Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review
Abstract
1. Introduction
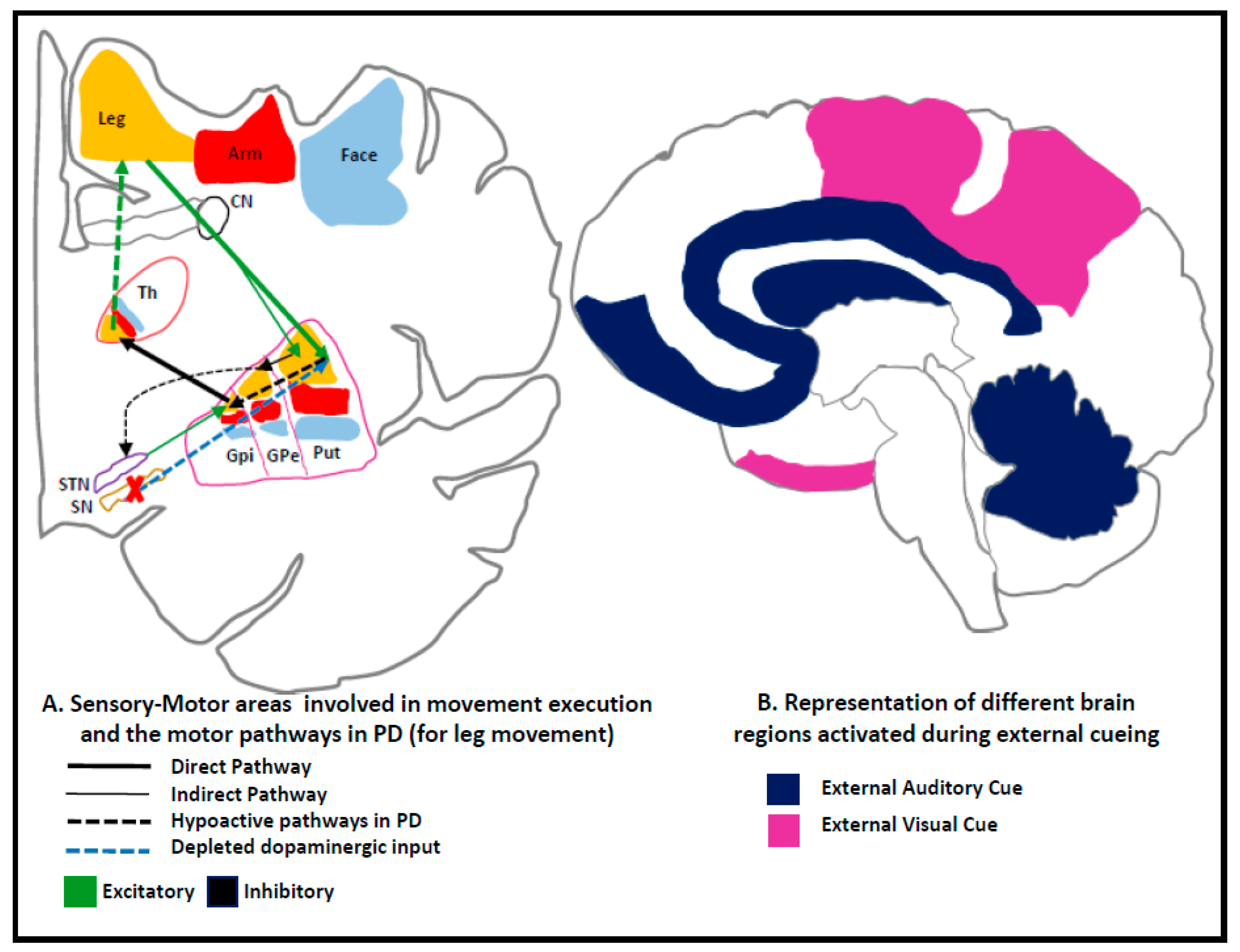
2. Pathophysiology of Motor Dysfunction in PD
3. Methodology
4. Cueing for Rehabilitation in PD
5. Benefits of Open-Loop Cueing on Gait in PD
6. Benefits of Closed-Loop Cueing on Gait in PD
7. Discussion
7.1. Different Cueing Types May Engage Different Mechanisms
7.2. Effect of Disease Stage on Cueing Strategy
7.3. Open-loop Cueing: Challenges and Limitations
7.4. Closed-loop Cueing: Challenges and Limitations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rogers, M.W. Disorders of posture, balance, and gait in Parkinson’s disease. Clin. Geriatr. Med. 1996, 12, 825–845. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson disease in 2015: Evolving basic, pathological and clinical concepts in PD. Nat. Rev. Neurol. 2016, 12, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Miller-Patterson, C.; Buesa, R.; McLaughlin, N.; Jones, R.; Akbar, U.; Friedman, J.H. Motor asymmetry over time in Parkinson’s disease. J. Neurol. Sci. 2018, 393, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Tomita, N.; Yano, M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J. Neurol. 2008, 255 (Suppl. 4), 19–29. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Hausdorff, J.M. The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Mov. Disord. 2008, 23 (Suppl. 2), S444–S450. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, T.A.; van der Kooij, H.; Munneke, M.; Bloem, B.R. Gait disorders and balance disturbances in Parkinson’s disease: Clinical update and pathophysiology. Curr. Opin. Neurol. 2008, 21, 461–471. [Google Scholar] [CrossRef]
- Hobert, M.A.; Nussbaum, S.; Heger, T.; Berg, D.; Maetzler, W.; Heinzel, S. Progressive Gait Deficits in Parkinson’s Disease: A Wearable-Based Biannual 5-Year Prospective Study. Front. Aging Neurosci. 2019, 11, 22. [Google Scholar] [CrossRef]
- Rochester, L.; Baker, K.; Nieuwboer, A.; Burn, D. Targeting dopa-sensitive and dopa-resistant gait dysfunction in Parkinson’s disease: Selective responses to internal and external cues. Mov. Disord. 2011, 26, 430–435. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Horak, F.B. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 267–274. [Google Scholar] [CrossRef]
- Beuter, A.; Hernandez, R.; Rigal, R.; Modolo, J.; Blanchet, P.J. Postural sway and effect of levodopa in early Parkinson’s disease. Can. J. Neurol. Sci. 2008, 35, 65–68. [Google Scholar] [CrossRef]
- King, L.A.; Horak, F.B. Lateral stepping for postural correction in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2008, 89, 492–499. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; St George, R.J.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B. Preparation for compensatory forward stepping in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2010, 91, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Potter-Nerger, M.; Volkmann, J. Deep brain stimulation for gait and postural symptoms in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Zhang, Y.Q.; Zhang, X.H.; Wang, Y.P.; Li, J.P.; Li, Y.J. Deep Brain Stimulation of Pedunculopontine Nucleus for Postural Instability and Gait Disorder After Parkinson Disease: A Meta-Analysis of Individual Patient Data. World Neurosurg. 2017, 102, 72–78. [Google Scholar] [CrossRef]
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M. Low-frequency deep brain stimulation for movement disorders. Parkinsonism Relat. Disord. 2016, 31, 14–22. [Google Scholar] [CrossRef]
- Xie, T.; Bloom, L.; Padmanaban, M.; Bertacchi, B.; Kang, W.; MacCracken, E.; Dachman, A.; Vigil, J.; Satzer, D.; Zadikoff, C.; et al. Long-term effect of low frequency stimulation of STN on dysphagia, freezing of gait and other motor symptoms in PD. J. Neurol. Neurosurg. Psychiatry 2018, 89, 989–994. [Google Scholar] [CrossRef]
- Debaere, F.; Wenderoth, N.; Sunaert, S.; Van Hecke, P.; Swinnen, S.P. Internal vs external generation of movements: Differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. NeuroImage 2003, 19, 764–776. [Google Scholar] [CrossRef]
- Cunnington, R.; Iansek, R.; Bradshaw, J.L.; Phillips, J.G. Movement-related potentials in Parkinson’s disease. Presence and predictability of temporal and spatial cues. Brain 1995, 118 Pt 4, 935–950. [Google Scholar] [CrossRef]
- Georgiou, N.; Iansek, R.; Bradshaw, J.L.; Phillips, J.G.; Mattingley, J.B.; Bradshaw, J.A. An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain 1993, 116 Pt 6, 1575–1587. [Google Scholar] [CrossRef]
- Ferrarin, M.; Rizzone, M.; Lopiano, L.; Recalcati, M.; Pedotti, A. Effects of subthalamic nucleus stimulation and L-dopa in trunk kinematics of patients with Parkinson’s disease. Gait Posture 2004, 19, 164–171. [Google Scholar] [CrossRef]
- Redgrave, P.; Rodriguez, M.; Smith, Y.; Rodriguez-Oroz, M.C.; Lehericy, S.; Bergman, H.; Agid, Y.; DeLong, M.R.; Obeso, J.A. Goal-directed and habitual control in the basal ganglia: Implications for Parkinson’s disease. Nat. Rev. Neurosci. 2010, 11, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Benninger, F.; Khlebtovsky, A.; Roditi, Y.; Keret, O.; Steiner, I.; Melamed, E.; Djaldetti, R. Beneficial effect of levodopa therapy on stooped posture in Parkinson’s disease. Gait Posture 2015, 42, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Schoellmann, A.; Fox, M.D.; Bohnen, N.I.; Factor, S.A.; Nieuwboer, A.; Hallett, M.; Lewis, S.J.G. Freezing of gait: Understanding the complexity of an enigmatic phenomenon. Brain 2019. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Berardelli, A. Sensorimotor integration in movement disorders. Mov. Disord. 2003, 18, 231–240. [Google Scholar] [CrossRef]
- Zia, S.; Cody, F.; O’Boyle, D. Joint position sense is impaired by Parkinson’s disease. Ann. Neurol. 2000, 47, 218–228. [Google Scholar] [CrossRef]
- Schubert, M.; Prokop, T.; Brocke, F.; Berger, W. Visual kinesthesia and locomotion in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2005, 20, 141–150. [Google Scholar] [CrossRef]
- Baroni, A.; Benvenuti, F.; Fantini, L.; Pantaleo, T.; Urbani, F. Human ballistic arm abduction movements: Effects of L-dopa treatment in Parkinson’s disease. Neurology 1984, 34, 868–876. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Benitez-Temino, B.; Blesa, F.J.; Guridi, J.; Marin, C.; Rodriguez, M. Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Mov. Disord. 2008, 23 (Suppl. 3), S548–S559. [Google Scholar] [CrossRef]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of Motor Dysfunction in Parkinson’s Disease as the Rationale for Drug Treatment and Rehabilitation. Parkinsons Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef]
- Sweeney, D.; Quinlan, L.R.; Browne, P.; Richardson, M.; Meskell, P.; ÓLaighin, G. A Technological Review of Wearable Cueing Devices Addressing Freezing of Gait in Parkinson’s Disease. Sensors 2019, 19, 1277. [Google Scholar] [CrossRef]
- Nieuwboer, A. Cueing for freezing of gait in patients with Parkinson’s disease: A rehabilitation perspective. Mov. Disord. 2008, 23 (Suppl. 2), S475–S481. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Kwakkel, G.; Rochester, L.; Jones, D.; van Wegen, E.; Willems, A.M.; Chavret, F.; Hetherington, V.; Baker, K.; Lim, I. Cueing training in the home improves gait-related mobility in Parkinson’s disease: The RESCUE trial. J. Neurol. Neurosurg. Psychiatry 2007, 78, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Amirnovin, R.; Williams, Z.M.; Cosgrove, G.R.; Eskandar, E.N. Visually guided movements suppress subthalamic oscillations in Parkinson’s disease patients. J. Neurosci. 2004, 24, 11302–11306. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.V.; Cheng, M.L.; Eden, U.; Williams, Z.; Brown, E.N.; Eskandar, E. The effects of cues on neurons in the basal ganglia in Parkinson’s disease. Front. Integr. Neurosci. 2012, 6, 40. [Google Scholar] [CrossRef]
- Glickstein, M.; Stein, J. Paradoxical movement in Parkinson’s disease. Trends Neurosci. 1991, 14, 480–482. [Google Scholar] [CrossRef]
- Ashoori, A.; Eagleman, D.M.; Jankovic, J. Effects of Auditory Rhythm and Music on Gait Disturbances in Parkinson’s Disease. Front. Neurol. 2015, 6, 234. [Google Scholar] [CrossRef]
- Ginis, P.; Nackaerts, E.; Nieuwboer, A.; Heremans, E. Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann. Phys. Rehabil. Med. 2018, 61, 407–413. [Google Scholar] [CrossRef]
- Griffin, H.J.; Greenlaw, R.; Limousin, P.; Bhatia, K.; Quinn, N.P.; Jahanshahi, M. The effect of real and virtual visual cues on walking in Parkinson’s disease. J. Neurol. 2011, 258, 991–1000. [Google Scholar] [CrossRef]
- Mancini, M.; Smulders, K.; Harker, G.; Stuart, S.; Nutt, J.G. Assessment of the ability of open-and closed-loop cueing to improve turning and freezing in people with Parkinson’s disease. Sci. Rep. 2018, 8, 12773. [Google Scholar] [CrossRef]
- Suteerawattananon, M.; Morris, G.S.; Etnyre, B.R.; Jankovic, J.; Protas, E.J. Effects of visual and auditory cues on gait in individuals with Parkinson’s disease. J. Neurol. Sci. 2004, 219, 63–69. [Google Scholar] [CrossRef]
- Lu, C.; Amundsen Huffmaster, S.L.; Tuite, P.J.; Vachon, J.M.; MacKinnon, C.D. Effect of Cue Timing and Modality on Gait Initiation in Parkinson Disease With Freezing of Gait. Arch. Phys. Med. Rehabil. 2017, 98, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Thaut, M.H.; McIntosh, G.C.; Rice, R.R.; Miller, R.A.; Rathbun, J.; Brault, J.M. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. 1996, 11, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Pili, R.; Corona, F.; Sors, F.; Agostini, T.A.; Bernardis, P.; Casula, C.; Cossu, G.; Guicciardi, M.; Pau, M. The Use of Footstep Sounds as Rhythmic Auditory Stimulation for Gait Rehabilitation in Parkinson’s Disease: A Randomized Controlled Trial. Front. Neurol. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Lowenthal, J.; Herman, T.; Gruendlinger, L.; Peretz, C.; Giladi, N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur. J. Neurosci. 2007, 26, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.C.; Brown, S.H.; Rice, R.R.; Thaut, M.H. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Howe, T.E.; Lovgreen, B.; Cody, F.W.; Ashton, V.J.; Oldham, J.A. Auditory cues can modify the gait of persons with early-stage Parkinson’s disease: A method for enhancing parkinsonian walking performance? Clin. Rehabil. 2003, 17, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Behrman, A.L.; Teitelbaum, P.; Cauraugh, J.H. Verbal instructional sets to normalise the temporal and spatial gait variables in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1998, 65, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.; Kelly, B.; Tunnicliffe, N.; Turnbull, G.I.; Walker, J.M. The effect of visual cues on the gait of independently mobile Parkinson’s patients. Phyiotherapy 1991, 77, 415–420. [Google Scholar] [CrossRef]
- Lewis, G.N.; Byblow, W.D.; Walt, S.E. Stride length regulation in Parkinson’s disease: The use of extrinsic, visual cues. Brain 2000, 123 Pt 10, 2077–2090. [Google Scholar] [CrossRef]
- Sidaway, B.; Anderson, J.; Danielson, G.; Martin, L.; Smith, G. Effects of long-term gait training using visual cues in an individual with Parkinson disease. Phys. Ther. 2006, 86, 186–194. [Google Scholar]
- Azulay, J.P.; Mesure, S.; Amblard, B.; Blin, O.; Sangla, I.; Pouget, J. Visual control of locomotion in Parkinson’s disease. Brain 1999, 122 Pt 1, 111–120. [Google Scholar] [CrossRef]
- Luessi, F.; Mueller, L.K.; Breimhorst, M.; Vogt, T. Influence of visual cues on gait in Parkinson’s disease during treadmill walking at multiple velocities. J. Neurol. Sci. 2012, 314, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bao, T.; Lee, U.H.; Kinnaird, C.; Carender, W.; Huang, Y.; Sienko, K.H.; Shull, P.B. Configurable, wearable sensing and vibrotactile feedback system for real-time postural balance and gait training: Proof-of-concept. J. Neuroeng. Rehabil. 2017, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Gopalai, A.A.; Senanayake, S.M.; Kiong, L.C.; Gouwanda, D. Real-time stability measurement system for postural control. J. Bodyw. Mov. Ther. 2011, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Van Wegen, E.; de Goede, C.; Lim, I.; Rietberg, M.; Nieuwboer, A.; Willems, A.; Jones, D.; Rochester, L.; Hetherington, V.; Berendse, H.; et al. The effect of rhythmic somatosensory cueing on gait in patients with Parkinson’s disease. J. Neurol. Sci. 2006, 248, 210–214. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Souza, C.; Callil Voos, M.; Fen Chien, H.; Ferreira Barbosa, A.; Brant Rodrigues, R.; Colucci Fonoff, F.; Caromano, F.A.; de Abreu, L.C.; Reis Barbosa, E.; Talamoni Fonoff, E. Combined auditory and visual cueing provided by eyeglasses influence gait performance in Parkinson Disease patients submitted to deep brain stimulation: A pilot study. Int. Arch. Med. 2015. [Google Scholar] [CrossRef]
- Lohnes, C.A.; Earhart, G.M. The impact of attentional, auditory, and combined cues on walking during single and cognitive dual tasks in Parkinson disease. Gait Posture 2011, 33, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Cubo, E.; Leurgans, S.; Goetz, C.G. Short-term and practice effects of metronome pacing in Parkinson’s disease patients with gait freezing while in the ‘on’ state: Randomized single blind evaluation. Parkinsonism Relat. Disord. 2004, 10, 507–510. [Google Scholar] [CrossRef]
- Del Olmo, M.F.; Cudeiro, J. Temporal variability of gait in Parkinson disease: Effects of a rehabilitation programme based on rhythmic sound cues. Parkinsonism Relat. Disord. 2005, 11, 25–33. [Google Scholar] [CrossRef]
- Van den Heuvel, M.R.; Kwakkel, G.; Beek, P.J.; Berendse, H.W.; Daffertshofer, A.; van Wegen, E.E. Effects of augmented visual feedback during balance training in Parkinson’s disease: A pilot randomized clinical trial. Parkinsonism Relat. Disord. 2014, 20, 1352–1358. [Google Scholar] [CrossRef]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Ability to modulate walking cadence remains intact in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1532–1534. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Mak, M.K. Balance and Gait Training with Augmented Feedback Improves Balance Confidence in People With Parkinson’s Disease: A Randomized Controlled Trial. Neurorehabil. Neural Repair 2014, 28, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Lim, I.; van Wegen, E.; de Goede, C.; Deutekom, M.; Nieuwboer, A.; Willems, A.; Jones, D.; Rochester, L.; Kwakkel, G. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: A systematic review. Clin. Rehabil. 2005, 19, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Baker, K.; Willems, A.M.; Jones, D.; Spildooren, J.; Lim, I.; Kwakkel, G.; Van Wegen, E.; Rochester, L. The short-term effects of different cueing modalities on turn speed in people with Parkinson’s disease. Neurorehabil. Neural Repair 2009, 23, 831–836. [Google Scholar] [CrossRef]
- Ford, M.P.; Malone, L.A.; Nyikos, I.; Yelisetty, R.; Bickel, C.S. Gait training with progressive external auditory cueing in persons with Parkinson’s disease. Arch. Phys. Med. Rehabil. 2010, 91, 1255–1261. [Google Scholar] [CrossRef]
- Marchese, R.; Diverio, M.; Zucchi, F.; Lentino, C.; Abbruzzese, G. The role of sensory cues in the rehabilitation of parkinsonian patients: A comparison of two physical therapy protocols. Mov. Disord. 2000, 15, 879–883. [Google Scholar] [CrossRef]
- Frazzitta, G.; Bertotti, G.; Ucellini, D.; Maestri, R. Parkinson’s Disease Rehabilitation-a pilot study with 1 year follow-up. Mov. Disord. 2010, 25, 1762–1763. [Google Scholar]
- Frazzitta, G.; Maestri, R.; Uccellini, D.; Bertotti, G.; Abelli, P. Rehabilitation treatment of gait in patients with Parkinson’s disease with freezing: A comparison between two physical therapy protocols using visual and auditory cues with or without treadmill training. Mov. Disord. 2009, 24, 1139–1143. [Google Scholar] [CrossRef]
- Jellish, J.; Abbas, J.J.; Ingalls, T.; Mahant, P.; Samanta, J.; Ospina, M.; Krishnamurthi, N. A System for Real-Time Feedback to Improve Gait and Posture in Parkinson’s Disease. IEEE J. Biomed. Health Inform. 2015, 19, 1809–1819. [Google Scholar] [CrossRef]
- Schlick, C.; Ernst, A.; Botzel, K.; Plate, A.; Pelykh, O.; Ilmberger, J. Visual cues combined with treadmill training to improve gait performance in Parkinson’s disease: A pilot randomized controlled trial. Clin. Rehabil. 2016, 30, 463–471. [Google Scholar] [CrossRef]
- Lee, N.Y.; Lee, D.K.; Song, H.S. Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J. Phys. Ther. Sci. 2015, 27, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Y.; Yang, Y.R.; Cheng, S.J.; Wu, Y.R.; Fuh, J.L.; Wang, R.Y. Virtual Reality-Based Training to Improve Obstacle-Crossing Performance and Dynamic Balance in Patients With Parkinson’s Disease. Neurorehabil. Neural Repair 2015, 29, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Pedreira, G.; Prazeres, A.; Cruz, D.; Gomes, I.; Monteiro, L.; Melos, A. Virtual games and quality of life in PD-a randomized controlled trial. Adv. Parkinson’s Dis. 2013, 2, 97–101. [Google Scholar] [CrossRef]
- Pompeu, J.E.; Mendes, F.A.; Silva, K.G.; Lobo, A.M.; Oliveira Tde, P.; Zomignani, A.P.; Piemonte, M.E. Effect of Nintendo Wii-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: A randomised clinical trial. Physiotherapy 2012, 98, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Wang, H.K.; Wu, R.M.; Lo, C.S.; Lin, K.H. Home-based virtual reality balance training and conventional balance training in Parkinson’s disease: A randomized controlled trial. J. Formos. Med. Assoc. 2016, 115, 734–743. [Google Scholar] [CrossRef]
- Yen, C.Y.; Lin, K.H.; Hu, M.H.; Wu, R.M.; Lu, T.W.; Lin, C.H. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: A randomized controlled trial. Phys. Ther. 2011, 91, 862–874. [Google Scholar] [CrossRef]
- Dockx, K.; Bekkers, E.M.; Van den Bergh, V.; Ginis, P.; Rochester, L.; Hausdorff, J.M.; Mirelman, A.; Nieuwboer, A. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst. Rev. 2016, 12, CD010760. [Google Scholar] [CrossRef]
- Zijlstra, W.; Rutgers, A.W.; Van Weerden, T.W. Voluntary and involuntary adaptation of gait in Parkinson’s disease. Gait Posture 1998, 7, 53–63. [Google Scholar] [CrossRef]
- Willems, A.M.; Nieuwboer, A.; Chavret, F.; Desloovere, K.; Dom, R.; Rochester, L.; Jones, D.; Kwakkel, G.; Van Wegen, E. The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study. Disabil. Rehabil. 2006, 28, 721–728. [Google Scholar] [CrossRef]
- McCoy, R.W.; Kohl, R.M.; Elliott, S.M.; Joyce, A.S. The impact of auditory cues on gait control of individuals with Parkinson’s disease. J. Hum. Mov. Stud. 2002, 42, 229–236. [Google Scholar]
- Rochester, L.; Baker, K.; Hetherington, V.; Jones, D.; Willems, A.M.; Kwakkel, G.; Van Wegen, E.; Lim, I.; Nieuwboer, A. Evidence for motor learning in Parkinson’s disease: Acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res. 2010, 1319, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, S.J.; Barber, B.; Colby, M.; Cormack, B.; Mick, T.; Jenkins, M.E. Cueing and gait improvement among people with Parkinson’s disease: A meta-analysis. Arch. Phys. Med. Rehabil. 2013, 94, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hallett, M. Neural correlates of dual task performance in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2008, 79, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Badarny, S.; Aharon-Peretz, J.; Susel, Z.; Habib, G.; Baram, Y. Virtual reality feedback cues for improvement of gait in patients with Parkinson’s disease. Tremor Other Hyperkinetic Mov. 2014, 4, 225. [Google Scholar] [CrossRef]
- Machado, J.P.F. Smartphone Based Closed-Loop Auditory Cueing System. 2014. Available online: https://repositorio-aberto.up.pt/bitstream/10216/75447/2/31866.pdf (accessed on 4 October 2019).
- Ginis, P.; Nieuwboer, A.; Dorfman, M.; Ferrari, A.; Gazit, E.; Canning, C.G.; Rocchi, L.; Chiari, L.; Hausdorff, J.M.; Mirelman, A. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Parkinsonism Relat. Disord. 2016, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Chomiak, T.; Sidhu, A.S.; Watts, A.; Su, L.; Graham, B.; Wu, J.; Classen, S.; Falter, B.; Hu, B. Development and Validation of Ambulosono: A Wearable Sensor for Bio-Feedback Rehabilitation Training. Sensors 2019, 19, 686. [Google Scholar] [CrossRef]
- Bartels, B.M.; Moreno, A.; Quezada, M.J.; Sivertson, H.; Abbas, J.; Krishnamurthi, N. Real-Time Feedback Derived from Wearable Sensors to Improve Gait in Parkinson’s Disease. Technol. Innov. 2018, 20, 37–46. [Google Scholar] [CrossRef]
- Thompson, E.; Agada, P.; Wright, W.G.; Reimann, H.; Jeka, J. Spatiotemporal gait changes with use of an arm swing cueing device in people with Parkinson’s disease. Gait Posture 2017, 58, 46–51. [Google Scholar] [CrossRef]
- Baskaran, D. Real-Time Feedback Training to Improve Gait and Posture in Parkinson’s Disease; Arizona State University: Tempe, AZ, USA, 2017. [Google Scholar]
- Krishnamurthi, N.; Baskaran, D.; Parikh, S.; Venugopal, V.; Muthukrishnan, N.; Driver-Dunckley, E.; Mahant, P.; Ospina, M.C.; Abbas, J.J. Real-Time Feedback during Treadmill Training for Individuals with Parkinson’s Disease; Society for Neuroscience: Chicago, IL, USA, 2019; p. 1. [Google Scholar]
- Morris, M.E.; Iansek, R.; Matyas, T.A.; Summers, J.J. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain 1996, 119 Pt 2, 551–568. [Google Scholar] [CrossRef]
- Schlick, C.; Struppler, A.; Boetzel, K.; Plate, A.; Ilmberger, J. Dynamic visual cueing in combination with treadmill training for gait rehabilitation in Parkinson disease. Am. J. Phys. Med. Rehabil. 2012, 91, 75–79. [Google Scholar] [CrossRef]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [CrossRef]
- Carpinella, I.; Cattaneo, D.; Bonora, G.; Bowman, T.; Martina, L.; Montesano, A.; Ferrarin, M. Wearable Sensor-Based Biofeedback Training for Balance and Gait in Parkinson Disease: A Pilot Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 622–630. [Google Scholar] [CrossRef]
- Espay, A.J.; Baram, Y.; Dwivedi, A.K.; Shukla, R.; Gartner, M.; Gaines, L.; Duker, A.P.; Revilla, F.J. At-home training with closed-loop augmented-reality cueing device for improving gait in patients with Parkinson disease. J. Rehabil. Res. Dev. 2010, 47, 573. [Google Scholar] [CrossRef]
- Karatsidis, A.; Richards, R.E.; Konrath, J.M.; van den Noort, J.C.; Schepers, H.M.; Bellusci, G.; Harlaar, J.; Veltink, P.H. Validation of wearable visual feedback for retraining foot progression angle using inertial sensors and an augmented reality headset. J. Neuroeng. Rehabil. 2018, 15, 78. [Google Scholar] [CrossRef]
- Young, W.R.; Shreve, L.; Quinn, E.J.; Craig, C.; Bronte-Stewart, H. Auditory cueing in Parkinson’s patients with freezing of gait. What matters most: Action-relevance or cue-continuity? Neuropsychologia 2016, 87, 54–62. [Google Scholar] [CrossRef]
- Pereira, M.P.; Gobbi, L.T.; Almeida, Q.J. Freezing of gait in Parkinson’s disease: Evidence of sensory rather than attentional mechanisms through muscle vibration. Parkinsonism Relat. Disord. 2016, 29, 78–82. [Google Scholar] [CrossRef]
- Rocha, P.A.; Porfirio, G.M.; Ferraz, H.B.; Trevisani, V.F. Effects of external cues on gait parameters of Parkinson’s disease patients: A systematic review. Clin. Neurol. Neurosurg. 2014, 124, 127–134. [Google Scholar] [CrossRef]
- Bhatt, T.; Yang, F.; Mak, M.K.; Hui-Chan, C.W.; Pai, Y.C. Effect of externally cued training on dynamic stability control during the sit-to-stand task in people with Parkinson disease. Phys. Ther. 2013, 93, 492–503. [Google Scholar] [CrossRef]
- Mak, M.K.; Hui-Chan, C.W. Audiovisual cues can enhance sit-to-stand in patients with Parkinson’s disease. Mov. Disord. 2004, 19, 1012–1019. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Mancini, M.; Horak, F.; Peterson, D. Anticipatory Postural Adjustment during Self-Initiated, Cued, and Compensatory Stepping in Healthy Older Adults and Patients With Parkinson Disease. Arch. Phys. Med. Rehabil. 2017, 98, 1316–1324. [Google Scholar] [CrossRef]
- Lopez, W.O.; Higuera, C.A.; Fonoff, E.T.; Souza Cde, O.; Albicker, U.; Martinez, J.A. Listenmee and Listenmee smartphone application: Synchronizing walking to rhythmic auditory cues to improve gait in Parkinson’s disease. Hum. Mov. Sci. 2014, 37, 147–156. [Google Scholar] [CrossRef]
- Muthukrishnan, N.; Turaga, P.; Abbas, J.J.; Ingalls, T.; Krishnamurthi, N. Gait and Balance Monitoring Using Wearable Technology for Real-Time Feedback in Parkinson’s Disease; Society for Neuroscience: Chicago, IL, USA, 2019; p. 1. [Google Scholar]
| Study | Intervention Type | Sensors; Feedback Mode | Outcome Measures | Study Protocol | Results | Limitations |
|---|---|---|---|---|---|---|
| Badarny et al. 2014 [84] | Visual | Wearable motion sensors; virtual reality based eye-glasses | Walking speed, stride length | Single-session study with cue and a follow-up evaluation (1 week later) | Increases in both walking speed and stride length, immediate effects and at follow-up | No control group; only assessed short-term effects |
| Jellish et al. 2015 [69] | Visual | Treadmill-based, video-based motion capture system and a feedback monitor | Step length, postural (back) angle measured during treadmill walking | Single-session study using multiple trials with and without cues | Increases in uprightness and step length | Utilized technology that is only available in research labs |
| Chomiak et al. 2019 [87] | Auditory | IMU sensors with a smartphone application-(Ambulosono sensor system) | Step length, walking distance, velocity, and cadence | Single-session study multiple trials | Evaluation of the sensor’s performance on healthy controls | Use of iPod Touch for feedback is not cost-effective and the system has not been evaluated on PD population |
| Bartels et al. 2018 [88] | Auditory | IMU sensors with a smartphone application | Stride length | Single-session study with multiple trials | Evaluation of the sensor’s performance on healthy controls | The system has not been evaluated on PD population |
| Young et al. 2014 [98] | Auditory-sonification of gait–swing phase | Video-based motion capture system with a smart phone application | Step length CoV | Single-session study in the lab with multiple trials | Reduction in step length variability | Utilized technology that is only available in research labs |
| Thompson et.al. 2017 [89] | Somatosensory | IMU sensors with a software application on the laptop and a vibratory device | Step length, lateral trunk sway, cadence, gait velocity, arm swing | Single-session study in the lab with multiple trials. | Increases in step length, arm swing magnitude, reduced cadence | Though somatosensory cues have been successful in helping with the rhythm of the movement, they are less effective in increasing the amplitude of the desired movement |
| Schlick et al. 2016 [70] | Visual | Treadmill-based pressure platform and video feedback monitor | Gait speed, stride length and cadence | Long-term training (5 weeks) at lab, RCT | Both the training and control group showed increases in gait speed and stride length post training, but sustained effects after 2 months were observed only in the case of feedback-based training | Small sample at follow-up because of attrition |
| Mirelman et al. 2016 [94] | Visual | Video-based motion capture system with virtual reality feedback | Fall incident rates | Long-term training (3 times/week for 6 weeks) at lab | Reduction in the rate of falls during the 6 month follow-up evaluation | No control group |
| Baskaran. 2017 [90] | Visual | Treadmill-based video-based motion capture system and a feedback monitor | Step length, postural (back) angle measured during treadmill walking | Long-term training (3 times/week for 6 weeks) at lab | Increases in uprightness and step length | No control group and a small sample size |
| Yang et al. 2016 [75] | Visual | Video-based motion capture system with virtual reality feedback | BBS, DGI, TUG test | Long-term training (2 times/week for 6 weeks) at lab, RCT | Increase in clinical score, BBS performance which was retained at 2 week follow-up | Small sample size |
| Van den Heuvel et al. 2014 [60] | Visual | Video-based augmented feedback system with treadmill and IMU sensors | FRT, BBS, UPDRS | Long-term training (2 times/week for 5 weeks) at lab, RCT | Improvements in balance scores in favor of the feedback system | Changes in scores were not statistically significant |
| Ginis et al. 2015 [86] | Auditory | IMU sensors with a smartphone application (CuPiD system) | Gait speed, cadence, stride length and stride length asymmetry | Long-term training (3 times/week for 6 weeks) at home, RCT | Increase in gait speed at post-training | Assessors were not blinded |
| Carpinella et al. 2016 [95] | Auditory and visual | IMU sensors and monitor for exercise therapy with a Gamepad (Gaming Experience in Parkinson’s Disease) | BBS and gait speed | Long-term training (3 times/week for 6 weeks) at lab, RCT | Increase in clinical score, BBS performance and retained effects at 1 month follow-up | Lack of online computation of gait measures and the use of technology that is only available in research labs |
| Frazzitta et al. 2009 [68] | Auditory and visual | Treadmill-based strain gauge and a visual feedback monitor | Stride length, gait speed | Long-term training (4 weeks) at lab | Greater increase in gait speed and stride length following treadmill-based cue training than with overground-based cue training | No control group and the study did not evaluate residual effect at follow-up |
| Rochester et al. 2010 [81] | Auditory, visual, and somatosensory | IMU-based rhythmical feedback system | Walking speed, step length, step frequency | Long-term training study for 6 weeks at lab | Increase in walking speed and step length with all cue types in both single and dual-tasking after training. | No control group |
| Espay et al. 2010 [96] | Auditory and visual | IMU sensors and a head-mounted display and headphones | Gait velocity, stride length and cadence | Home-based training for 2 weeks | Increase in gait velocity and stride length after training | No control group |
| Pompeu et al. 2012 [74] | Auditory and visual | Wii Fit games | UPDRS | Long-term training (2 times/week for 7 weeks) with exercise therapy at lab | Decrease (improvement) in UPDRS post-training and at 2 month follow-up evaluation | No control group |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthukrishnan, N.; Abbas, J.J.; Shill, H.A.; Krishnamurthi, N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors 2019, 19, 5468. https://doi.org/10.3390/s19245468
Muthukrishnan N, Abbas JJ, Shill HA, Krishnamurthi N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors. 2019; 19(24):5468. https://doi.org/10.3390/s19245468
Chicago/Turabian StyleMuthukrishnan, Niveditha, James J. Abbas, Holly A. Shill, and Narayanan Krishnamurthi. 2019. "Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review" Sensors 19, no. 24: 5468. https://doi.org/10.3390/s19245468
APA StyleMuthukrishnan, N., Abbas, J. J., Shill, H. A., & Krishnamurthi, N. (2019). Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors, 19(24), 5468. https://doi.org/10.3390/s19245468




