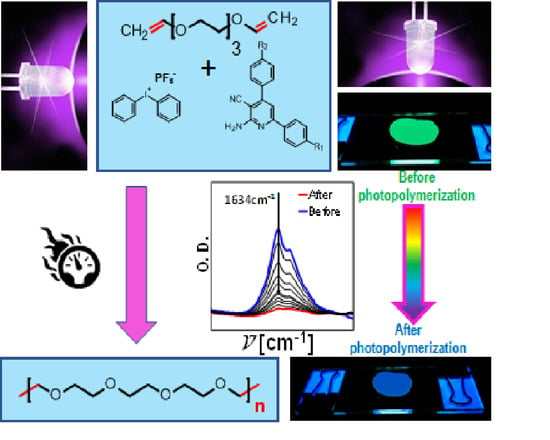
The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Absorption and Fluorescence Characteristics
2.3. Electrochemical Characteristics Determination of Oxidation Potentials
2.4. Preparation of Thin-Layer Samples for Monitoring the Photopolymerization Processes by FPT
2.5. Monitoring the Photopolymerization Processes by FPT
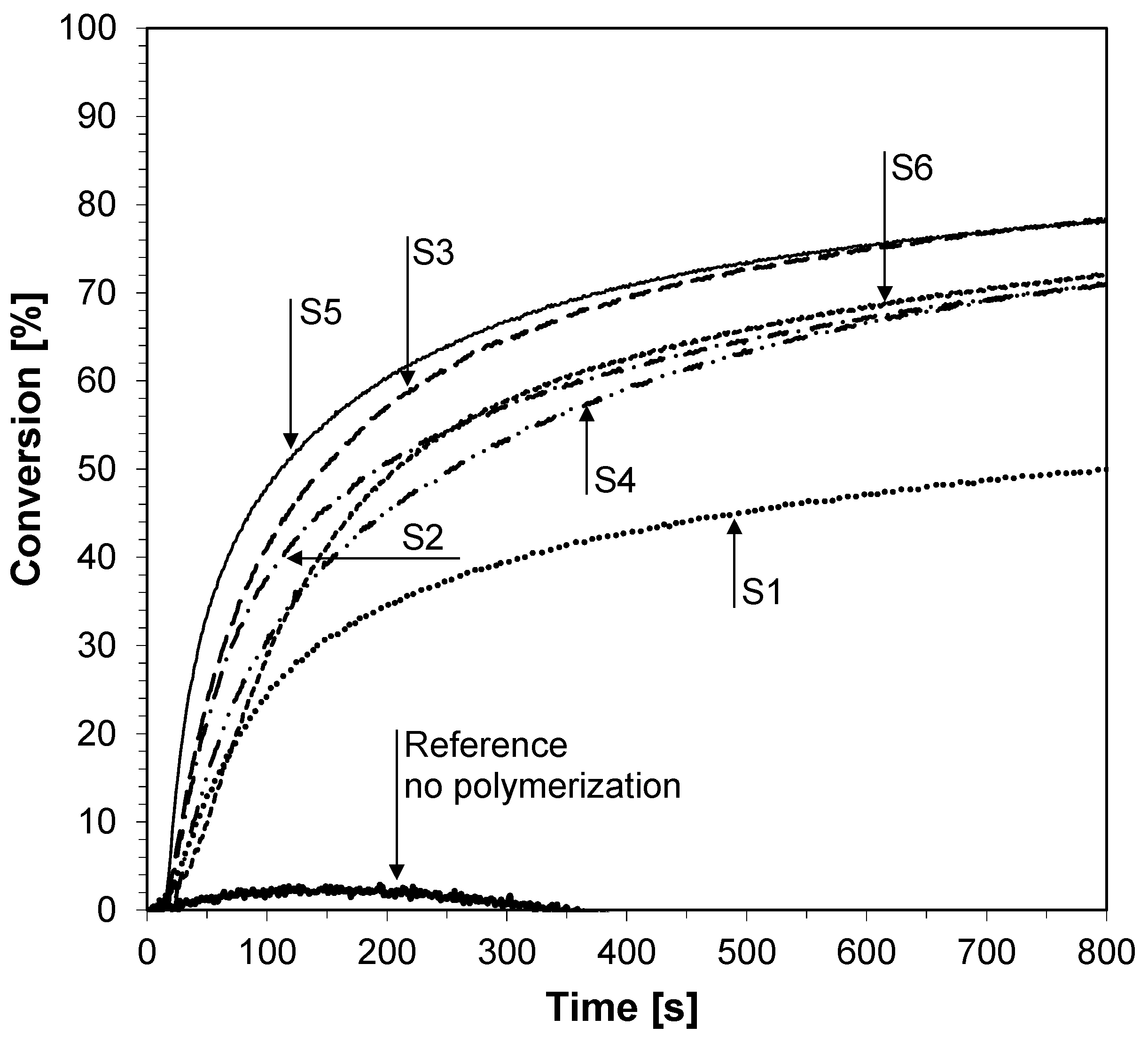
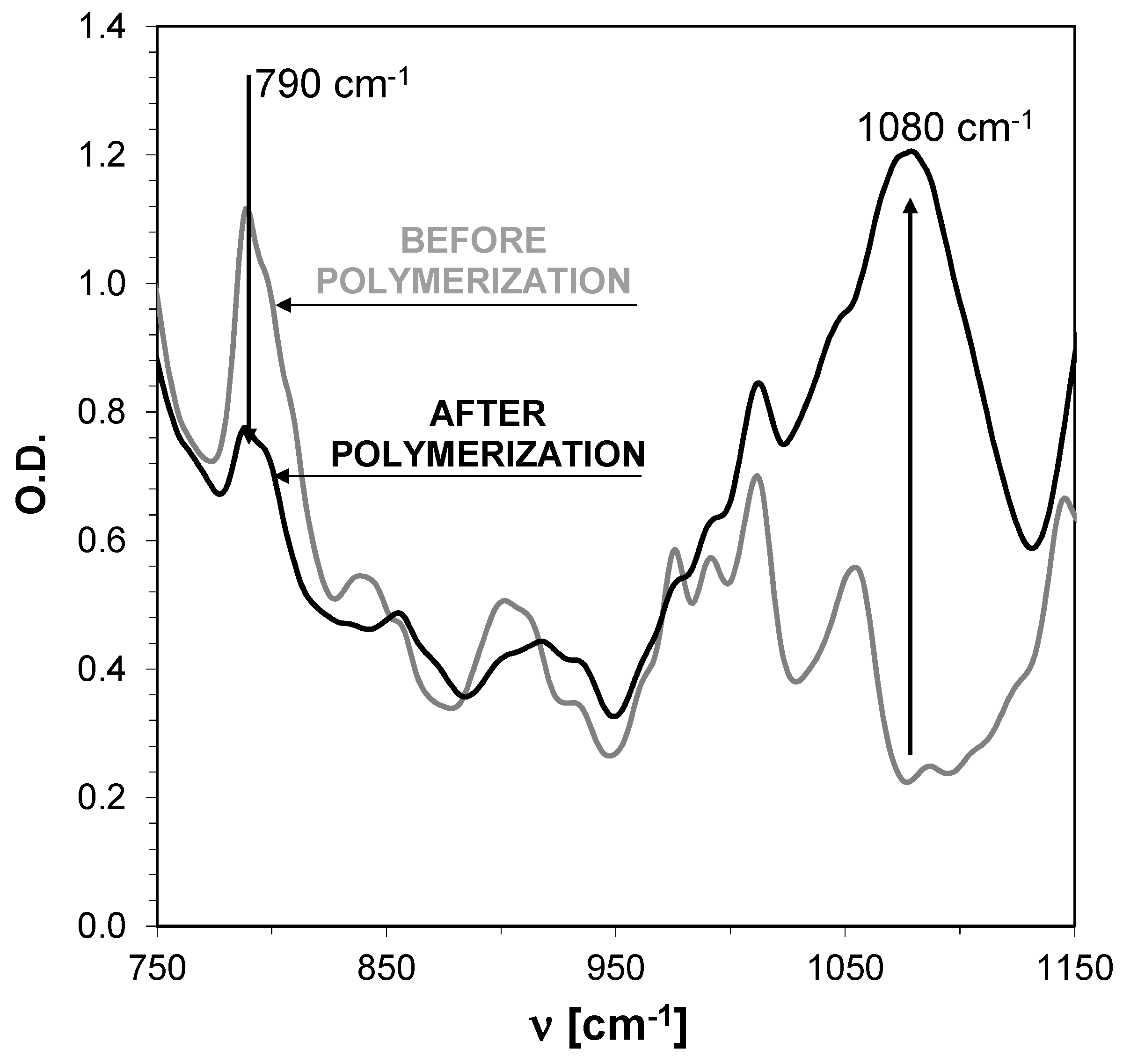
2.6. Monitoring the Photopolymerization Processes by Real-Time FT-IR
2.6.1. Cationic Photopolymerization (CP) Experiments
2.6.2. Free-Radical Photopolymerization (FRP) Experiments
2.6.3. Thiol-ene Photopolymerization (FRP) Experiments
2.6.4. Source of Light for Real-Time Experiments
3. Results
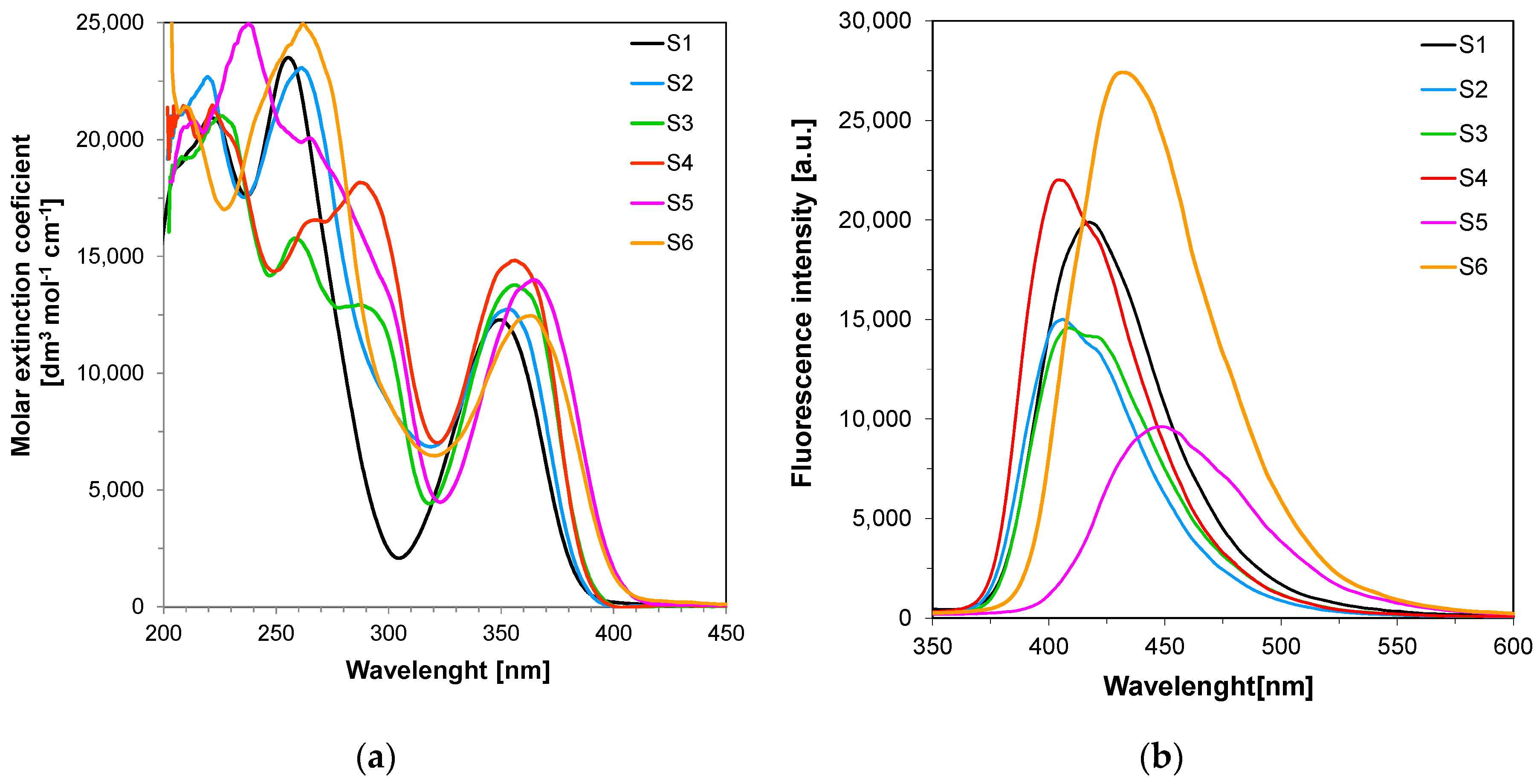
3.1. Spectral Characteristics of the Sensors/Co-Initators
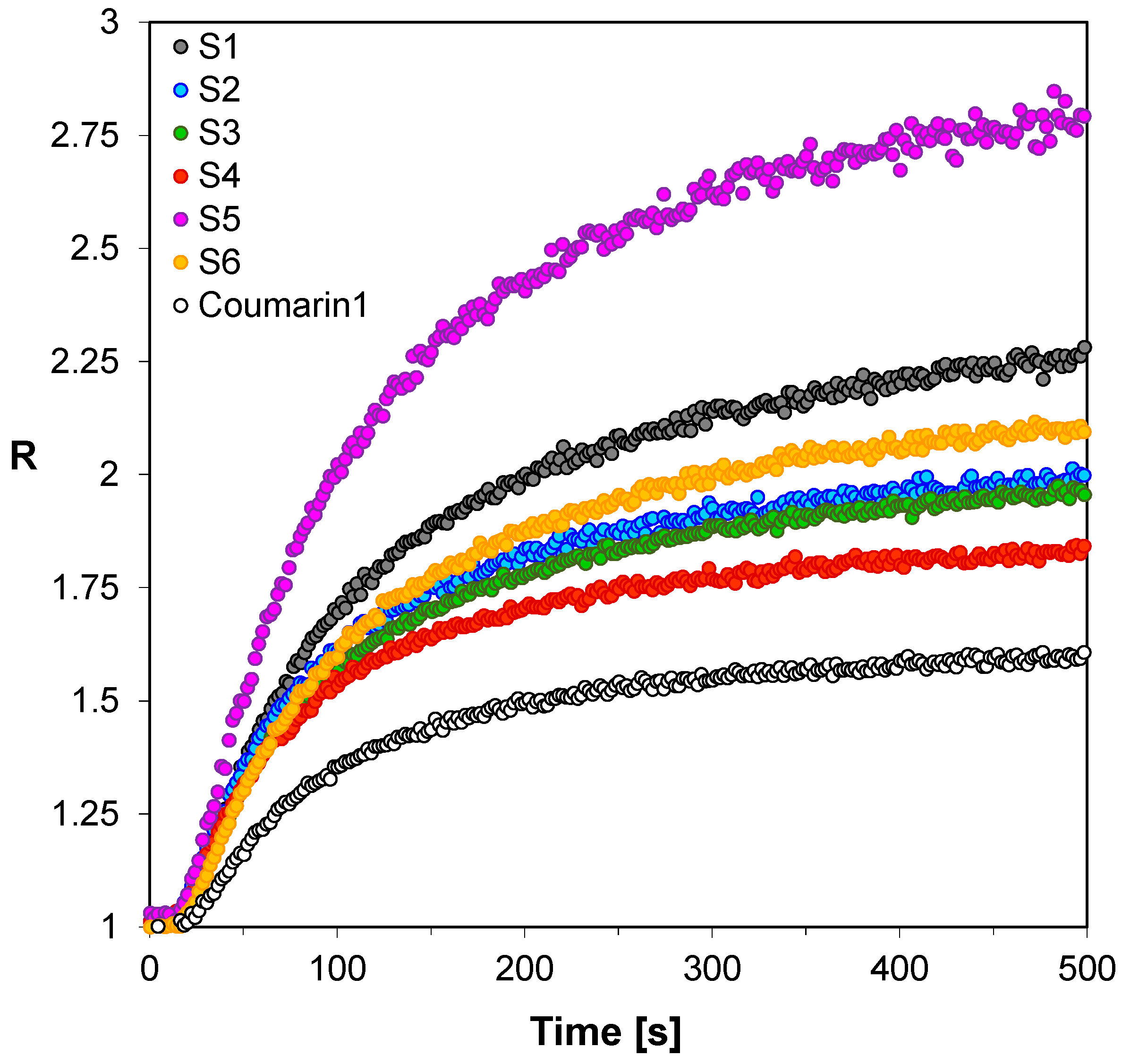
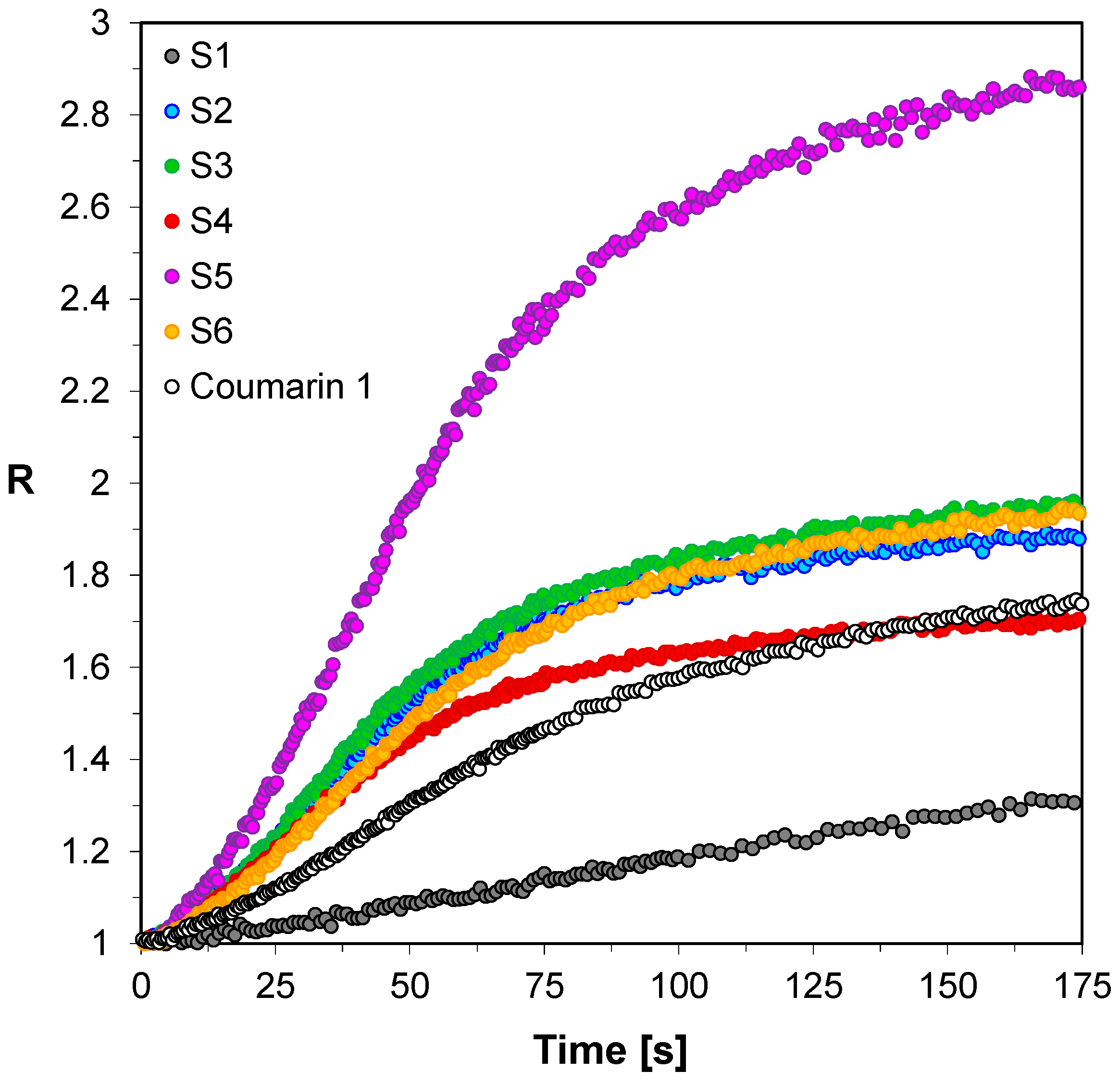
3.2. Performance of the 2-amino-4,6-diphenyl-pyridine-3-carbonitrile derivatives in the Role of Sensors in FPT
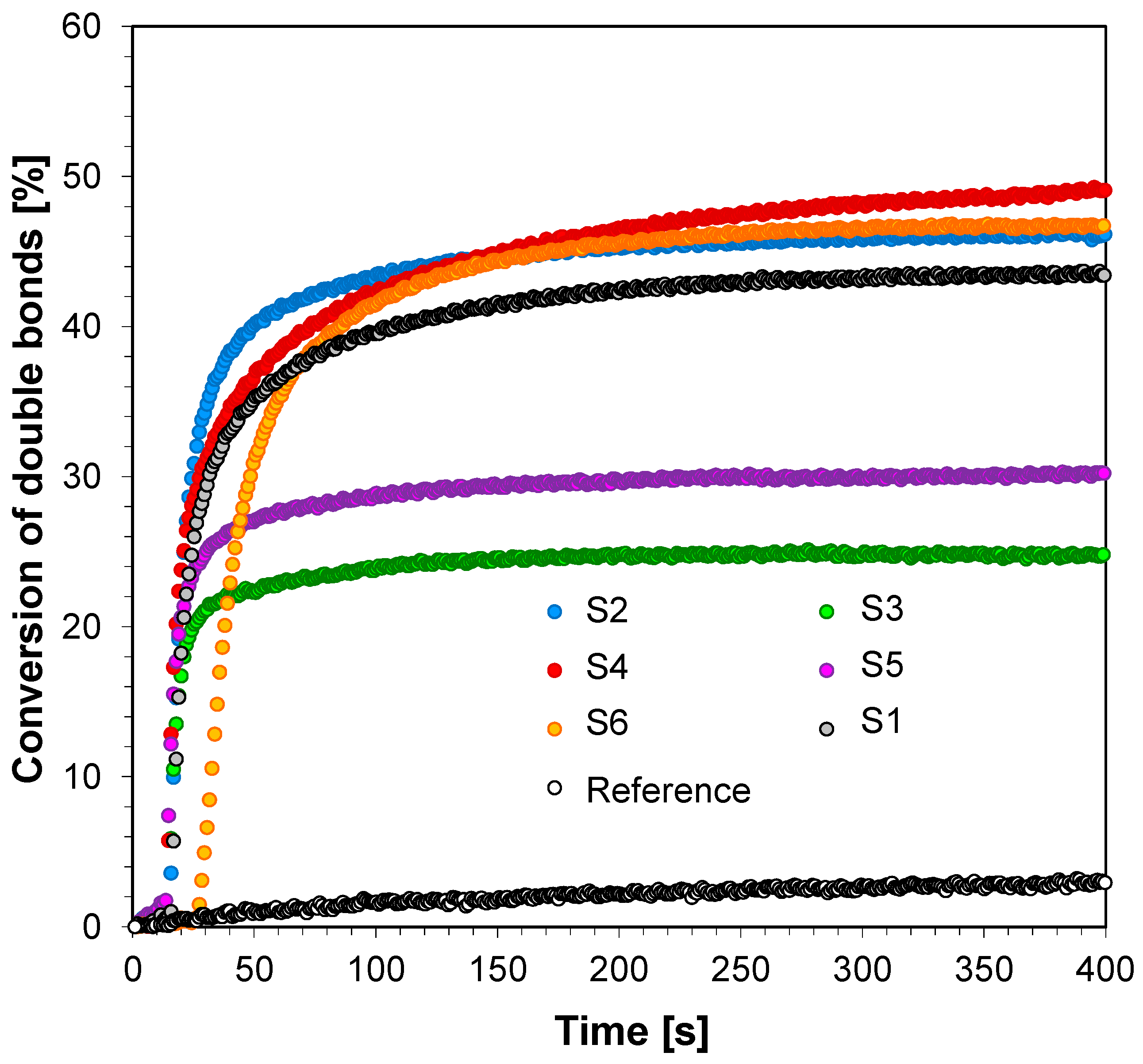
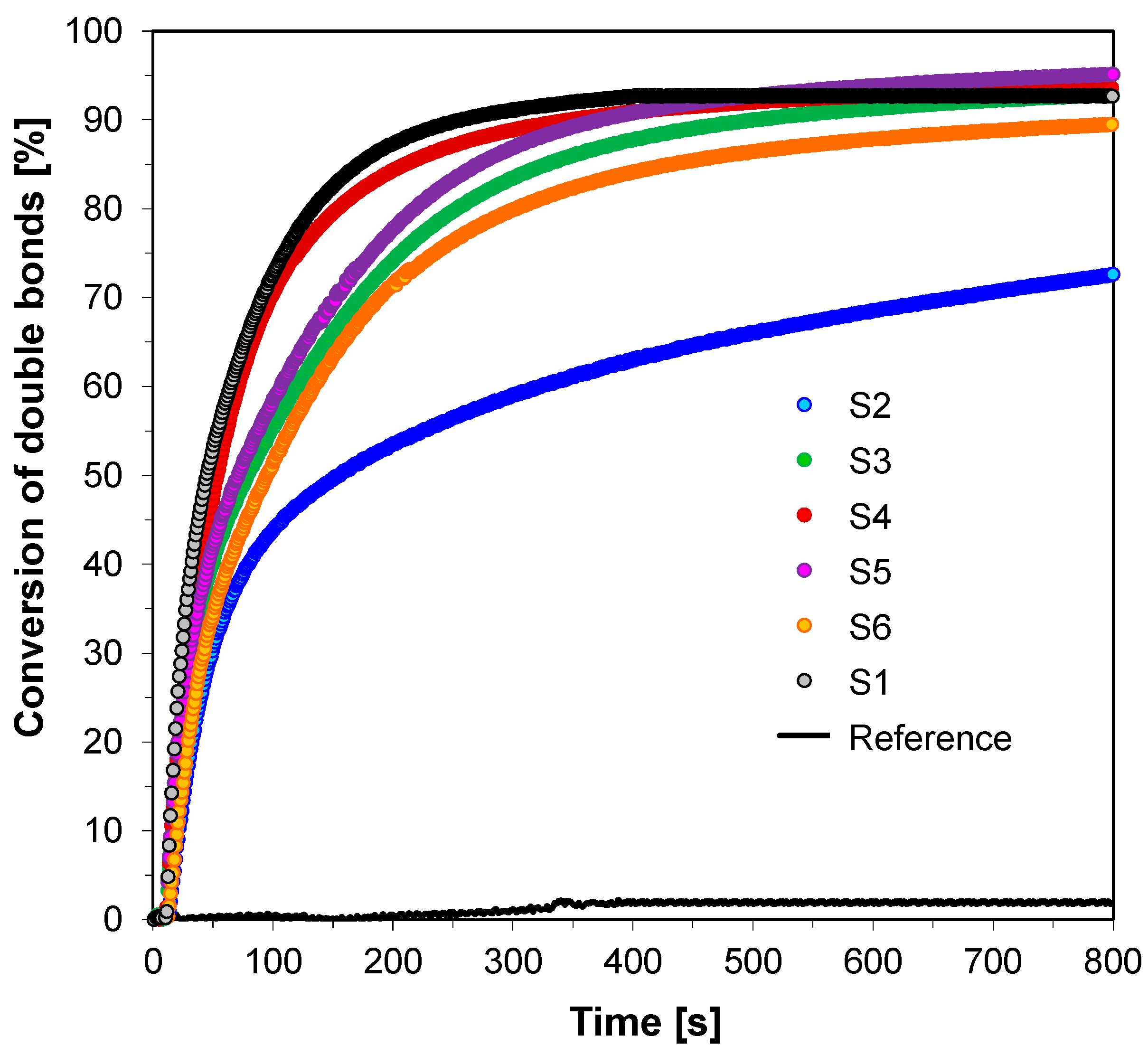
3.3. Performance of the 2-amino-4,6-diphenyl-pyridine-3-carbonitrile derivatives in the Role of Co-Initiators in Bimolecular Photoinitiating Systems for Cationic Photopolymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ambrose, W.P.; Goodwin, P.M.; Jett, J.H.; Van Orden, A.; Werner, J.H.; Keller, R.A. Single molecule fluorescence spectroscopy at ambient temperature. Chem. Rev. 1999, 99, 2929–2956. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Plenum Press: New York, NY, USA, 1983; pp. 78–90. [Google Scholar]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001; pp. 200–348. [Google Scholar]
- Bosch, P.; Catalina, F.; Corrales, T.; Peinado, C. Fluorescent Probes for Sensing Processes in Polymers. Chem. Eur. J. 2005, 11, 4314–4325. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Applicability of quinolizino-coumarins for monitoring free radical photopolymerization by fluorescence spectroscopy. Polym. Test. 2015, 42, 99–107. [Google Scholar]
- Ortyl, J.; Sawicz, K.; Popielarz, R. Performance of amidocoumarins as probes for monitoring of cationic photopolymerization of monomers by fluorescence probe technology. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4522–4528. [Google Scholar] [CrossRef]
- Ortyl, J.; Galek, M.; Milart, P.; Popielarz, R. Aminophthalimide probes for monitoring of cationic photopolymerization by fluorescence probe technology and their effect on the polymerization kinetics. Polym. Test. 2012, 31, 466–473. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Brady, T.P.; Lichlyter, D.; Theodorakis, E.A. A Ratiometric Fluorescent Viscosity Sensor. J. Am. Chem. Soc. 2006, 128, 398–399. [Google Scholar] [CrossRef]
- Lee, S.-C.; Heo, J.; Woo, H.C.; Lee, J.-A.; Seo, Y.H.; Lee, C.-L.; Kim, S.; Kwon, O.-P. Fluorescent Molecular Rotors for Viscosity Sensors. Chem. Eur. J. 2018, 24, 13706–13718. [Google Scholar]
- Zhou, K.; Ren, M.; Deng, B.; Lin, W. Development of a viscosity sensitive fluorescent probe for real-time monitoring of mitochondria viscosity. New J. Chem. 2017, 41, 11507–11511. [Google Scholar] [CrossRef]
- Lou, J.; Hatton, T.A.; Laibinis, P.E. Fluorescent Probes for Monitoring Temperature in Organic Solvents. Anal. Chem. 1997, 69, 1262–1264. [Google Scholar] [CrossRef]
- Peng, H.; Stich, M.I.J.; Yu, J.; Sun, L.; Fischer, L.H.; Wolfbeis, O.S. Luminescent Europium(III) Nanoparticles for Sensing and Imaging of Temperature in the Physiological Range. Adv. Mater. 2010, 22, 716–719. [Google Scholar] [CrossRef]
- Kozak, M.; Kalota, B.; Tkaczyk, S.; Tsvirko, M. Luminescent Temperature Sensor Material Based on an Eu(III) β-Diketonate Complex Incorporated into Cellulose Triacetate. J. Appl. Spectrosc. 2014, 81, 678–683. [Google Scholar] [CrossRef]
- Lam, H.; Rao, G.; Loureiro, J.; Tolosa, L. Dual Optical Sensor for Oxygen and Temperature Based on the Combination of Time Domain and Frequency Domain Techniques. Talanta 2011, 84, 65–70. [Google Scholar] [CrossRef][Green Version]
- Baleizao, C.; Nagl, S.; Schaferling, M.; Berberan-Santos, M.N.; Wolfbeis, O.S. Dual Fluorescence Sensor for Trace Oxygen and Temperature with Unmatched Range and Sensitivity. Anal. Chem. 2008, 80, 6449–6457. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, J.; Zhou, L.; Zhou, X.; Xiang, H. Ratiometric optical oxygen sensing: A review in respect of material design. Analyst 2012, 137, 4885–4901. [Google Scholar] [CrossRef] [PubMed]
- Jonaghani, M.Z.; Zali-Boeini, H.; Taheri, R.; Rudbari, H.A.; Askari, B. Naphthothiazole-based highly selective and sensitive fluorescent and colorimetric chemosensor for detection of pollutant metal ions. RSC Adv. 2016, 6, 34940–34945. [Google Scholar] [CrossRef]
- Owicki, J.C. Fluorescence polarization and anisotropy in high throughput screening: Perspectives and primer. J. Biomol. Screen. 2000, 5, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.S.; Jolley, M.E. Fluorescence polarization: An analytical tool for immunoassay and drug discovery. Comb. Chem. High Throughput Screen. 1999, 2, 177–190. [Google Scholar]
- Martinić, I.; Eliseeva, S.V.; Petoud, S. Near-infrared emitting probes for biological imaging: Organic fluorophores, quantum dots, fluorescent proteins, lanthanide(III) complexes and nanomaterials. J. Lumin. 2017, 189, 19–43. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Ortyl, J.; Galica, M.; Popielarz, R.; Bogdał, D. Application of a carbazole derivative as a spectroscopic fl uorescent probe for real time monitoring of cationic photopolymerization. Pol. J. Chem. Tech. 2014, 16, 75–80. [Google Scholar] [CrossRef]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Mechanism of interaction of coumarin-based fluorescent molecular probes with polymerizing medium during free radical polymerization of a monomer. Polym. Test. 2016, 55, 310–317. [Google Scholar] [CrossRef]
- Kaur, N.; Chopra, S.; Singh, G.; Raj, P.; Bhasin, A.; Sahoo, S.K.; Kuwar, A.; Singh, N. Chemosensors for biogenic amines and biothiols. J. Mater. Chem. B 2018, 6, 4872–4902. [Google Scholar] [CrossRef]
- Gao, Q.; Du, J.; Liu, H.; Lu, S.; Zhou, X.; Yang, C. A hemicyanine-based optical probe for biomembranes and intracellular pH sensing. J. Lumin. 2018, 202, 246–252. [Google Scholar] [CrossRef]
- Khan, R.I.; Pitchumani, K. β-Cyclodextrin included coumarin derivatives as selective fluorescent sensors for Cu2+ ions in HeLa cells. RSC Adv. 2016, 6, 20269–20275. [Google Scholar] [CrossRef]
- Kumar, N.; Bhalla, V.; Kumar, M. Resonance energy transfer-based fluorescent probes for Hg2+, Cu2+ and Fe2+/Fe3+ ions. Analyst 2014, 139, 543–558. [Google Scholar] [CrossRef]
- Szalai, A.M.; Armando, N.G.; Barabas, F.M.; Stefani, F.D.; Giordano, L.; Bari, S.E.; Cavasotto, C.N.; Silberstein, S.; Aramendía, P.F. A fluorescence nanoscopy marker for corticotropin-releasing hormone type 1 receptor: Computer design, synthesis, signaling effects, super-resolved fluorescence imaging, and in situ affinity constant in cells. Phys. Chem. Chem. Phys. 2018, 20, 29212–29220. [Google Scholar] [CrossRef]
- Lin, Q.; Buccella, D. Highly selective, red emitting BODIPY-based fluorescent indicators for intracellular Mg2+ imaging. J. Mater. Chem. B 2018, 6, 7247–7256. [Google Scholar] [CrossRef]
- McLachlan, B.G.; Bell, J.H.; Espina, J.; Gallery, J.; Gouterman, M.; No Demandante, C.G.; Bjarke, L. Flight Testing of a Luminescent Surface Pressure Sensor; NASA Technical Memorandum 103970; Ames Research Center: Mountain View, CA, USA, 1992; pp. 94035–100000.
- Stich, M.I.J.; Fischer, L.H.; Wolfbeis, O.S. Multiple fluorescent chemical sensing and imaging. Chem. Soc. Rev. 2010, 39, 3102–3114. [Google Scholar] [CrossRef]
- Li, M.; Tian, R.; Yan, D.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. A luminescent ultrathin film with reversible sensing toward pressure. Chem. Commun. 2016, 52, 4663–4666. [Google Scholar] [CrossRef]
- Pączkowski, J. Fluorescent probes as a research tool in polymer chemistry. Polimery 2005, 50, 520–529. [Google Scholar] [CrossRef]
- Józefowicz, M.; Bajorek, A.; Pietrzak, M.; Jędrzejewska, B.; Heldt, J.R.; Heldt, J. Influence of degree of methyl methacrylate polymerization on spectroscopic properties of ethyl 5-(4-aminophenyl)- and 5-(4-dimethylaminophenyl)-3-amino-2,4-dicyanobenzoate. J. Lumin. 2013, 134, 414–422. [Google Scholar] [CrossRef]
- Ortyl, J.; Popielarz, R. The performance of 7-hydroxycoumarin-3-carbonitrile and 7-hydroxycoumarin-3-carboxylic acid as fluorescent probes for monitoring of cationic photopolymerization processes by FPT. J. Appl. Pol. Sci. 2012, 128, 1974–1978. [Google Scholar] [CrossRef]
- Ortyl, J.; Wilamowski, J.; Milart, P.; Galek, M.; Popielarz, R. Relative Sensitization Efficiency of Fluorescent Probes/sensitizers for Monitoring and Acceleration of Cationic Photopolymerization of Monomers. Polym. Test. 2015, 48, 151–159. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J.; Chachaj-Brekiesz, A.; Pilch, M.; Popielarz, R. Applicability of samarium(III) complexes for the role of luminescent molecular sensors for monitoring progress of photopolymerization processes and control of the thickness of polymer coatings. Spectrochim. Acta Part A 2018, 199, 30–440. [Google Scholar] [CrossRef]
- Ortyl, J.; Milart, P.; Popielarz, R. Applicability of aminophthalimide probes for monitoring and acceleration of cationic photopolymerization of epoxides. Polym. Test. 2013, 32, 708–715. [Google Scholar] [CrossRef]
- Peinado, C.; Salvador, E.F.; Catalina, F.; Lozano, A.E. Solvatochromic and rigidochromic fluorescent probes based on D–π-A diaryl ethylene and butadiene derivatives for UV-curing monitoring. Polymer 2001, 42, 2815–2825. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers: Part I: Comparison of the performance of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers, Part II: Determination of relative quantum efficiency of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2018, 67, 144–150. [Google Scholar] [CrossRef]
- Itagaki, H.; Horie, K.; Mitra, I. Luminescent probe studies of the microstructure and mobility of solid polymers. Prog. Polym. Sci. 1990, 15, 361–424. [Google Scholar] [CrossRef]
- Wang, Z.J.; Song, J.C.; Bao, R.; Neckers, D.C. Fluorescence probes for monitoring polymerization processes. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 325–333. [Google Scholar] [CrossRef]
- Khudyakov, I.V.; Legg, J.C.; Purvis, M.B.; Overton, B.J. Kinetics of Photopolymerization of Acrylates with Functionality of 1-6. Ind. Eng. Chem. Res. 1999, 38, 3353–3359. [Google Scholar] [CrossRef]
- Hu, S.; Popielarz, R.; Neckers, D.C. Fluorescence Probe Techniques (FPT) for Measuring the Relative Efficiencies of Free-Radical Photoinitiators. Macromolecules 1998, 31, 4107–4113. [Google Scholar] [CrossRef]
- Ortyl, J.; Topa, M.; Kamińska-Borek, I.; Popielarz, R. Mechanism of interaction of aminocoumarins with reaction medium during cationic photopolymerization of triethylene glycol divinyl ether. Eur. Polym. J. 2019, 116, 45–55. [Google Scholar] [CrossRef]
- Lees, A.J. Organometallic complexes as luminescence probes in monitoring thermal and photochemical polymerizations. Coord. Chem. Rev. 1998, 177, 3–35. [Google Scholar] [CrossRef]
- Strehmel, B.; Malpert, J.H.; Sarker, A.M.; Neckers, D.C. New Intramolecular Fluorescence Probes That Monitor Photoinduced Radical and Cationic Cross-Linking. Macromolecules 1999, 32, 7476–7482. [Google Scholar] [CrossRef]




| Structures of the Derivatives of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile | |||||
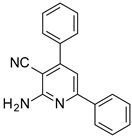 |  | 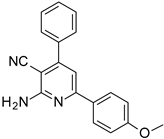 | |||
| S1 | S2 | S3 | |||
 |  |  | |||
| S4 | S5 | S6 | |||
| Reference Molecular Fluorescent Sensors | |||||
 |  | ||||
| C1 | 25ST | ||||
| Compound | Concentration [mol·dm−3] | λab-max [nm] | ε@λab-max [dm3·mol−1·cm−1] | ε@320 nm [dm3·mol−1·cm−1] | λfl @λex-320nm [nm] | Intensity @λfl-max [a. u.] |
|---|---|---|---|---|---|---|
| S1 | 7.38 × 10−5 | 349 | 15,890 | 4835 | 418 | 19,890 |
| S2 | 6.84 × 10−5 | 354 | 12,750 | 6874 | 407 | 15,000 |
| S3 | 7.77 × 10−5 | 357 | 13,780 | 4532 | 409 | 14,580 |
| S4 | 7.37 × 10−5 | 357 | 14,830 | 7068 | 404 | 22,010 |
| S5 | 7.18 × 10−5 | 364 | 13,990 | 4668 | 448 | 9610 |
| S6 | 6.99 × 10−5 | 363 | 12,460 | 6473 | 432 | 27,420 |
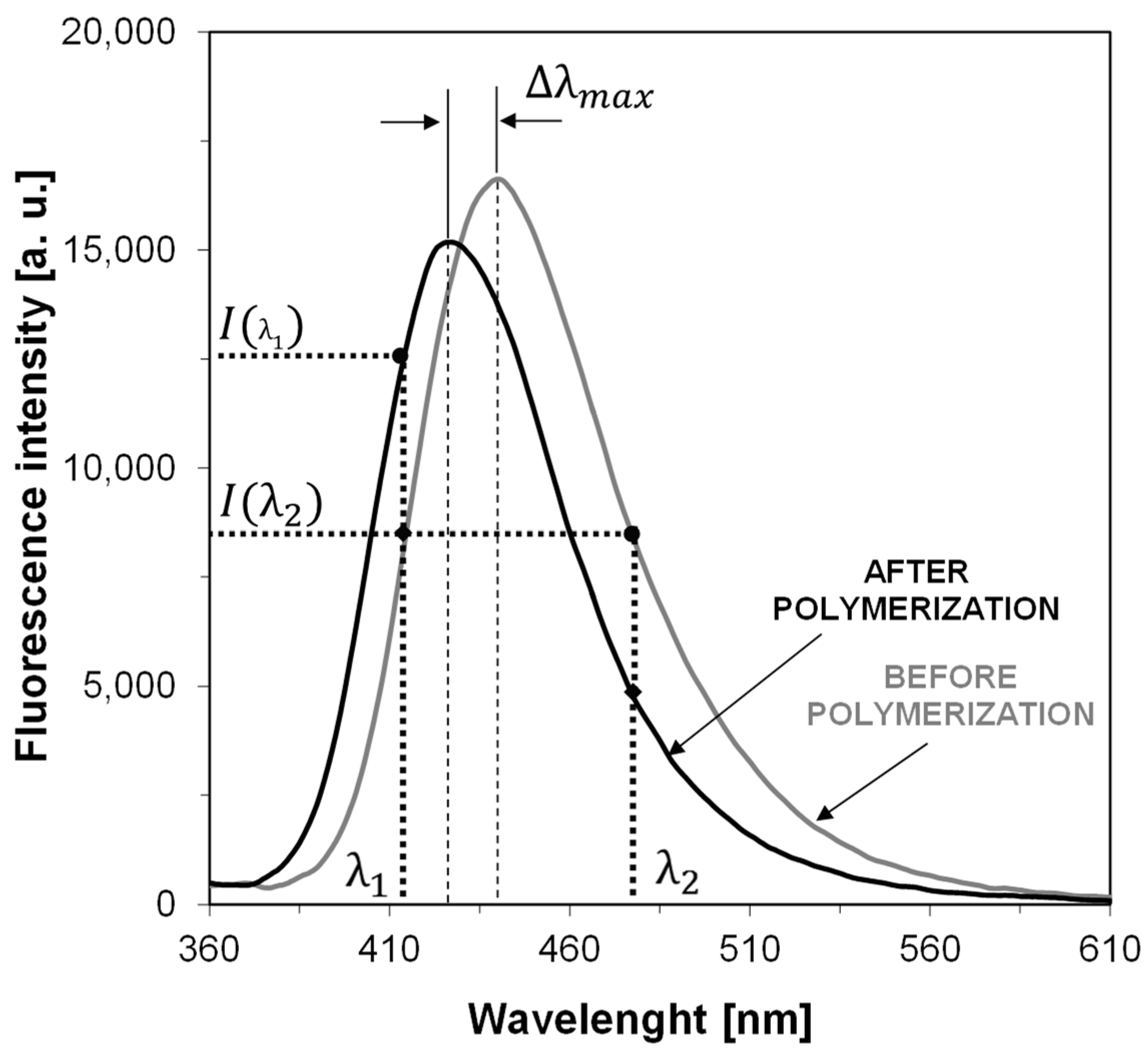
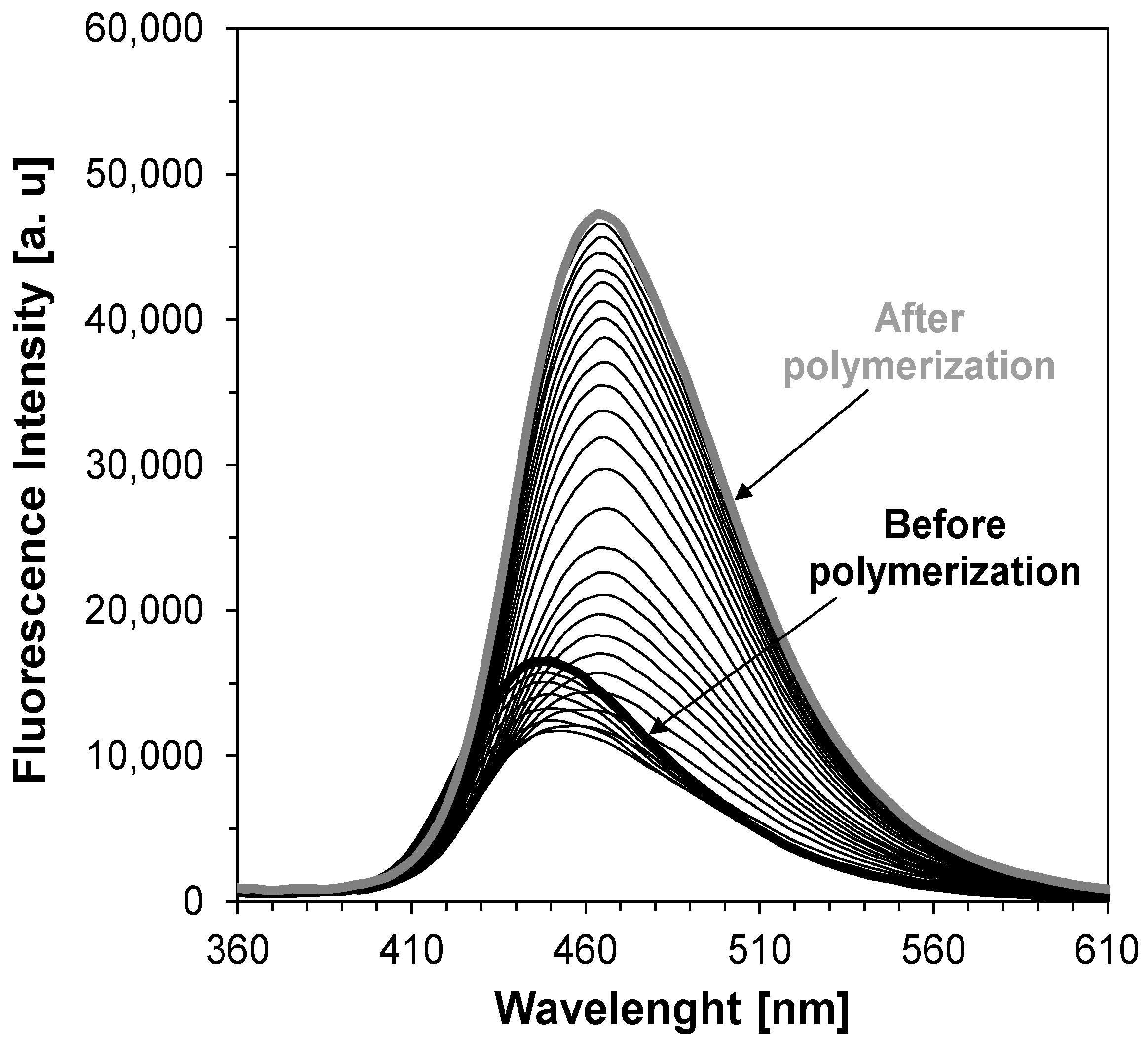
| Sensor | λmax-BEFORE [nm] | Intensity @λmax-BEFORE [a.u.] | λmax-AFTER POL [nm] | Intensity @λmax-AFTER [a.u.] | |ΔImax| [a.u.] | ΔImax a [%] | Δλmax [nm] | Relative Sensitivity b |
| Free-radical photopolymerization process of TMPTMA under 320 nm | ||||||||
| S1 | 413 | 35,770 | 404 | 29,800 | 5970 | 17 | 9 | 2.15 |
| S2 | 407 | 36,530 | 401 | 27,220 | 9310 | 25 | 6 | 1.56 |
| S3 | 413 | 63,550 | 405 | 54,390 | 9160 | 14 | 8 | 1.55 |
| S4 | 406 | 48,980 | 402 | 37,910 | 11,070 | 23 | 4 | 1.32 |
| S5 | 440 | 16,630 | 427 | 15,180 | 1450 | 9 | 13 | 2.76 |
| S6 | 426 | 39,030 | 420 | 28,200 | 10,830 | 28 | 6 | 1.77 |
| C1-ref. | 423 | 22,900 | 418 | 12,920 | 9980 | 44 | 5 | 1.00 |
| Sensor | λmax-BEFORE [nm] | Intensity λmax-BEFORE [a.u.] | λmax-AFTER [nm] | Intensity @λmax-AFTER [a.u.] | |ΔImax| [a.u.] | ΔImax a [%] | Δλmax [nm] | Relative Sensitivity b |
| Thiol-ene photopolymerization process of TMPTMA/MERCAPTO under 320 nm | ||||||||
| S1 | 413 | 21,950 | 405 | 24,290 | 2340 | 11 | 8 | 0.53 |
| S2 | 409 | 38,060 | 403 | 42,850 | 4790 | 13 | 6 | 1.39 |
| S3 | 414 | 33,000 | 406 | 39,340 | 6340 | 19 | 8 | 1.49 |
| S4 | 407 | 56,550 | 403 | 65,970 | 9420 | 17 | 4 | 1.12 |
| S5 | 442 | 19,030 | 428 | 25,280 | 6250 | 33 | 14 | 2.93 |
| S6 | 428 | 50,860 | 423 | 51,330 | 470 | 1 | 5 | 1.44 |
| C1-ref. | 426 | 26,910 | 421 | 41,190 | 14,280 | 53 | 5 | 1.00 |
| Sensor | λmax-BEFORE [nm] | Intensity λmax-BEFORE [a.u.] | λmax-AFTER [nm] | Intensity @λmax-AFTER [a.u.] | |ΔImax| [a.u.] | ΔImax a [%] | Δλmax [nm] | |
| Cationic photopolymerization process of TEGDVE under 320 nm | ||||||||
| S1 | 420 | 11,760 | 423 | 40,050 | 28,290 | 241 | 3 | |
| S2 | 415 | 12,580 | 426 | 17,210 | 4630 | 37 | 11 | |
| S3 | 417 | 12,220 | 447 | 38,940 | 26,720 | 219 | 30 | |
| S4 | 412 | 16,110 | 442 | 26,270 | 10,160 | 63 | 30 | |
| S5 | 450 | 16,540 | 463 | 54,140 | 37,600 | 227 | 13 | |
| S6 | 438 | 11,910 | 441 | 23,090 | 11,180 | 94 | 3 | |
| 25ST-ref. | 459 | 11,180 | 440 | 9790 | 1390 | 12 | 19 | |
| Fluorescent Sensor/ Co-initiator | ε @λab-365 [dm3·mol−1·cm−1] | Eox1/2 [mV] | E00 [eV] | ΔGet [eV] | Conversion of Double Bonds in TEGDVE @365 nm (95 mW/cm2) | Conversion of CADE Epoxy Monomer @365 nm (190 mW/cm2) |
|---|---|---|---|---|---|---|
| S1 | 8410 | 1584 | 3.22 | −0.92 | 83 | 50 |
| S2 | 10,330 | 1635 | 3.10 | −0.74 | 83 | 71 |
| S3 | 12,970 | 1521 | 3.06 | −0.82 | 82 | 78 |
| S4 | 13,820 | 1602 | 3.09 | −0.77 | 83 | 71 |
| S5 | 13,990 | 1157 | 3.06 | −1.18 | 85 | 78 |
| S6 | 12,410 | 1664 | 3.10 | −0.71 | 82 | 72 |
| Fluorescent Sensor/ Co-Initiator | Free-Radical Photopolymerization | Thiol-ene Photopolymerization | |
|---|---|---|---|
| Conversion of Double Bonds in TMPTA @365 nm (95 mW/cm2) | Conversion of Double Bonds in TMPTMA in thiol-ene Polymerization @365 nm (95 mW/cm2) | Conversion of thiol Bonds in Mercapto in thiol-ene Polymerization @365 nm (95 mW/cm2) | |
| S1 | 44 | 93 | 45 |
| S2 | 46 | 73 | 35 |
| S3 | 25 | 93 | 46 |
| S4 | 49 | 92 | 45 |
| S5 | 30 | 95 | 45 |
| S6 | 47 | 89 | 44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortyl, J.; Fiedor, P.; Chachaj-Brekiesz, A.; Pilch, M.; Hola, E.; Galek, M. The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light. Sensors 2019, 19, 1668. https://doi.org/10.3390/s19071668
Ortyl J, Fiedor P, Chachaj-Brekiesz A, Pilch M, Hola E, Galek M. The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light. Sensors. 2019; 19(7):1668. https://doi.org/10.3390/s19071668
Chicago/Turabian StyleOrtyl, Joanna, Paweł Fiedor, Anna Chachaj-Brekiesz, Maciej Pilch, Emilia Hola, and Mariusz Galek. 2019. "The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light" Sensors 19, no. 7: 1668. https://doi.org/10.3390/s19071668
APA StyleOrtyl, J., Fiedor, P., Chachaj-Brekiesz, A., Pilch, M., Hola, E., & Galek, M. (2019). The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization Under Near UV Light. Sensors, 19(7), 1668. https://doi.org/10.3390/s19071668