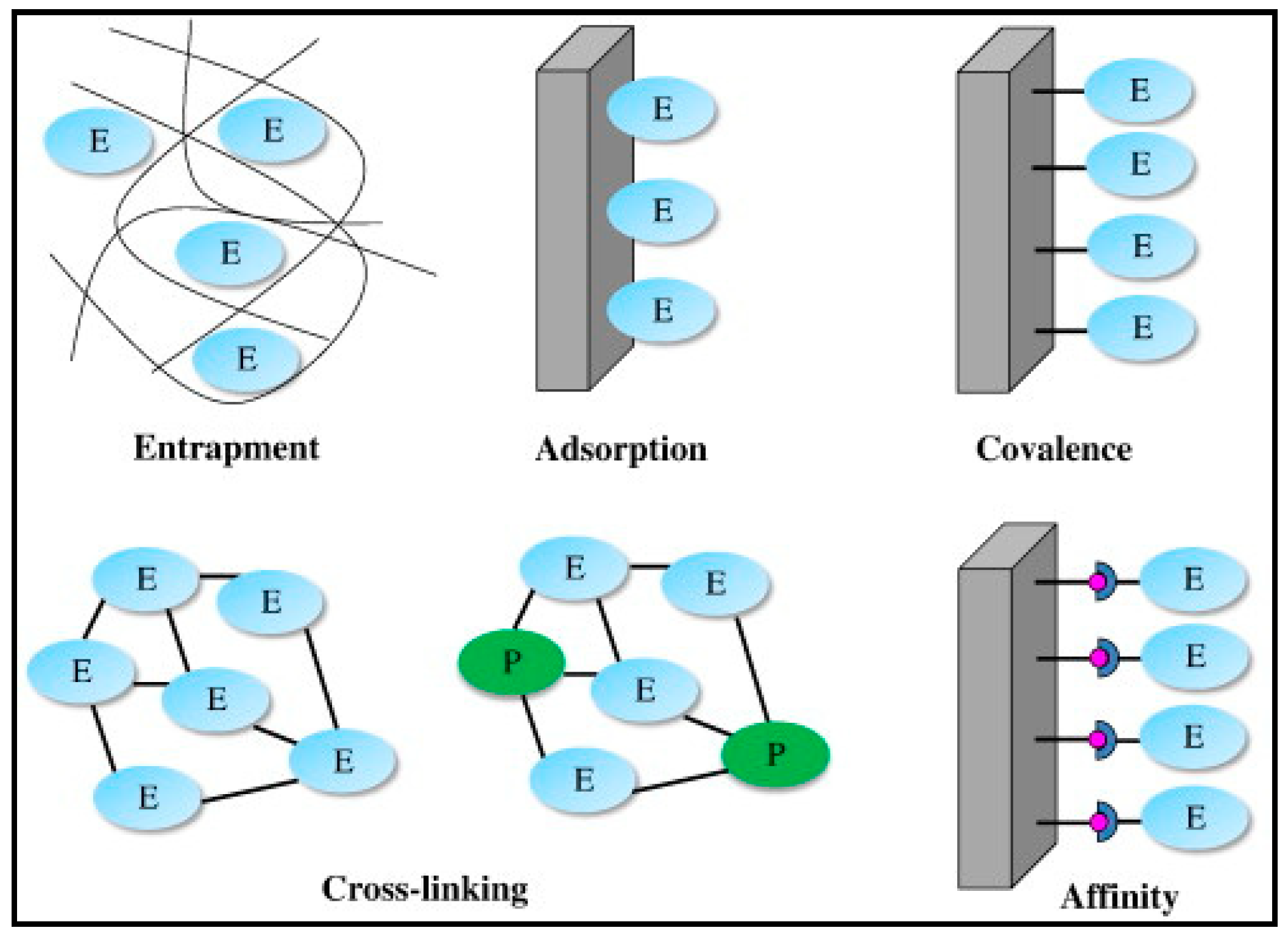
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: A literature survey. In Methods in Molecular Biology; Guisan, J., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 1051, pp. 15–31. [Google Scholar]
- Choi, M.M.F. Progress in enzyme-based biosensors using optical transducers. Microchim. Acta 2004, 148, 107–132. [Google Scholar] [CrossRef]
- Arya, S.K.; Datta, M.; Malhotra, B.D. Recent advances in cholesterol biosensor. Biosens. Bioelectron. 2008, 23, 1083–1100. [Google Scholar] [CrossRef]
- Andreescu, S.; Marty, J.L. Twenty years research in cholinesterase biosensors: From basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. New electrochemiluminescent biosensors combining polyluminol and an enzymatic matrix. Anal. Bioanal. Chem. 2009, 394, 971–980. [Google Scholar] [CrossRef]
- Tembe, S.; Karve, M.; Inamdar, S.; Haram, S.; Melo, J.; D’Souza, S.F. Development of electrochemical biosensor based on tyrosinase immobilized in composite biopolymeric film. Anal. Biochem. 2006, 349, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hanko, M.; Bruns, N.; Tiller, J.C.; Heinze, J. Optical biochemical sensor for determining hydroperoxides in nonpolar organic liquids as archetype for sensors consisting of amphiphilic conetworks as immobilisation matrices. Anal. Bioanal. Chem. 2006, 386, 1273–1283. [Google Scholar] [CrossRef]
- Galezowska, A.; Sikora, T.; Istamboulie, G.; Trojanowicz, M.; Polec, I.; Nunes, G.S.; Noguer, T.; Marty, J.L. Application of Genetically Engineered Acetylcholinesterases in Screen-Printed Amperometric Biosensor for Detection of Organophosphorus Insecticides. Sens. Mater. 2017, 20, 299. [Google Scholar] [CrossRef][Green Version]
- Valdés-Ramírez, G.; Cortina, M.; Ramírez-Silva, M.T.; Marty, J.L. Acetylcholinesterase-based biosensors for quantification of carbofuran, carbaryl, methylparaoxon, and dichlorvos in 5% acetonitrile. Anal. Bioanal. Chem. 2008, 392, 699–707. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhury, N.K. Entrapment of biomolecules in sol-gel matrix for applications in biosensors: Problems and future prospects. Biosens. Bioelectron. 2007, 22, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, P.C.A.; Araújo, A.N.; Conceição, M. Optical sensors and biosensors based on sol-gel films. Talanta 2007, 72, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Talat, M.; Hasan, S.H.; Pandey, R.K. Enzymatic detection of heavy metal ions in aqueous solution from vegetable wastes by immobilizing pumpkin (Cucumis melo) urease in calcium alginate beads. Biotechnol. Bioprocess Eng. 2008, 13, 210–216. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon paste electrodes in facts, numbers, and notes: A review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Mousty, C. Biosensing applications of clay-modified electrodes: A review. Anal. Bioanal. Chem. 2010, 396, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, M.M.; Ciniciato, G.P.M.K.; Ibrahim, S.A.; Phang, S.M.; Yunus, K.; Fisher, A.C.; Iwamoto, M.; Vengadesh, P. Preparation of a Three-Dimensional Reduced Graphene Oxide Film by Using the Langmuir-Blodgett Method. Langmuir 2015, 31, 10426–10434. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Yorganci, E.; Asav, E. Do copper ions activate tyrosinase enzyme? A biosensor model for the solution. Bioelectrochemistry 2010, 78, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.C.S.; Daniel-da-Silva, A.L.; Xavier, A.M.R.B.; Tavares, A.P.M. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem. Eng. Process. Process Intensif. 2017, 117, 1–8. [Google Scholar] [CrossRef]
- Cao, S.; Xu, P.; Ma, Y.; Yao, X.; Yao, Y.; Zong, M.; Li, X.; Lou, W. Recent advances in immobilized enzymes on nanocarriers. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 1814–1823. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Vakhshiteh, F.; Barkhi, M.; Baharifar, H.; Poor-Akbar, E.; Zari, N.; Stamatis, H.; Bordbar, A.K. Immobilization of cellulase enzyme onto magnetic nanoparticles: Applications and recent advances. Mol. Catal. 2017, 442, 66–73. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.; Kim, S.H.; Kim, J.H.; Yu, H.; Kim, H.J.; Yang, Y.H.; Kan, E.; Kim, Y.H.; Lee, S.H. Biocompatible cellulose nanocrystals as supports to immobilize lipase. J. Mol. Catal. B Enzym. 2015, 122, 170–178. [Google Scholar] [CrossRef]
- Deng, X.; Cao, S.; Li, N.; Wu, H.; Smith, T.J.; Zong, M.; Lou, W. A magnetic biocatalyst based on mussel-inspired polydopamine and its acylation of dihydromyricetin. Cuihua Xuebao/Chin. J. Catal. 2016, 37, 584–595. [Google Scholar] [CrossRef]
- Cao, S.; Huang, Y.; Li, X.; Xu, P.; Wu, H.; Li, N.; Lou, W. Preparation and Characterization of Immobilized Lipase from Pseudomonas Cepacia onto Magnetic Cellulose Nanocrystals. Nat. Publ. Gr. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Meng, L.; Wu, P.; Chen, G.; Cai, C.; Sun, Y.; Yuan, Z. Low potential detection of glutamate based on the electrocatalytic oxidation of NADH at thionine/single-walled carbon nanotubes composite modified electrode. Biosens. Bioelectron. 2009, 24, 1751–1756. [Google Scholar] [CrossRef]
- Ammam, M.; Fransaer, J. Highly sensitive and selective glutamate microbiosensor based on cast polyurethane/AC-electrophoresis deposited multiwalled carbon nanotubes and then glutamate oxidase/electrosynthesized polypyrrole/Pt electrode. Biosens. Bioelectron. 2010, 25, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Gourine, A.V.; Huckstepp, R.T.R.; Dale, N. A microelectrode biosensor for real time monitoring of l-glutamate release. Anal. Chim. Acta 2009, 645, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Pemberton, R.M.; Fielden, P.R.; Hart, J.P. Development of a novel reagentless, screen-printed amperometric biosensor based on glutamate dehydrogenase and NAD+, integrated with multi-walled carbon nanotubes for the determination of glutamate in food and clinical applications. Sens. Actuators B Chem. 2015, 216, 614–621. [Google Scholar] [CrossRef]
- Akhtar, M.H.; Hussain, K.K.; Gurudatt, N.G.; Shim, Y.B. Detection of Ca2+-induced acetylcholine released from leukemic T-cells using an amperometric microfluidic sensor. Biosens. Bioelectron. 2017, 98, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.T.C.; Sale, M.G.F.; Di Lorenzo, M. Towards timely Alzheimer diagnosis: A self-powered amperometric biosensor for the neurotransmitter acetylcholine. Biosens. Bioelectron. 2017, 87, 607–614. [Google Scholar] [CrossRef] [PubMed]
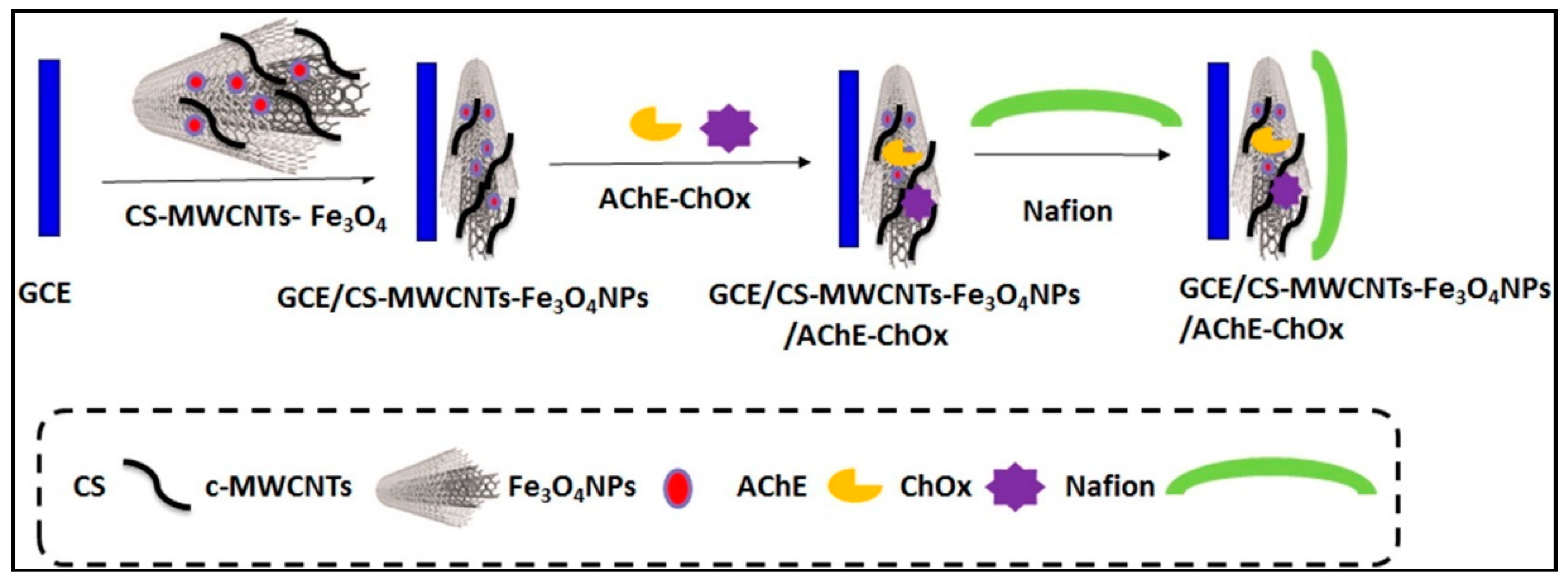
- Bolat, E.Ö.; Tığ, G.A.; Pekyardımcı, Ş. Fabrication of an amperometric acetylcholine esterase-choline oxidase biosensor based on MWCNTs-Fe3O4NPs-CS nanocomposite for determination of acetylcholine. J. Electroanal. Chem. 2017, 785, 241–248. [Google Scholar] [CrossRef]
- De Souza Ribeiro, F.A.; Tarley, C.R.T.; Borges, K.B.; Pereira, A.C. Development of a square wave voltammetric method for dopamine determination using a biosensor based on multiwall carbon nanotubes paste and crude extract of Cucurbita pepo L. Sens. Actuators B Chem. 2013, 185, 743–754. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Jha, S.K. Dopamine biosensor based on surface functionalized nanostructured nickel oxide platform. Biosens. Bioelectron. 2016, 84, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Le Goff, A.; Putaux, J.L.; Tertis, M.; Cristea, C.; Səndulescu, R.; Cosnier, S. Design of a reduced-graphene-oxide composite electrode from an electropolymerizable graphene aqueous dispersion using a cyclodextrin-pyrrole monomer. Application to dopamine biosensing. Electrochim. Acta 2015, 178, 108–112. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, Q.; Zhou, T.; Zhu, M.; Jin, L.; Shi, G. On-line microdialysis system with poly(amidoamine)-encapsulated Pt nanoparticles biosensor for glutamate sensing in vivo. Bioelectrochemistry 2011, 81, 53–57. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Shahrokhian, S.; Iraji zad, A.; Mohajerzadeh, S.; Vosoughi, M.; Darbari, S.; Sanaee, Z. Mediator-less highly sensitive voltammetric detection of glutamate using glutamate dehydrogenase/vertically aligned CNTs grown on silicon substrate. Biosens. Bioelectron. 2012, 31, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, V.M.; Wassum, K.M.; Maidment, N.T.; Monbouquette, H.G. Electrochemically deposited iridium oxide reference electrode integrated with an electroenzymatic glutamate sensor on a multi-electrode array microprobe. Biosens. Bioelectron. 2013, 42, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Özel, R.E.; Ispas, C.; Ganesana, M.; Leiter, J.C.; Andreescu, S. Glutamate oxidase biosensor based on mixed ceria and titania nanoparticles for the detection of glutamate in hypoxic environments. Biosens. Bioelectron. 2014, 52, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Salazar, P.; Martín, M.; O’Neill, R.D.; González-Mora, J.L. Glutamate microbiosensors based on Prussian Blue modified carbon fiber electrodes for neuroscience applications: In-vitro characterization. Sens. Actuators B Chem. 2016, 235, 117–125. [Google Scholar] [CrossRef]
- Batra, B.; Yadav, M.; Pundir, C.S. l-Glutamate biosensor based on l-glutamate oxidase immobilized onto ZnO nanorods/polypyrrole modified pencil graphite electrode. Biochem. Eng. J. 2016, 105, 428–436. [Google Scholar] [CrossRef]
- Cui, Y.; Barford, J.P.; Renneberg, R. Development of an interference-free biosensor for l-glutamate using a bienzyme salicylate hydroxylase/l-glutamate dehydrogenase system. Enzyme Microb. Technol. 2007, 41, 689–693. [Google Scholar] [CrossRef]
- Cui, Y.; Barford, J.P.; Renneberg, R. Development of an l-glutamate biosensor using the coimmobilization of l-glutamate dehydrogenase and p-hydroxybenzoate hydroxylase on a Clark-type electrode. Sens. Actuators B Chem. 2007, 127, 358–361. [Google Scholar] [CrossRef]
- Jamal, M.; Xu, J.; Razeeb, K.M. Disposable biosensor based on immobilisation of glutamate oxidase on Pt nanoparticles modified Au nanowire array electrode. Biosens. Bioelectron. 2010, 26, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Pemberton, R.M.; Fielden, P.R.; Hart, J.P. A reagentless, screen-printed amperometric biosensor for the determination of glutamate in food and clinical applications. Methods Mol. Biol. 2017, 1572, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Pundir, C.S. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef]
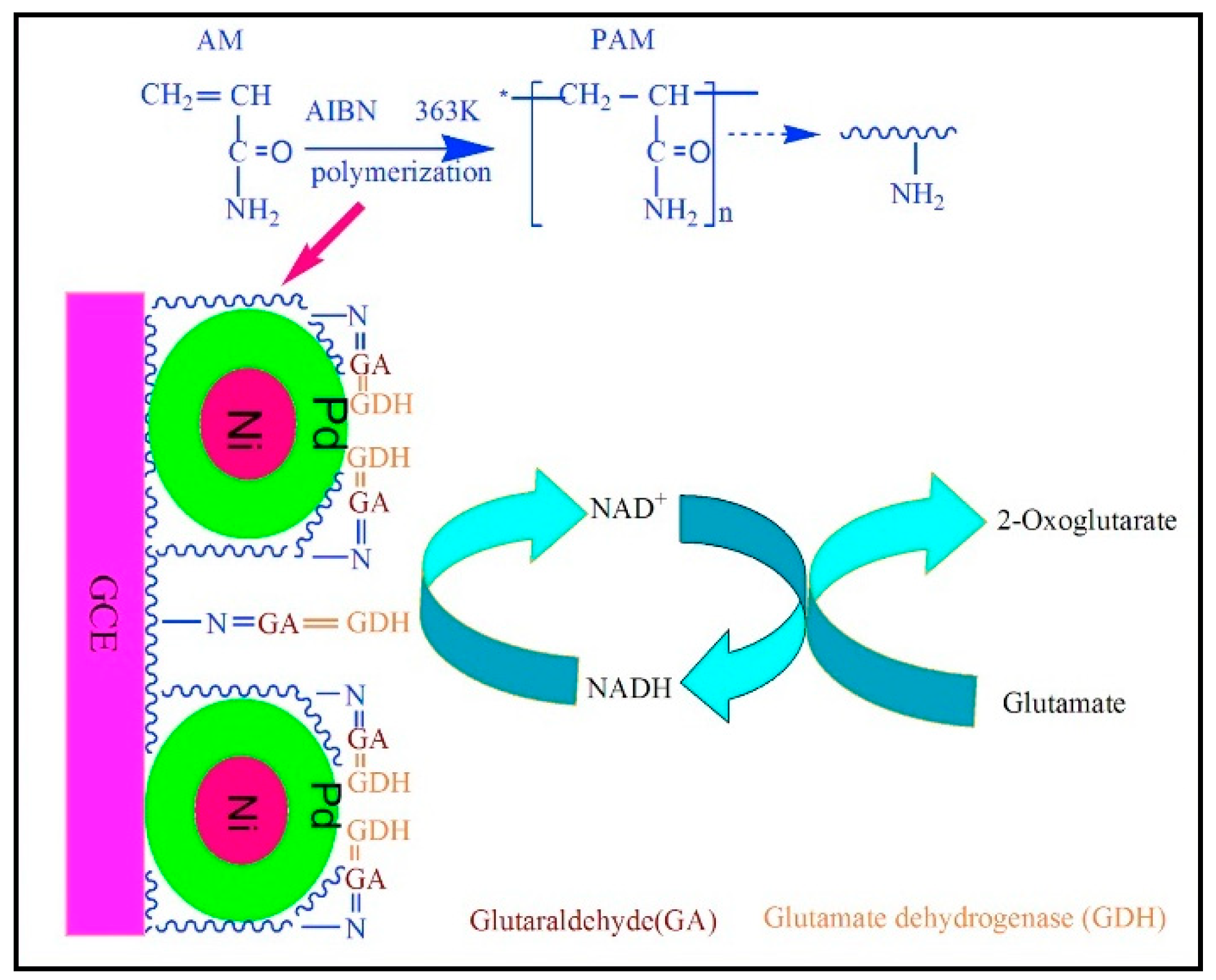
- Yu, H.; Ma, Z.; Wu, Z. Immobilization of Ni-Pd/core-shell nanoparticles through thermal polymerization of acrylamide on glassy carbon electrode for highly stable and sensitive glutamate detection. Anal. Chim. Acta 2015, 896, 137–142. [Google Scholar] [CrossRef]
- Batra, B.; Kumari, S.; Pundir, C.S. Construction of glutamate biosensor based on covalent immobilization of glutmate oxidase on polypyrrole nanoparticles/polyaniline modified gold electrode. Enzyme Microb. Technol. 2014, 57, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kashyap, S.; Kumar, S.; Abraham, S.; Gupta, T.K.; Kayastha, A.M.; Malhotra, B.D.; Saxena, P.S.; Srivastava, A.; Singh, R.K. Excellent storage stability and sensitive detection of neurotoxin quinolinic acid. Biosens. Bioelectron. 2017, 90, 224–229. [Google Scholar] [CrossRef]
- Fritea, L.; Tertiș, M.; Cosnier, S.; Cristea, C.; Săndulescu, R. ELECTROCHEMICAL SCIENCE A Novel Reduced Graphene Oxide/β-Cyclodextrin/Tyrosinase Biosensor for Dopamine Detection. Int. J. Electrochem. Sci. 2015, 10, 7292–7302. [Google Scholar]
- Taly, A.; Corringer, P.J.; Guedin, D.; Lestage, P.; Changeux, J.P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef]
- Lozier, B.K.; Haven, T.R.; Astill, M.E.; Hill, H.R. Detection of acetylcholine receptor modulating antibodies by flow cytometry. Am. J. Clin. Pathol. 2015, 143, 186–192. [Google Scholar] [CrossRef]
- Luchicchi, A.; Bloem, B.; Viaña, J.N.M.; Mansvelder, H.D.; Role, L.W. Illuminating the role of cholinergic signaling in circuits of attention and emotionally salient behaviors. Front. Synaptic Neurosci. 2014, 6, 1–10. [Google Scholar] [CrossRef]
- Colliver, T.L.; Ewing, A.G. Neurotransmitters, Electrochemical Detection of. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar] [CrossRef]
- Picollo, F.; Battiato, A.; Bernardi, E.; Marcantoni, A.; Pasquarelli, A.; Carbone, E.; Olivero, P.; Carabelli, V. Microelectrode Arrays of Diamond-Insulated Graphitic Channels for Real-Time Detection of Exocytotic Events from Cultured Chromaffin Cells and Slices of Adrenal Glands. Anal. Chem. 2016, 88, 7493–7499. [Google Scholar] [CrossRef]
- Lin, Y.; Trouillon, R.; Svensson, M.I.; Keighron, J.D.; Cans, A.S.; Ewing, A.G. Carbon-ring microelectrode arrays for electrochemical imaging of single cell exocytosis: Fabrication and characterization. Anal. Chem. 2012, 84, 2949–2954. [Google Scholar] [CrossRef]
- Yakushenko, A.; Schnitker, J.; Wolfrum, B. Printed carbon microelectrodes for electrochemical detection of single vesicle release from PC12 cells. Anal. Chem. 2012, 84, 4613–4617. [Google Scholar] [CrossRef]
- Liu, X.; Tong, Y.; Fang, P.P. Recent development in amperometric measurements of vesicular exocytosis. TrAC Trends Anal. Chem. 2019, 113, 13–24. [Google Scholar] [CrossRef]
- Sangubotla, R.; Kim, J. Recent trends in analytical approaches for detecting neurotransmitters in Alzheimer’s disease. TrAC Trends Anal. Chem. 2018, 105, 240–250. [Google Scholar] [CrossRef]
- Emran, M.Y.Y.; Mekawy, M.; Akhtar, N.; Shenashen, M.A.A.; EL-Sewify, I.M.M.; Faheem, A.; El-Safty, S.A.A. Broccoli-shaped biosensor hierarchy for electrochemical screening of noradrenaline in living cells. Biosens. Bioelectron. 2018, 100, 122–131. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Wilson, C.M.; Brabazon, F.; Von Leden, R.; Jurgens, J.S.; Oakes, T.R.; Selwyn, R.G. FDG-PET imaging in mild traumatic brain injury: A critical review. Front. Neuroenergetics 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.M.; Robbins, E.M.; Catt, K.A.; Cody, P.A.; Happe, C.L.; Cui, X.T. Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosens. Bioelectron. 2017, 89, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Trikantzopoulos, E.; Nguyen, M.D.; Jacobs, C.B.; Wang, Y.; Mahjouri-Samani, M.; Ivanov, I.N.; Venton, B.J. Laser Treated Carbon Nanotube Yarn Microelectrodes for Rapid and Sensitive Detection of Dopamine in Vivo. ACS Sens. 2016, 1, 508–515. [Google Scholar] [CrossRef]
- Park, J.W.; Bhimani, R.V.; Park, J. Noradrenergic Modulation of Dopamine Transmission Evoked by Electrical Stimulation of the Locus Coeruleus in the Rat Brain. ACS Chem. Neurosci. 2017, 8, 1913–1924. [Google Scholar] [CrossRef]
- Van Schoors, J.; Viaene, J.; Van Wanseele, Y.; Smolders, I.; Dejaegher, B.; Vander Heyden, Y.; Van Eeckhaut, A. An improved microbore UHPLC method with electrochemical detection for the simultaneous determination of low monoamine levels in in vivo brain microdialysis samples. J. Pharm. Biomed. Anal. 2016, 127, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.H.; Nolan, J.K.; Park, H.; Lam, S.; Fattah, M.; Page, J.C.; Joe, H.E.; Jun, M.B.G.; Lee, H.; Kim, S.J.; et al. Facile fabrication of flexible glutamate biosensor using direct writing of platinum nanoparticle-based nanocomposite ink. Biosens. Bioelectron. 2019, 131, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Xu, S.; Song, Y.; Zhang, Y.; Li, Z.; Gao, F.; Xie, J.; Sha, L.; Xu, Q.; Shen, Y.; et al. In situ detection of neurotransmitters and epileptiform electrophysiology activity in awake mice brains using a nanocomposites modified microelectrode array. Sens. Actuators B Chem. 2019, 288, 601–610. [Google Scholar] [CrossRef]
- Ferreira, N.R.; Ledo, A.; Laranjinha, J.; Gerhardt, G.A.; Barbosa, R.M. Simultaneous measurements of ascorbate and glutamate in vivo in the rat brain using carbon fiber nanocomposite sensors and microbiosensor arrays. Bioelectrochemistry 2018, 121, 142–150. [Google Scholar] [CrossRef]
- Oh, Y.; Heien, M.L.; Park, C.; Kang, Y.M.; Kim, J.; Boschen, S.L.; Shin, H.; Cho, H.U.; Blaha, C.D.; Bennet, K.E.; et al. Tracking tonic dopamine levels in vivo using multiple cyclic square wave voltammetry. Biosens. Bioelectron. 2018, 121, 174–182. [Google Scholar] [CrossRef]
- Baker, K.L.; Bolger, F.B.; Lowry, J.P. Development of a microelectrochemical biosensor for the real-time detection of choline. Sens. Actuators B Chem. 2017, 243, 412–420. [Google Scholar] [CrossRef]
- Baker, K.L.; Bolger, F.B.; Lowry, J.P. A microelectrochemical biosensor for real-time in vivo monitoring of brain extracellular choline. Analyst 2015, 140, 3738–3745. [Google Scholar] [CrossRef]
- Gu, H.; Liu, Y.; Ren, T.; Xia, W.; Guo, Y.; Shi, G. An electrochemical biosensor based on double molecular recognition for selective monitoring of cerebral dopamine dynamics at 4 min interval. Sens. Actuators B Chem. 2019, 287, 356–363. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Borisova, T.; Kucherenko, D.; Soldatkin, O.; Kucherenko, I.; Pastukhov, A.; Nazarova, A.; Galkin, M.; Borysov, A.; Krisanova, N.; Soldatkin, A.; et al. An amperometric glutamate biosensor for monitoring glutamate release from brain nerve terminals and in blood plasma. Anal. Chim. Acta 2018, 1022, 113–123. [Google Scholar] [CrossRef]
- Ganesana, M.; Trikantzopoulos, E.; Maniar, Y.; Lee, S.T.; Venton, B.J. Development of a novel micro biosensor for in vivo monitoring of glutamate release in the brain. Biosens. Bioelectron. 2019, 130, 103–109. [Google Scholar] [CrossRef]
- Zestos, A.G.; Jacobs, C.B.; Trikantzopoulos, E.; Ross, A.E.; Venton, B.J. Polyethylenimine carbon nanotube fiber electrodes for enhanced detection of neurotransmitters. Anal. Chem. 2014, 86, 8568–8575. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.; Zhang, Y.; Xu, S.; Xu, H.; Wang, M.; Wang, Y.; Cai, X. A microelectrode array electrodeposited with reduced graphene oxide and Pt nanoparticles for norepinephrine and electrophysiological recordings. J. Micromech. Microeng. 2017, 27, 115001. [Google Scholar] [CrossRef]
| Category | Neurotransmitter | Biological Function | Chemical Structure |
|---|---|---|---|
| Amino acid | Glutamate | cognition, memory and learning processes | 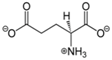 |
| Tyrosine | regulation of energy balance, memory, learning |  | |
| Biogenic amines | Dopamine | responsible for feelings of pleasure | 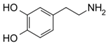 |
| Epinephrine | leading to a physical boost and heightened awareness | 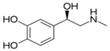 | |
| Norepinephrine | improving attention and the speed at which responsive actions occur |  | |
| Serotonin | regulating mood, sleep, emesis, sexuality, appetite, pain | 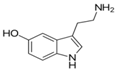 | |
| Tryptamine | acting in central nervous system and gastrointestinal tract | 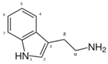 | |
| Acetyl choline | Acetylcholine | thought, learning and memory |  |
| Soluble gases | Nitric oxide | cognitive functions, homeostatic functions, neurosecretion and synaptic plasticity | 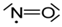 |
| Hydrogen sulphide | neuromodulator in the brain |  |
| Neurological Biomarker | Electrode Surface | Linear Range; LOD (µM) | Sensitivity | Technique | Electrolyte | Real Samples/Storage | Interferences | Ref. |
|---|---|---|---|---|---|---|---|---|
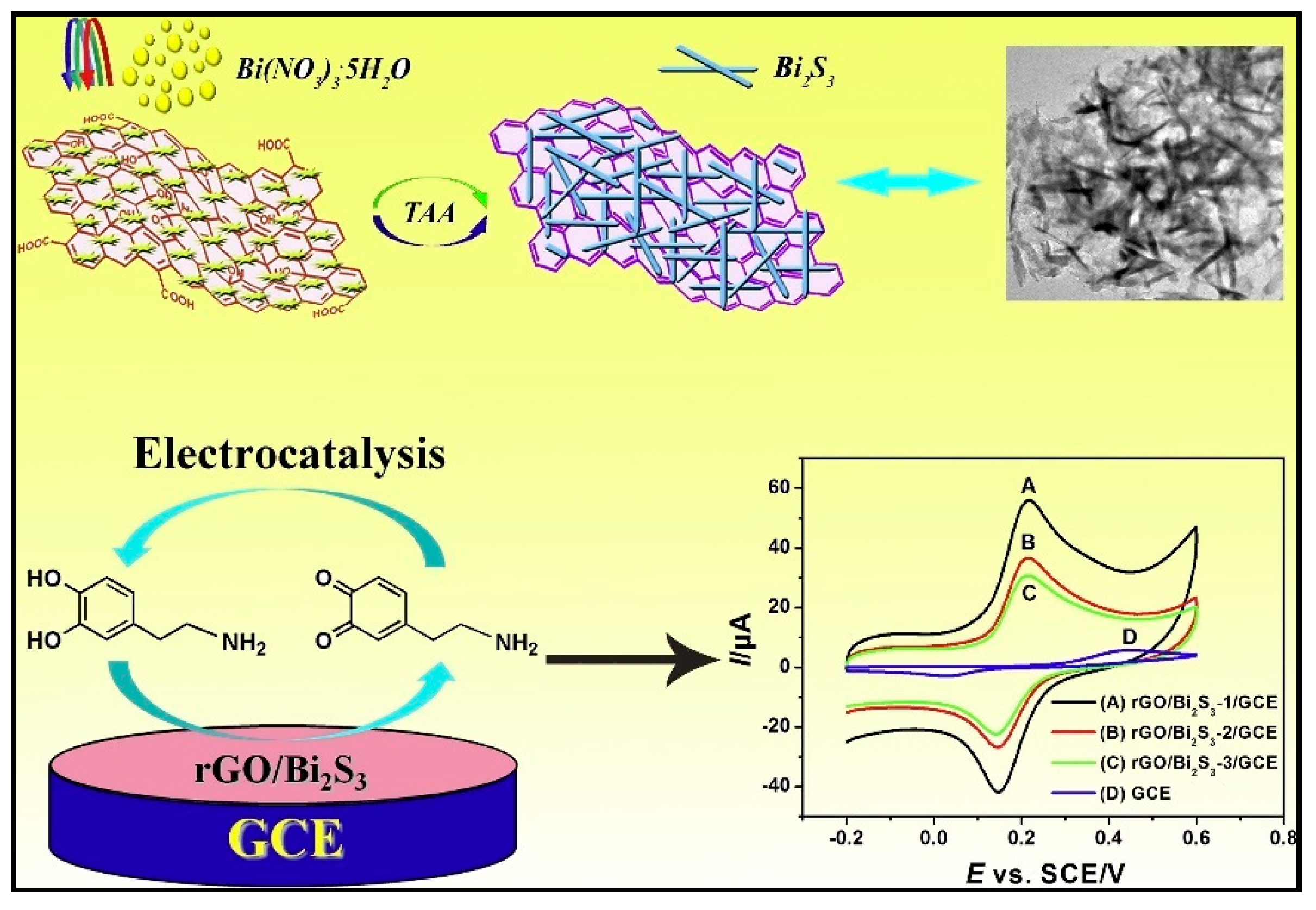
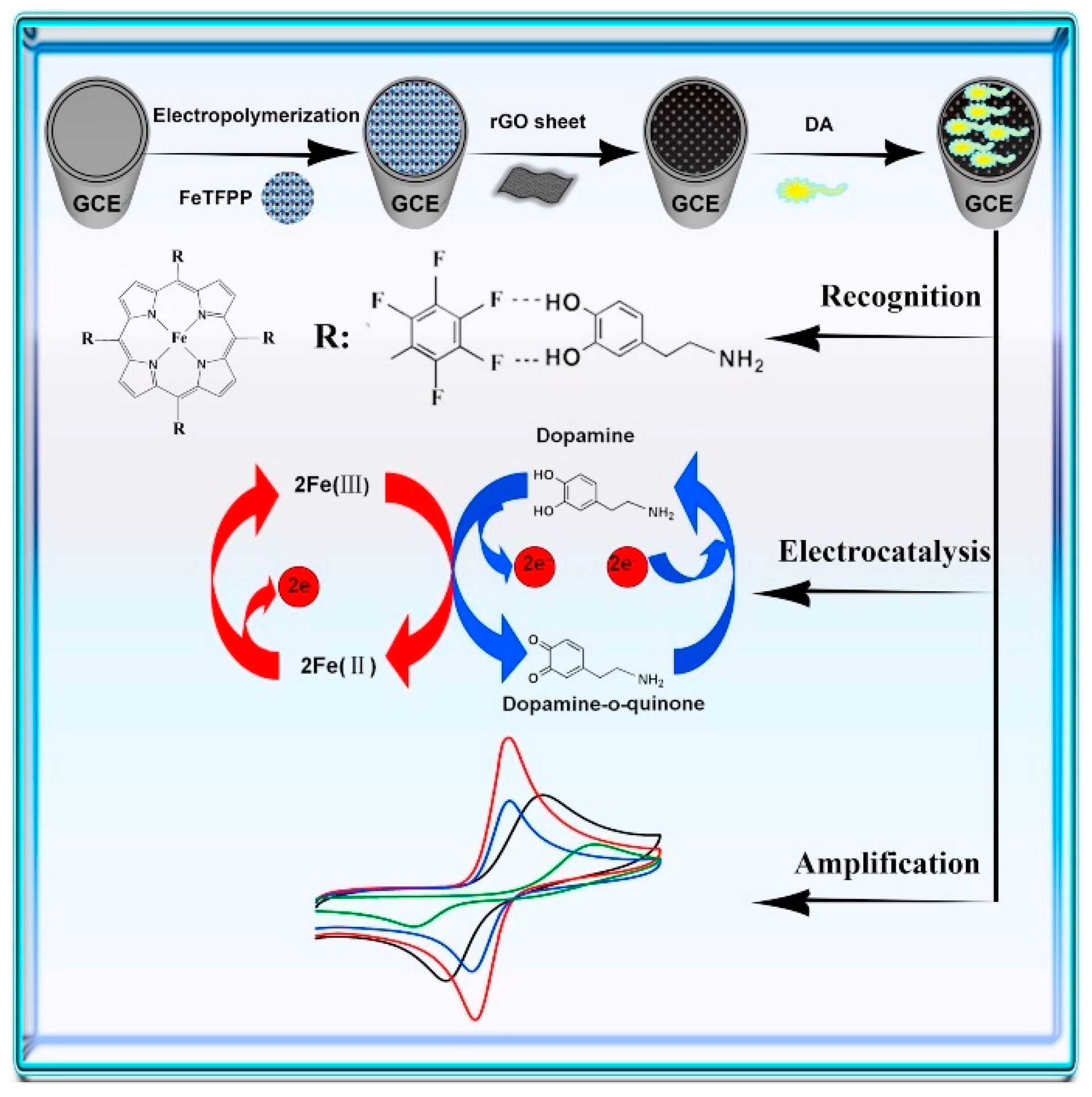
| Dopamine | rGO/Bi2S3/GCE | 0.01–40; 1.23 × 10−2 | 2.046 µA µM−1 | CV–DPV | 0.1 M PBS pH 6.0 | Urine samples/30 days (91.6%) | Ca2+, Na+, Li+, Cu2+, Cl−, SO42−, phenacetin, Glu, Fru, caffeine, APAP, Cys, Tyr, proline, AA, UA | [70] |
| rGO-poly(FeTFPP)/GCE | 0.05–300; 2.3 × 10−2 | 0.039 µA µM−1 | CV–DPV-EIS | 0.1 M PBS pH 6.0 | Lake water; urine samples/long-term stability (90.2–93.6%) | Na+, Li+, Ca2+, Cl−, SO42−, Glu, Mal, Fru, Lac, AA, UA | [71] | |
| rGO/PU | 1 × 10−4–11.5 × 10−4; 1 × 10−6 | 0.011 µA pM−1 | CV-DPV | 0.05 M PBS pH 7.0 | Human serum, urine/15 days (96.4%) | Fe3+, Zn2+, 4-NP, AA, UA, Tyr, Trp, GSH, Glu | [72] | |
| PGr/GCE | 0.01–50; 1 × 10−3 | 0.478 µA µM−1 | CV-DPV | 0.1 M PBS pH 7.0 | Human blood/30 days (89%) | AA, UA | [73] | |
| 0.005–1; 1 × 10−3 | 0.004 µA µM−1 | Simultaneous detection of DA and AA | ||||||
| rGO-Cu2O/GCE | 10–900; 5 × 10−2 | 0.520 μA μM−1 cm−2 | CV-DPV | 0.1 M PBS pH 7.0 | Human blood; urine/15 days (85%) | UA, AA, Glu, K+, Na+, Cl−, SO42− | [74] | |
| rGO/ZIF-8/GCE | 0.1–100; 3 × 10−2 | 0.153 μA μM−1 | CV-DPV | 0.1 M PBS pH 7.0 | Human serum/15 days (95.7%) | AA | [75] | |
| HNP-AuAg/GCE | 5–335; 2 × 10−1 | 0.399 μA μM−1 cm−2 | CV-DPV-Amp | 0.1 M PBS pH 7.0 | -/ 20 days (99.3%) | AA / Simultaneous detection of DA and UA | [76] | |
| N-G/NiTsPC/GCE | 0.1–200; 1 × 10−1 | 0.089 μA μM−1 | Amp-CV-EIS | 0.1 M PBS pH 7.4 | - / 30 days (93.21%) | AA, UA | [77] | |
| poly-FA/MWCNT/GCE | 5.00–120.0; 2.21 | 0.037 μA μM−1 | Amp-CV | 0.1 M PBS pH 7.0 | Pharmaceutical samples/- | 5-HT, AA, UA/Simultaneous detection of DA, NADH and EP | [78] | |
| NiO NP-MWCNT-DHP/GCE | 0.07–4.8; 5 × 10−2 | 3.800 μA μM−1 | DPV-SWV | 0.2 M PBS pH 7.0 | Cerebrospinal fluid, human serum and lung fluid/- | - / Simultaneous detection of DA, and EP | [79] | |
| HNP-PtTi/GCE | 0.004–500; 3.2 | 0.186 μA μM−1 cm−2 | CV-DPV-Amp | 0.1 M PBS pH 7.0 | Human serum/- | Na+, K+, Fe3+, Cu2+, Al3+, Glu, and H2O2 / Simultaneous detection of DA, UA and AA | [80] | |
| CPE/GO | 0.08–2.30; 8.6 × 10−3 | 0.489 μA μM−1 | CV-DPV-LSV | 0.2 M Britton–Robinson buffer pH 4.0 | Human blood/15 days (95.75%) | Na+, NH4+, NO3−, Cl−, CO32−, K+, I−, phenylalanine, Cys, Trp/Simultaneous detection of DA in the presence of Tyr | [81] | |
| CE | 0.4 – 100; 2 × 10−1 | 2.292 μA μM−1 cm−2 | CV-DPV | 0.1 M PBS pH 7.0 | Human serum/14 days (95%) | Citric acid, Glu, Cys, l-glycine, Lys, Tyr, ANI, catechol, hydroquinone, phenol, resorcinol, Ca2+, K+, Mg2+, Na+, Zn2+/ Simultaneous detection of DA in the presence of AA, UA, Trp, and nitrite (NO2−) | [82] | |
| NiO-CuO/GR/GCE | 0.5–20; 0.17 | 9.406 μA μM−1 cm−2 | EIS-SWV | 0.1 M PBS pH 8.0 | Human serum, blood, pharmaceutical samples/30 days (95%) | K+, Na+, Zn2+, NO3−, Cl−, SO42−/ Simultaneous detection of DA in the presence of APAP and Trp | [83] | |
| GR/p-AHNSA/SPCs | 0.05–100; 2 × 10−3 | 0.099 μA μM−1 | CV-EIS-SWV | 0.1 M PBS pH 7.2 | Human plasma, urine, pharmaceutical samples/- | AA, UA, Trp/Simultaneous detection of DA and 5-HT | [84] | |
| [AMIM][BF4]/CCE | 0.1–20; 6.8 × 10−2 | 1.356 μA μM−1 | CV-DPV | 0.1 M PBS pH 7.0 | Human blood serum, urine, pharmaceutical samples/20 days (96.6%) | Ca2+, Mg2+, Zn2+, Fe3+, K+, NO2−/ Simultaneous detection of DA and APAP | [85] | |
| CB-chit/GCE | 0.1–1400; 1 × 10−2 | 0.132 μA μM−1 | CV-DPV | 0.1 M PBS pH 7.4 | Human urine, pharmaceutical samples/- | -/ Simultaneous detection of DA and AA | [86] | |
| Epinephrine | Paraffin/MWCNT/CoPc | 1.33–5.50; 1.56 × 10−2 | 5.920 μA μM−1 | DPV | 0.2 M PBS pH 6.0 | Human urine samples/(1000 determinations) | UA | [87] |
| poly-FA/MWCNT/GCE | 73.0–1406; 22.28 | 0.004 μA μM−1 | Amp-DPV | 0.1 M PBS pH 7.0 | Pharmaceutical samples/- | 5-HT, AA, UA/Simultaneous detection of DA, NADH and EP | [78] | |
| EDDPT/GO/CPE | 1.5–600; 0.65 | 0.076 μA μM−1 | DPV-Amp | 0.1 M alkaline solution pH 7.0 | Human serum, pharmaceutical samples/7 days (92%) | K+, Na+, Mg2+, Cl−, Glu, Fru, folic acid | [88] | |
| NiONP-MWCNT-DHP/GCE | 0.3–9.5; 8.2 × 10−2 | 0.390 μA μM−1 | DPV-SWV | 0.2 M PBS pH 7.0 | Cerebrospinal fluid, human serum and lung fluid/- | -/Simultaneous detection of DA, and EP | [79] | |
| Glutamate | Pt/NiNAE | 500–800; 83 | 0.096 μA μM−1 cm−2 | Amp-CV | 1 M NaOH | -/60 days (90%) | AA, UA, Glu | [68] |
| Norepinephrine | AuNPs/ITO | 0.1–25; 8.7 × 10−2 | 1.011 μA μM−1 | SWV-CV | 0.1 M PBS pH 7.2 | Human blood, urine/7 days (96.3%) | DA, UA, AA | [89] |
| Oxytocin | BDDE | 1–10; 5 × 10−2 | - | Amp-CV | 0.1 M PBS pH 7.4 | - | -/Selective detection of oxytocin and vasopresin | [90] |
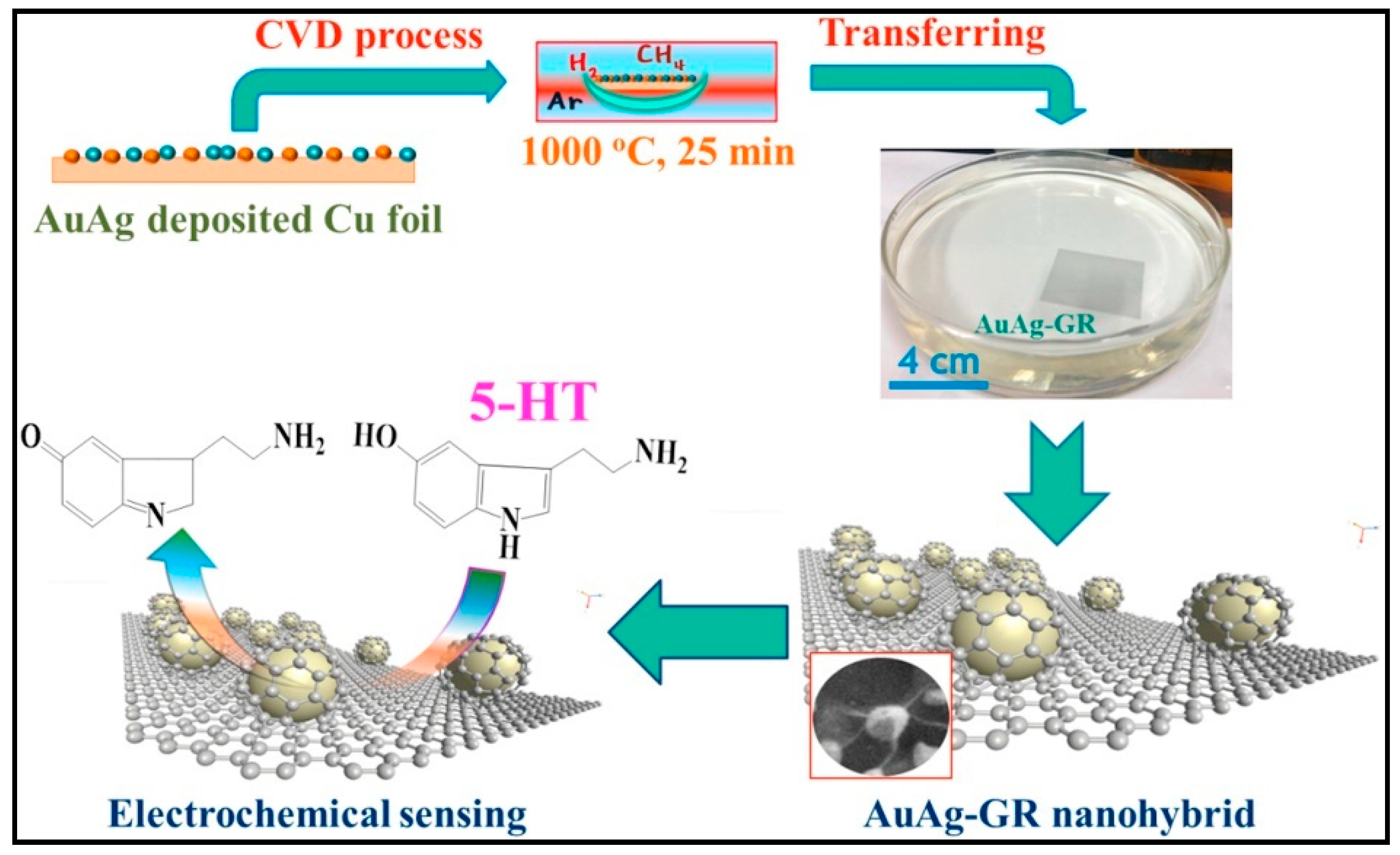
| Serotonin | GR/p-AHNSA/SPCs | 0.05–150; 3 × 10−3 | 0.101 μA μM−1 | CV-EIS SWV | 0.1 M PBS pH 7.2 | Human plasma, urine, pharmaceutical samples/- | AA, UA, Trp/Simultaneous detection of DA and 5-HT | [84] |
| PEDOTNTs/rGO/Ag NPs/GCE | 0.01– 500; 1 × 10−4 | 0.0143 μA μM−1 cm−2 | Amp-CV-DPV | 0.1 M PBS pH 7.4 | Bovine assayed multi-sera/30 days (97%) | Cys, Trp, Ala, Glu, DA, EP and NE/ Simultaneous detection of 5-HT in the presence of AA, UA, Tyr | [91] | |
| AuAgNPs/GR/ITO | 0.0027–4.82 1.6 × 10−3 | 0.766 μA μM−1 cm−2 | Amp-CV | 0.1 M PBS pH 7.4 | Human serum/19 days (88%) | Glu, K+, Cl-, UA, AA/- | [92] | |
| Tryptamine | GCE | 0.047–0.545 0.8 × 10−3 | 3.1 μA μM−1 | SWADdSV | 0.1 M Acetate buffer pH 5.3 | Food samples/- | Putrescine/- | [93] |
| Neurological Biomarker | Electrode Surface | Linear Range; LOD (µM) | Sensitivity | Technique | Electrolyte | Real Samples/Storage | Interferences | Ref. |
|---|---|---|---|---|---|---|---|---|
| Acetylcholine | SPCE/AuNPs/pTTBA-AChE | 7 × 10−4 −60; 6 × 10−4 | 0.019 µA µM−1 | Amp-CV-EIS | 0.1 M PBS pH 7.4 | Human plasma samples and cell line/60 days (91%) | AA, UA, catechol, GABA, APAP, DA, EP, glutamine | [139] |
| AChE/hPG/ Pt | 240–1900; 10 | 0.003 μA µM−1cm−2 | CV-Amp-DPV-SWV | 0.01 M Glycine pH 7.4 + 0.1 M of NaCl | - | - | [140] | |
| GCE/Chit-MWCNTs-Fe3O4NPs/AChE-ChOx | 0.02–0.11; 6.1 × 10−4 | 5.890 µA µM−1 | Amp-CV-EIS | 0.05 M PBS pH 7.5 | Human serum samples/30 days (60%) | AA, UA, APAP, Cys, Glu | [141] | |
| Dopamine | HRP/MWCNTs | 32–44; 2 | 1.980 µA µM−1 | CV-DPV-SWV | 0.25 M PBS pH 6.5 | Pharmaceutical samples/Freezing 48 h (99.66%) | AA, UA | [142] |
| Tyr/ NiONPs/ITO | 2–200; 1.038 | 0.060 µA µM−1 | CV | 0.05M PBS pH 6.5 | Fetal bovine serum samples/45 days (77%) | AA, UA | [143] | |
| rGO/β-CD-Py/GCE | 0.027–38.6; 0.027 | 0.012 μA µM−1cm−2 | Amp | 0.1M PBS pH 6.5 | - | AA, UA, Glu | [144] | |
| Glutamate | GlDH-Th-SWCNTs/GCE | 0.5–400; 0.1 | 0.137 μA µM−1 cm−2 | CV-Amp | 0.1 M PBS pH 8.3 | -/14 days (93%) | AA, UA, APAP | [135] |
| GlOx/ MWCNT/PPy/Pt | 0.3–140; 0.3 | 0.384 μA µM−1 cm−2 | Amp | 0.1 M PBS pH 7.4 | -/30 days (70%) | AA, UA, APAP | [136] | |
| GlOx/MWCNT/PAMAM/Pt/ Nafion | 1.0–50.0; 0.5 | 0.002 μA µM−1 | Amp-LSV | aCSF pH 7.4 | Artificial cerebrospinal fluid/14 days (86%) | AA, DA/In vivo measurement of glutamate in the striatum of rats | [145] | |
| GlDH/VACNTs | 0.1–20; 0.057 | 0.976 µA µM−1 cm−2 | CV-DPV | 0.1 M PBS pH 7.0 | -/14 days (80.5%) | AA, UA | [146] | |
| GlOx/IrOx-MEA | 5–300; 0.32 | 0.007 × 10−3 µA µM−1 | Amp | PBS pH 7.2 | -/14 days (71%) | AA, DA/In vitro and in vivo glutamate sensing | [147] | |
| CeO2/TiO2/GlOx/Chit/oPD/Pt | 5–50; 0.493 | 0.793 × 10−3 µA µM−1 | Amp | 0.1 M PBS pH 7.4 | Artificial cerebrospinal fluid/20 days (55%) | AA, DA, l-DOPA, 5-HT | [148] | |
| CFE/PoPD/GlOx/Gluth | 0–150; 1.5 | 0.135 µA µM−1 cm−2 | Amp | 0.1 M PBS pH 7.4 | -/30 days (90%) | Glu, lactate, 5-HT, glutamine, UA, AA | [149] | |
| GlOx/ZnONRs/PPy/PGE | 0.02–500; 1.8 × 10−4 | - | CV | 0.1 M Tris–HCl pH 8.5 | Food samples/90 days (70%) | - | [150] | |
| MWCNT-Chit-Mel B/GLDH-NAD+-Chit/MWCNT-Chit | 7.5–105; 3.0 | 0.390 × 10−3 µA μM−1 | CV-Amp | 0.075 M PBS pH 7.0 | Fetal bovine serum sample, food samples; / - | AA | [138] | |
| SHL-GlDH/oxygen electrode | 10–1500; 3.0 | 0.087 × 10−3 µA μM−1 | Amp | 0.1 M Tris–HCl pH 8.0 | - /14 days (≈100%) | AA, UA, 19 amino acids | [151] | |
| HBH-GlDH/oxygen electrode | 10–1500; 5.0 | 0.089 × 10−3 µA μM−1 | Amp | 0.1 M PBS pH 6.5 | - /7 days (70%) | AA, UA, 19 amino acids | [152] | |
| GlOx/PtNP/NAE | Up to 800; 14 | 0.011 μA μM−1 cm−2 | Amp | 0.01 M PBS pH 7.4 | -/14 days (98%) | - | [153] | |
| GlDH-Chit-MelB/SPCE | 12.5–150; 1.5 | 0.037 μA μM−1 | Amp | 0.075 M PBS pH 7.0 | Fetal bovine serum sample, food samples; /- | - | [154] | |
| GlOx/cMWCNTs/AuNPs/Chit/ AuE | 5–500; 1.6 | 0.155 μA μM−1 cm−2 | CV-EIS | 0.1 M PBS pH 7.5 | Human serum samples;/4 months | AA, UA, Glu, bilirubin, urea, triglycerides | [155] | |
| GlDH/Ni-Pd-PAM/GCE | 5–500; 0.052 | 4.768 μA μM−1 cm−2 | CV-EIS-DPV | 0.1 M PBS pH 7.4 | Food samples; / 60 days (94.85%) | AA, Cys, l-aspartate | [156] | |
| GlOx-PPyNPs/PANI/AuE | 0.02–400; 0.1 × 10−3 | 0.533 μA μM−1 cm−2 | CV-EIS | 0.1 M PBS pH 7.5 | Food samples; / 60 days (70%) | AA, Glu, citric acid, Cys, methionine, lysine, aspartic acid, NaCl, glycine | [157] | |
| GlOx/PPD/Pt microelectrode | 0.5–100 5 × 10−3 | 0.279 μA μM−1 | Amp | 0.1 M PBS pH 7.4 | Artificial cerebrospinal fluid;/5 months (95%) | l-glutamine, l-aspartic acid, AA, DA, UA, 5-HT, catechol/In vivo glutamate sensing | [137] | |
| Quinolinic acid | BSA/QPRT/rGO/ITO | 6.5–65,000 6.5 | 7.860 × 103 μA μM−1 cm−2 | CV-DPV | PBS pH 7.0 | Human serum samples;/30 days (95%) | - | [158] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakolian-Ardakani, Z.; Hosu, O.; Cristea, C.; Mazloum-Ardakani, M.; Marrazza, G. Latest Trends in Electrochemical Sensors for Neurotransmitters: A Review. Sensors 2019, 19, 2037. https://doi.org/10.3390/s19092037
Tavakolian-Ardakani Z, Hosu O, Cristea C, Mazloum-Ardakani M, Marrazza G. Latest Trends in Electrochemical Sensors for Neurotransmitters: A Review. Sensors. 2019; 19(9):2037. https://doi.org/10.3390/s19092037
Chicago/Turabian StyleTavakolian-Ardakani, Zahra, Oana Hosu, Cecilia Cristea, Mohammad Mazloum-Ardakani, and Giovanna Marrazza. 2019. "Latest Trends in Electrochemical Sensors for Neurotransmitters: A Review" Sensors 19, no. 9: 2037. https://doi.org/10.3390/s19092037
APA StyleTavakolian-Ardakani, Z., Hosu, O., Cristea, C., Mazloum-Ardakani, M., & Marrazza, G. (2019). Latest Trends in Electrochemical Sensors for Neurotransmitters: A Review. Sensors, 19(9), 2037. https://doi.org/10.3390/s19092037








