Convolutional Neural Network for Breathing Phase Detection in Lung Sounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sets
2.2. Manual Annotation of Breathing Phases
2.3. Developed Algorithm
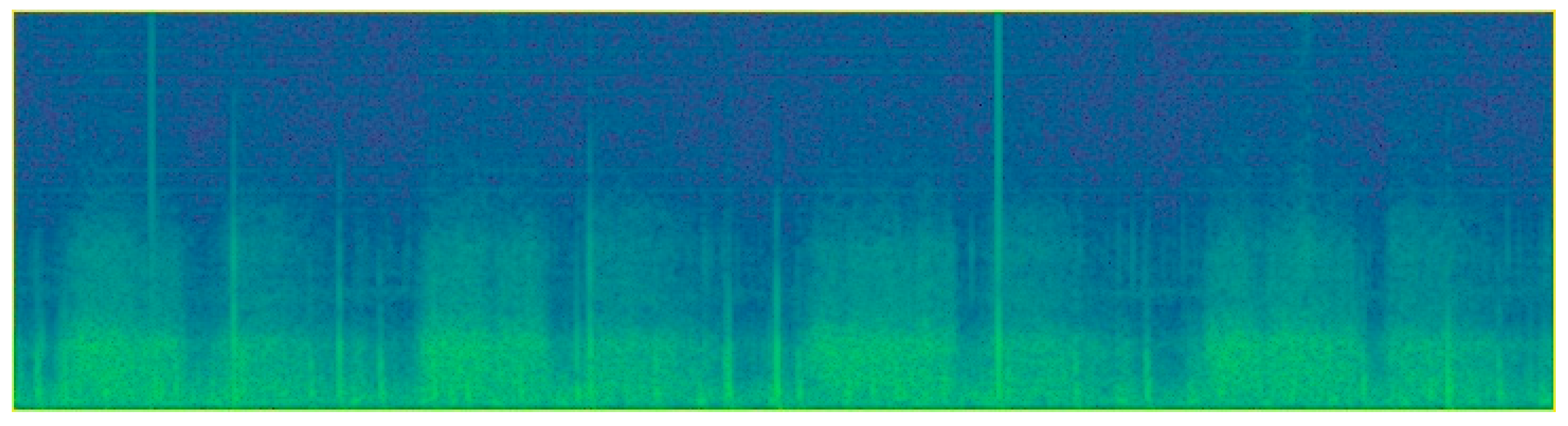
2.3.1. Data Pre-Processing
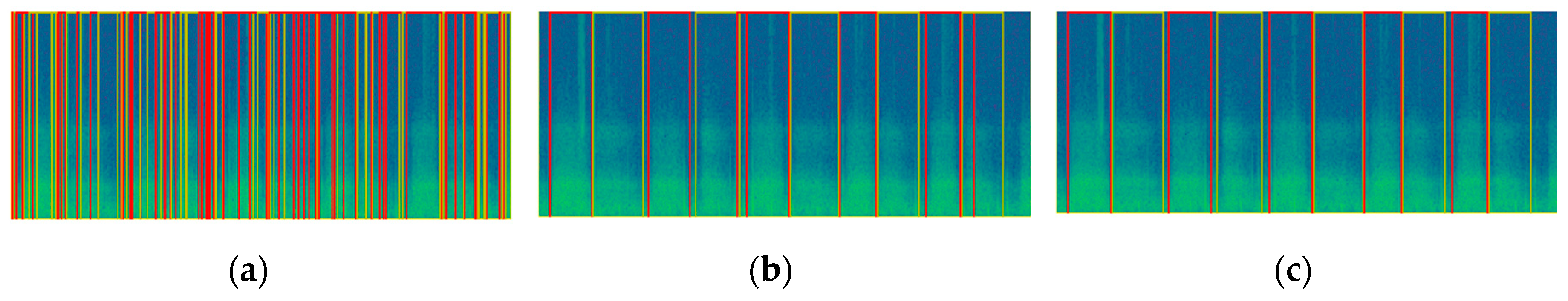
2.3.2. Object Detection
2.3.3. Post-Processing
2.4. Evaluation of the Algorithm
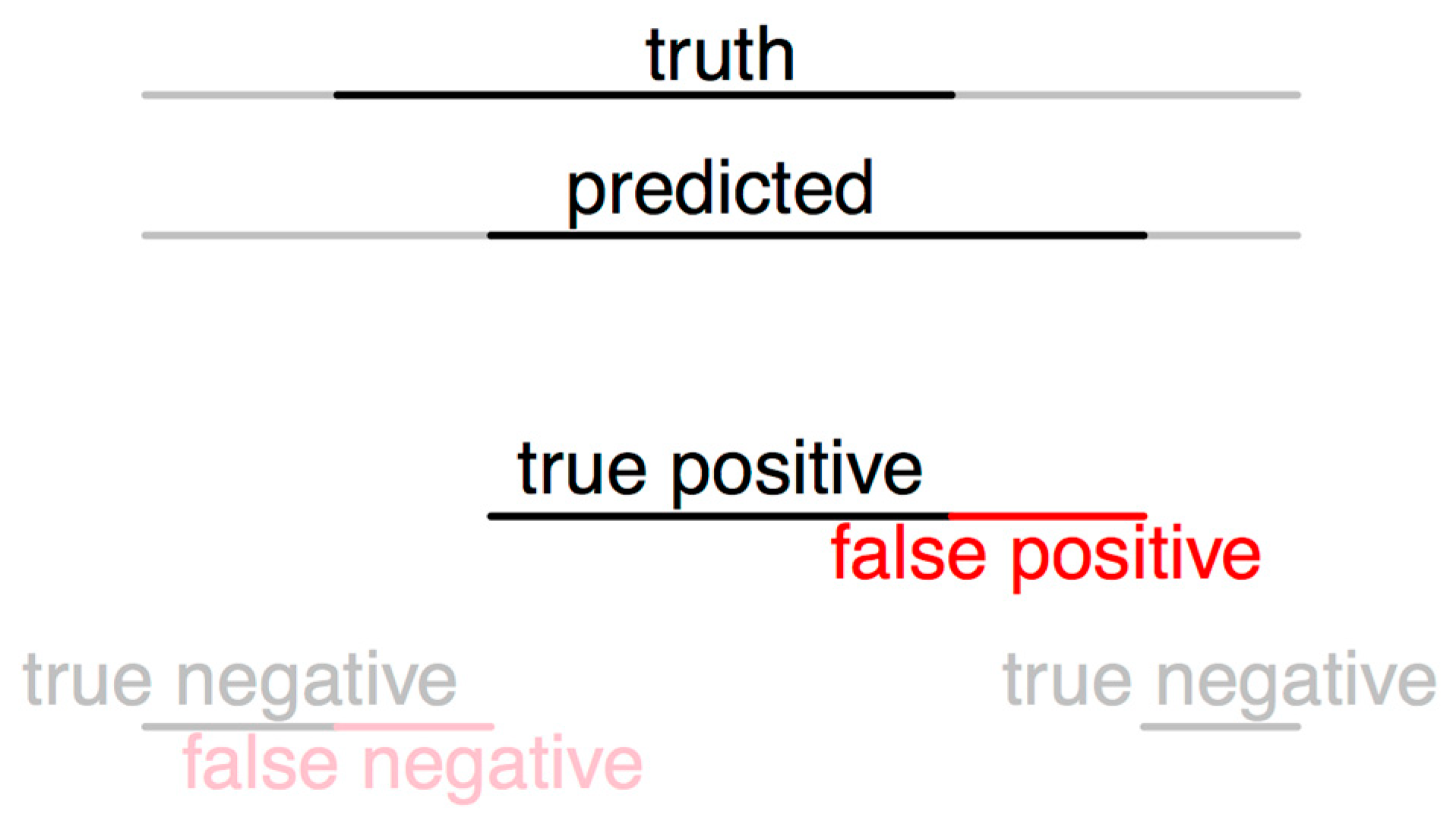
2.4.1. Evaluation Method 1
2.4.2. Evaluation Method 2
3. Results
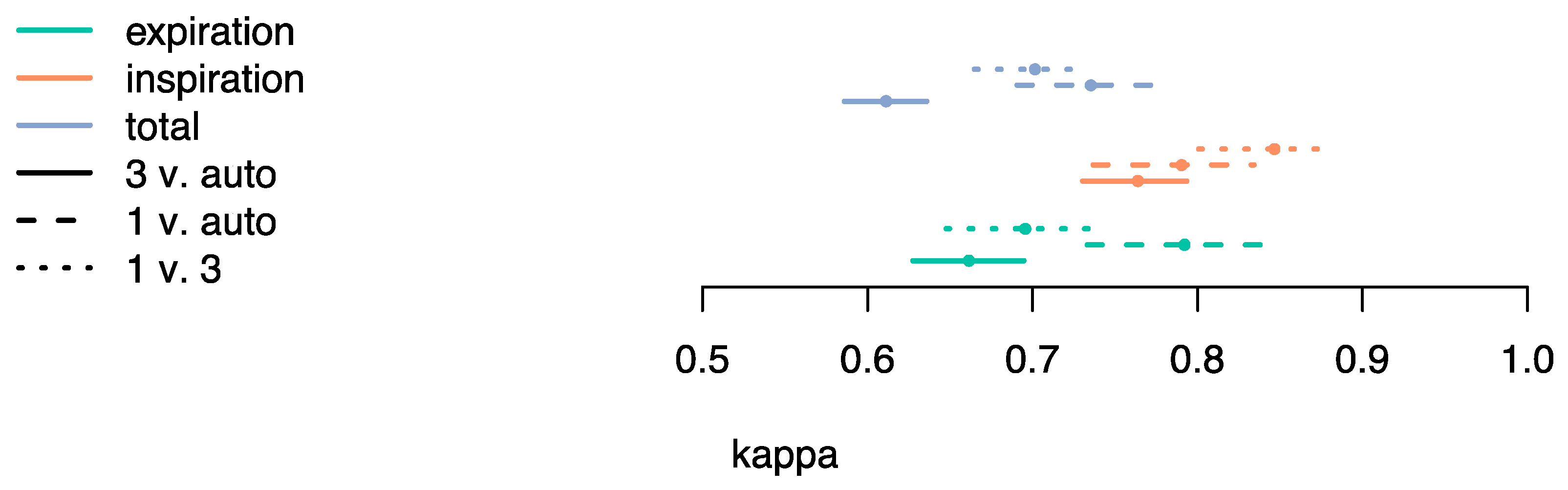
3.1. Evaluation Method 1
3.2. Evaluation Method 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bohadana, A.; Izbicki, G.; Kraman, S.S. Fundamentals of Lung Auscultation. N. Engl. J. Med. 2014, 370, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Piirila, P.; Sovijarvi, A. Crackles: Recording, analysis and clinical significance. Eur. Respir. J. 1995, 8, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Jácome, C.; Marques, A. Computerized Respiratory Sounds: Novel Outcomes for Pulmonary Rehabilitation in COPD. Respir. Care 2017, 62, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Chuah, J.; Moussavi, Z. Automated Respiratory Phase Detection by Acoustical Means. Available online: https://www.researchgate.net/profile/Zahra_Moussavi/publication/228724229_Automated_respiratory_phase_detection_by_acoustical_means/links/55085b5a0cf27e990e0a83ce/Automated-respiratory-phase-detection-by-acoustical-means.pdf (accessed on 14 April 2019).
- Huq, S.; Moussavi, Z. Acoustic breath-phase detection using tracheal breath sounds. Med. Biol. Eng. Comput. 2012, 50, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Reyes, B.A.; Reljin, N.; Kong, Y.; Nam, Y.; Ha, S.; Chon, K.H. Towards the Development of a Mobile Phonopneumogram: Automatic Breath-Phase Classification Using Smartphones. Ann. Biomed. Eng. 2016, 44, 2746–2759. [Google Scholar] [CrossRef] [PubMed]
- Sovijärvi, A.R.A.; Malmberg, L.P.; Charbonneau, G.; Vanderschoot, J.; Dalmasso, F.; Sacco, C.; Rossi, M.; Earis, J.E. Characteristics of breath sounds and adventitious respiratory sounds. Eur. Respir. Rev. 2000, 10, 591–596. [Google Scholar]
- Todd, S.; Walsted, E.S.; Grillo, L.; Livingston, R.; Menzies-Gow, A.; Hull, J.H. Novel assessment tool to detect breathing pattern disorder in patients with refractory asthma. Respirology 2018, 23, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Dellweg, D.; Haidl, P.; Siemon, K.; Appelhans, P.; Kohler, D. Impact of breathing pattern on work of breathing in healthy subjects and patients with COPD. Respir. Physiol. Neurobiol. 2008, 161, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wu, L.; Alleva, F.; Droppo, J.; Huang, X.; Stolcke, A. The Microsoft 2017 Conversational Speech Recognition System. In Proceedings of the 2018 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Calgaria, AB, Canada, 15–20 April 2018; pp. 5934–5938. [Google Scholar]
- Saon, G.; Kurata, G.; Sercu, T.; Audhkhasi, K.; Thomas, S.; Dimitriadis, D.; Cui, X.; Ramabhadran, B.; Picheny, M.; Lim, L.-L.; et al. English Conversational Telephone Speech Recognition by Humans and Machines. arXiv, 2017; arXiv:1703.02136. [Google Scholar]
- Jaitly, N.; Hinton, G.E. A New Way to Learn Acoustic Events. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwjh_a3X987hAhUIIIgKHa5_D4cQFjAAegQIABAC&url=https%3A%2F%2Fdeeplearningworkshopnips2011.files.wordpress.com%2F2011%2F12%2F24.pdf&usg=AOvVaw0s46muyf44MGKBfkbPf-Ez (accessed on 14 April 2019).
- Aviles-Solis, J.C.; Vanbelle, S.; Halvorsen, P.A.; Francis, N.; Cals, J.W.L.; Andreeva, E.A.; Marques, A.; Piirilä, P.; Pasterkamp, H.; Melbye, H. International perception of lung sounds: A comparison of classification across some European borders. BMJ Open Respir. Res. 2017, 4, e000250. [Google Scholar] [CrossRef] [PubMed]
- Dinis, J.; Campos, G.; Rodrigues, J.; Marques, A. Respiratory Sound Annotation Software. In Proceedings of the International Conference on Health Informatics, Vilamoura, Portugal, 7–9 November 2013; pp. 183–188. [Google Scholar]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster R-CNN: Towards Real-Time Object Detection with Region Proposal Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Uijlings, J.R.; Sande, K.E.; Gevers, T.; Smeulders, A.W. Selective Search for Object Recognition. Int. J. Comput. Vis. 2013, 104, 154–171. [Google Scholar] [CrossRef]
- Alexe, B.; Deselaers, T.; Ferrari, V. Measuring the Objectness of Image Windows. IEEE Trans. Pattern Anal. Mach. Intell. 2012, 34, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Russakovsky, O.; Deng, J.; Su, H.; Krause, J.; Satheesh, S.; Ma, S.; Huang, Z.; Karpathy, A.; Khosla, A.; Bernstein, M.; et al. ImageNet Large Scale Visual Recognition Challenge. Int. J. Comput. Vis. 2015, 115, 211–252. [Google Scholar] [CrossRef]
- Everingham, M.; Eslami, S.M.A.; Van Gool, L.; Williams, C.K.I.; Winn, J.; Zisserman, A. The Pascal Visual Object Classes Challenge: A Retrospective. Int. J. Comput. Vis. 2015, 111, 98–136. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Efron, B. Bootstrap Methods: Another Look at the Jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Melbye, H.; Garcia-Marcos, L.; Brand, P.; Everard, M.; Priftis, K.; Pasterkamp, H. Wheezes, crackles and rhonchi: Simplifying description of lung sounds increases the agreement on their classification: A study of 12 physicians’ classification of lung sounds from video recordings. BMJ Open Respir. Res. 2016, 3, e000136. [Google Scholar] [CrossRef] [PubMed]
- Pinho, C.; Oliveira, A.; Jácome, C.; Rodrigues, J.; Marques, A. Automatic Crackle Detection Algorithm Based on Fractal Dimension and Box Filtering. Procedia Comput. Sci. 2015, 64, 705–712. [Google Scholar] [CrossRef]
| Datasets | Annotation | N of Files | Duration | N of Inspiration Identified | N of Expiration Identified |
|---|---|---|---|---|---|
| Subset 1 (training) | Annotator 1 | 1022 | 10 s | 3212 | 2842 |
| Subset 2 (training) | Algorithm (inspected by Annotator 2) | 112 | 15 s | 447 | 418 |
| Subset 3 (test) | Annotator 1 | 120 | 15 s | 479 | 436 |
| Annotator 3 | 120 | 15 s | 499 | 459 |
| Agreement Using Boxes | Inspiration | Expiration | Both Phases |
|---|---|---|---|
| Annotator 1 vs. Algorithm | 98% | 95% | 96% |
| Annotator 3 vs. Algorithm | 95% | 79% | 87% |
| Annotator 1 vs. Annotator 3 | 95% | 84% | 90% |
| Sensitivity | Specificity | |||||
|---|---|---|---|---|---|---|
| Inspiration | Expiration | Both Phases | Inspiration | Expiration | Both Phases | |
| Algorithm (Annotator 1) | 97% | 94% | 96% | 86% | 87% | 87% |
| Algorithm (Annotator 3) | 98% | 97% | 98% | 84% | 78% | 81% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jácome, C.; Ravn, J.; Holsbø, E.; Aviles-Solis, J.C.; Melbye, H.; Ailo Bongo, L. Convolutional Neural Network for Breathing Phase Detection in Lung Sounds. Sensors 2019, 19, 1798. https://doi.org/10.3390/s19081798
Jácome C, Ravn J, Holsbø E, Aviles-Solis JC, Melbye H, Ailo Bongo L. Convolutional Neural Network for Breathing Phase Detection in Lung Sounds. Sensors. 2019; 19(8):1798. https://doi.org/10.3390/s19081798
Chicago/Turabian StyleJácome, Cristina, Johan Ravn, Einar Holsbø, Juan Carlos Aviles-Solis, Hasse Melbye, and Lars Ailo Bongo. 2019. "Convolutional Neural Network for Breathing Phase Detection in Lung Sounds" Sensors 19, no. 8: 1798. https://doi.org/10.3390/s19081798
APA StyleJácome, C., Ravn, J., Holsbø, E., Aviles-Solis, J. C., Melbye, H., & Ailo Bongo, L. (2019). Convolutional Neural Network for Breathing Phase Detection in Lung Sounds. Sensors, 19(8), 1798. https://doi.org/10.3390/s19081798





