A Sensitive Pyrimethanil Sensor Based on Electrospun TiC/C Film
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Apparatus
2.3. Preparation of TiC/C
2.4. Electrode Preparation
3. Results and Discussion
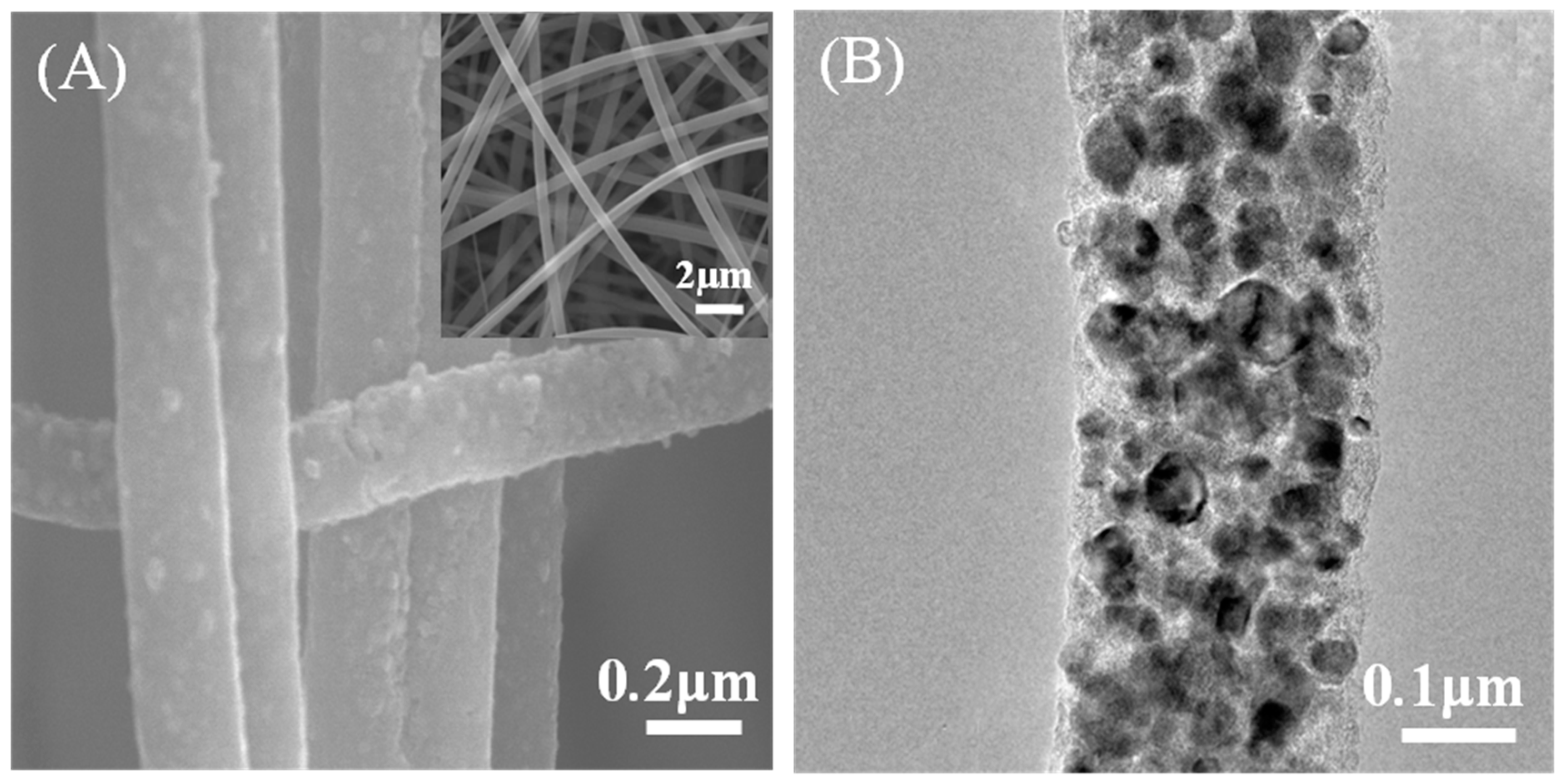
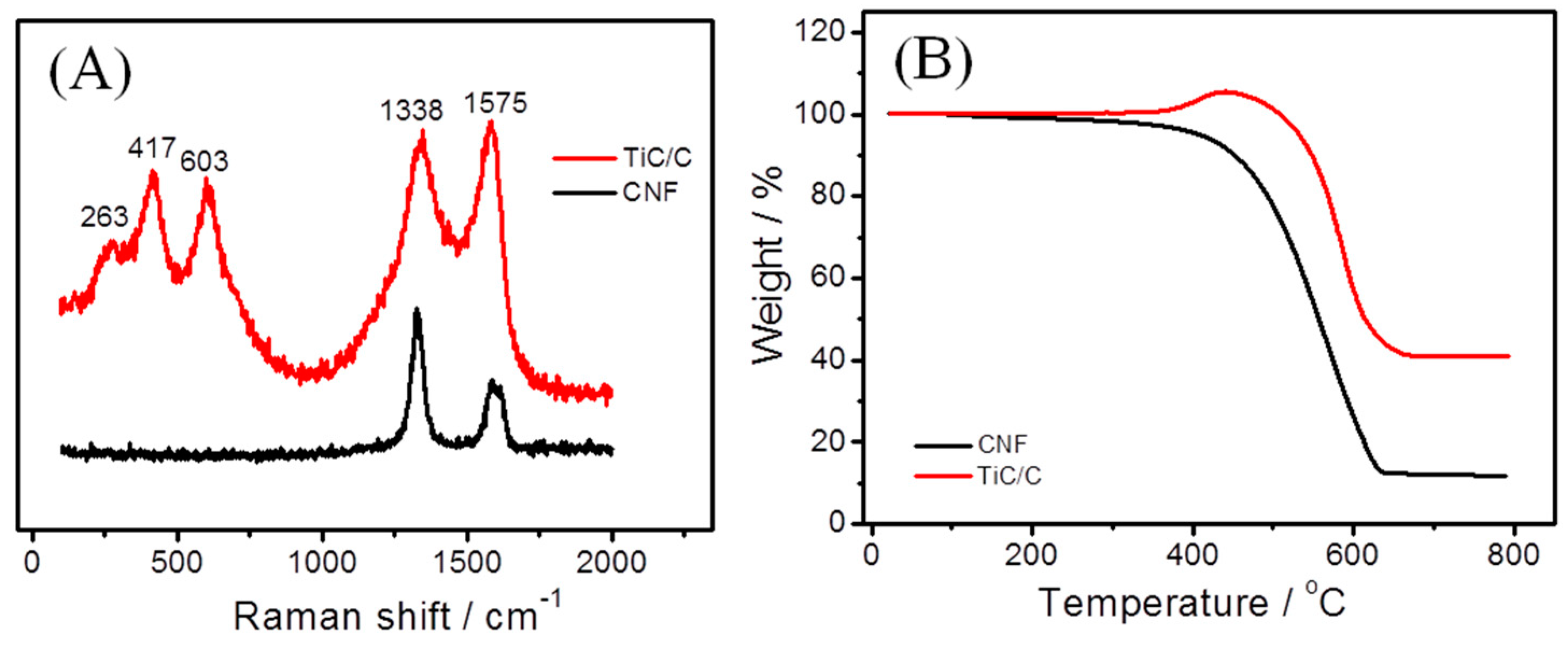
3.1. Characterization
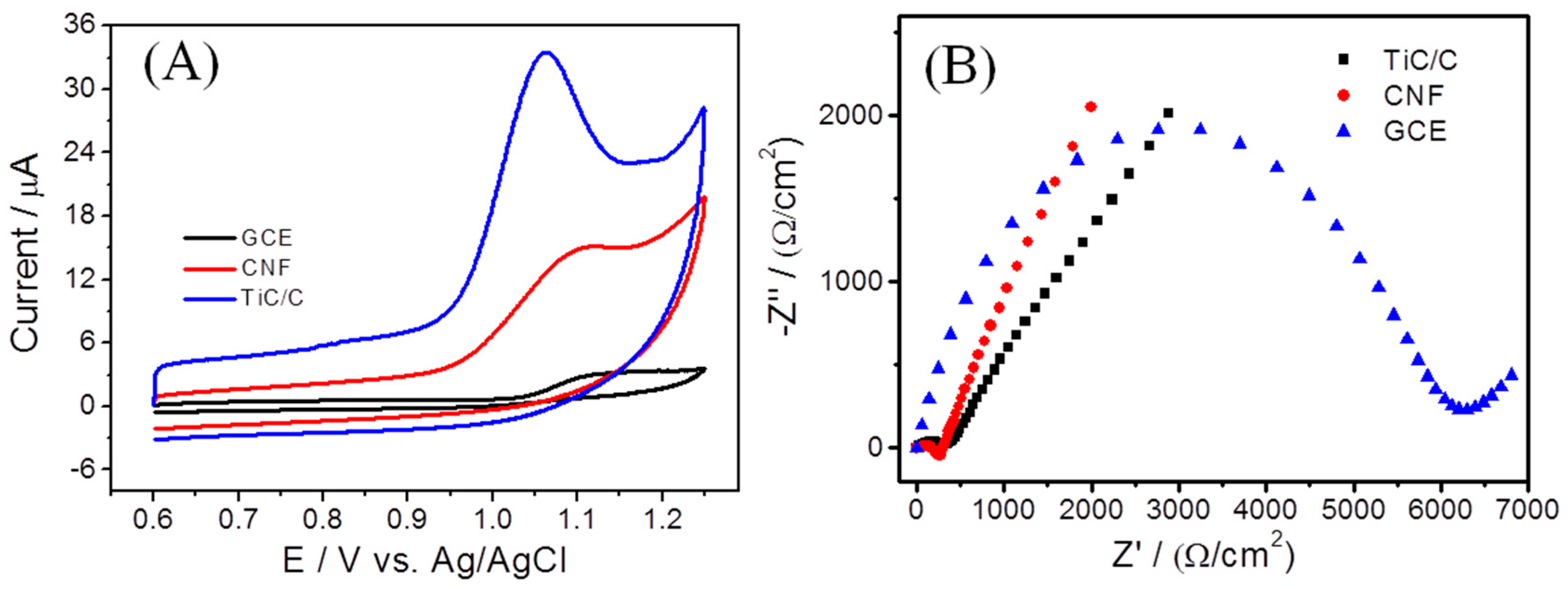
3.2. Electrochemical Behavior
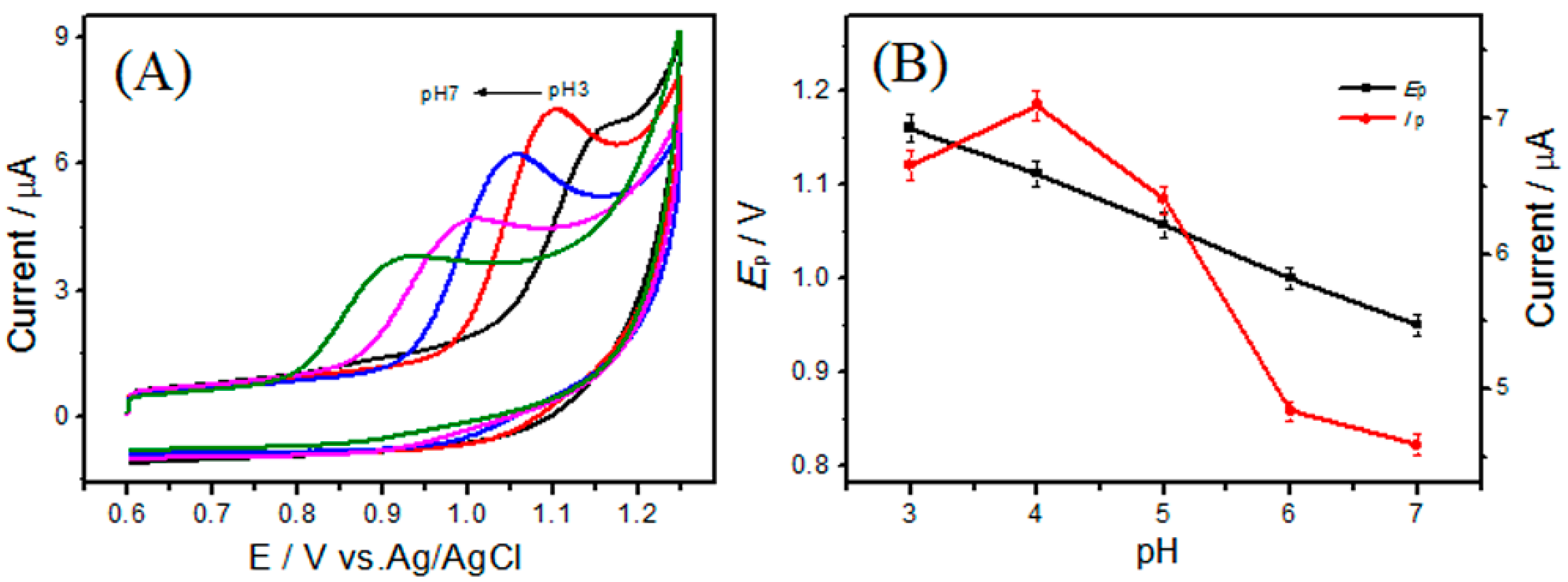
3.3. Effect of pH
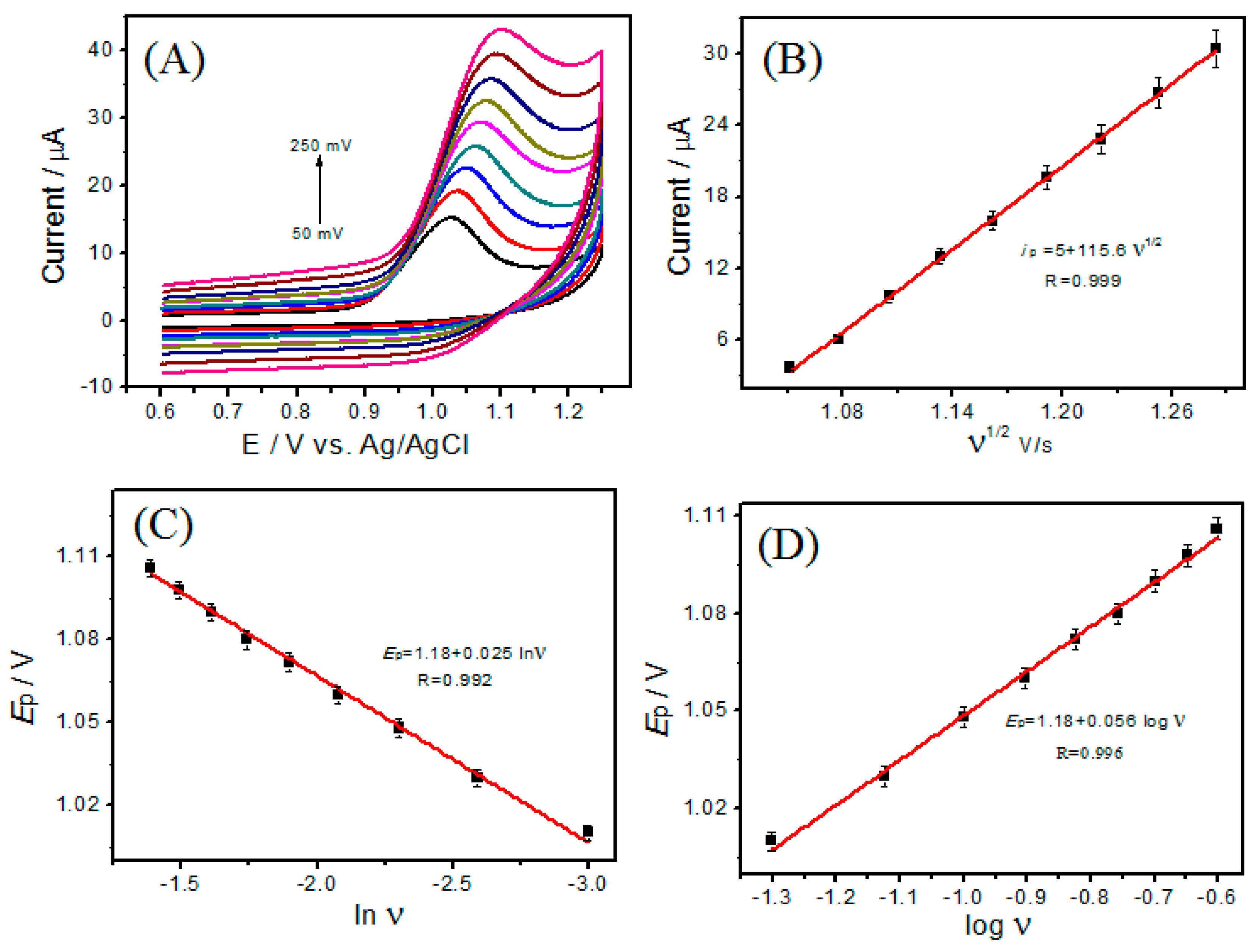
3.4. Effect of Scan Rate
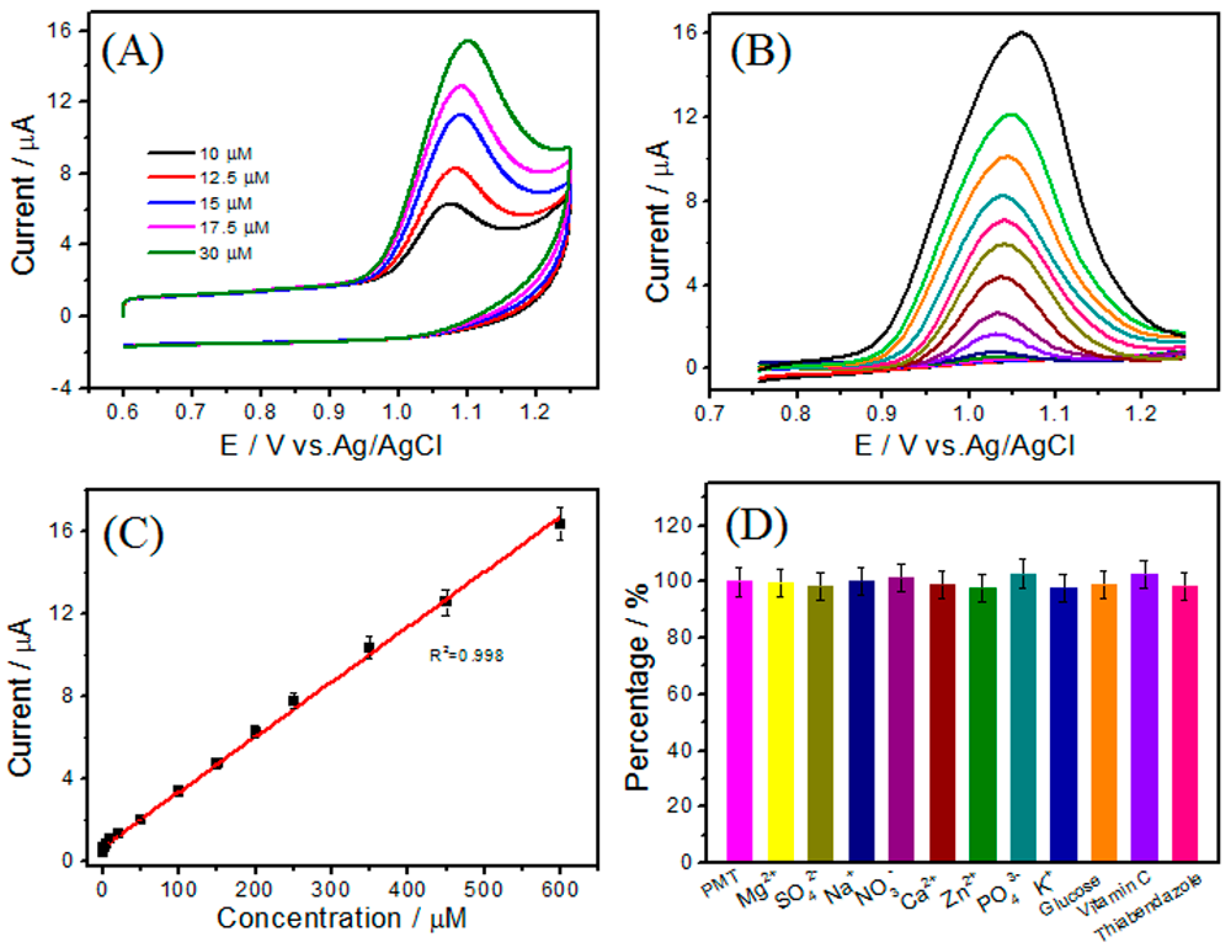
3.5. Determination of PMT by DPV
3.6. Reproducibility, Stability, and Interference Study
3.7. Natural Sample Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, J.; Wang, S.; Wang, P.; Ma, L.; Chen, X.; Xu, R. Toxicity assessment of 40 herbicides to the green alga Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2006, 64, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Miquel, A.S.; Arben, M.; Salvador, A. Pesticide determination in tap water and juice samples using disposable amperometric biosensors made using thick film technology. Anal. Chim. Acta 2001, 442, 35–44. [Google Scholar]
- Baggiani, C.; Baravalle, P.; Giraudi, G.; Tozzi, C. Molecularly imprinted solid-phase extraction method for the high-performance liquid chromatographic analysis of fungicide pyrimethanil in wine. J. Chromatogr. A 2007, 1141, 158–164. [Google Scholar] [CrossRef]
- Cheng, J.; Xia, Y.T.; Zhou, Y.W.; Guo, F.; Chen, G. Application of an ultrasound-assisted surfactant-enhanced emulsification microextraction method for the analysis of diethofencarb and pyrimethanil fungicides in water and fruit juice samples. Anal. Chim. Acta 2011, 701, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cheng, G.; Guo, F.; Wang, X.; Cheng, J. In-syringe demulsified dispersive liquid-liquid microextraction and high performance liquid chromatography-mass spectrometry for the determination of trace fungicides in environmental water samples. Anal. Chim. Acta 2012, 724, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pan, L.; Ying, G.; Kookana, R.S. Enhanced and irreversible sorption of pesticide pyrimethanil by soil amended with biochars. J. Environ. Sci. 2010, 22, 615–620. [Google Scholar] [CrossRef]
- You, X.; Wang, S.; Liu, F.; Shi, K. Ultrasound-assisted surfactant-enhanced emulsification microextraction based on the solidification of a floating organic droplet used for the simultaneous determination of six fungicide residues in juices and red wine. J. Chromatogr. A 2013, 1300, 64–69. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L.; Cheng, J.; Guo, F.; Zhi, X.; Hu, H.; Chen, G. Dispersive liquid-liquid microextraction based on the solidification of a floating organic droplet for simultaneous analysis of diethofencarb and pyrimethanil in apple pulp and peel. Anal. Bioanal. Chem. 2011, 399, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Li, L.B.; Liu, D.; Shi, A.P.; You, T.Y. Simultaneous stripping determination of cadmium and lead ions based on the N-doped carbon quantum dots-graphene oxide hybrid. Sens. Actuators B Chem. 2018, 255, 1762–1770. [Google Scholar] [CrossRef]
- Li, L.B.; Liu, D.; Wang, K.; Mao, H.P.; You, T.Y. Quantitative detection of nitrite with N-doped graphene quantum dots decorated N-doped carbon nanofibers composite-based electrochemical sensor. Sens. Actuators B Chem. 2017, 251, 17–23. [Google Scholar] [CrossRef]
- Li, L.B.; Yu, B.; You, T.Y. Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg(Ⅱ) ions. Biosens. Bioelectron. 2015, 74, 263–269. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Xiong, T.R.; He, S.J.; Li, Y.H.; Zhu, Y.M.; Hou, H.Q. Asymmetric supercapacitor based on flexible TiC/CNF felt supported interwoven nickel-cobalt binary hydroxide nanosheets. J. Power Sources 2016, 317, 57–64. [Google Scholar] [CrossRef]
- Zhao, Y.; Thapa, A.; Feng, Q.; Xi, M.; Qiao, Q.; Fong, H. Electrospun TiC/C nano-felt surface-decorated with Pt nanoparticles as highly efficient and cost-effective counter electrode for dye-sensitized solar cells. Nanoscale 2013, 5, 11742–11747. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, F.; Ye, K.; Zhang, Y.; Jiang, X.; Yin, J.; Wang, G.; Cao, D. Highly porous Fe3O4–Fe nanowires grown on C/TiC nanofiber arrays as the high performance anode of lithium-ion batteries. J. Power Sources 2014, 258, 260–265. [Google Scholar] [CrossRef]
- Yao, Y.; Huo, K.; Hu, L.; Liu, N.; Cha, J.J.; McDowell, M.T.; Chu, P.K.; Cui, Y. Highly conductive, mechanically robust, and electrochemically inactive TiC/C nanofiber scaffold for high-performance silicon anode batteries. ACS Nano 2011, 5, 8346–8351. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Du, J.; Yang, Y.; Li, Y.; Xia, Y.; Gan, Y.; Huang, H.; Zhang, W.; Li, X. TiC nanorods derived from cotton fibers: chloride-assisted VLS growth, structure, and mechanical properties. Cryst. Growth Des. 2011, 11, 4422–4426. [Google Scholar] [CrossRef]
- Zhou, G.; Xiong, T.; Jiang, S.; Jian, S.; Zhou, Z.; Hou, H. Flexible titanium carbide–carbon nanofibers with high modulus and high conductivity by electrospinning. Mater. Lett. 2016, 165, 91–94. [Google Scholar] [CrossRef]
- Guo, Q.H.; Liu, L.J.; Wu, T.T.; Wang, Q.Q.; Wang, H.H.; Liang, J.Y.; Chen, S.L. Flexible and conductive titanium carbide carbon nanofibers for high-performance glucose biosensing. Electrochim. Acta 2018, 281, 517–524. [Google Scholar] [CrossRef]
- Jiang, S.H.; Uch, B.; Agarwal, S.; Greiner, A. Ultralight, thermally insulating, compressible polyimide fiber assembled sponges. ACS Appl. Mat. Interfaces 2017, 9, 32308–32315. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, D.W.; May, R.A.; Berglund, S.P. Low temperature synthesis and characterization of nanocrystalline titanium carbide with tunable porous architectures. Chem. Mater. 2010, 22, 319–329. [Google Scholar] [CrossRef]
- Guo, Q.H.; Wu, T.T.; Liu, L.J.; Hou, H.Q.; Chen, S.L.; Wang, L. Flexible and conductive titanium carbide–carbon nanofibers for the simultaneous determination of ascorbic acid, dopamine and uric acid. J. Mater. Chem. B 2018, 6, 4610–4617. [Google Scholar] [CrossRef]
- Agarwal, S.; Jiang, S.H.; Chen, Y.M. Progress in the field of water-and/or temperature-triggered polymer actuators. Macromol. Mater. Eng. 2019, 304, 1800548–1800566. [Google Scholar]
- Esmaili, R.; Nematollahi, D. Kinetic study of the oxidation of 4-Morpholinoaniline and N,N-Dialkyl-p-phenylenediamines in the presence of barbituric acids derivatives by digital simulation of cyclic voltammograms. J. Electrochem. Soc. 2012, 159, 792–799. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Talemi, R.P. A new methodology for electrostatic immobilization of a non-labeled single strand DNA onto a self-assembled diazonium modified gold electrode and detection of its hybridization by differential pulse voltammetry. Talanta 2013, 103, 344–348. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Biparva, P. Electrochemical determination of sulfite and phenol using a carbon paste electrode modified with ionic liquids and graphene nanosheets: application to determination of sulfite and phenol in real samples. Measurement 2014, 56, 170–177. [Google Scholar] [CrossRef]
- Zargar, B.; Parham, H.; Hatamie, A. Electrochemical investigation and stripping voltammetric determination of captopril at CuO nanoparticles/multi-wall carbon nanotube nanocomposite electrode in tablet and urine samples. Anal. Methods 2015, 7, 1026–1035. [Google Scholar] [CrossRef]
- Liang, P.; Wang, F.; Wan, Q. Ionic liquid-based ultrasound-assisted emulsification microextraction coupled with high performance liquid chromatography for the determination of four fungicides in environmental water samples. Talanta 2013, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Q.; Zhang, M.; Zhang, S.; Zhang, L. An electrochemical fungicide pyrimethanil sensor based on carbon nanotubes/ionic-liquid construction modified electrode. Food Chem. 2015, 187, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.M.P.J.; Rahemi, V.; Borges, F.; Brett, C.M.A.; Garrido, E.M.P.G. Carbon nanotube b-cyclodextrin modified electrode as enhanced sensing platform for the determination of fungicide pyrimethanil. Food Control 2016, 60, 7–11. [Google Scholar] [CrossRef]
- Yang, L.J.; Hu, Y.D.; Wang, Q.; Dong, Y.Y.; Zhang, L. Ionic liquid-assisted electrochemical determination of pyrimethanil using reduced graphene oxide conjugated to flower-like NiCo2O4. Anal. Chim. Acta 2016, 935, 104–112. [Google Scholar] [CrossRef] [PubMed]

| Methods | Linear Range (μM) | LOD (nM) | Ref. |
|---|---|---|---|
| Liquid chromatography | 0.025–25 | 9.0 | [28] |
| MWCNTs-[BMIM][PF6] | 0.1–100 | 16.0 | [29] |
| PANI-β-CD/fMWCNT | 10–80 | 1040 | [30] |
| NiCo2O4/RGO/[OMIM]PF6 | 0.1–140 | 11.0 | [31] |
| TiC/C | 0.1–600 | 33.0 | This work |
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSDa (%) |
|---|---|---|---|---|
| Water | 0 | Nd b | – | – |
| 100.00 | 99.54 | 99.54 | 2.8 | |
| 200.00 | 202.15 | 101.07 | 2.5 | |
| Apple | 0 | Nd b | – | – |
| 50.00 | 48.25 | 96.50 | 3.2 | |
| 100.00 | 98.06 | 98.06 | 2.8 | |
| Cucumber | 0 | Nd b | – | – |
| 200.00 | 202.05 | 101.03 | 3.1 | |
| 300.00 | 301.21 | 100.40 | 3.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, L.; Wu, T.; Liu, L.; Wang, H.; Wang, Q.; Hou, H.; Guo, Q. A Sensitive Pyrimethanil Sensor Based on Electrospun TiC/C Film. Sensors 2019, 19, 1531. https://doi.org/10.3390/s19071531
Sui L, Wu T, Liu L, Wang H, Wang Q, Hou H, Guo Q. A Sensitive Pyrimethanil Sensor Based on Electrospun TiC/C Film. Sensors. 2019; 19(7):1531. https://doi.org/10.3390/s19071531
Chicago/Turabian StyleSui, Ling, Tingting Wu, Lijuan Liu, Honghong Wang, Qingqing Wang, Haoqing Hou, and Qiaohui Guo. 2019. "A Sensitive Pyrimethanil Sensor Based on Electrospun TiC/C Film" Sensors 19, no. 7: 1531. https://doi.org/10.3390/s19071531
APA StyleSui, L., Wu, T., Liu, L., Wang, H., Wang, Q., Hou, H., & Guo, Q. (2019). A Sensitive Pyrimethanil Sensor Based on Electrospun TiC/C Film. Sensors, 19(7), 1531. https://doi.org/10.3390/s19071531




