A New Model and Its Application for the Dynamic Response of RGO Resistive Gas Sensor
Abstract
:1. Introduction
2. Experimental
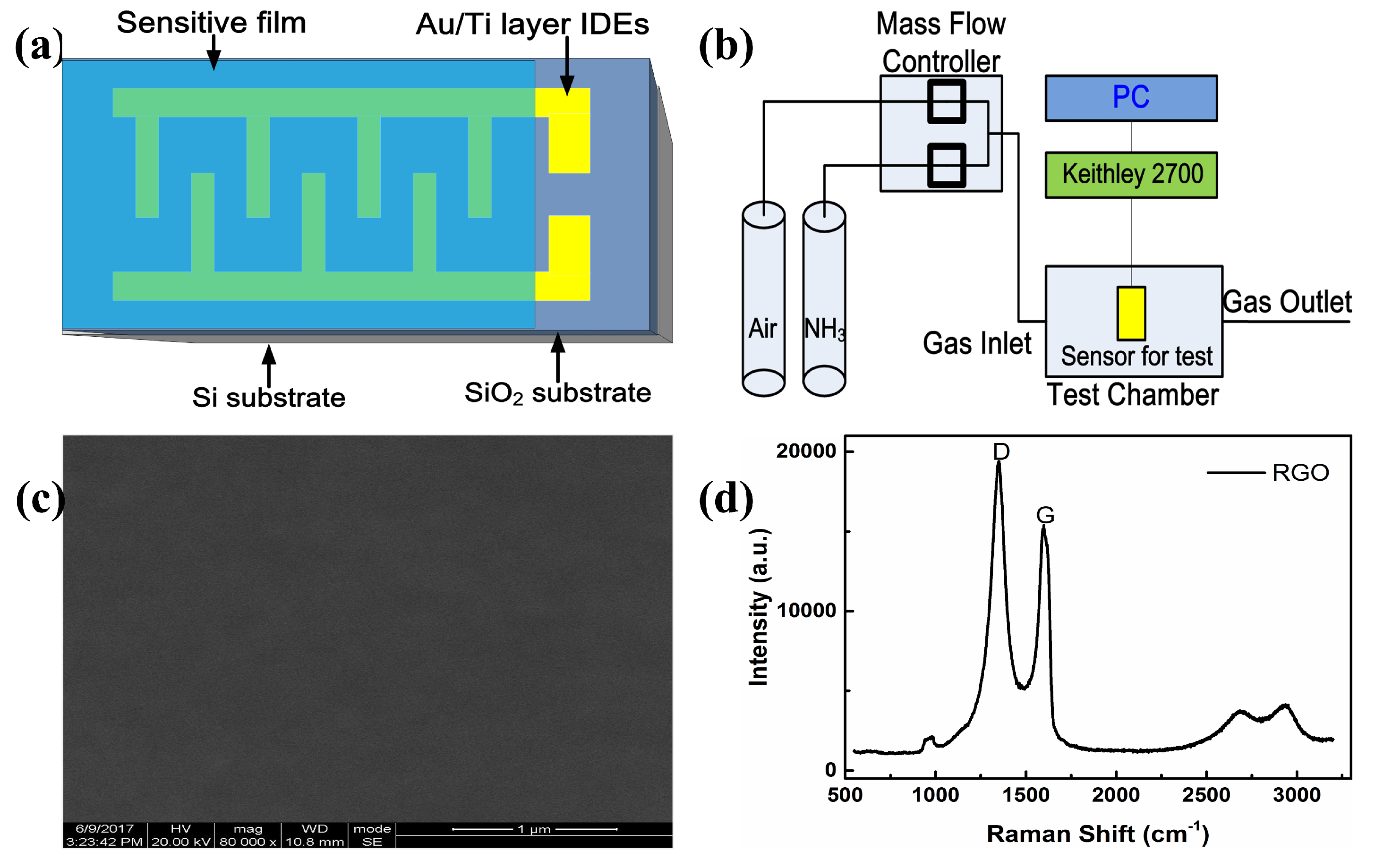
2.1. Sensor Preparation
2.2. Test Instrument and Measurement Procedure
2.3. The Characteristics of Film
2.4. The Dynamic Response and Preliminary Analysis
3. Theory
3.1. Influence of Adsorbed Gas Molecules
3.2. Basic Assumptions
- Surface approximation. The surface of the film is homogeneous and the adsorption process is deemed as monolayer adsorption. This assumption implies that multilayer and microporous adsorption, etc., are not considered.
- Gas concentration effect. The adsorption and desorption of gas molecules occur simultaneously, and their numbers are influenced by the number of molecules contacting the surface of the film per unit time. This assumption means that the main factor that affects the adsorption and desorption is the gas concentration under the conditions of constant temperature and pressure, with no illumination.
- Response process approximation. When gas concentration is changed, the number of molecules colliding on the surface of the film changes greatly at the beginning, thereafter the adsorption and desorption occur simultaneously and at last reach a balance state. The main factor that affects the adsorption and desorption is the gas intermolecular force.
3.3. Intermolecular Forces Based Model of Adsorption and Desorption
| Total molecules |
| = Free molecules + molecules hit on the film surface () |
| = Free molecules + A-type molecules + NA-type molecules |
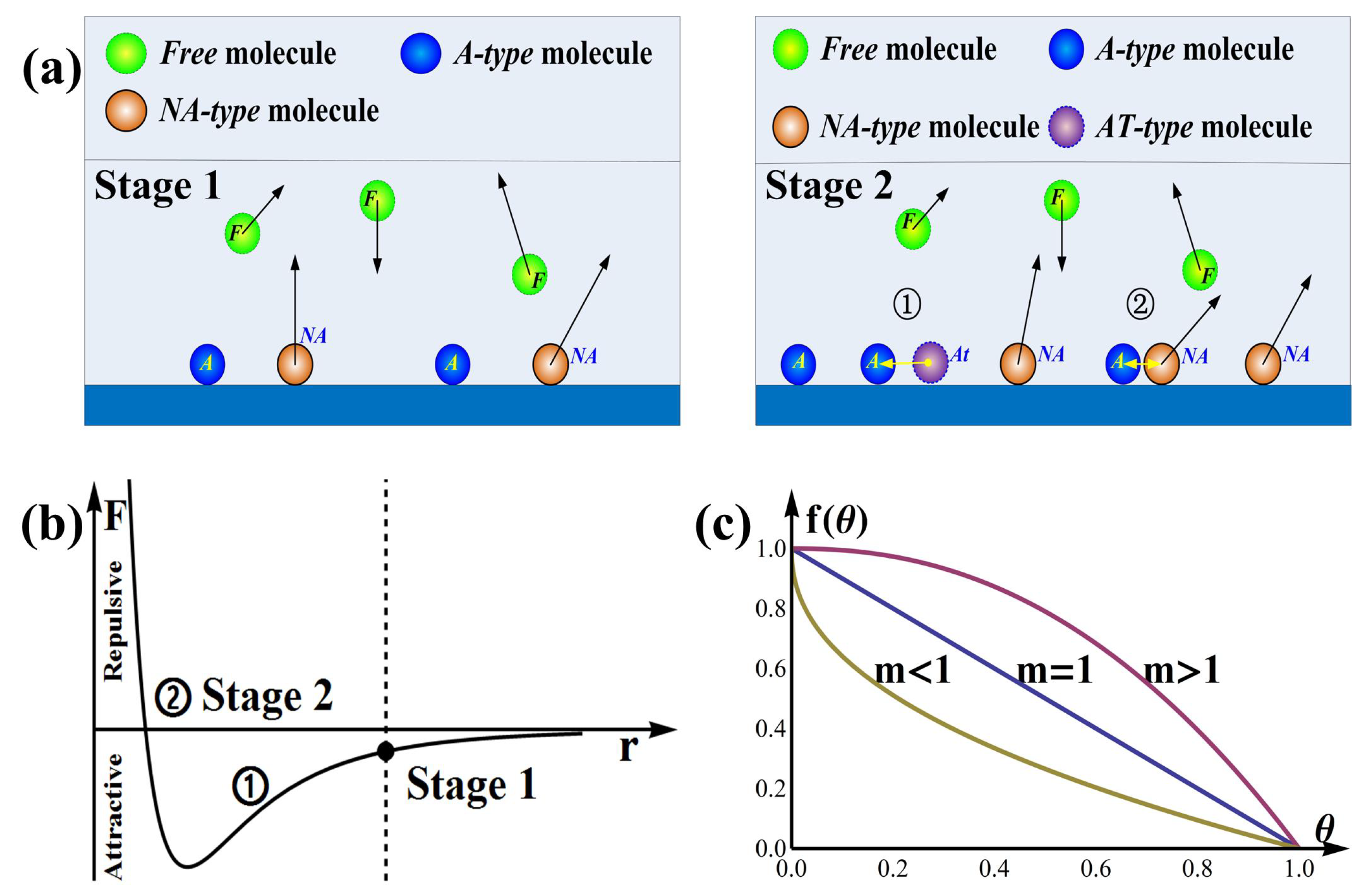
- Stage 1: The Beginning StateThis stage can finish instantaneously under ideal conditions.The randomly moving gas molecules collide constantly with each other and with the surface of sensor, of them hitting on the surface of sensor, and molecules are adsorbed on the surface. The adsorption ratio is mainly affected by the sensitive material, surface morphology, temperature, humidity, pressure, illumination, and gas species, etc. This state is shown in Figure 3a stage 1 and Figure 3b stage 1. .
- Stage 2: Adsorption ProcessAdsorption and desorption occur simultaneously, however, we can focus only on the combined effect, which can be regarded as net increment of adsorption.(1) With the increment of A-type molecules, the probability that NA-type molecules meet A-type molecules increases. Meanwhile, the average intermolecular distance decreases and the attractive force increases as shown in Figure 3b stage 2 ①. Consequently, some of the NA-type molecules approaching A-type molecules are more likely to be attracted to the film and transferred to AT-type molecules as shown in Figure 3a stage 2 ①. Note that the number of the NA-type molecules is proportional to molecules hitting on the film surface (), then the rate of adsorption is affected by and a functionwhere is an increasing function of .(2) With the increment of the A-type molecules number, the average distance between some A-type molecules decreases, resulting in the weakening of the attractive forces and the strengthening of the repulsive forces as shown in Figure 3b stage 2 ②. As a result, the number of AT-type molecules will decrease, which means the adsorption rate will slow downwhere is a decreasing function of .
3.4. Analysis of Intermolecular Forces Adsorption Model
4. Results and Discussion
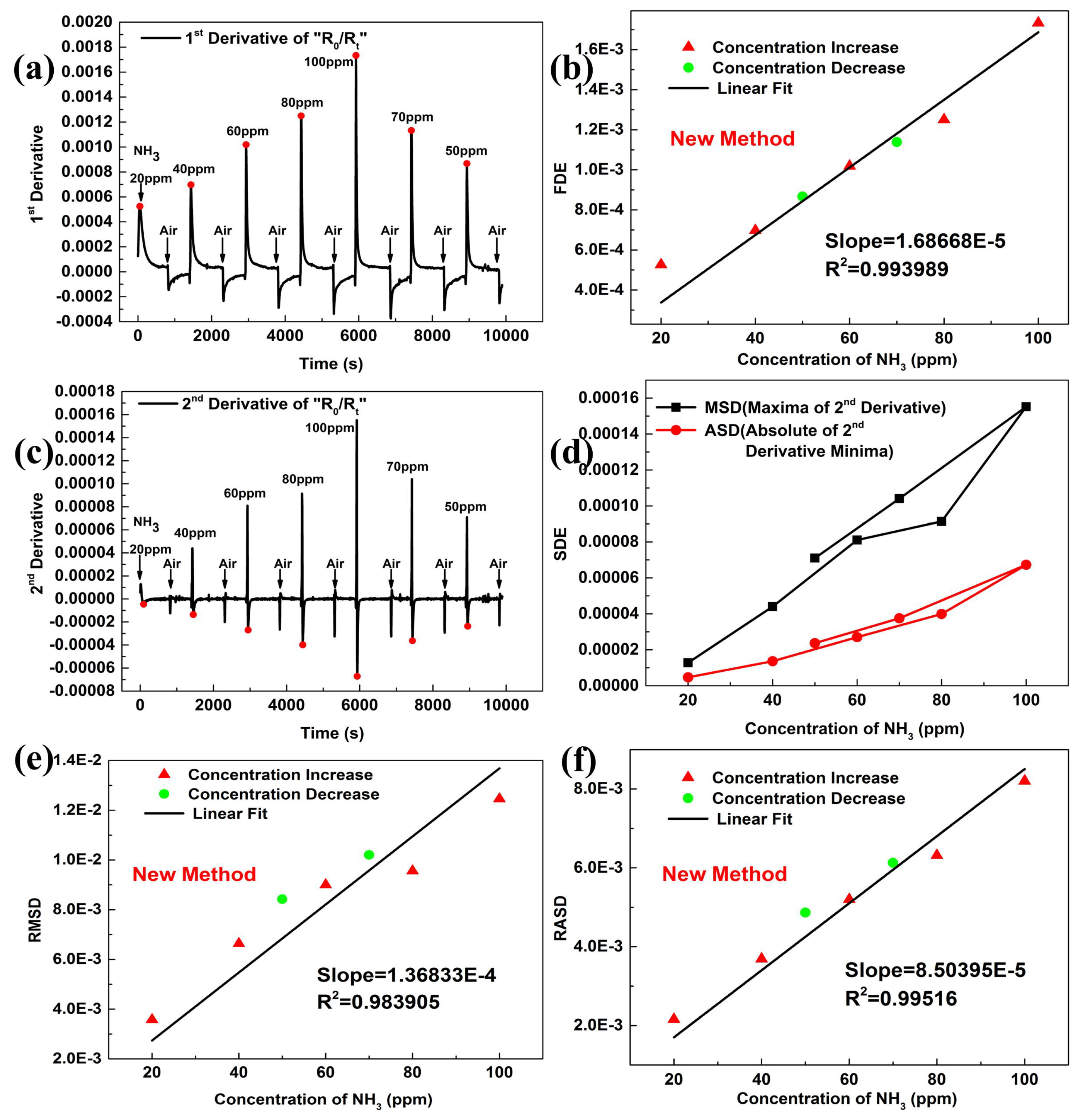
4.1. New Calibration Method of Gas Concentration
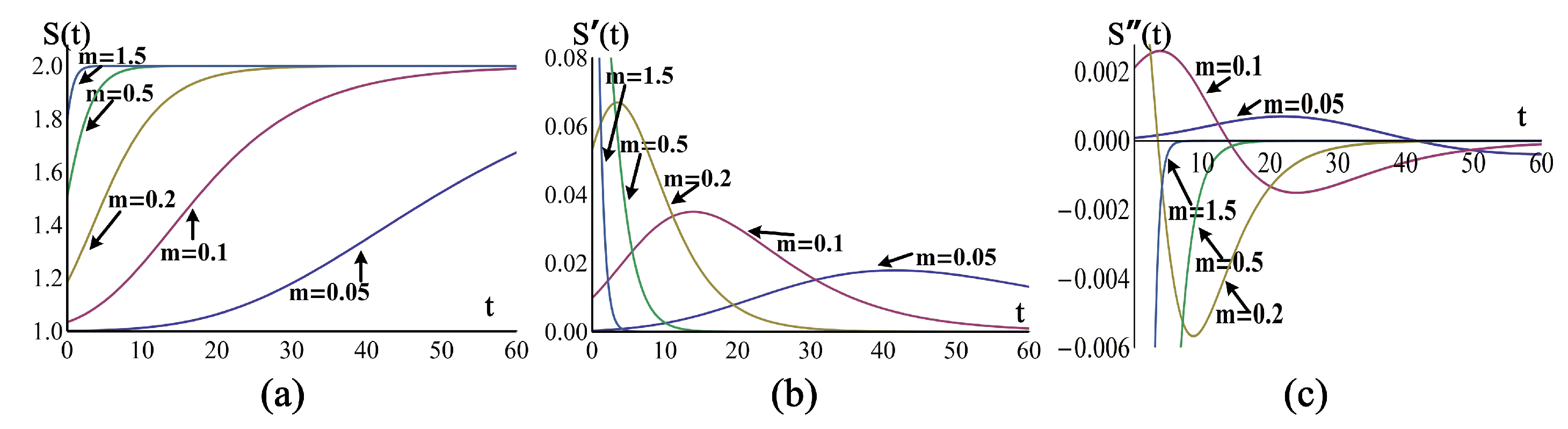
4.2. Influence of Parameter m
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Ratinac, K.R.; Yang, W.; Ringer, S.P.; Braet, F. Toward ubiquitous environmental gas sensors-capitalizing on the promise of graphene. Environ. Sci. Technol. 2010, 44, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Snow, A.W.; Perkins, F.K.; Ancona, M.G.; Robinson, J.T.; Snow, E.S.; Foos, E.E. Disordered nanomaterials for chemielectric vapor sensing: A review. IEEE Sens. J. 2015, 15, 1301–1320. [Google Scholar] [CrossRef]
- Toda, K.; Furue, R.; Hayami, S. Recent progress in applications of graphene oxide for gas sensing: A review. Anal. Chim. Acta 2015, 878, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, X.; Wang, Y.; Liu, G.; Zhu, X.; Huang, Y.; Guo, Y.; Gao, C.; Zhou, M. Study on gas sensing of reduced graphene oxide/ZnO thin film at room temperature. Sens. Actuators B Chem. 2017, 240, 870–880. [Google Scholar] [CrossRef]
- Lu, G.; Park, S.; Yu, K.; Ruoff, R.S.; Ocola, L.E.; Rosenmann, D.; Chen, J. Toward practical gas sensing with highly reduced graphene oxide: A new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano 2011, 5, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical chemical sensors from chemically derived graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, S.; Liu, G.; Potyrailo, R.A.; Balandin, A.A.; Shur, M.S. Selective Sensing of Individual Gases Using Graphene Devices. IEEE Sens. J. 2013, 13, 2818–2822. [Google Scholar] [CrossRef]
- Langmuir, I. The evaporation, condensation and reflection of molecules and the mechanism of adsorption. J. Frankl. Inst. 1916, 183, 101–102. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Aranovich, G.L.; Donohue, M.D. Adsorption compression: An important new aspect of adsorption behavior and capillarity. Langmuir 2003, 19, 2722–2735. [Google Scholar] [CrossRef]
- Hu, H.; Trejo, M.; Nicho, M.E.; Saniger, J.M.; García-Valenzuela, A. Adsorption kinetics of optochemical NH3 gas sensing with semiconductor polyaniline films. Sens. Actuators B Chem. 2002, 82, 14–23. [Google Scholar] [CrossRef]
- Tételin, A.; Pellet, C.; Laville, C.; N’Kaoua, G. Fast response humidity sensors for a medical microsystem. Sens. Actuators B Chem. 2003, 91, 211–218. [Google Scholar] [CrossRef]
- Liang, S.Z.; Chen, G.; Harutyunyan, A.R.; Cole, M.W.; Sofo, J.O. Analysis and optimization of carbon nanotubes and graphene sensors based on adsorption-desorption kinetics. Appl. Phys. Lett. 2013, 103, 233108. [Google Scholar] [CrossRef]
- Hasan, N.; Zhang, W.; Radadia, A.D. Few-flakes reduced graphene oxide sensors for organic vapors with a high signal-to-noise ratio. Nanomaterials 2017, 7, 339. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Ye, Q.; Zhang, S.L.; Wu, D. Assessing kinetics of surface adsorption–desorption of gas molecules via electrical measurements. Sens. Actuators B Chem. 2016, 223, 791–798. [Google Scholar] [CrossRef]
- Kisuuk, P. The sticking probabilities of gases chemisorbed on the surfaces of solids—II. J. Phys. Chem. Solids 1958, 5, 78–84. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Wu, Q. A highly sensitive room-temperature sensing material for NH3: SnO2-nanorods coupled by rGO. Sens. Actuators B Chem. 2017, 242, 1216–1226. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, C.; Han, T.; Zhang, Y.; Liu, S.; Fei, T.; Lu, G.; Zhang, T. High-performance reduced graphene oxide-based room-temperature NO2 sensors: A combined surface modification of SnO2 nanoparticles and nitrogen doping approach. Sens. Actuators B Chem. 2017, 242, 269–279. [Google Scholar] [CrossRef]
- Hu, N.; Yang, Z.; Wang, Y.; Zhang, L.; Wang, Y.; Huang, X.; Wei, H.; Wei, L.; Zhang, Y. Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology 2014, 25, 025502. [Google Scholar] [CrossRef] [PubMed]
- Andrea, C.; Alberto, F.; Luca, S.; Andrea, G.; Silvio, M. Recognizing physisorption and chemisorption in carbon nanotubes gas sensors by double exponential fitting of the response. Sensors 2016, 16, 731. [Google Scholar]
- Newton, M.I.; Starke, T.K.H.; Willis, M.R.; Mchale, G. NO2 detection at room temperature with copper phthalocyanine thin film devices. Sens. Actuators B Chem. 2000, 67, 307–311. [Google Scholar] [CrossRef]
- Mazein, P.; Zimmermann, C.; Rebière, D.; Déjous, C.; Pistré, J.; Planade, R. Dynamic analysis of Love waves sensors responses: Application to organophosphorus compounds in dry and wet air. Sens. Actuators B Chem. 2003, 95, 51–57. [Google Scholar] [CrossRef]
- Wu, C.H.; Jiang, G.J.; Chiu, C.C.; Chong, P.; Jeng, C.C.; Wu, R.J.; Chen, J.H. Fast gas concentration sensing by analyzing the rate of resistance change. Sens. Actuators B Chem. 2015, 209, 906–910. [Google Scholar] [CrossRef]
- Roussel, S.; Forsberg, G.; Steinmetz, V.; Grenier, P.; Bellon-Maurel, V. Optimisation of electronic nose measurements. Part I: Methodology of output feature selection. J. Food Eng. 1998, 37, 207–222. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, C.; Zeng, D.; Zhang, Q.; Li, H.; Bi, Z. A feature extraction method and a sampling system for fast recognition of flammable liquids with a portable E-nose. Sens. Actuators B Chem. 2007, 124, 437–443. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Xie, G.; Su, Y.; Tai, H.; Du, X.; Yu, H.; Zhang, Q. A New Model and Its Application for the Dynamic Response of RGO Resistive Gas Sensor. Sensors 2019, 19, 889. https://doi.org/10.3390/s19040889
Du H, Xie G, Su Y, Tai H, Du X, Yu H, Zhang Q. A New Model and Its Application for the Dynamic Response of RGO Resistive Gas Sensor. Sensors. 2019; 19(4):889. https://doi.org/10.3390/s19040889
Chicago/Turabian StyleDu, Hongfei, Guangzhong Xie, Yuanjie Su, Huiling Tai, Xiaosong Du, He Yu, and Qiuping Zhang. 2019. "A New Model and Its Application for the Dynamic Response of RGO Resistive Gas Sensor" Sensors 19, no. 4: 889. https://doi.org/10.3390/s19040889
APA StyleDu, H., Xie, G., Su, Y., Tai, H., Du, X., Yu, H., & Zhang, Q. (2019). A New Model and Its Application for the Dynamic Response of RGO Resistive Gas Sensor. Sensors, 19(4), 889. https://doi.org/10.3390/s19040889





