Bed-Embedded Heart and Respiration Rates Detection by Longitudinal Ballistocardiography and Pattern Recognition
Abstract
1. Introduction
2. Materials and Methods
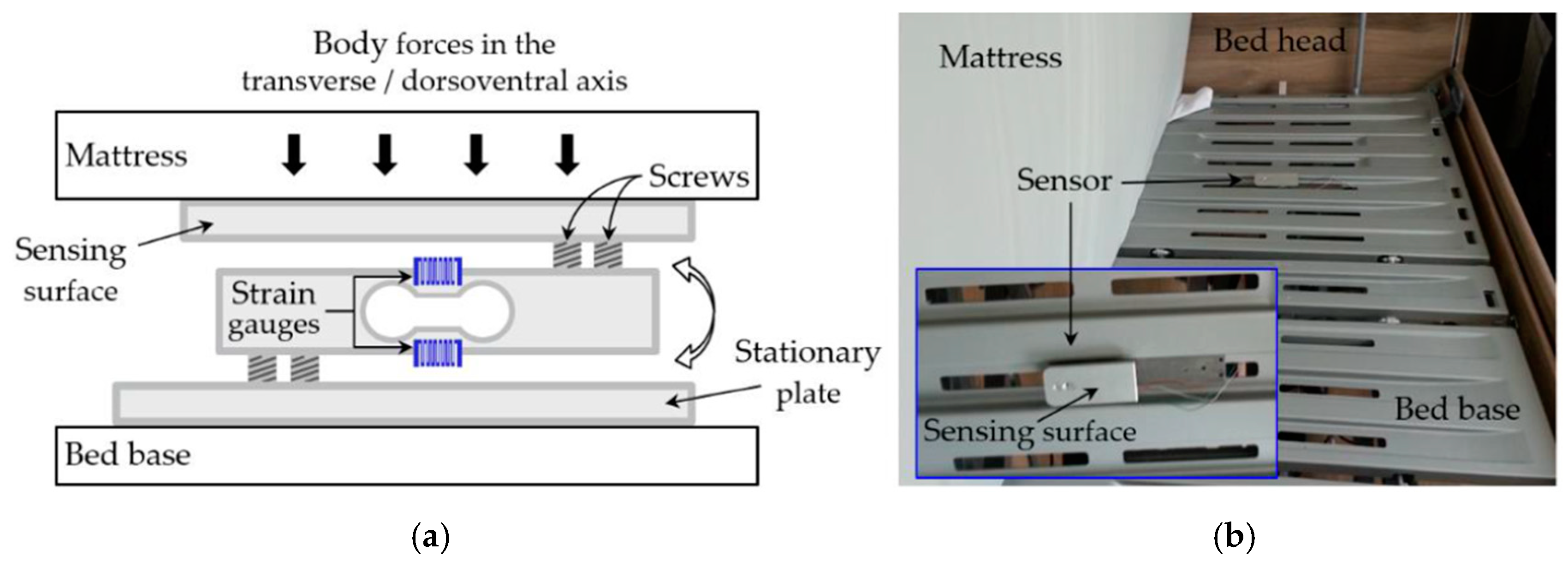
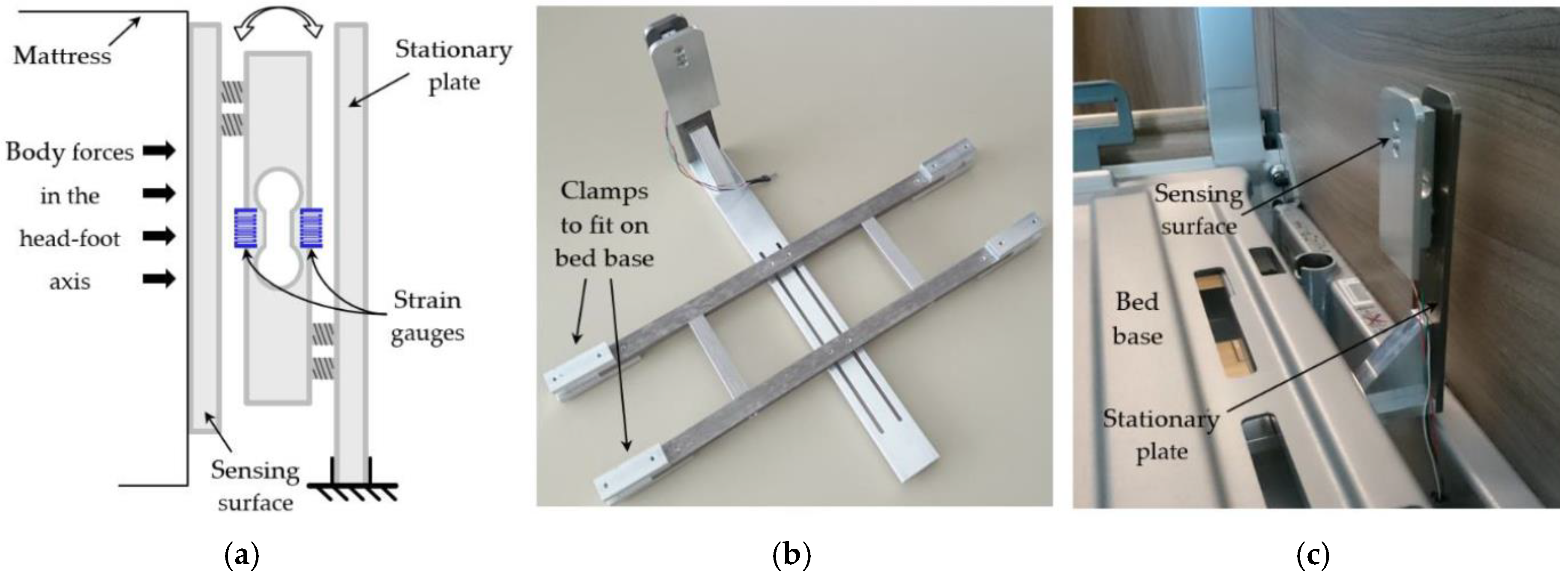
2.1. Measurement Setup
2.1.1. Force Sensor
2.1.2. ECG Sensor
2.1.3. Data Acquisition
2.2. Signal Preprocessing
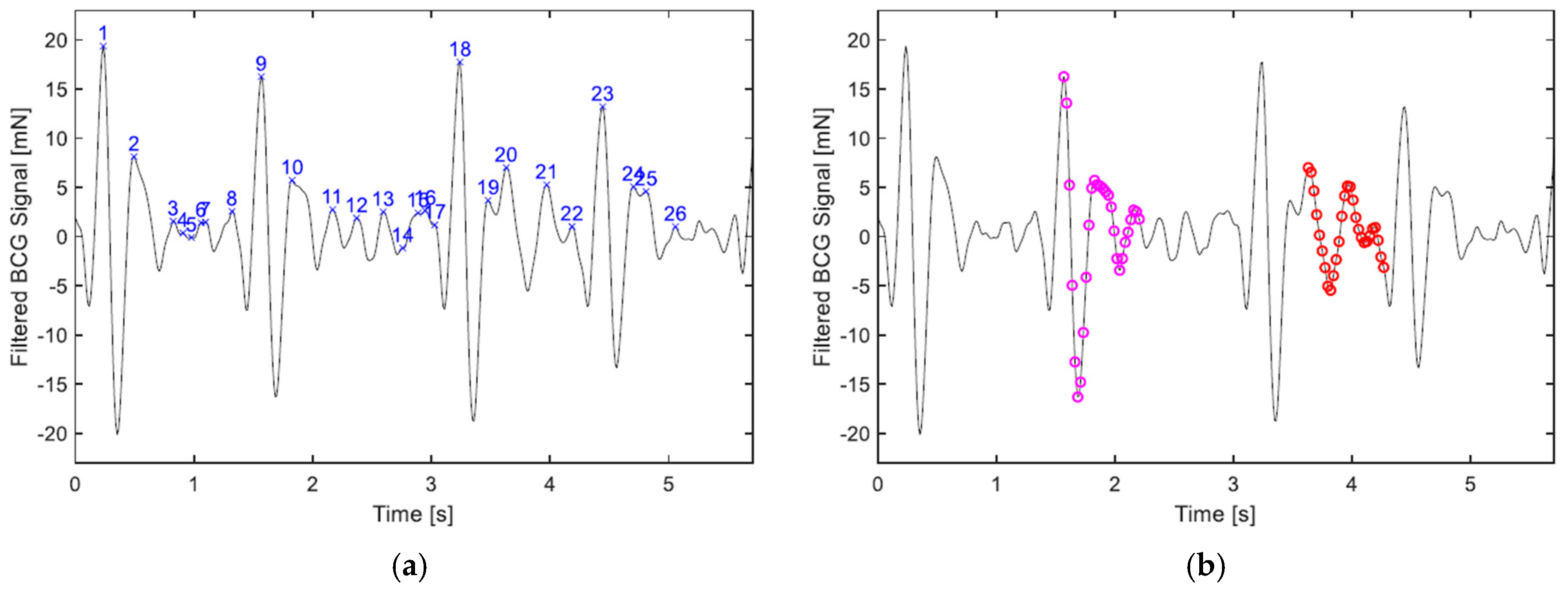
2.3. Heart and Respiration Rates Detection Algorithms
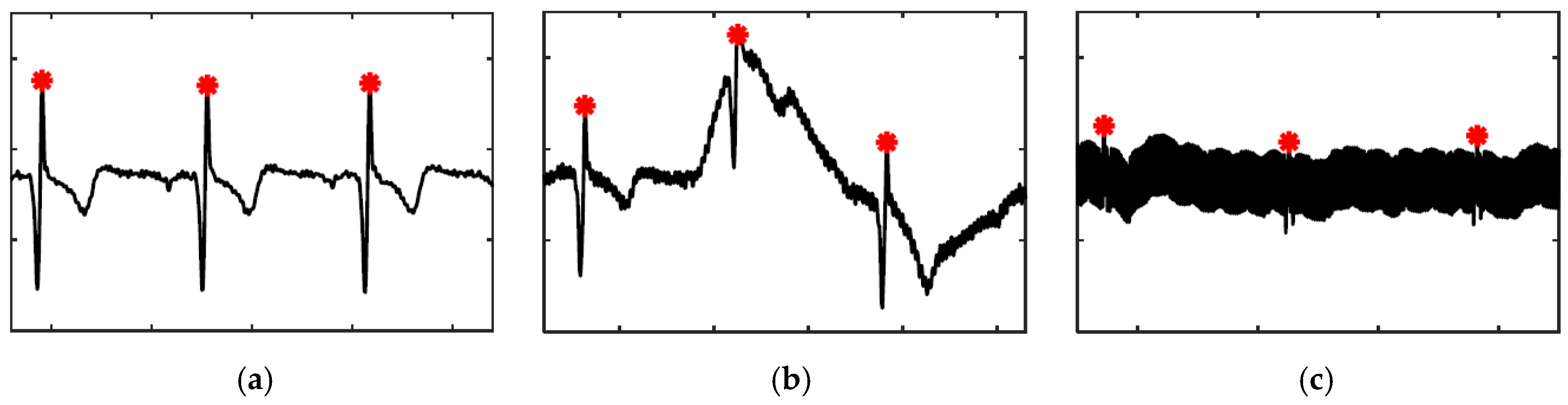
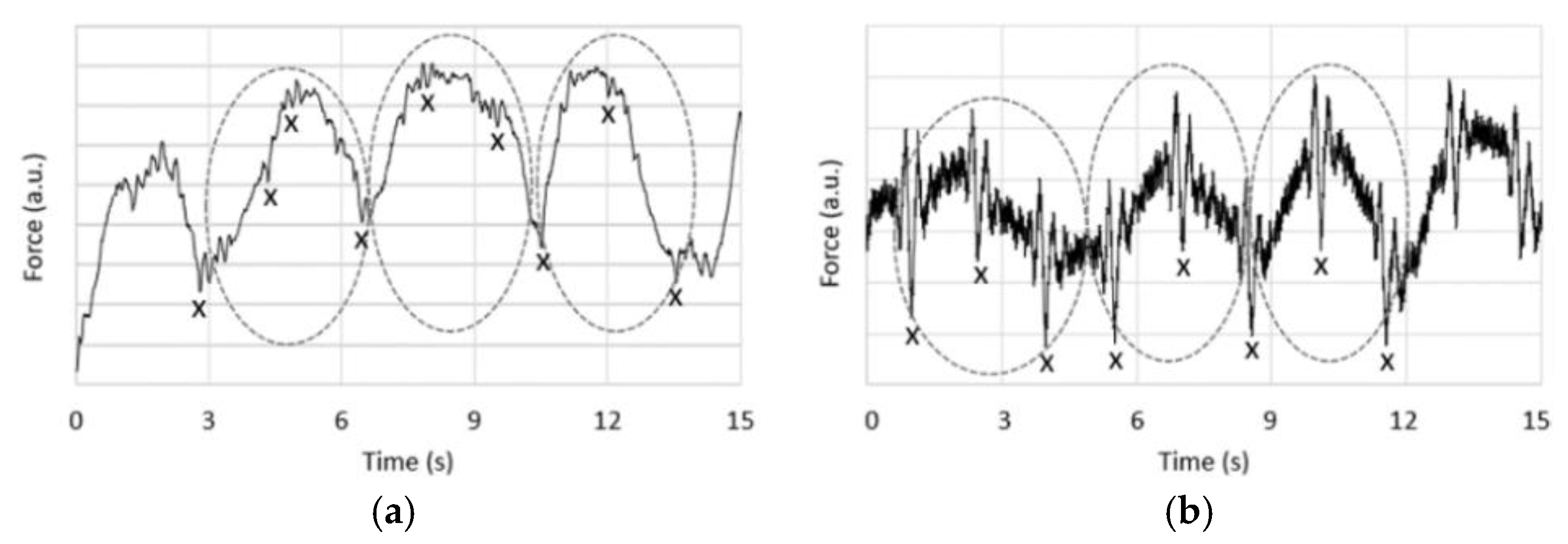
2.3.1. Heart Rate in BCG
- If the ratio of the feature vectors’ magnitudes to one another is larger than α, or, as the case may be, smaller than 1/α, where α is set to 3.
- If the difference between the timestamps of the respective vectors’ first elements is less than tmin, which is set to 0.33 s, making the algorithm only suitable for heart rates lower than 180 bpm.
- If the absolute difference in the chronological order of the vectors is less than i (index).
- The size of the cluster. The cluster containing the largest number of vectors is chosen.
- The mean of dissimilarity. If the largest number of vectors contained in a cluster is equally matched by more than one cluster, the one with the lowest mean of dissimilarity among its vectors is picked.
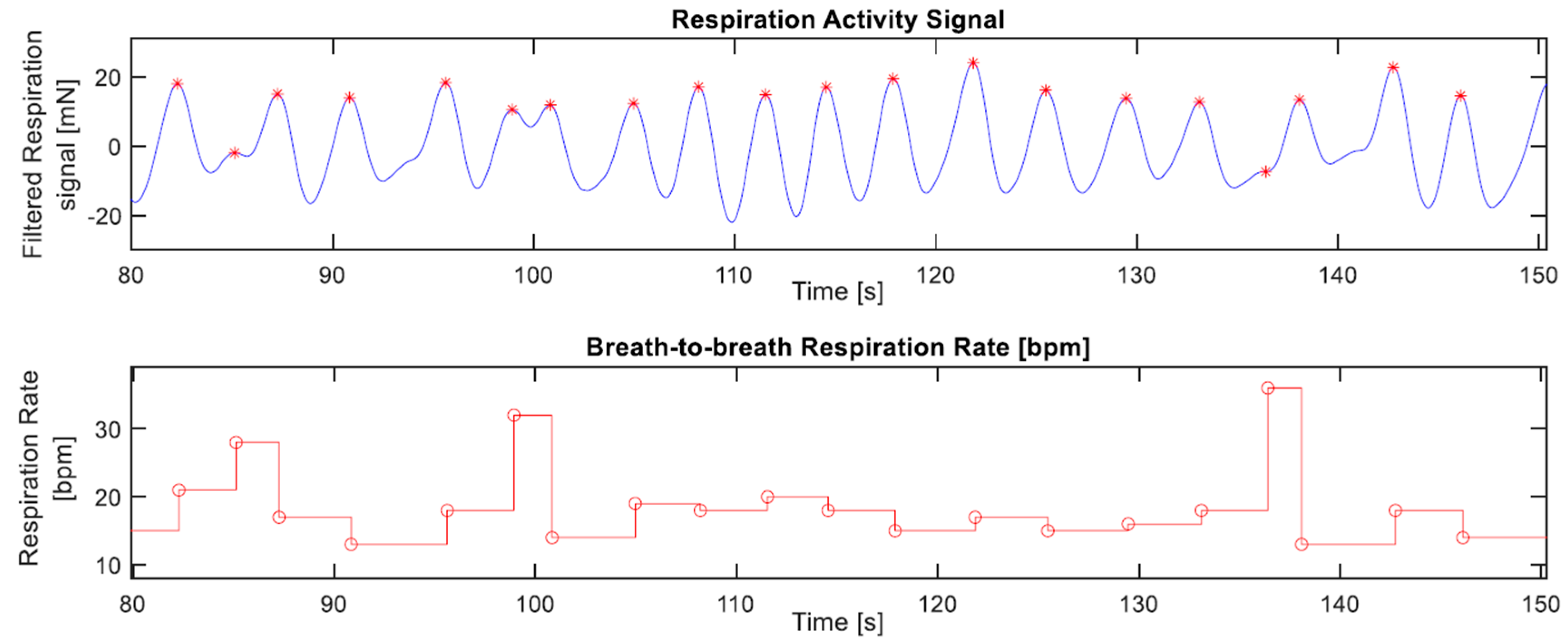
2.3.2. Respiration Rate in BCG
2.3.3. Heart Rate in ECG
2.4. Measurements
2.5. Evaluation
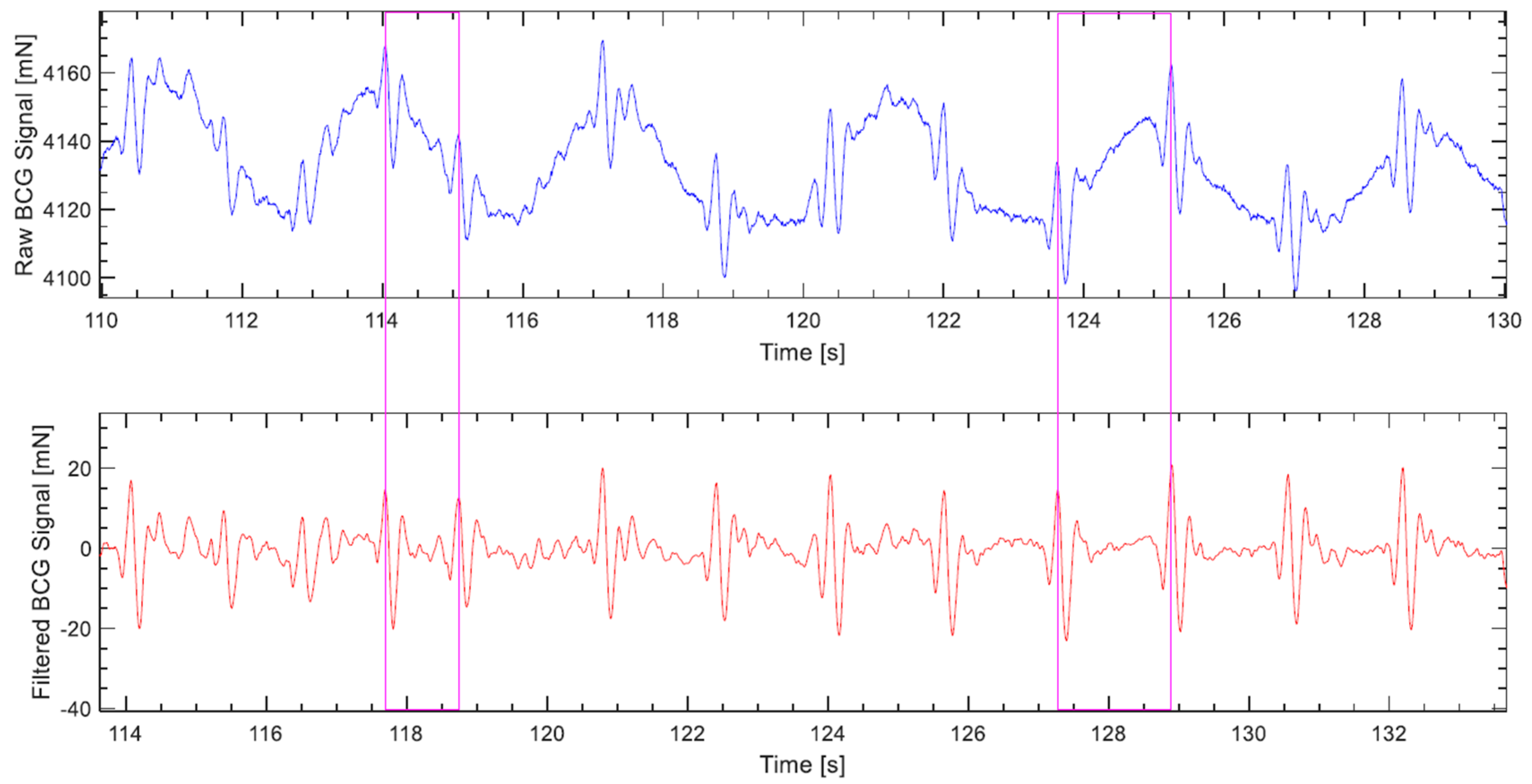
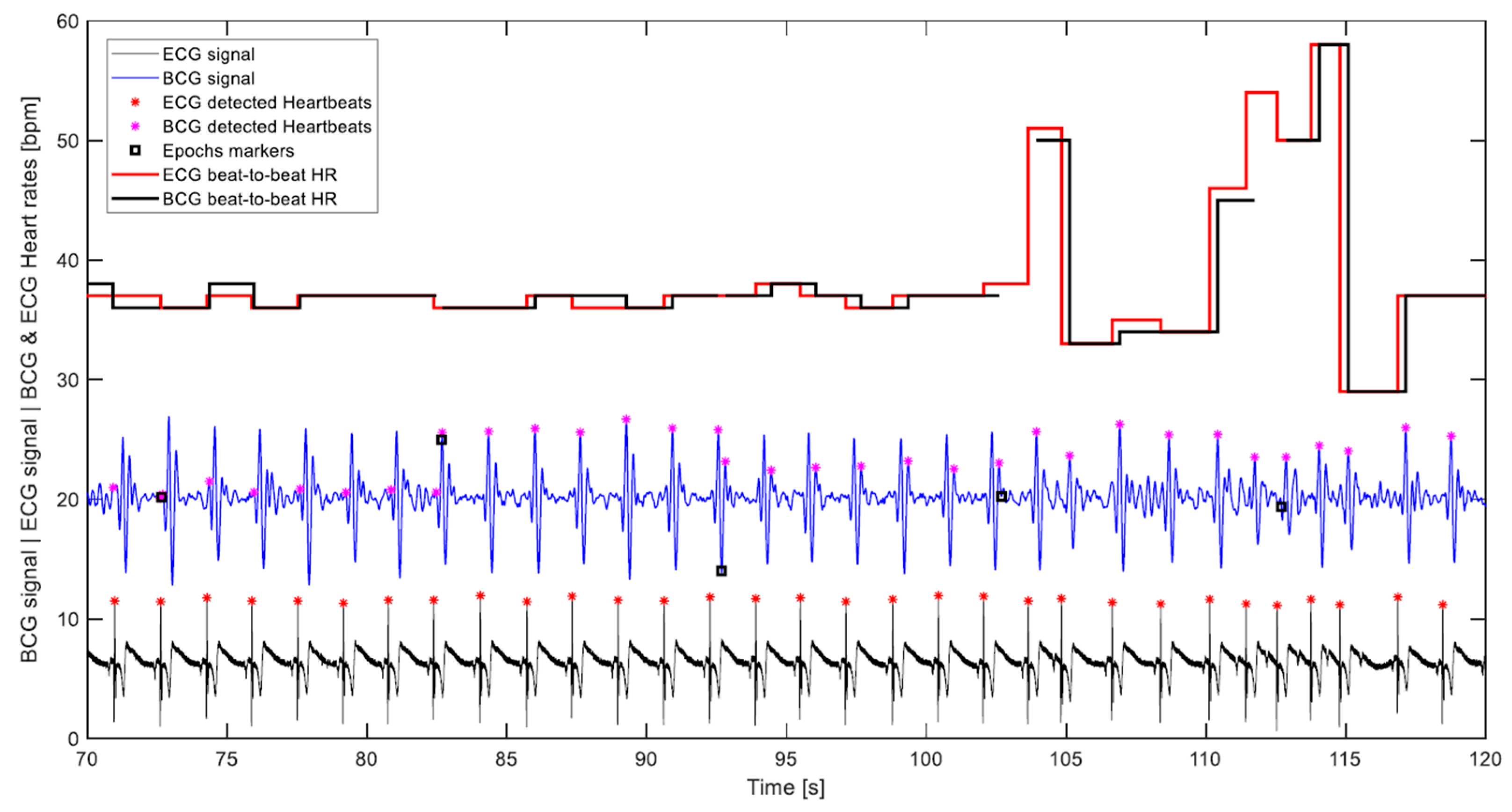
- The correct detection was evaluated visually, which was carried out by plotting the BCG and ECG signals with their respective heart cycles’ detection markings and corresponding calculated heart rates. Figure 11 displays part of a signal undergoing visual assessment.
- The calculated heart rates were rounded to integers. Furthermore, a discrepancy of up to 5 bpm between the ECG and corresponding BCG beat-to-beat heart rates was considered a correct detection, as long as it was visually determined that the detected BCG waves of the consecutive heart cycles are of the same type, which meant that the discrepancy was not a result of wrong detection, but rather a time shift of the wave due to an artifact, for instance. Such high discrepancies happen infrequently, and mostly with higher heart rates.
- Given that the algorithm does not single out any specific wave to represent the cardiac cycle, like the J wave for instance, but rather looks for the waveform most consistently occurring in the 10-s segment being processed. It follows that the calculation of the beat-to-beat heart rate of the cardiac cycle that lies between two consecutive segments based on potentially different waves is not warranted. In Figure 11, which depicts 5 transitions between 10-s segments, it can be noticed that the algorithm was first detecting the H wave, then in the next segment the J wave, and later the L wave. Consequently, the 29 intermediate cardiac cycles, which lie between the 30 segments of a 5-min measurement are subtracted from the number of cycles assumed to be detectable by the algorithm, and the correct detection percentage is calculated accordingly. This can be observed in the plot of Figure 11 by the cuts in the BCG heart rate step diagram. It is worth pointing out that this exclusion was not entertained in the previous paper, namely Reference [33], and all heart cycles contained in a measurement were considered detectable.
- Despite the fact that artifacts caused by volunteers’ movements occurred, since it was not that frequent, no exclusion of heartbeats that might have been undetected because of such artifacts was made in this evaluation.
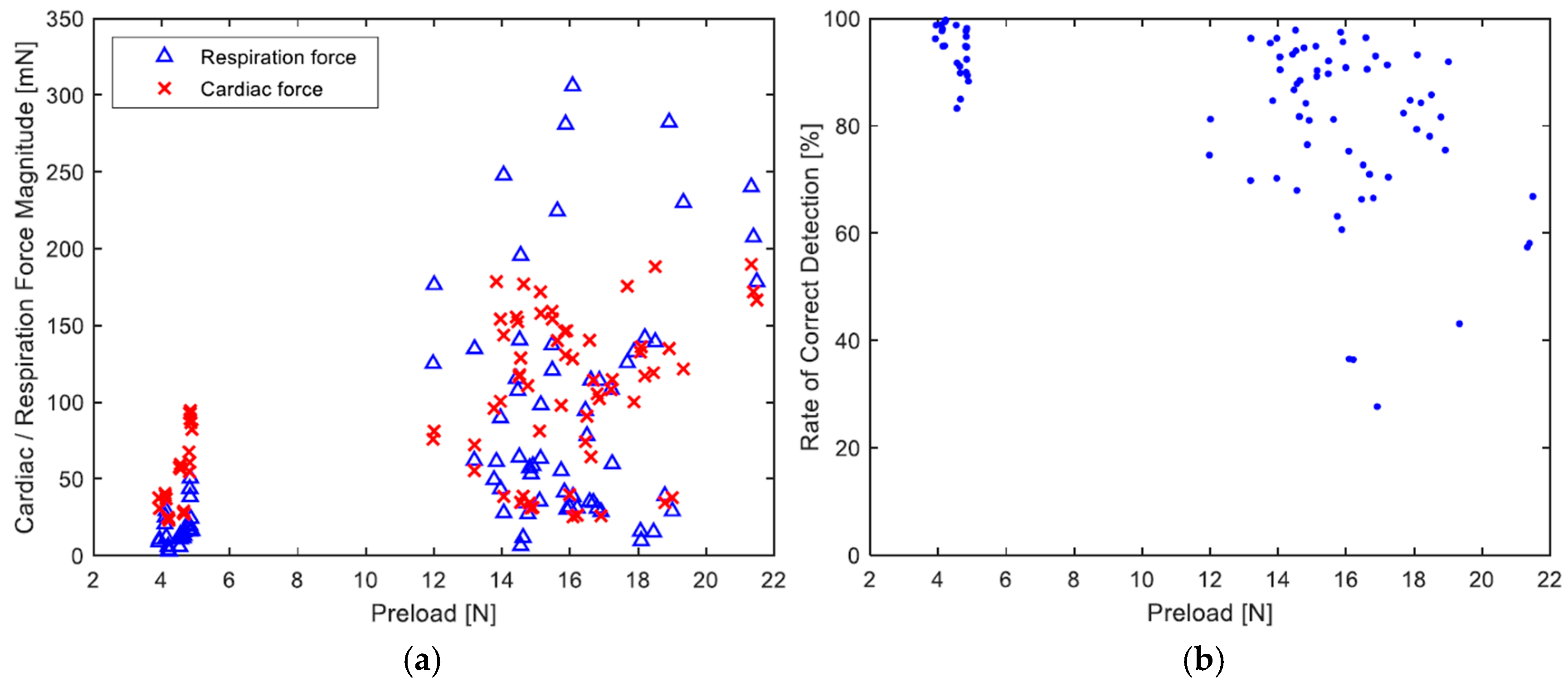
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- Unsupervised pattern recognition:
- Agglomerative clustering:
- Features and feature vectors:
- Proximity measure:
References
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Nijhof, N.; van Gemert-Pijnen, L.J.E.W.C.; Woolrych, R.; Sixsmith, A. An evaluation of preventive sensor technology for dementia care. J. Telemed. Telecare 2013, 19, 95–100. [Google Scholar] [CrossRef]
- Gordon, J.W. Certain Molar Movements of the Human Body produced by the Circulation of the Blood. J. Anat. Physiol. 1877, 11, 533–536. [Google Scholar] [PubMed]
- Pinheiro, E.; Postolache, O.; Girão, P. Theory and developments in an unobtrusive cardiovascular system representation: Ballistocardiography. Open Biomed. Eng. J. 2010, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Ballistocardiography, a Bibliography; National Aeronautics and Space Administration: Washington, DC, USA, 1965.
- Starr, I.; Rawson, A.J.; Schroeder, H.A.; Joseph, N.R. Studies on the estimation of cardiac output in man, and of abnormalities in cardiac function from the heart’s recoil and the blood’s impact; the ballistocardiogram. Am. J. Physiol. 1939, 127, 1–28. [Google Scholar] [CrossRef]
- Starr, I.; Braunstein, J.R.; Dock, W.; Gubner, R.; Hamilton, W.F.; Nickerson, J.L.; Rappaport, M.B.; Scarborough, W.R.; Smith, J.E. First Report of the Committee on Ballistocardiographic Terminology. Circulation 1953, 7, 929–931. [Google Scholar] [CrossRef]
- Scarborough, W.R.; Talbot, S.A. Proposals for ballistocardiographic nomenclature and conventions: Revised and extended report of Committee on Ballistocardiographic Terminology. Circulation 1956, 14, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.B.; Rappaport, M.B.; Sprague, H.B. Ballistocardiography. II. The normal ballistocardiogram. Circulation 1953, 7, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gubner, R.S.; Rodstein, M.; Ungerleider, H.E. Ballistocardiography; an appraisal of technic, physiologic principles, and clinical value. Circulation 1953, 7, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Giovangrandi, L.; Inan, O.T.; Wiard, R.M.; Etemadi, M.; Kovacs, G.T.A. Ballistocardiography—A Method Worth Revisiting. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 4279–4282. [Google Scholar]
- Starr, I.; Wood, F.C. Twenty-Year Studies with the Ballistocardiograph. Circulation 1961, 23, 714–732. [Google Scholar] [CrossRef]
- Soames, R.W.; Atha, J. Three-dimensional ballistocardiographic responses to changes of posture. Clin. Phys. Physiol. Meas. 1982, 3, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Brüser, C.; Stadlthanner, K.; de Waele, S.; Leonhardt, S. Adaptive beat-to-beat heart rate estimation in ballistocardiograms. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Henderson, Y. The mass-movements of the Circulation as shown by a Recoil Curve. Am. J. Physiol.-Leg. Content 1905, 14, 287–298. [Google Scholar] [CrossRef]
- Nickerson, J.L.; Curtis, H.J. The design of the Ballistocardiograph. Am. J. Physiol.-Leg. Content 1944, 142, 1–11. [Google Scholar] [CrossRef]
- Dock, W.; Taubman, F. Some technics for recording the ballistocardiogram directly from the body. Am. J. Med. 1949, 7, 751–755. [Google Scholar] [CrossRef]
- Inan, O.T.; Etemadi, M.; Widrow, B.; Kovacs, G.T.A. Adaptive cancellation of floor vibrations in standing ballistocardiogram measurements using a seismic sensor as a noise reference. IEEE Trans. Bio-Med. Eng. 2010, 57, 722–727. [Google Scholar] [CrossRef] [PubMed]
- González-Landaeta, R.; Casas, O.; Pallàs-Areny, R. Heart rate detection from an electronic weighing scale. Physiol. Meas. 2008, 29, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, S.; Gómez-Clapers, J.; Casanella, R.; Pallas-Areny, R. Heart and respiratory rate detection on a bathroom scale based on the ballistocardiogram and the continuous wavelet transform. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 2557–2560. [Google Scholar]
- Baek, H.J.; Chung, G.S.; Kim, K.K.; Park, K.S. A smart health monitoring chair for nonintrusive measurement of biological signals. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Koivistoinen, T.; Junnila, S.; Värri, A.; Kööbi, T. A new method for measuring the ballistocardiogram using EMFi sensors in a normal chair. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–4 September 2004; pp. 2026–2029. [Google Scholar]
- Zhu, Y.; Zhang, H.; Jayachandran, M.; Ng, A.K.; Biswas, J.; Chen, Z. Ballistocardiography with fiber optic sensor in headrest position: A feasibility study and a new processing algorithm. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5203–5206. [Google Scholar]
- Pinheiro, E.; Postolache, O.; Girão, P. Study on Ballistocardiogram Acquisition in a Moving Wheelchair with Embedded Sensors. Metrol. Meas. Syst. 2012, 19, 739–750. [Google Scholar] [CrossRef]
- Jung, D.W.; Hwang, S.H.; Yoon, H.N.; Lee, Y.-J.G.; Jeong, D.-U.; Park, K.S. Nocturnal awakening and sleep efficiency estimation using unobtrusively measured ballistocardiogram. IEEE Trans. Bio-Med. Eng. 2014, 61, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rosales, L.; Skubic, M.; Heise, D.; Devaney, M.J.; Schaumburg, M. Heartbeat detection from a hydraulic bed sensor using a clustering approach. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2383–2387. [Google Scholar]
- Paalasmaa, J.; Ranta, M. Detecting Heartbeats in the Ballistocardiogram with Clustering. In Proceedings of the ICML/UAI/COLT 2008 Workshop on Machine Learning for Health-Care Applications, Helsinki, Finland, 9 July 2008. [Google Scholar]
- Watanabe, K.; Watanabe, T.; Watanabe, H.; Ando, H.; Ishikawa, T.; Kobayashi, K. Noninvasive measurement of heartbeat, respiration, snoring and body movements of a subject in bed via a pneumatic method. IEEE Trans. Bio-Med. Eng. 2005, 52, 2100–2107. [Google Scholar] [CrossRef]
- Chee, Y.; Han, J.; Youn, J.; Park, K. Air mattress sensor system with balancing tube for unconstrained measurement of respiration and heart beat movements. Physiol. Meas. 2005, 26, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, W.; Nemoto, T.; Kanemitsu, Y.; Kitamura, K.-i.; Yamakoshi, K.-i.; Wei, D. Real-time monitoring of respiration rhythm and pulse rate during sleep. IEEE Trans. Bio-Med. Eng. 2006, 53, 2553–2563. [Google Scholar]
- Jin, J.; Wang, X.; Li, S.; Wu, Y. A Novel Heart Rate Detection Algorithm in Ballistocardiogram Based on Wavelet Transform. In Proceedings of the 2009 Second International Workshop on Knowledge Discovery and Data Mining, Moscow, Russia, 23–25 January 2009; Luo, Q., Gong, M., Eds.; Conference Pub. Services/IEEE Computer Society: Los Alamitos, CA, USA, 2009; pp. 76–79. [Google Scholar]
- Gomez-Clapers, J.; Serra-Rocamora, A.; Casanella, R.; Pallas-Areny, R. Towards the standardization of ballistocardiography systems for J-peak timing measurement. Measurement 2014, 58, 310–316. [Google Scholar] [CrossRef]
- Lima, F.; Albukhari, A.; Zhu, R.; Mescheder, U. Contactless Sleep Monitoring Measurement Setup. Proceedings 2018, 2, 1083. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, A.; Narvaiza, L.; Ruiz, J.; Gálvez-Barrón, C. Normal respiratory rate and peripheral blood oxygen saturation in the elderly population. J. Am. Geriatr. Soc. 2013, 61, 2238–2240. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, S.; Koutroumbas, K. Pattern Recognition, 4th ed.; Elsevier/Academic Press: Amsterdam, The Netherlands; London, UK, 2009. [Google Scholar]





| Volunteer Details | Lying Position | Average Heart Rate [bpm] | No. Theoretically Detectable Beat-to-Beat Cycles | No. Cycles Not Correctly Detected 3 | No. False Positive Detections 4 | Correct Detection (%) | Avg. Corr. Detection (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age [yr] | Wt. [kg] | Ht. [cm] | |||||||
| M | 31 | 83 | 180 | Supine | 76 | 1050 | 19 | 0 | 98.2 | 95.4 |
| Prone | 64 | 872 | 100 | 0 | 88.6 | |||||
| Left side | 69 | 944 | 32 | 0 | 96.6 | |||||
| Right side | 68 | 928 | 18 | 0 | 98.0 | |||||
| M 1 | 35 | 78 | 180 | Supine | 39 | 502 | 29 | 8 | 94.3 | 93.0 |
| Prone | 41 | 524 | 19 | 4 | 96.4 | |||||
| Left side | 38 | 486 | 43 | 8 | 91.2 | |||||
| Right side | 43 | 551 | 55 | 7 | 90.0 | |||||
| M | 28 | 75 | 170 | Supine | 53 | 700 | 65 | 1 | 90.7 | 85.3 |
| Prone | 56 | 756 | 149 | 5 | 80.5 | |||||
| Left side | 57 | 758 | 37 | 2 | 95.0 | |||||
| Right side | 61 | 822 | 204 | 3 | 74.8 | |||||
| M | 30 | 78 | 170 | Supine | 87 | 1211 | 200 | 0 | 83.5 | 86.9 |
| Prone | 83 | 1151 | 285 | 0 | 75.2 | |||||
| Left side | 79 | 1089 | 67 | 1 | 93.9 | |||||
| Right side | 82 | 1142 | 59 | 0 | 94.9 | |||||
| M | 26 | 65 | 170 | Supine | 59 | 802 | 75 | 1 | 90.7 | 86.4 |
| Prone | 61 | 828 | 29 | 0 | 96.5 | |||||
| Left side | 63 | 853 | 94 | 1 | 88.9 | |||||
| Right side | 61 | 822 | 252 | 5 | 69.3 | |||||
| M | 30 | 95 | 173 | Supine | 84 | 1177 | 383 | 0 | 67.4 | 68.2 |
| Prone | 88 | 1235 | 500 | 1 | 59.8 | |||||
| Left side | 83 | 1150 | 173 | 0 | 84.9 | |||||
| Right side | 86 | 1206 | 473 | 0 | 60.8 | |||||
| F 2 | 70 | 67 | 158 | Supine | 68 | 926 | 615 | 9 | 33.6 | 72.2 |
| Prone | 70 | 958 | 186 | 4 | 80.6 | |||||
| Left side | 65 | 879 | 117 | 36 | 86.7 | |||||
| Right side | 67 | 921 | 109 | 12 | 88.1 | |||||
| Lying Position | Correct Detection (%) |
|---|---|
| Supine | 79.8 |
| Prone | 82.5 |
| Left side | 91.0 |
| Right side | 82.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albukhari, A.; Lima, F.; Mescheder, U. Bed-Embedded Heart and Respiration Rates Detection by Longitudinal Ballistocardiography and Pattern Recognition. Sensors 2019, 19, 1451. https://doi.org/10.3390/s19061451
Albukhari A, Lima F, Mescheder U. Bed-Embedded Heart and Respiration Rates Detection by Longitudinal Ballistocardiography and Pattern Recognition. Sensors. 2019; 19(6):1451. https://doi.org/10.3390/s19061451
Chicago/Turabian StyleAlbukhari, Almothana, Frederico Lima, and Ulrich Mescheder. 2019. "Bed-Embedded Heart and Respiration Rates Detection by Longitudinal Ballistocardiography and Pattern Recognition" Sensors 19, no. 6: 1451. https://doi.org/10.3390/s19061451
APA StyleAlbukhari, A., Lima, F., & Mescheder, U. (2019). Bed-Embedded Heart and Respiration Rates Detection by Longitudinal Ballistocardiography and Pattern Recognition. Sensors, 19(6), 1451. https://doi.org/10.3390/s19061451





