Correlation and Similarity between Cerebral and Non-Cerebral Electrical Activity for User’s States Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
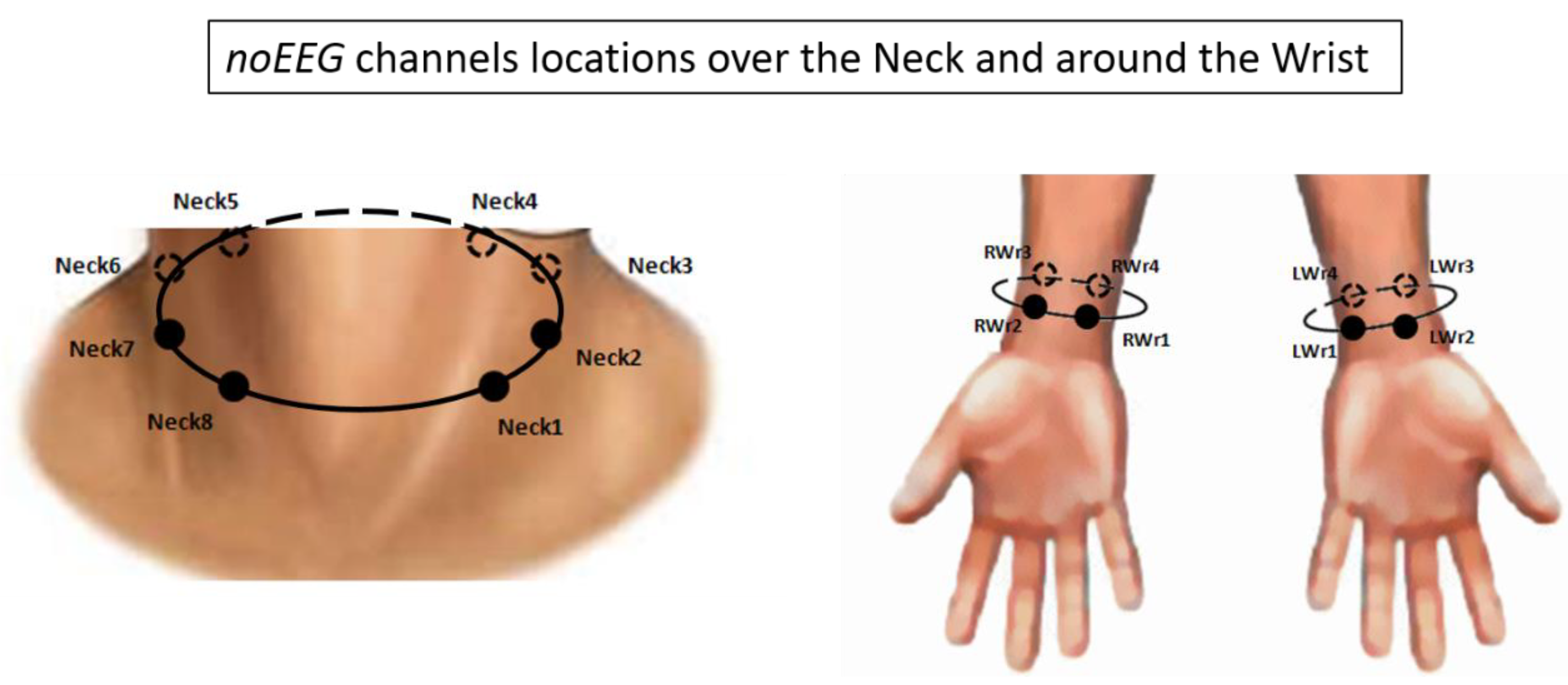
2.3. Brain and Body Electrical Activity Recording and Analysis
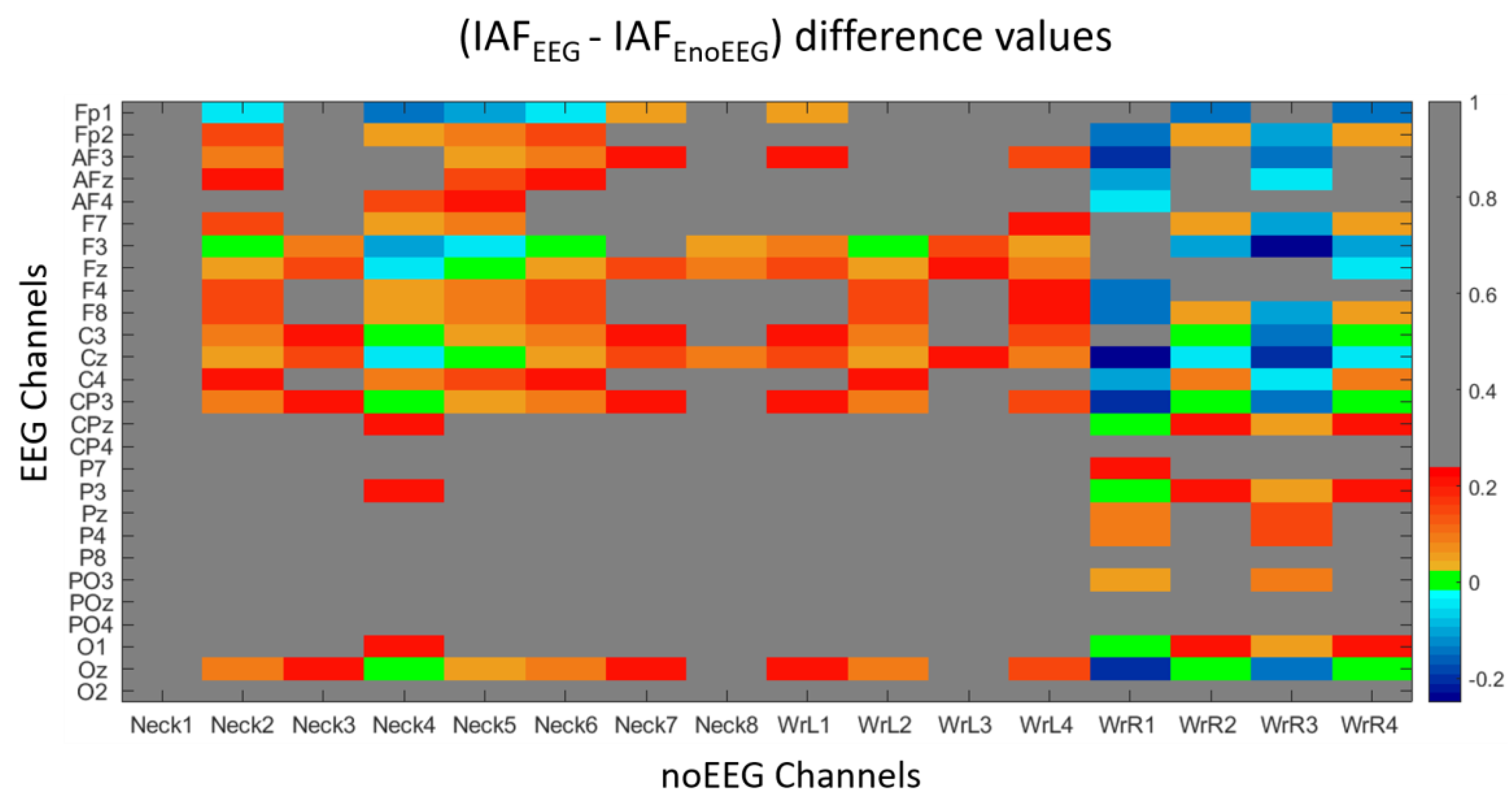
2.4. Correlation Analysis and IAF Comparison
2.5. OA versus OC Discrimination Analysis
3. Results
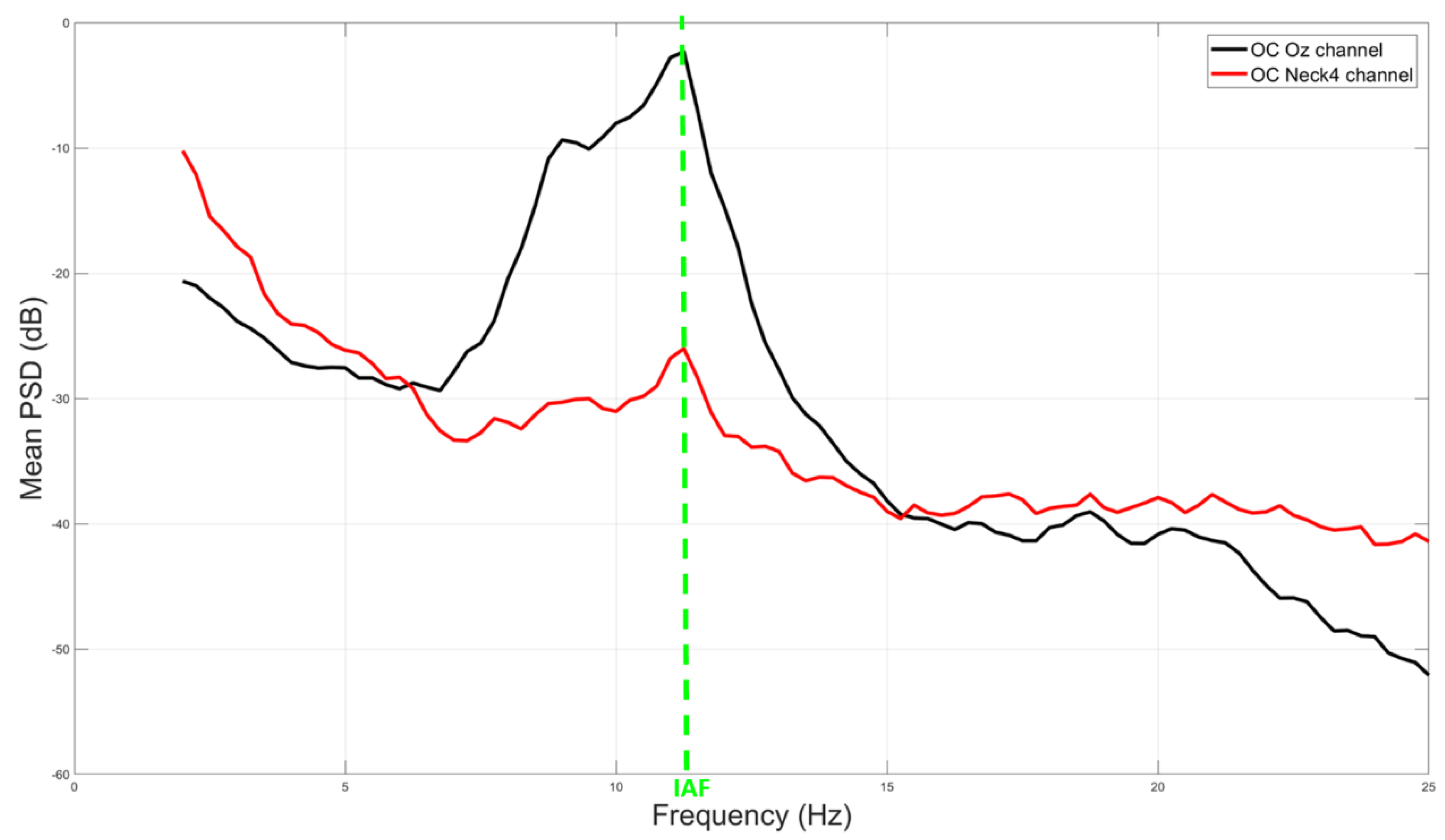
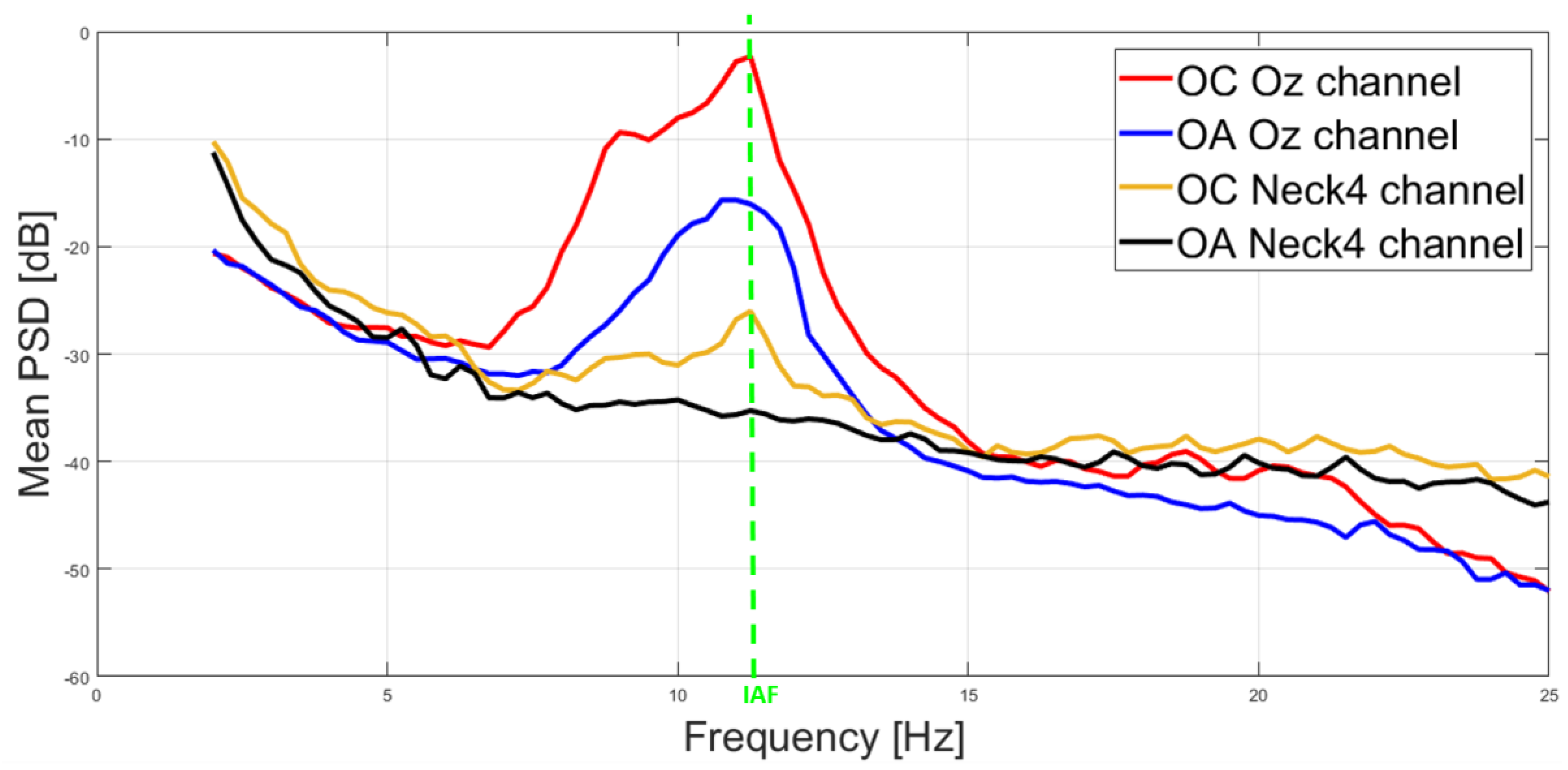
3.1. Correlation and IAF Estimation
3.2. OA and OC Discrimination
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M. Essentials of Neural Science and Behavior; Appleton & Lange: Norwalk, CT, USA, 1995. [Google Scholar]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology; Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Willis, M. Dielectric and Electronic Properties of Biological Materials. Biochem. Educ. 1980, 8, 31. [Google Scholar] [CrossRef]
- Cooper, R. The electrical properties of salt-water solutions over the frequency range 1–4000 Mc/s. J. Inst. Electr. Eng. Part I Gen. 1946, 93, 358. [Google Scholar]
- Borghini, G.; Astolfi, L.; Vecchiato, G.; Mattia, D.; Babiloni, F. Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neurosci. Biobehav. Rev. 2014, 44, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.C. The Brain’s Alpha Rhythms and the Mind: A Review of Classical and Modern Studies of the Alpha Rhythm Component of the Electroencephalogram with Commentaries on Associated Neuroscience and Neuropsychology; Elsevier: Boston, MA, USA, 2003. [Google Scholar]
- Borghini, G.; Aricò, P.; Graziani, I.; Sun, Y.; Taya, F.; Bezerianos, A.; Thakor, N.V.; Babiloni, F. Quantitative Assessment of the Training Improvement in a Motor-Cognitive Task by Using EEG, ECG and EOG Signals. Brain Topogr. 2015, 29, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Borghini, G.; Aricò, P.; Di Flumeri, G.; Cartocci, G.; Colosimo, A.; Bonelli, S.; Golfetti, A.; Imbert, J.P.; Granger, G.; Benhacene, R.; et al. EEG-Based Cognitive Control Behaviour Assessment: An Ecological Study with Professional Air Traffic Controllers. Sci. Rep. 2017, 7, 547. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, M. Executive control over unconscious cognition: Attentional sensitization of unconscious information processing. Front. Hum. Neurosci. 2012, 6, 61. [Google Scholar] [CrossRef]
- Kuldas, S.; Abu Bakar, Z.; Nizam Ismail, H. The role of unconscious information processing in the acquisition and learning of instructional messages. Electron. J. Res. Educ. Psychol. 2012, 10, 907–940. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef]
- Henning, S.; Merboldt, K.-D.; Frahm, J. Task- and EEG-correlated analyses of BOLD MRI responses to eyes opening and closing. Brain Res. 2006, 1073–1074, 359–364. [Google Scholar] [CrossRef]
- Fumoto, M.; Sato-Suzuki, I.; Seki, Y.; Mohri, Y.; Arita, H. Appearance of high-frequency alpha band with disappearance of low-frequency alpha band in EEG is produced during voluntary abdominal breathing in an eyes-closed condition. Neurosci. Res. 2004, 50, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Roesler, O.; Bader, L.; Forster, J.; Hayashi, Y.; Heßler, S.; Suendermann-Oeft, D. Comparison of EEG Devices for Eye State Classification. In Proceedings of the International Conference on Applied Informatics for Health and Life Sciences (AIHLS), Kusadasi, Turkey, 19–22 October 2014. [Google Scholar]
- Arico, P.; Borghini, G.; Di Flumeri, G.; Sciaraffa, N.; Colosimo, A.; Babiloni, F. Passive BCI in Operational Environments: Insights, Recent Advances and Future trends. IEEE Trans. Biomed. Eng. 2017, 64, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Toppi, J.; Borghini, G.; Astolfi, L.; Petti, M.; He, E.; De Giusti, V.; He, B.; Babiloni, F. Investigating Cooperative Behavior in Ecological Settings: An EEG Hyperscanning Study. PLoS ONE 2016, 11, e0154236. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, G.; Maglione, A.G.; Vecchiato, G.; Di Flumeri, G.; Colosimo, A.; Scorpecci, A.; Marsella, P.; Giannantonio, S.; Malerba, P.; Borghini, G.; et al. Mental workload estimations in unilateral deafened children. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milano, Italy, 25–29 August 2015; pp. 1654–1657. [Google Scholar]
- Vecchiato, G.; Borghini, G.; Aricò, P.; Graziani, I.; Maglione, A.G.; Cherubino, P.; Babiloni, F. Investigation of the effect of EEG-BCI on the simultaneous execution of flight simulation and attentional tasks. Med. Biol. Eng. Comput. 2016, 54, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Borghini, G.; Aricò, P.; Di Flumeri, G.; Colosimo, A.; Herrero Ezquerro, M.T.; Salinari, S.; Bezerianos, A.; Thakor, N.V.; Babiloni, F. A New Perspective for the Training Assessment: Machine Learning-Based Neurometric for Augmented User’s Evaluation. Front. Neurosci. 2017, 11, 325. [Google Scholar] [CrossRef]
- Di Flumeri, G.; Aricò, P.; Borghini, G.; Sciaraffa, S.; Maglione, A.G.; Rossi, D.; Modica, E.; Trettel, A.; Babiloni, F.A.; et al. Colosimo EEG-based Approach-Withdrawal index for the pleasantness evaluation during taste experience in realistic settings. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Jeju Island, Korea, 11–15 July 2017. [Google Scholar]
- Di Flumeri, G.; Aricò, P.; Borghini, G.; Colosimo, A.; Babiloni, F. A new regression-based method for the eye blinks artifacts correction in the EEG signal, without using any EOG channel. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Gratton, G.; Coles, M.G.; Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Tcheslavski, G.V. Techniques to Assess Stationarity and Gaussianity of EEG: An Overview. Int. J. Bioautomotion 2012, 16, 135–142. [Google Scholar]
- Blanco, S.; Garcia, H.; Quiroga, R.Q.; Romanelli, L.; Rosso, O.A. Stationarity of the EEG series. IEEE Eng. Med. Biol. Mag. 1995, 14, 395–399. [Google Scholar] [CrossRef]
- Klonowski, W. Everything you wanted to ask about EEG but were afraid to get the right answer. Nonlinear Biomed. Phys. 2009, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Feshchenko, V.A. The way to EEG-classification: transition from language of patterns to language of systems. Int. J. Neurosci. 1994, 79, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Draper, N.R. Applied regression analysis. Commun. Stat. Theory Methods 1998, 27, 2581–2623. [Google Scholar] [CrossRef]
- Bamber, D. The area above the ordinal dominance graph and the area below the receiver operating characteristic graph. J. Math. Psychol. 1975, 12, 387–415. [Google Scholar] [CrossRef]
- Kübler, A.; Birbaumer, N. Brain-computer interfaces and communication in paralysis: extinction of goal directed thinking in completely paralysed patients? Clin. Neurophysiol. 2008, 119, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. An Introduction to ROC Analysis. Pattern Recogn. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]

| EEG Band | f1 | f2 |
|---|---|---|
| Delta | 0 | IAF − 6 |
| Theta | IAF − 6 | IAF − 2 |
| Alpha | IAF − 2 | IAF + 2 |
| Beta | IAF + 2 | IAF + 16 |
| Gamma | IAF + 16 | IAF + 25 |
| Channel Type | Delta | Theta | Alpha | Beta |
|---|---|---|---|---|
| EEG | 72 | 144 | 144 | 360 |
| noEEG | 64 | 128 | 128 | 320 |
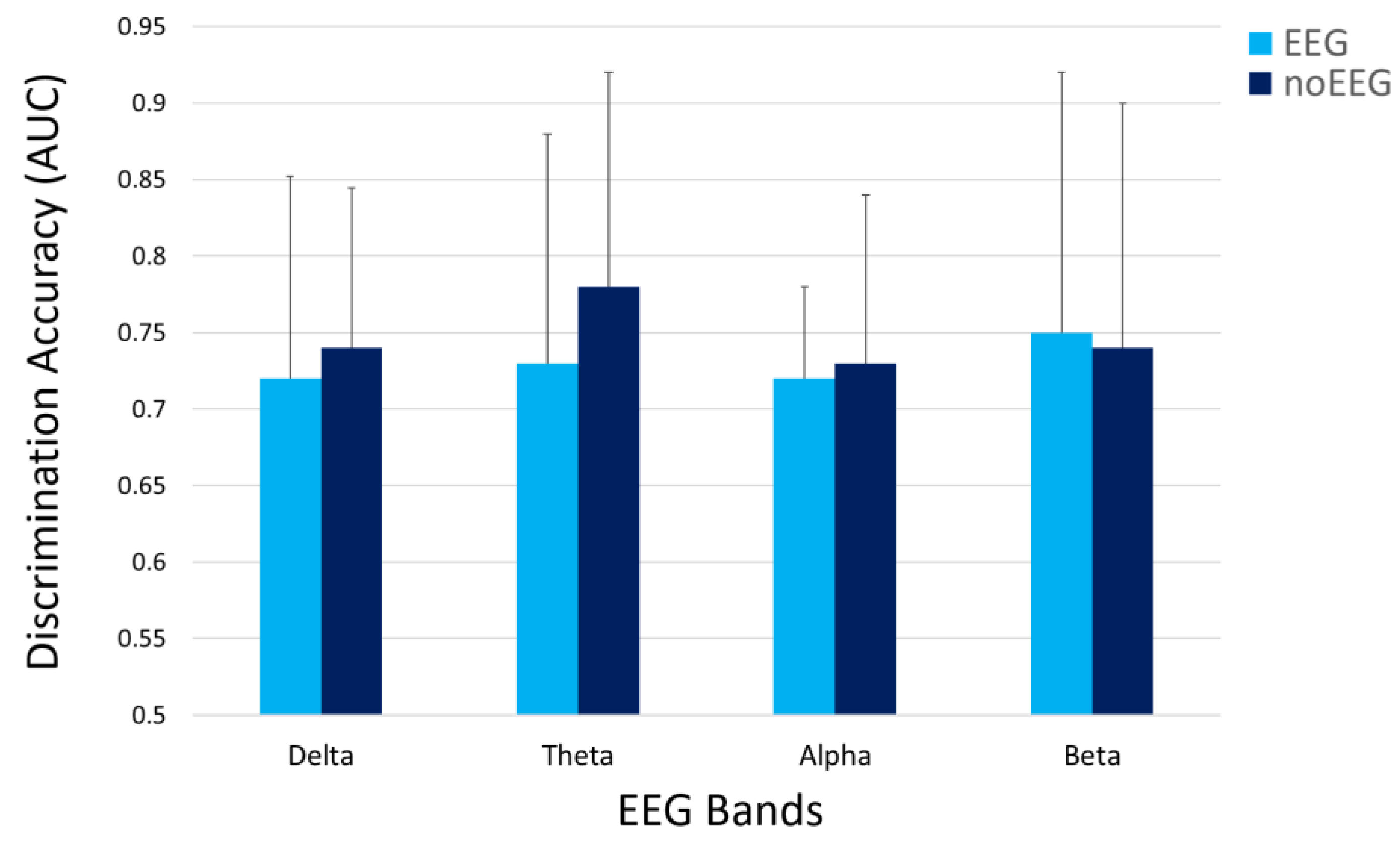
| Features Type | Delta (p = 0.72) | Theta (p = 0.47) | Alpha (p = 0.88) | Beta (p = 0.93) |
|---|---|---|---|---|
| EEG | 0.72 | 0.73 | 0.72 | 0.75 |
| noEEG | 0.74 | 0.78 | 0.73 | 0.74 |
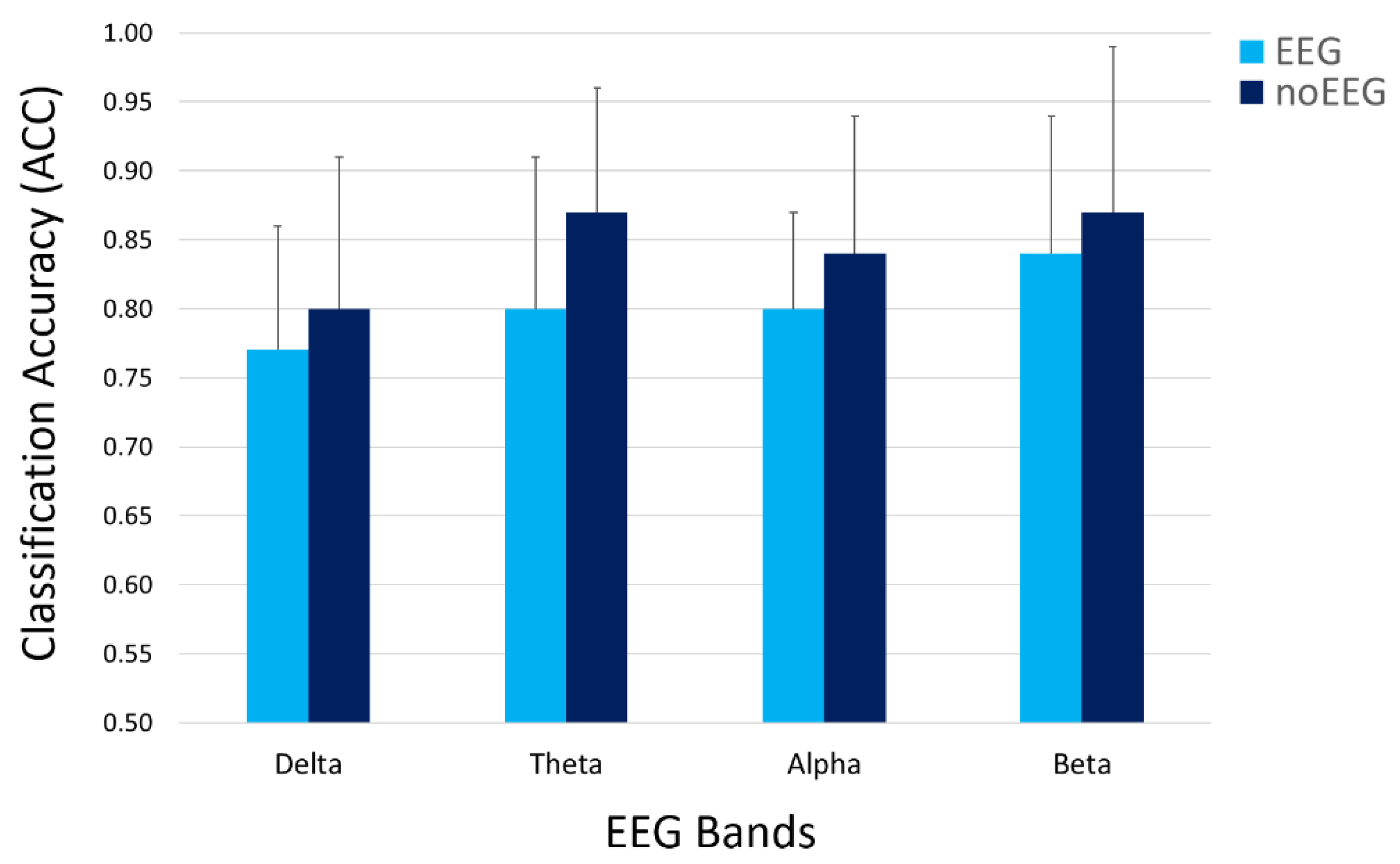
| Features Type | Delta (p = 0.53) | Theta (p = 0.16) | Alpha (p = 0.42) | Beta (p = 0.55) |
|---|---|---|---|---|
| EEG | 0.77 | 0.80 | 0.80 | 0.84 |
| noEEG | 0.80 | 0.87 | 0.84 | 0.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghini, G.; Aricò, P.; Di Flumeri, G.; Sciaraffa, N.; Babiloni, F. Correlation and Similarity between Cerebral and Non-Cerebral Electrical Activity for User’s States Assessment. Sensors 2019, 19, 704. https://doi.org/10.3390/s19030704
Borghini G, Aricò P, Di Flumeri G, Sciaraffa N, Babiloni F. Correlation and Similarity between Cerebral and Non-Cerebral Electrical Activity for User’s States Assessment. Sensors. 2019; 19(3):704. https://doi.org/10.3390/s19030704
Chicago/Turabian StyleBorghini, Gianluca, Pietro Aricò, Gianluca Di Flumeri, Nicolina Sciaraffa, and Fabio Babiloni. 2019. "Correlation and Similarity between Cerebral and Non-Cerebral Electrical Activity for User’s States Assessment" Sensors 19, no. 3: 704. https://doi.org/10.3390/s19030704
APA StyleBorghini, G., Aricò, P., Di Flumeri, G., Sciaraffa, N., & Babiloni, F. (2019). Correlation and Similarity between Cerebral and Non-Cerebral Electrical Activity for User’s States Assessment. Sensors, 19(3), 704. https://doi.org/10.3390/s19030704









