Monolithic Microwave-Microfluidic Sensors Made with Low Temperature Co-Fired Ceramic (LTCC) Technology
Abstract
1. Introduction
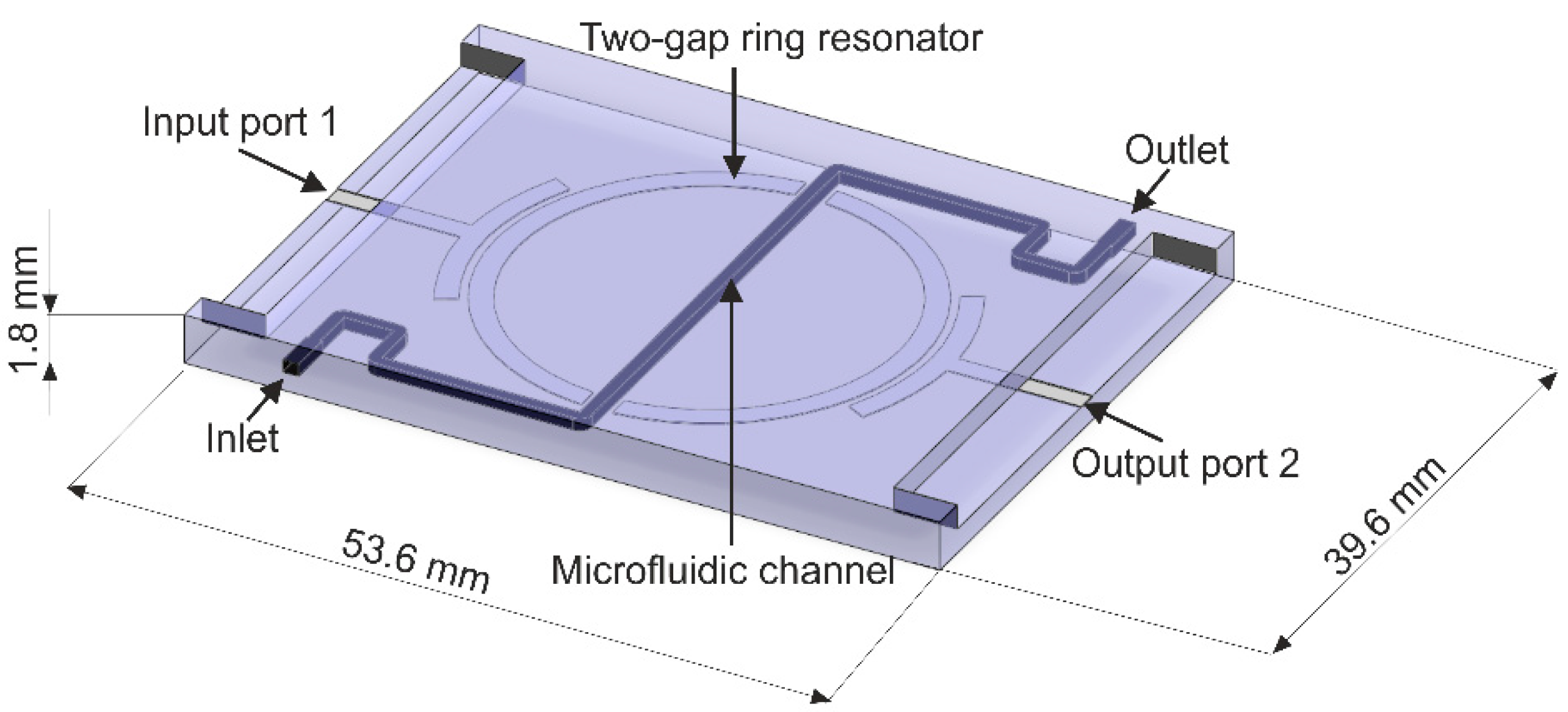
2. Sensor Construction and Principle of Operation
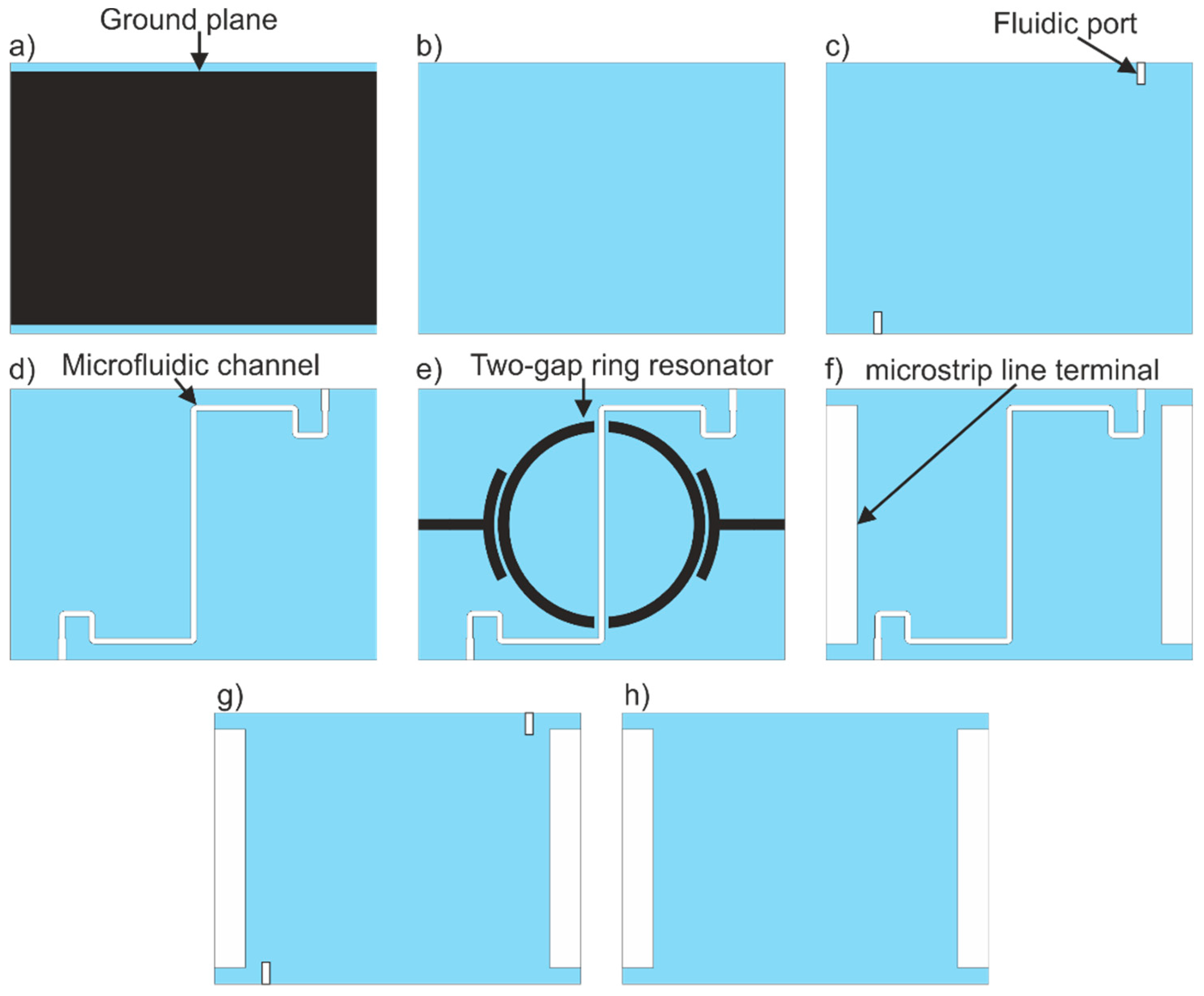
2.1. Resonator-Based Sensor
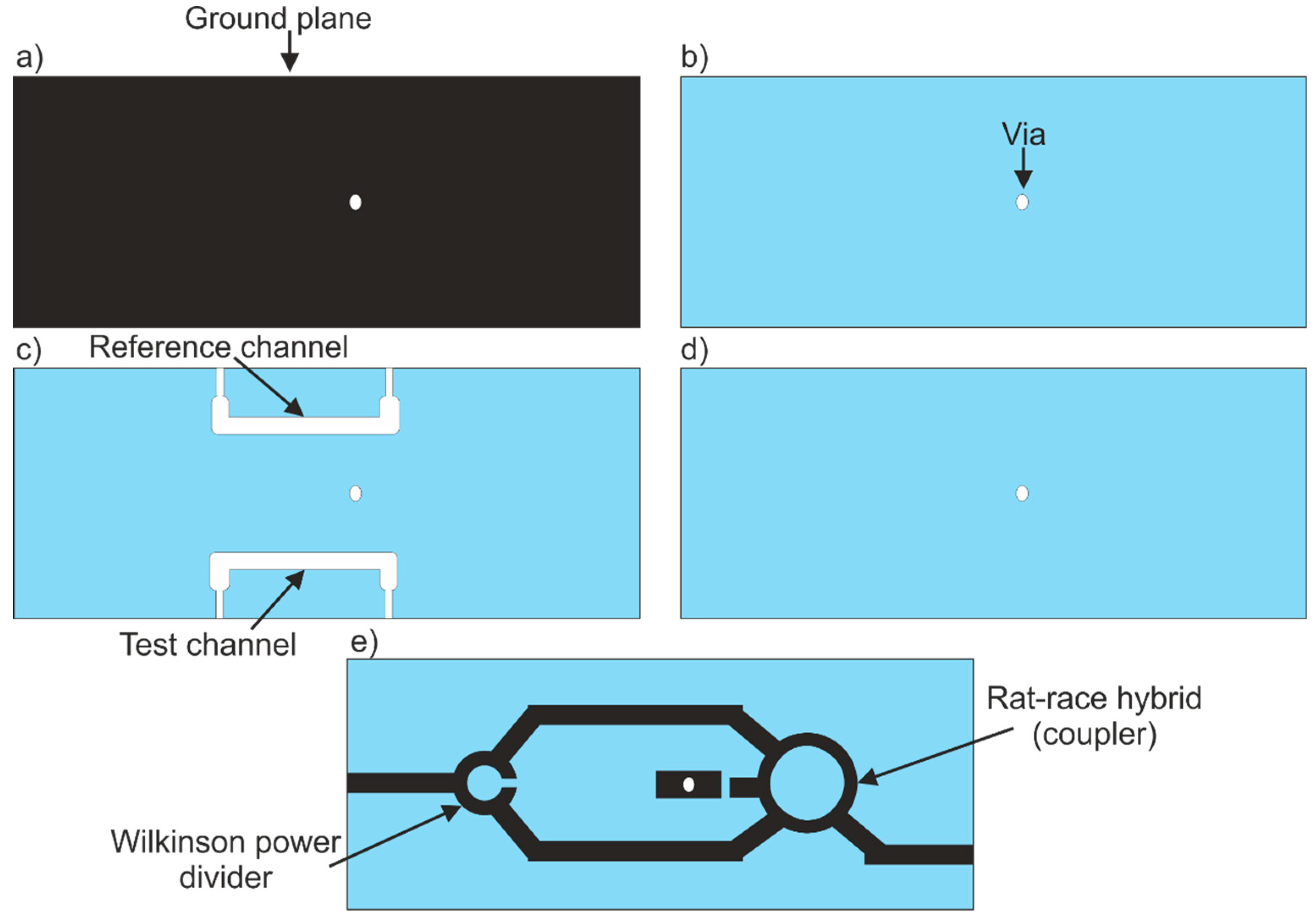
2.2. Coupler-Based Sensor
3. Technology
3.1. Resonator-Based Sensor
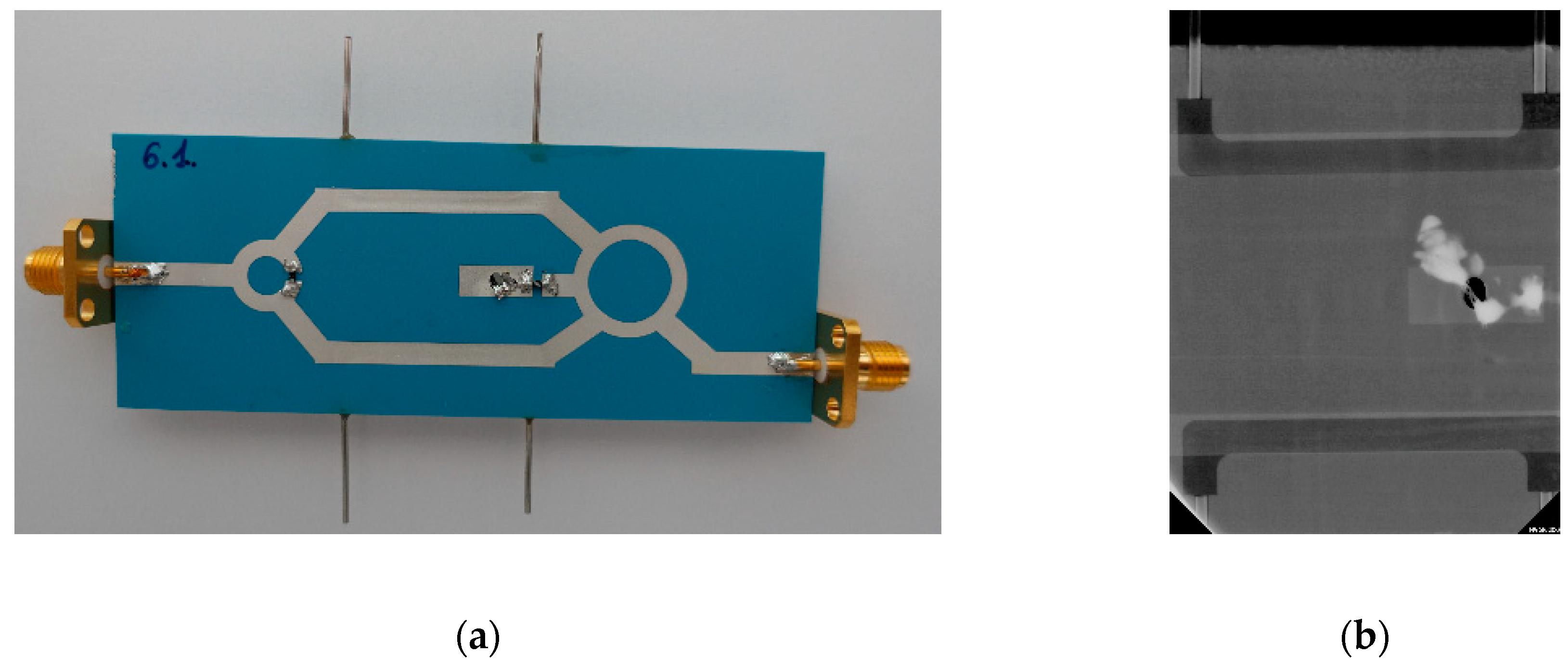
3.2. Coupler-Based Sensor
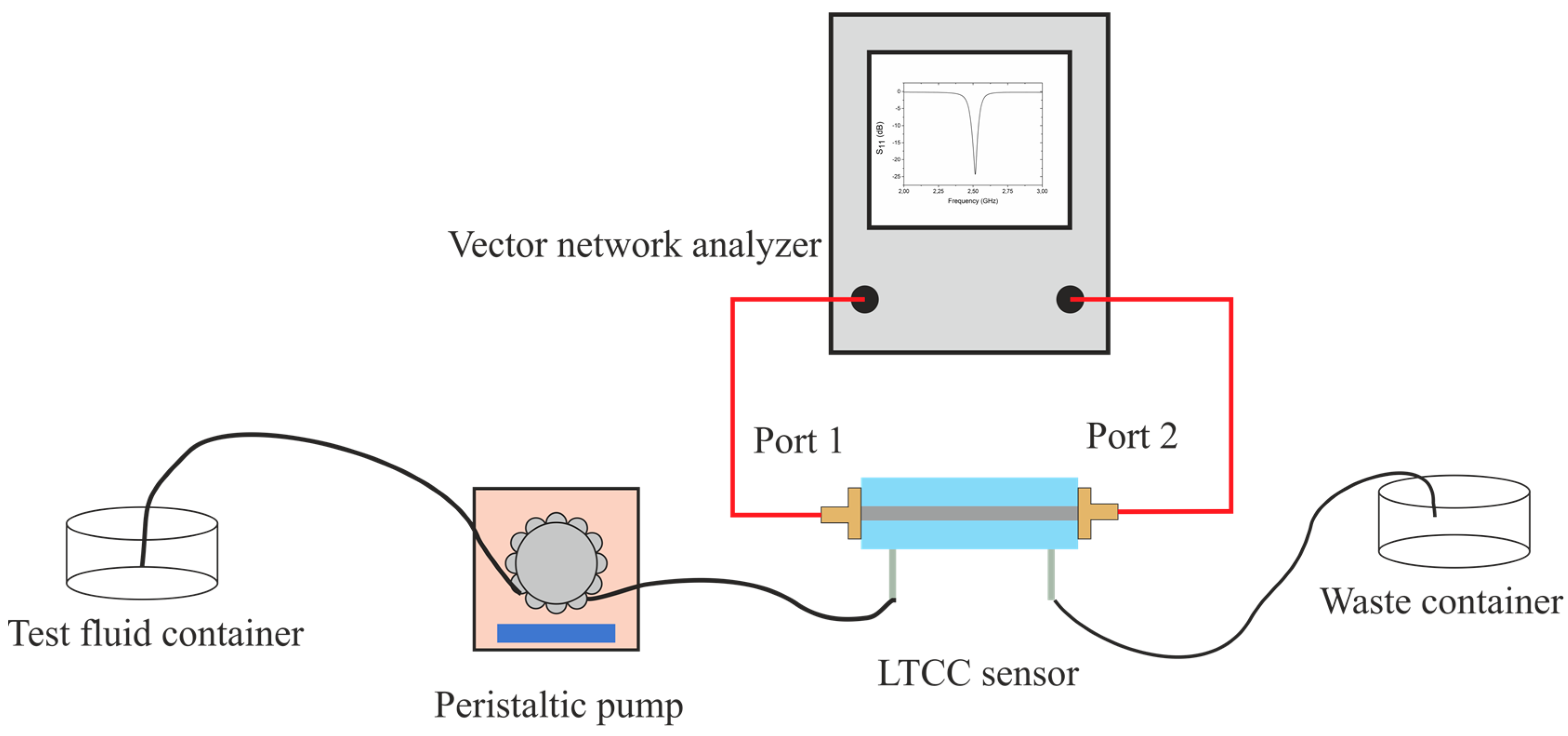
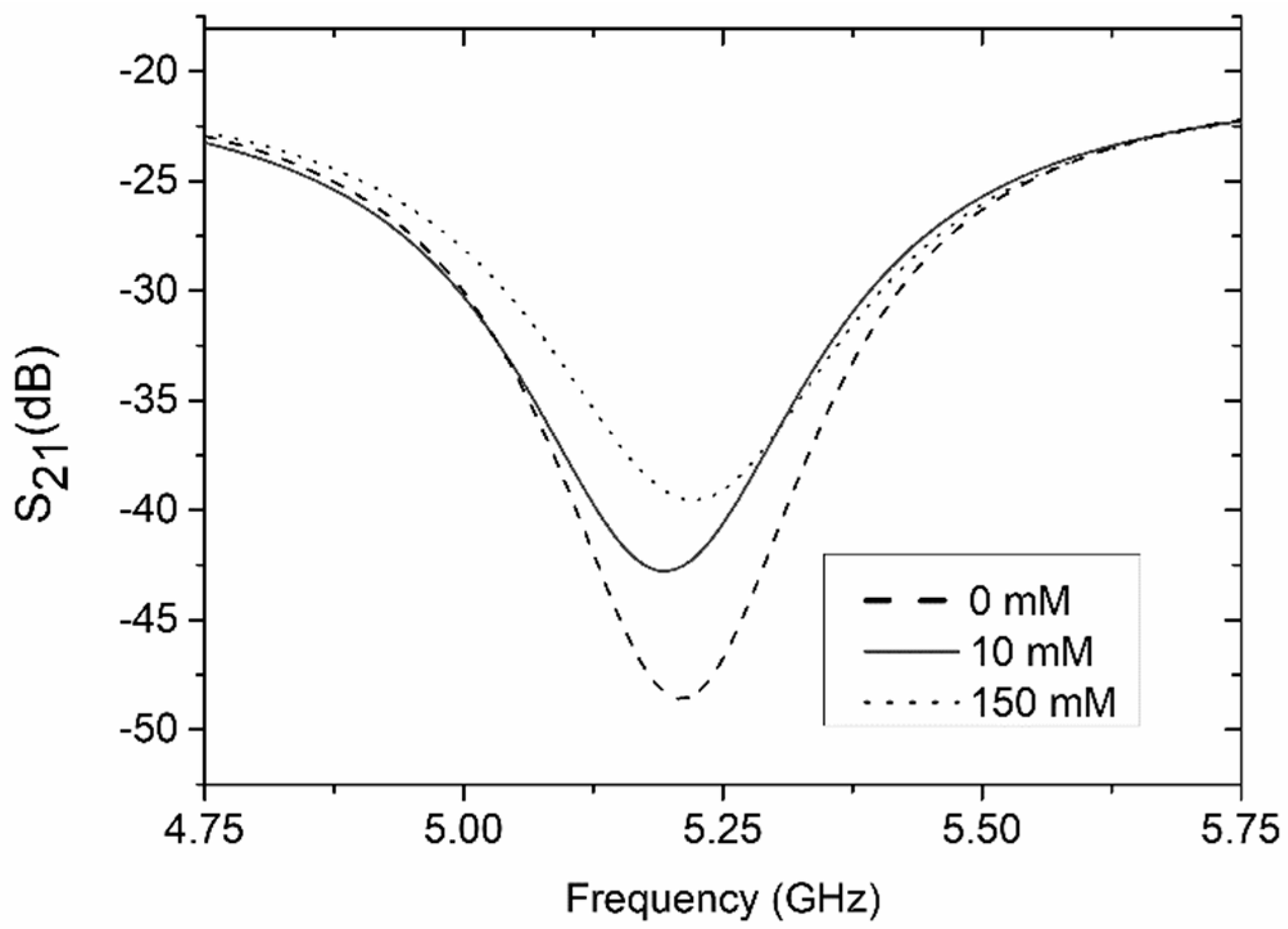
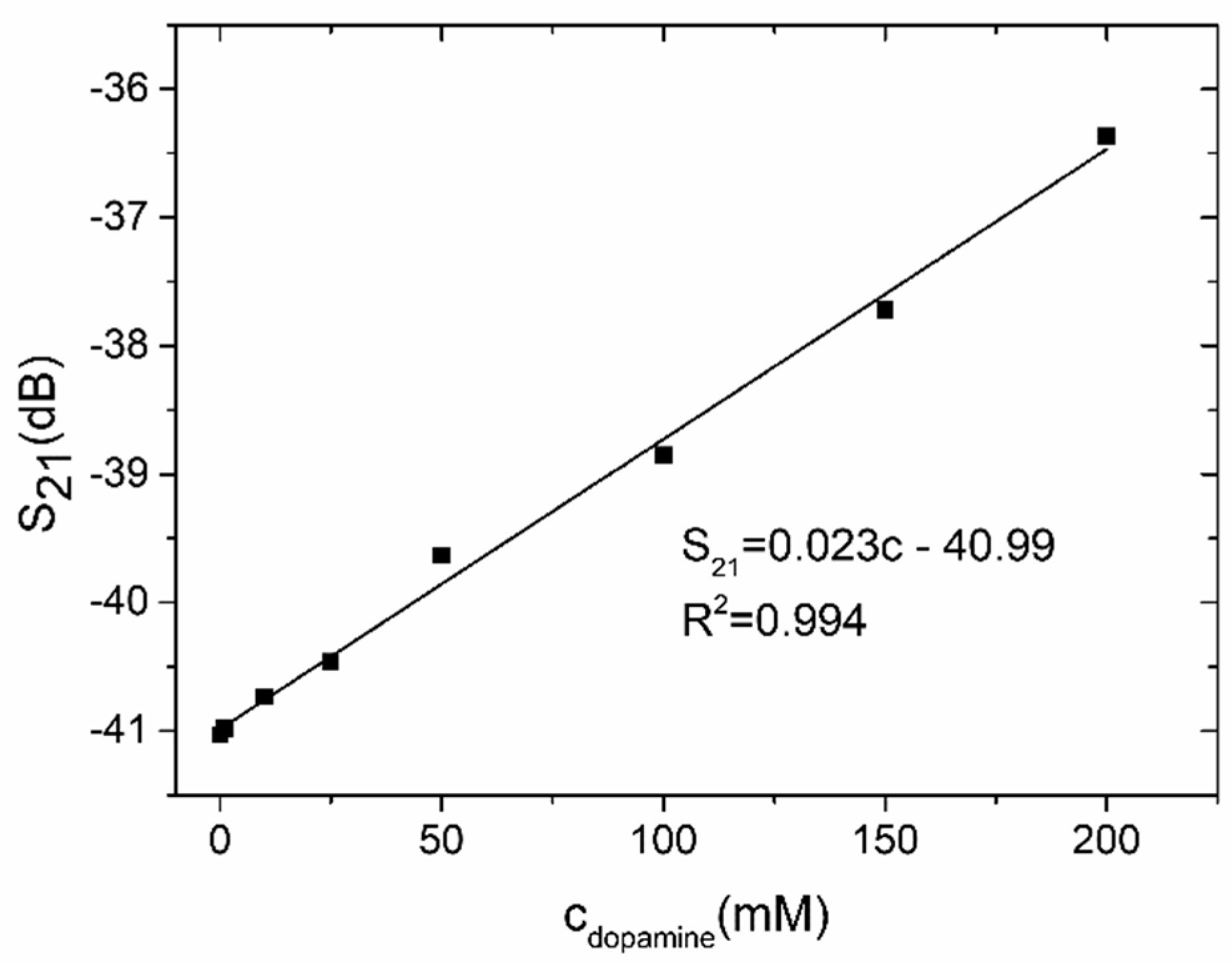
4. Experimental Verification and Discussion
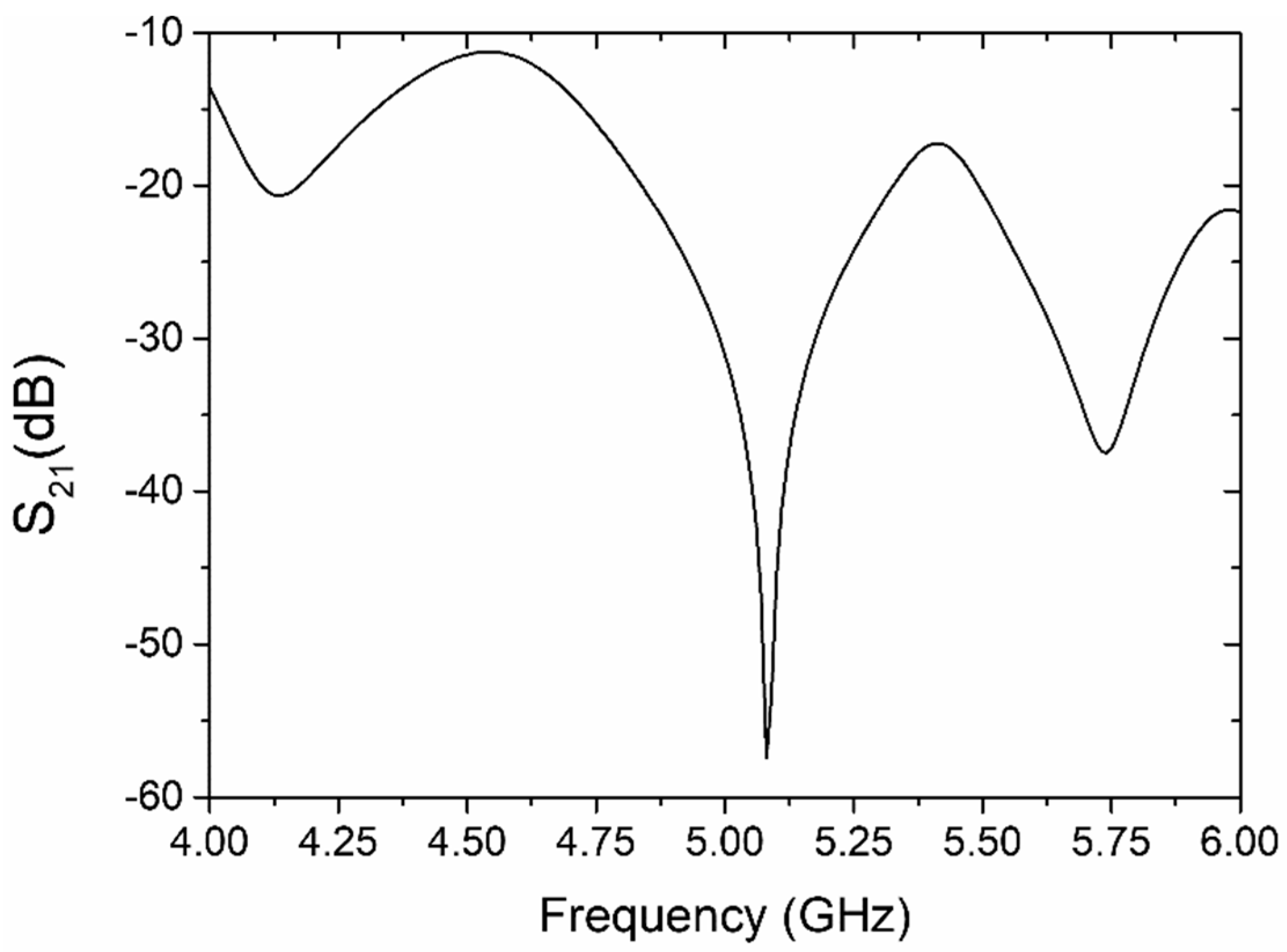
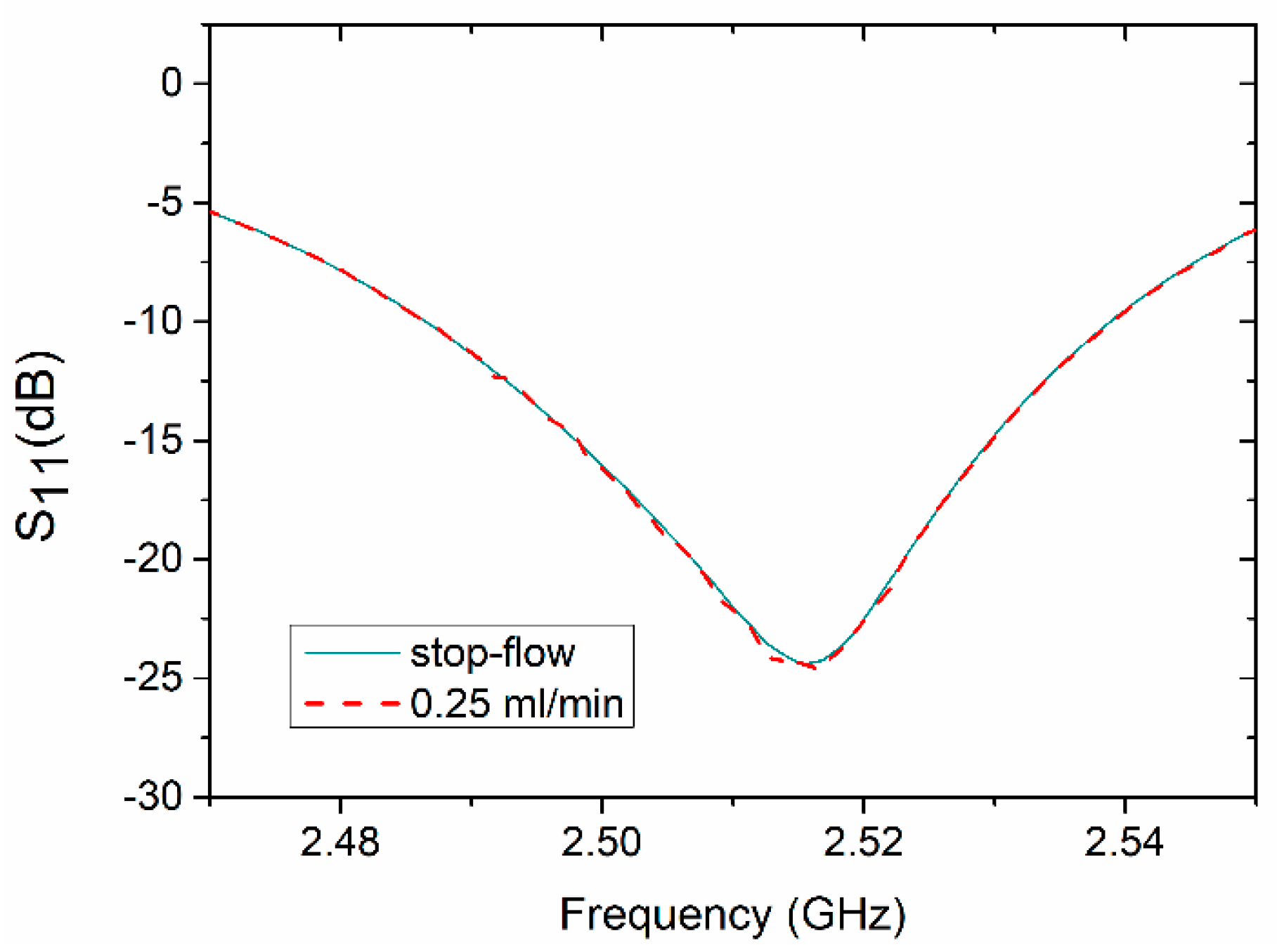
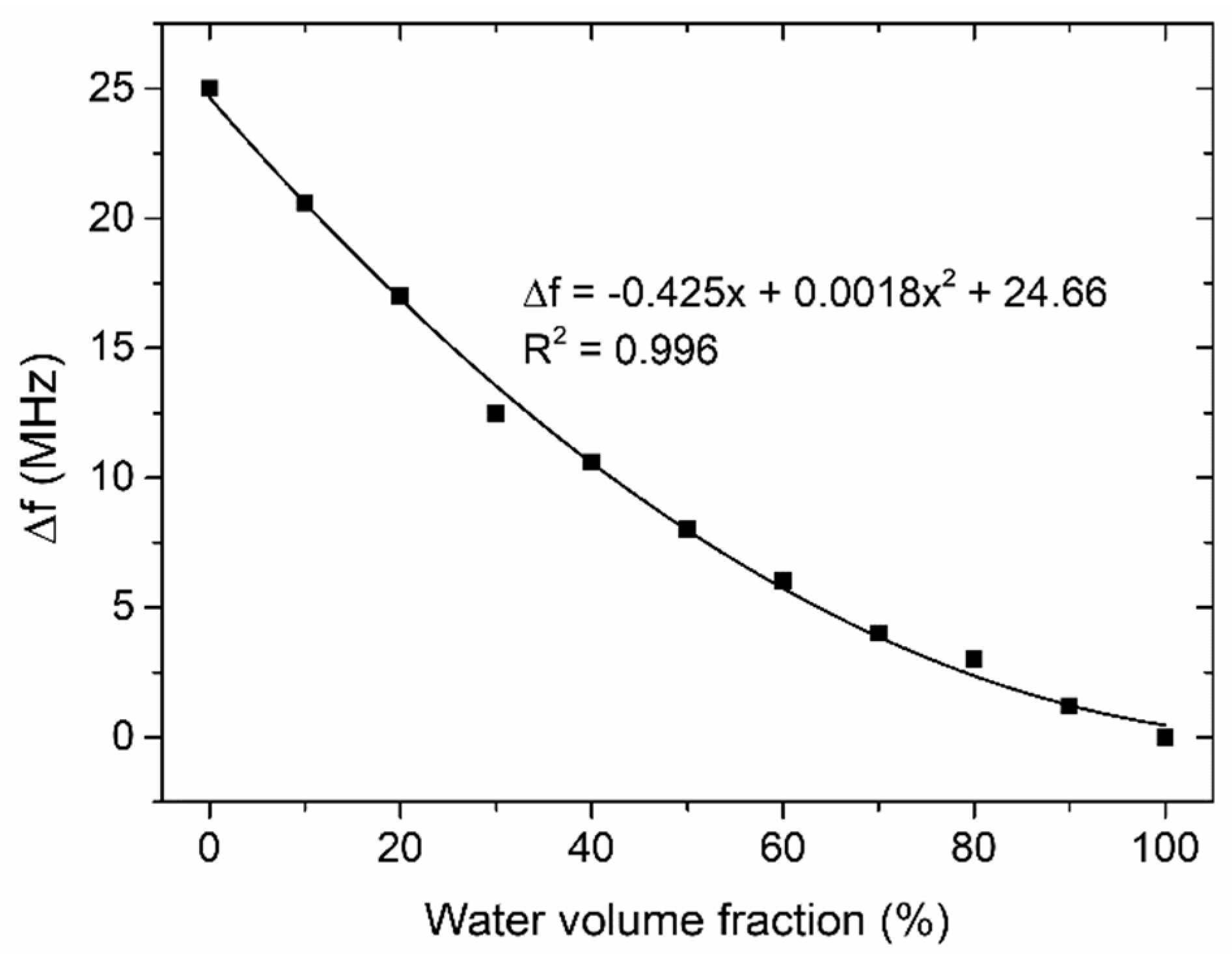
4.1. Resonator-Based Sensor
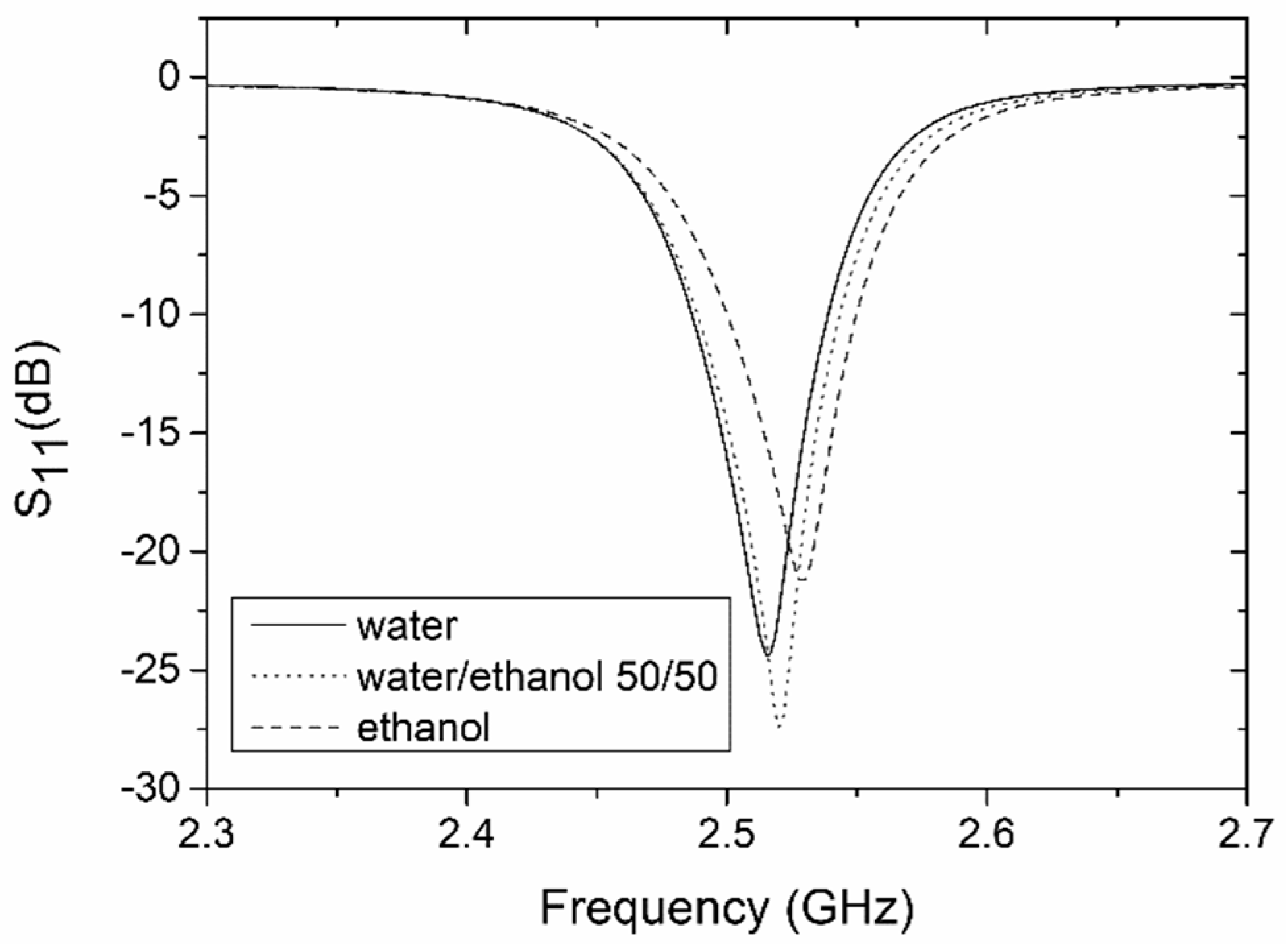
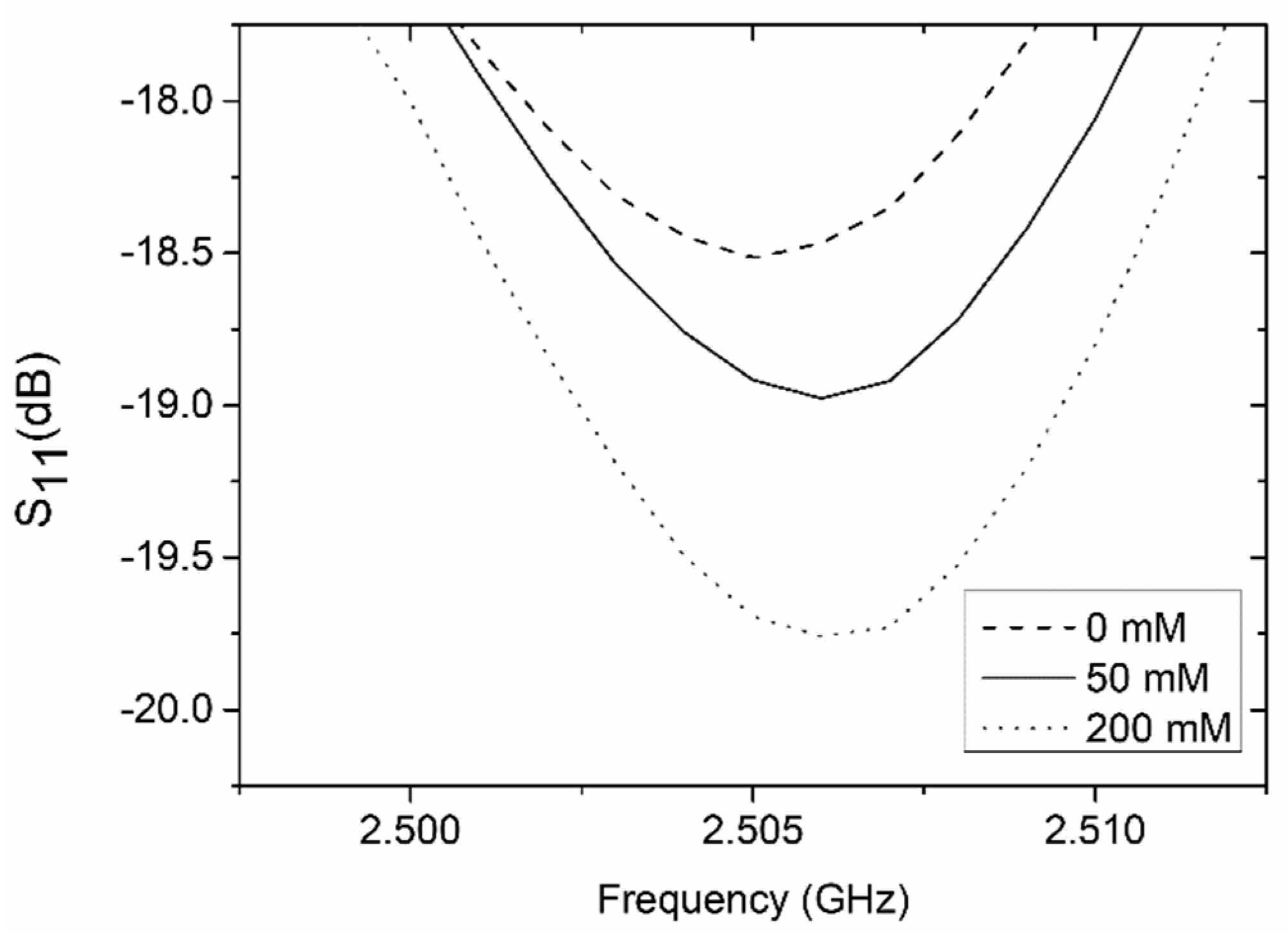
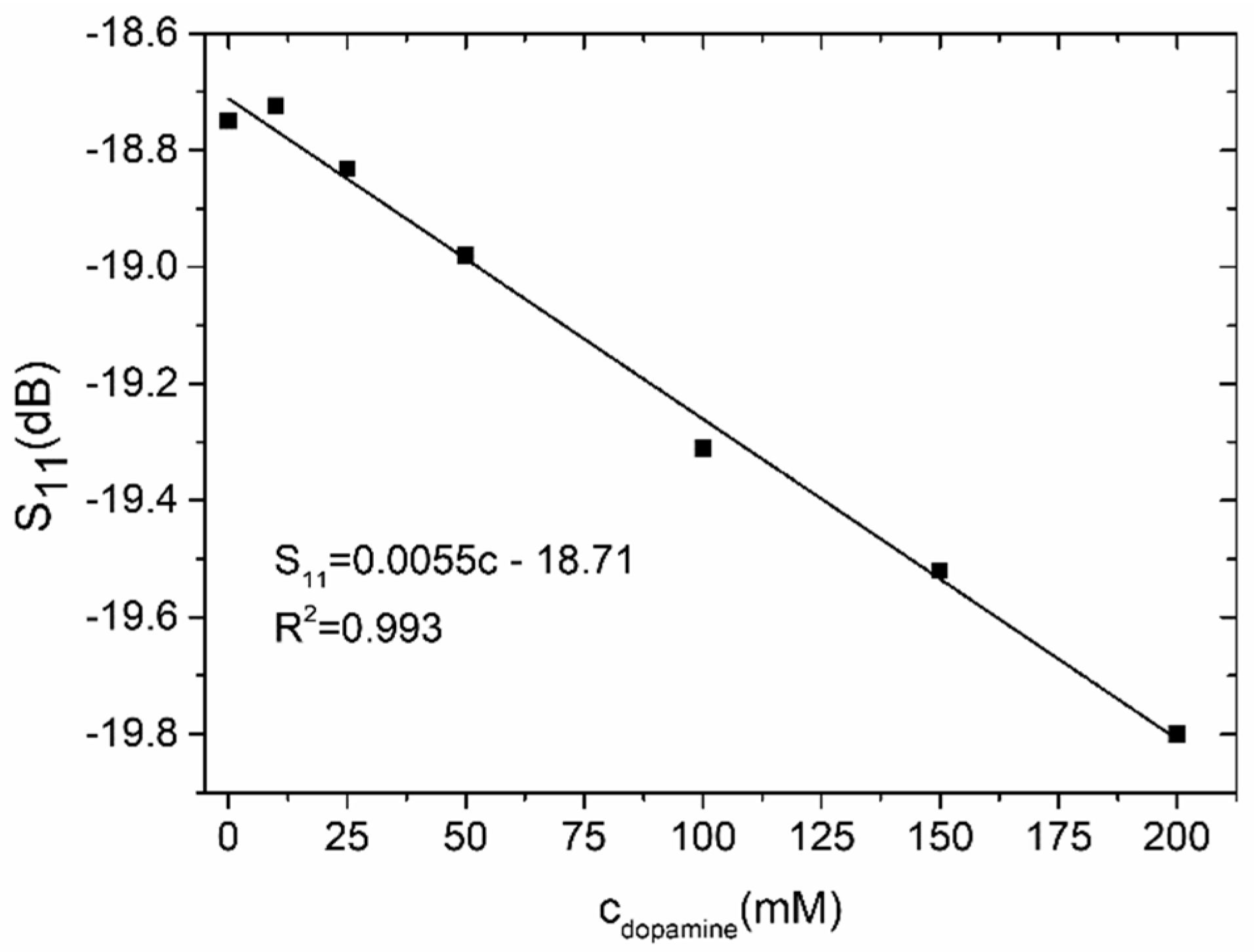
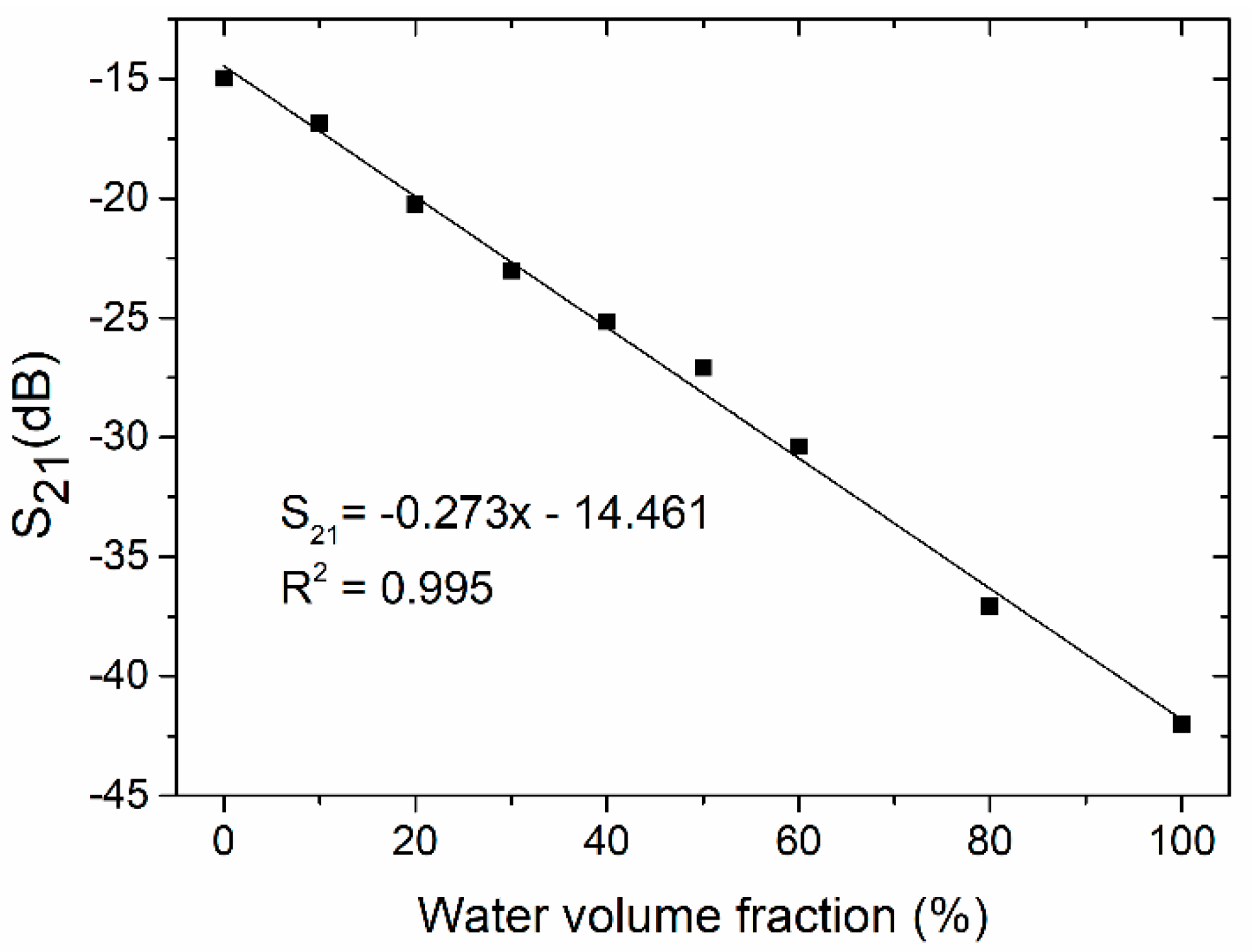
4.2. Coupler-Based Sensor
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuswandi, B.; Nuriman; Huskens, J.; Verboom, W. Optical sensing for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, W.; Drzozga, K.; Baluta, S.; Cabaj, J.; Malecha, K. A fluorescent biosensors for detection vital body fluids’ agents. Sensors 2018, 18, 2357. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidic integrated biosensors: A leading technology towards lab-on-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Ishii, R.; Itoh, T.; Hamakawa, S.; Tsunoda, T.; Hanaoka, T.; Mizukami, F. Immobilization of enzyme-encapsulated nanoporous material in a microreactor and reaction analysis. Chem. Eng. J. 2011, 167, 744–749. [Google Scholar] [CrossRef]
- Pijanowska, D.G.; Remiszewska, E.; Pederzolli, C.; Lunelli, L.; Vendano, V.; Canteri, R.; Dudziński, K.; Kruk, J.; Torbicz, W. Surface modification for microreactor fabrication. Sensors 2006, 6, 370–379. [Google Scholar] [CrossRef]
- Malecha, K.; Remiszewska, E.; Pijanowska, D.G. Surface modification of low and high temperature co-fired ceramics for enzymatic microreactor fabrication. Sens. Actuators B Chem. 2014, 190, 873–880. [Google Scholar] [CrossRef]
- Marchiarullo, D.J.; Sklavounos, A.H.; Oh, K.; Poe, B.L.; Barker, N.S.; Landers, J.P. Low-power microwave-mediated heating for microchip-based PCR. Lab Chip 2013, 13, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Issadore, D.; Humphry, K.J.; Brown, K.A.; Sandberg, L.; Weitz, D.A.; Westervelt, R.M. Microwave dielectric heating of drops in microfluidic devices. Lab Chip 2009, 9, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.J.; Sundaresan, S.G.; Geist, J.; Reyes, D.R.; Booth, J.C.; Rao, M.V.; Gaitan, M. Microwave dielectric heating of fluids in an integrated microfluidic device. J. Micromec. Microeng. 2007, 17, 2224–2230. [Google Scholar] [CrossRef]
- Malecha, K.; Macioszczyk, J.; Słobodzian, P.; Sobków, J. Application of microwave heating in ceramic-based microfluidic module. Microelectr. Int. 2018, 35, 126–132. [Google Scholar] [CrossRef]
- He, P.; Haswell, S.J.; Fletcher, P.D. Efficiency, monitoring and control of microwave heating within a continuous flow capillary reactor. Sens. Actuators B Chem. 2005, 105, 516–520. [Google Scholar] [CrossRef]
- Macioszczyk, J.; Rac-Rumijowska, O.; Słobodzian, P.; Teterycz, H.; Malecha, K. Microfluidical microwave reactor for synthesis of gold nanoparticles. Micromachines 2017, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Manadhar, G.; Arigong, B.; Zhang, H. Dual-band microwave device for sensing dielectric property changes in microfluidic channels. Microw. Opt. Technol. Lett. 2012, 54, 2691–2694. [Google Scholar] [CrossRef]
- Landoulsi, A.; Leroy, J.; Dalmay, C.; Pothier, A.; Bessaudou, A.; Blondy, P. A microfluidic sensor dedicated to microwave dielectric spectroscopy of liquids medium and flowing colloidal suspension. Procedia Eng. 2014, 87, 504–507. [Google Scholar] [CrossRef]
- Cui, Y.; Sun, J.; He, Y.; Wang, Z.; Wang, P. A simple, tunable, and highly sensitive radio-frequency sensor. App. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef] [PubMed]
- Withayachumnankul, W.; Jaruwongrungsee, K.; Tuantranont, A.; Fumeaux, C.; Abbott, D. Metamaterial-based microfluidic sensor for dielectric characterization. Sens. Actuators A Phys. 2013, 189, 233–237. [Google Scholar] [CrossRef]
- Abduljabar, A.A.; Rowe, D.J.; Porch, A.; Barrow, D.A. Novel microwave microfluidic sensor using a microstrip split-ring resonator. IEEE Trans. Microw. Theory Tech. 2014, 62, 679–688. [Google Scholar] [CrossRef]
- Boybay, M.; Jiao, A.; Glawdel, T.; Ren, C.L. Microwave sensing and heating of individual droplets in microfluidic devices. Lab Chip 2013, 13, 3840–3846. [Google Scholar] [CrossRef]
- Yesiloz, G.; Boybay, M.; Ren, C.L. Effective thermos-capillary mixing in droplet microfluidic integrated with a microwave heater. Anal. Chem. 2017, 89, 1978–1984. [Google Scholar] [CrossRef]
- Song, C.; Wang, P. A radio frequency device for measurement of minute dielectric property changes in microfluidic channels. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Wessel, J.; Schmalz, K.; Cahill, B.; Gastrock, G. Microwave Biosensor for Characterization of Compartments in Teflon Capillaries. In Proceedings of the IEEE 42nd European Microwave Conference, Amsterdam, The Netherlands, 29 October–1 November 2012. [Google Scholar] [CrossRef]
- Ibanez-Garcia, N.; Martinez-Cisneros, C.S.; Valdes, F.; Alonso, J. Green-tape ceramics. New technological approach for integrating electronics and fluidics in microsystems. Trends Anal. Chem. 2008, 27, 24–32. [Google Scholar] [CrossRef]
- Golonka, L.J. Technology and applications of Low Temperature Cofired Ceramic (LTCC) based sensors and microsystems. Bull. Pol. Acad. Sci. Tech. Sci. 2006, 54, 221–231. [Google Scholar]
- Shafique, M.F.; Laister, A.; Clark, M.; Miles, R.E.; Robertson, I.D. Fabrication of embedded microfluidic channels in low temperature co-fired ceramic technology using laser machining and progressive lamination. J. Eur. Ceram. Soc. 2011, 31, 2199–2204. [Google Scholar] [CrossRef]
- Barlow, F.; Wood, J.; Elshabini, A.; Stephens, E.F.; Feeler, R.; Kemner, G.; Junghans, J. Fabrication of precise fluidic structures in LTCC. Int. J. Appl. Ceram. Technol. 2009, 6, 18–23. [Google Scholar] [CrossRef]
- Andrijasevic, D.; Smetana, W.; Zehetner, J.; Zoppel, S.; Brenner, W. Aspects of micro structuring low temperature co-fired ceramic (LTCC) for realization complex 3D objects by embossing. Microelectron. Eng. 2007, 84, 1198–1201. [Google Scholar] [CrossRef]
- Bartsch, H.; Welker, T.; Welker, K.; Witte, H.; Müller, J. LTCC based bioreactors for cell cultivation. IOP Conf. Series Mater. Sci. Eng. 2015, 104, 012001. [Google Scholar] [CrossRef]
- Barteczka, B.; Słobodzian, P.; Dąbrowski, A.; Golonka, L. Influence of firing process quality on dielectric constant of microwave LTCC substrates. Microelectron. Int. 2014, 31, 169–175. [Google Scholar] [CrossRef]
- Baluta, S.; Malecha, K.; Zając, D.; Sołoducho, J.; Cabaj, J. Dopamine sensing with fluorescence strategy based on low temperature co-fired ceramic technology modified with conducting polymers. Sens. Actuators B Chem. 2017, 252, 803–812. [Google Scholar] [CrossRef]
- Dervisevic, M.; Senel, M.; Cevik, E. Novel impedimetric dopamine biosensor based on boronic acid functional poythiophene modified electrodes. Mater. Sci. Eng. C 2017, 72, 641–649. [Google Scholar] [CrossRef]




| Parameter | Water | LTCC |
|---|---|---|
| Conductivity (S/m) | 5.5 × 10−6 | 10−12 |
| Relative permittivity, εr | 80.1 | 7.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malecha, K.; Jasińska, L.; Grytsko, A.; Drzozga, K.; Słobodzian, P.; Cabaj, J. Monolithic Microwave-Microfluidic Sensors Made with Low Temperature Co-Fired Ceramic (LTCC) Technology. Sensors 2019, 19, 577. https://doi.org/10.3390/s19030577
Malecha K, Jasińska L, Grytsko A, Drzozga K, Słobodzian P, Cabaj J. Monolithic Microwave-Microfluidic Sensors Made with Low Temperature Co-Fired Ceramic (LTCC) Technology. Sensors. 2019; 19(3):577. https://doi.org/10.3390/s19030577
Chicago/Turabian StyleMalecha, Karol, Laura Jasińska, Anna Grytsko, Kamila Drzozga, Piotr Słobodzian, and Joanna Cabaj. 2019. "Monolithic Microwave-Microfluidic Sensors Made with Low Temperature Co-Fired Ceramic (LTCC) Technology" Sensors 19, no. 3: 577. https://doi.org/10.3390/s19030577
APA StyleMalecha, K., Jasińska, L., Grytsko, A., Drzozga, K., Słobodzian, P., & Cabaj, J. (2019). Monolithic Microwave-Microfluidic Sensors Made with Low Temperature Co-Fired Ceramic (LTCC) Technology. Sensors, 19(3), 577. https://doi.org/10.3390/s19030577





