Cu(I) Coordination Complex Precursor for Randomized CuOx Microarray Loaded on Carbon Nanofiber with Excellent Electrocatalytic Performance for Electrochemical Glucose Detection
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Preparation of the Cu–Carbon Nanofiber Paste Electrode (Cu-C18/CNF)
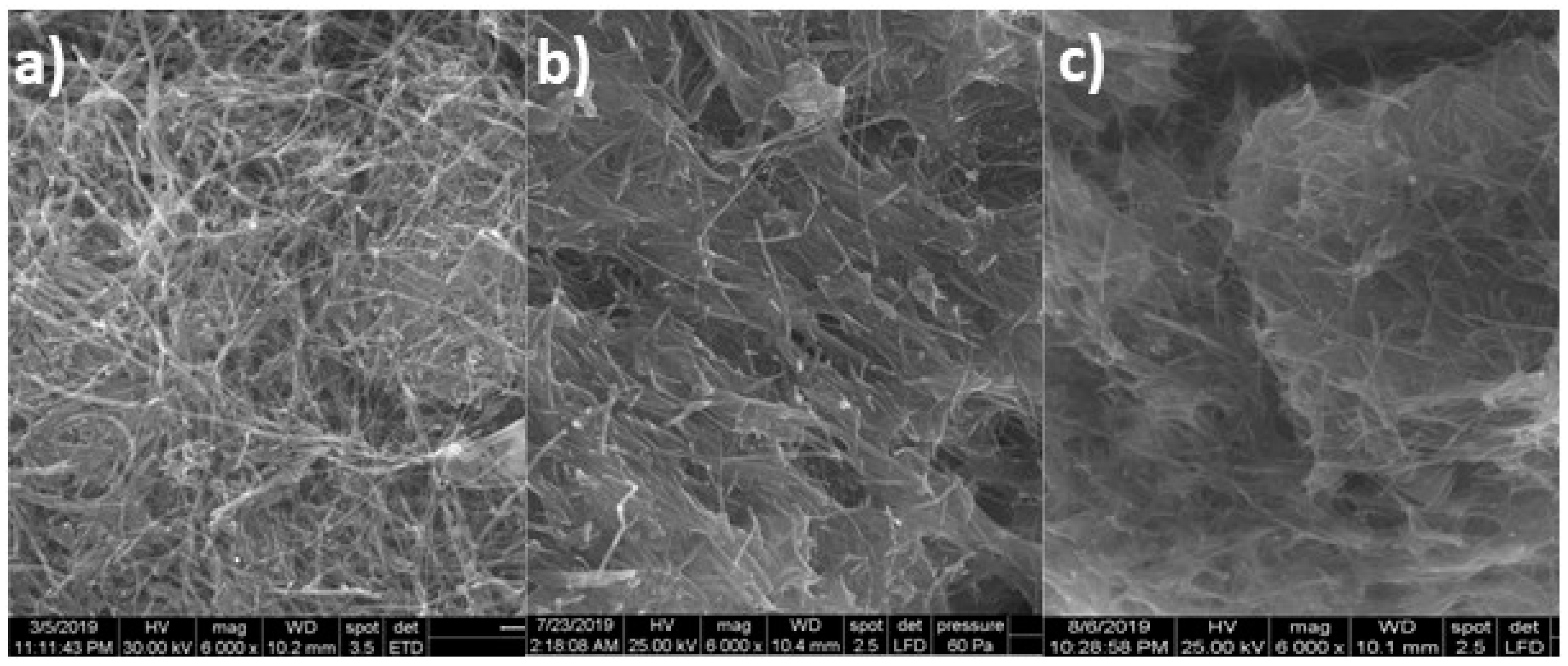
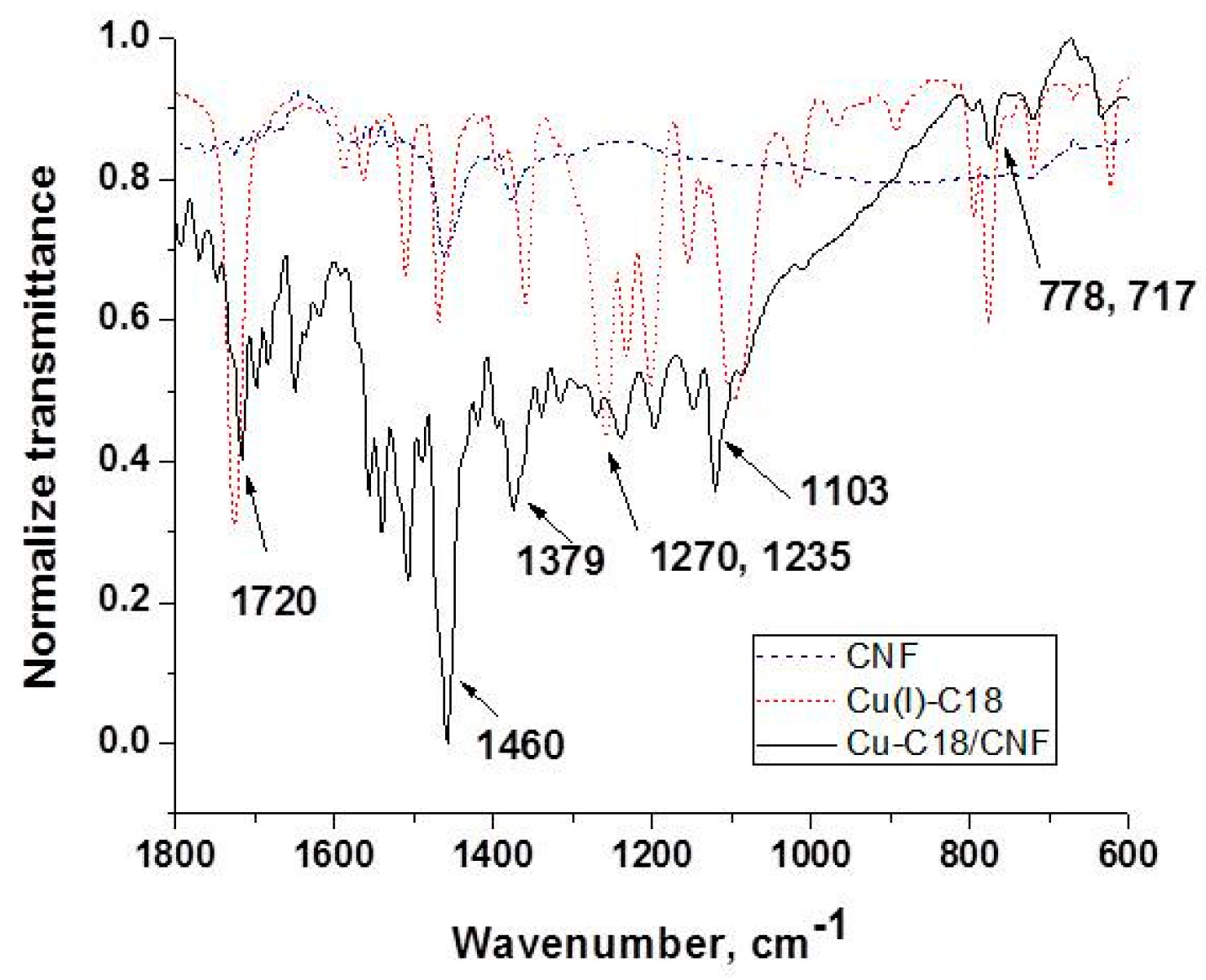
3.2. Structural and Morphological Characterization
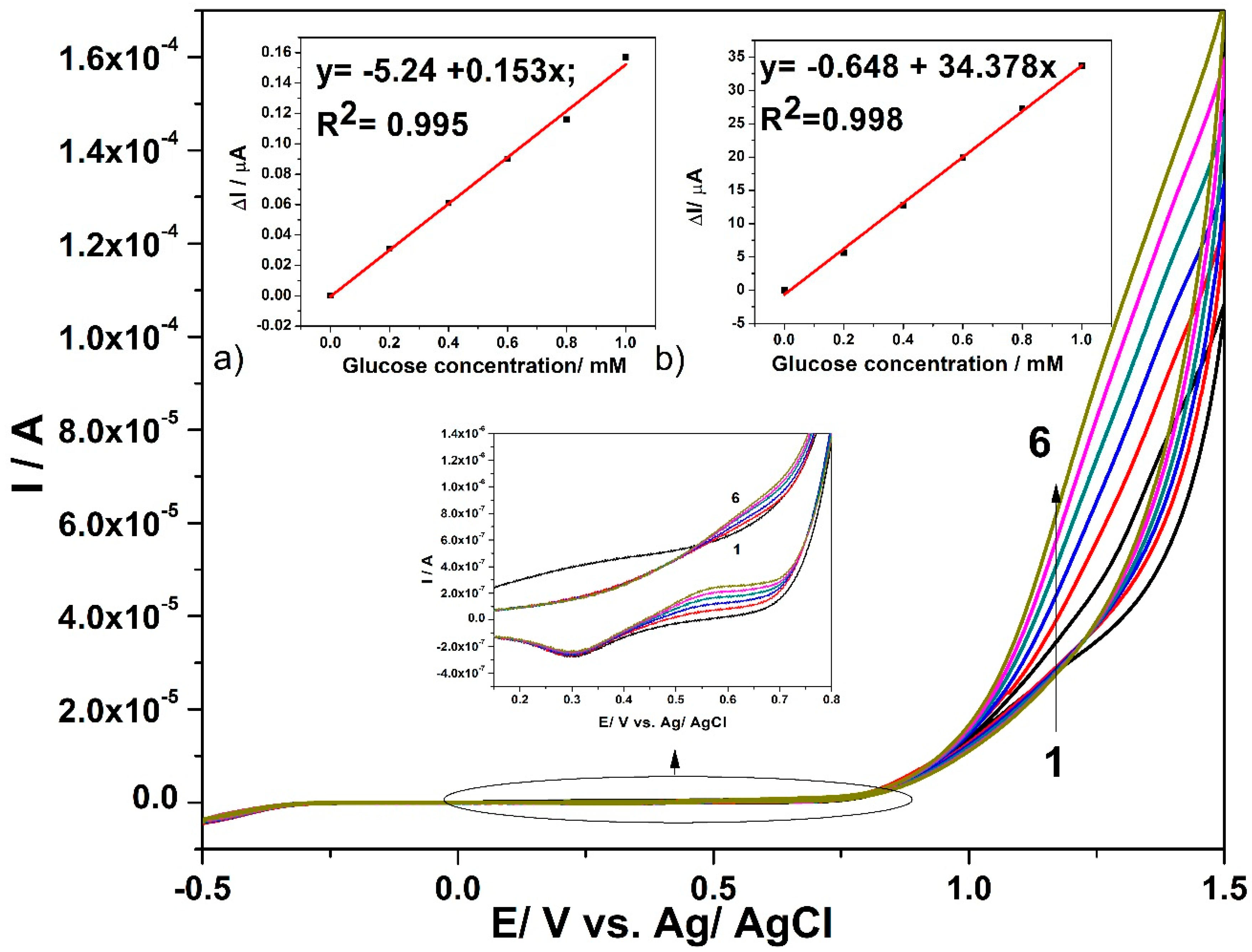
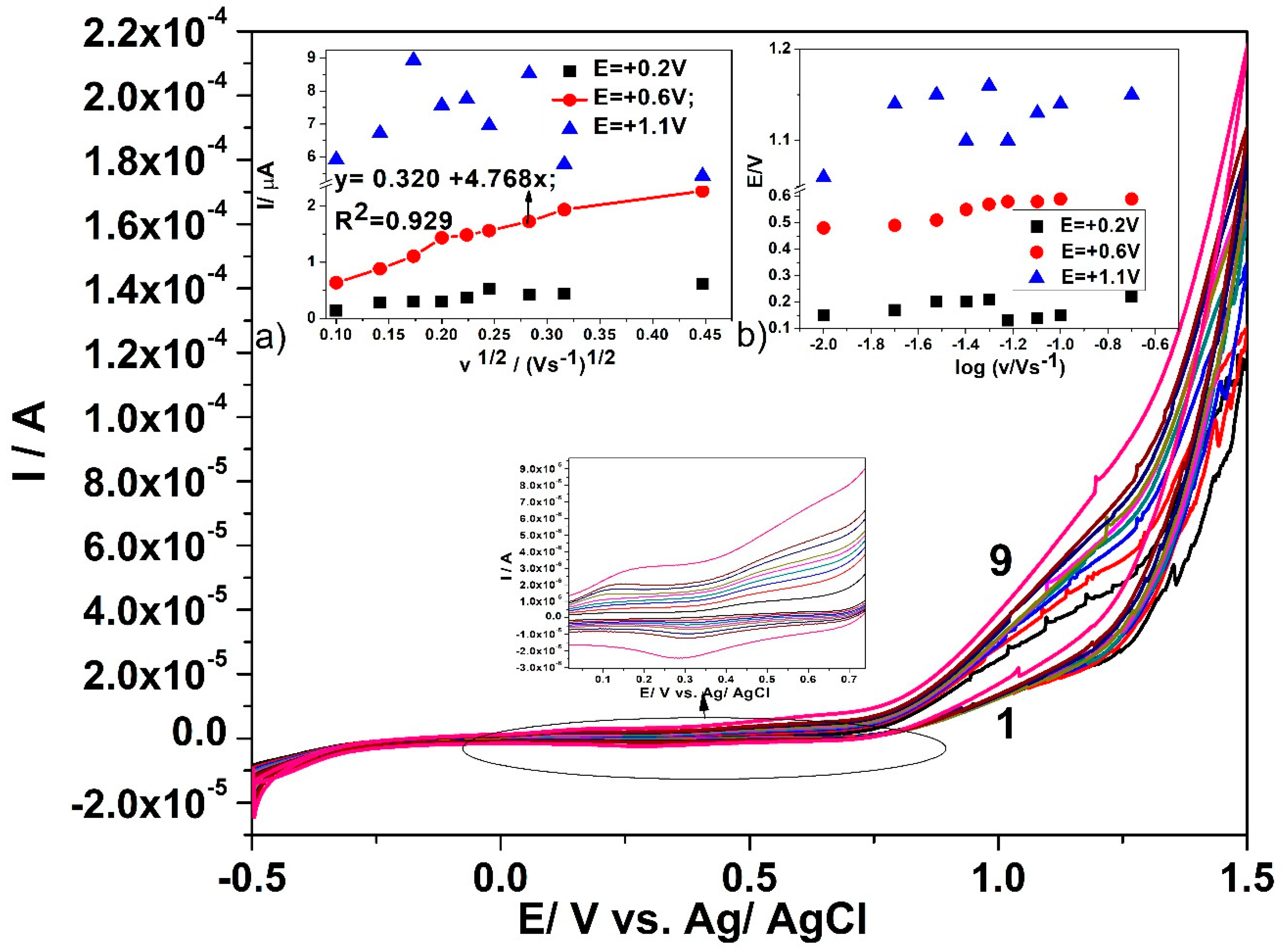
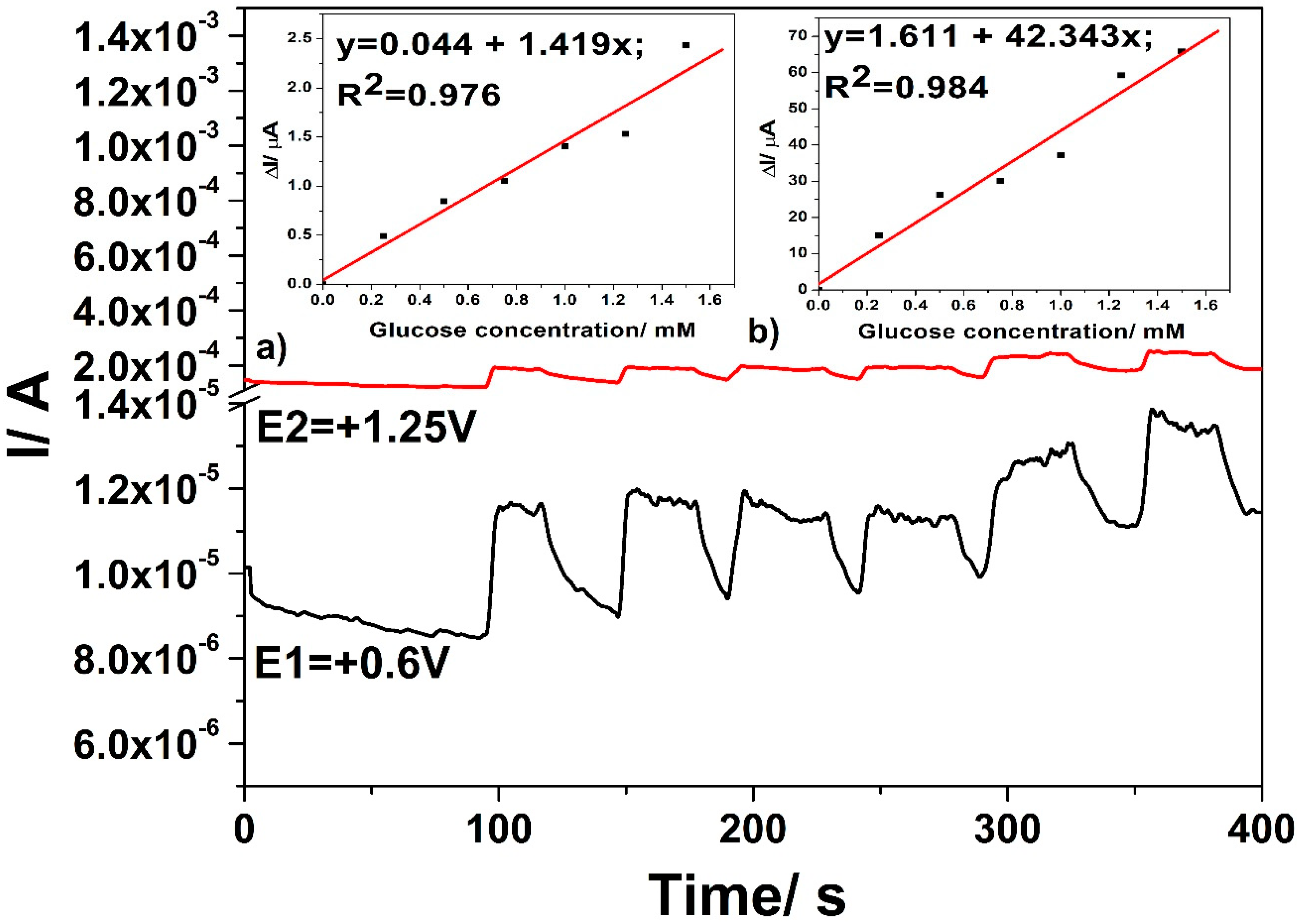
3.3. Electrochemical Characterization for Glucose Detection
3.4. Detection Results
- +0.6 V vs. Ag/AgCl for a time duration of 50 ms, where glucose is oxidized to gluconolactone;
- +1.25 V vs. Ag/AgCl for a time duration of 100 ms, where glucose is further oxidized under oxygen evolution reaction generation; and,
- +0.75 V vs. Ag/AgCl for a time duration of 100 ms, to assure Cu(II)/Cu(III) system generation.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, K.; Kim, S.; Lee, H.N.; Park, Y.M.; Bae, Y.-S.; Kim, H.-J. Electrochemically derived CuO nanorod from copper-based metal-organic framework for non-enzymatic detection of glucose. Appl. Surf. Sci. 2019, 479, 720–726. [Google Scholar] [CrossRef]
- Mohammadi, S.; Taheri, A.; Rezayati-zad, Z. Ultrasensitive and selective non-enzymatic glucose detection based on pt electrode modified by carbon nanotubes@ graphene oxide/nickel hydroxide-Nafion hybrid composite in alkaline media. Prog. Chem. Biochem. Res. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Campana, A.L.; Florez, S.L.; Noguera, M.J.; Fuentes, O.P.; Puentes, P.R.; Cruz, J.C.; Osma, J.F. Enzyme-Based Electrochemical Biosensors for Microfluidic Platforms to Detect Pharmaceutical Residues in Wastewater. Biosensors 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef]
- Calandra, P.; Caschera, D.; Liveri, V.T.; Lombardo, D. How self-assembly of amphiphilic molecules can generate complexityin the nanoscale. Colloids Surf. A 2015, 484, 164–183. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Li, Y.P.; He, Z.Y.; Yan, Z.F. Facile preparation of Cu-Cu2O nanoporous nanoparticles as a potential catalyst for non-enzymatic glucose sensing. RSC Adv. 2013, 3, 2178–2181. [Google Scholar] [CrossRef]
- Wang, A.J.; Feng, J.J.; Li, Z.H.; Liao, Q.C.; Wang, Z.Z.; Chen, J.R. Solvothermal synthesis of Cu/Cu2O hollow microspheres for non-enzymatic amperometric glucose sensing. CrystEngComm 2012, 14, 1289–1295. [Google Scholar] [CrossRef]
- Foroughi, F.; Rahsepar, M.; Hadianfard, M.J.; Kim, H. Microwave-assisted synthesis of graphene modified CuO nanoparticles for voltammetric enzyme-free sensing of glucose at biological pH values. Microchim. Acta 2017, 185, 57. [Google Scholar] [CrossRef]
- Raziq, A.; Tariq, M.; Hussain, R.; Mehmood, M.H.; Ullah, I.; Khan, J.; Muhammad, M. Highly sensitive, non-enzymatic and precious metal-free electrochemical glucose sensor based on a Ni-Cu/TiO2 modifiedglassy carbon electrode. J. Serb. Chem. Soc. 2018, 83, 733–744. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, W.; Zhao, Q.; Wu, W.; Wei, X.; Lu, Q. Enhancing the sensitivity of hexachlorobenzene electrochemicalsensor based on nitrogen–doped graphene. Sens. Actuat. B-Chem. 2016, 235, 439–446. [Google Scholar] [CrossRef]
- Bott, A.W. Electrochemical Methods for the Determination of Glucose. Curr. Sep. 1998, 17, 25–31. [Google Scholar]
- Chung, R.-J.; Wang, A.-N.; Liao, Q.-L.; Chuang, K.-Y. Non-Enzymatic Glucose Sensor Composed of Carbon-Coated Nano-Zinc Oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef]
- Sakamoto, M.; Takamura, K. 522—Catalytic oxidation of biological components on platinum electrodes modified by adsorbed metals. Anodic oxidation of glucose. Bioelectrochem. Bioenerg. 1982, 9, 571–582. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Wang, X.; Yang, S.; Wang, R. Electrochemical Oxidation and Determination of Glucose in Alkaline Media based on Au (111)-like Nanoparticle Array on Indium Tin Oxide Electrode. Electrochim. Acta 2014, 138, 174–186. [Google Scholar] [CrossRef]
- Luo, M.Z.; Baldwin, R.P. Characterization of carbohydrate oxidation at copper electrodes. J. Electroanal. Chem. 1995, 387, 87–94. [Google Scholar] [CrossRef]
- Larew, L.A.; Johnson, D.C. Concentration dependence of the mechanism of glucose oxidation at gold electrodes in alkaline media. J. Electroanal. Chem. 1989, 262, 167–182. [Google Scholar] [CrossRef]
- Karra, S.; Wooten, M.; Griffith, W.; Gorski, W. Morphology of Gold Nanoparticles and Electrocatalysis of Glucose Oxidation. Electrochim. Acta 2016, 218, 8–14. [Google Scholar] [CrossRef]
- Tominaga, M.; Shimazoe, T.; Nagashima, M.; Taniguchi, I. Electrocatalytic oxidation of glucose at gold nanoparticle-modified carbon electrodes in alkaline and neutral solutions. Electrochem. Commun. 2005, 7, 189–193. [Google Scholar] [CrossRef]
- Pop, A.; Manea, F.; Orha, C.; Motoc, S.; Ilinoiu, E.; Vaszilcsin, N.; Schoonman, J. Copper-decorated carbon nanotubes-based composite electrodes for nonenzymatic detection of glucose. Nanoscale Res. Lett. 2012, 7, 266–270. [Google Scholar] [CrossRef]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Manea, F.; Motoc, S.; Pop, A.; Remes, A.; Schoonman, J. Silver functionalized carbon nanofiber composite electrodes for ibuprofen detection. Nanoscale Res. Lett. 2012, 7, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Vamvakaki, V.; Tsagaraki, K.; Chaniotakis, N. Carbon Nanofiber-Based Glucose Biosensor. Anal. Chem. 2006, 78, 5538–5542. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Orha, C.; Pop, A. Enhanced Electrochemical Response of Diclofenac at a Fullerene-Carbon Nanofiber Paste Electrode. Sensors 2019, 19, 1332. [Google Scholar] [CrossRef]
- Mugweru, A.; Mahmud, R.; Ghosh, K.; Wanekaya, A.K. Carbon nanofiber modified with osmium based redox polymer for glucose sensing. J. Electrochem. Sci. Eng. 2017, 7, 181–191. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Li, L.; You, T. Direct Electrochemistry of Glucose Oxidase on Novel Free-Standing Nitrogen-Doped Carbon Nanospheres@Carbon Nanofibers Composite Film. Sci. Rep. 2015, 5, 09885. [Google Scholar] [CrossRef]
- Armaroli, N.; Accorsi, G.; Cardinali, F.; Listorti, A. Photochemistry and Photophysics of Coordination Compounds: Copper. Top. Curr. Chem. 2007, 280, 69–115. [Google Scholar]
- Fengzhao, T.S.; Xia, N. Metal Complexes as Molecular Electrocatalysts for Water Oxidation: A Mini-Review. Int. J. Electrochem. Sci. 2018, 13, 4601–4612. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Yu, G.-Q.; Wang, F.F.; Wei, P.-J.; Liu, J.-G. Bioinspired Transition-Metal Complexes as Electrocatalysts for the Oxygen Reduction Reaction. Chem. Eur. J. 2019, 25, 3726–3739. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Varnell, J.A.; DiAscro, A.M. Nonprecious Metal Catalysts for Oxygen Reduction in Heterogeneous Aqueous Systems. Chem. Rev. 2018, 118, 2313–2339. [Google Scholar] [CrossRef]
- Thorseth, A.; Tornow, C.E.; Tse, E.C.M.; Gewirth, A.A. Cu complexes that catalyze the oxygen reduction reaction. Coord. Chem. Rev. 2013, 257, 130–139. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Lee, Y.-M.; Nam, W. Mechanisms of Two-Electron versus Four-Electron Reduction of Dioxygen Catalyzed by Earth-Abundant Metal Complexes. ChemCatChem 2018, 10, 9–28. [Google Scholar] [CrossRef]
- Cariati, E.; Lucenti, E.; Botta, C.; Giovanella, U.; Marinotto, D.; Righetto, S. Cu(I) hybrid inorganic–organic materials with intriguing stimuli responsive and optoelectronic properties. Coord. Chem. Rev. 2016, 306, 566–614. [Google Scholar] [CrossRef]
- Ilmi, R.; Al-Busaidi, I.J.; Haque, A.; Khan, M.S. REVIEW: Recent progress in coordination chemistry, photo-physical properties and applications of pyridine-based Cu(I) complexes. J. Coord. Chem. 2018, 71, 3045–3076. [Google Scholar] [CrossRef]
- Crispini, A.; Cretu, C.; Aparaschivei, D.; Andelescu, A.A.; Sasca, V.; Badea, V.; Aiello, I.; Szerb, E.I.; Costisor, O. Influence of the counterion on the geometry of Cu(I) and Cu(II) complexes with 1,10-phenanthroline. Inorg. Chim. Acta 2018, 470, 342–351. [Google Scholar] [CrossRef]
- Fransted, K.A.; Jackson, N.E.; Zong, R.; Mara, M.W.; Huang, J.; Harpham, M.R.; Shelby, M.L.; Thummel, R.P.; Chen, L.X. Ultrafast Structural Dynamics of Cu(I)-Bicinchoninic Acid and Their Implications for Solar Energy Applications. J. Phys. Chem. A 2014, 118, 10497–10506. [Google Scholar] [CrossRef] [PubMed]
- Cretu, C.; Andelescu, A.A.; Candreva, A.; Crispini, A.; Szerb, E.I.; La Deda, M. Bisubstituted-biquinoline Cu(I) complexes: Synthesis, mesomorphism and photophysical studies in solution and condensed states. J. Mater. Chem. C 2018, 6, 10073–10082. [Google Scholar] [CrossRef]
- Nie, C.; Tong, X.; Wu, S.; Gong, S.; Peng, D. Paraffin confined in carbon nanotubes as nanoencapsulated phase change materials: Experimental and molecular dynamics studies. RSC Adv. 2015, 5, 92812–92817. [Google Scholar] [CrossRef]
- Marioli, J.M.; Kuwana, T. Electrochemical characterization of carbohydrate oxidation at copper electrodes. Electrochim. Acta 1992, 37, 1187–1197. [Google Scholar] [CrossRef]
- Manea, F.; Radovan, C.; Schoonman, J. Amperometric determination of thiourea in alkaline media on a copper oxide–copper electrode. J. Appl. Electrochem. 2006, 36, 1075–1081. [Google Scholar] [CrossRef]
- Ramirez-Garcia, S.; Alegret, S.; Cespedes, F.; Forster, R.J. Carbon composite electrodes: Surface and electrochemical properties. Analyst 2002, 127, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Stulik, K.; Amatore, C.; Holub, K.; Marecek, V.; Kutner, W. Microelectrodes. Definitions, characterization and applications. Pure Appl. Chem. 2000, 72, 1483–1492. [Google Scholar] [CrossRef]
- Simm, A.O.; Banks, C.E.; Ward-Jones, S.; Davies, T.J.; Lawrence, N.S.; Jones, T.G.J.; Jiang, L. Boron-doped diamond microdisc arrays: Electrochemical characterisation and their use as a substrate for the production of microelectrode arrays of diverse metals (Ag, Au, Cu) via electrodeposition. Analyst 2005, 130, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.J.; Compton, R.G. The cyclic and linear sweep voltammetry of regular and random arrays of microdisc electrodes: Theory. J. Electroanal. Chem. 2005, 585, 63–82. [Google Scholar] [CrossRef]
- Cieciwa, A.; Wuthrich, R.; Comninellis, C. Electrochemical characterization of mechanically implanted boron-doped diamond electrodes. Electrochem. Commun. 2006, 8, 375–382. [Google Scholar] [CrossRef]
- Na, W.; Lee, J.; Jun, J.; Kim, W.; Kim, Y.K.; Jang, J. Highly sensitive copper nanowire conductive electrode for nonenzymatic glucose detection. J. Ind. Eng. Chem. 2019, 69, 358–363. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion coefficients of ferri- and ferrocyanide ions in aqueous media, using twin-electrode thin-layer electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Jiang, J. Synthesis of the crystalline porous copper oxide architectures derived from metal-organic framework for electrocatalytic oxidation and sensitive detection of glucose. J. Ind. Eng. Chem. 2019, 70, 330–337. [Google Scholar] [CrossRef]
- Wu, C.-H.; Onno, E.; Lin, C.-Y. CuO nanoparticles decorated nano-dendrite-structured CuBi2O4 for highly sensitive and selective electrochemical detection of glucose. Electrochim. Acta 2017, 229, 129–140. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Ki, H.-Y.; Kim, B.-S. A novel CuS microflower superstructure based sensitive and selective nonenzymatic glucose detection. Sens. Actuators B 2016, 233, 93–99. [Google Scholar] [CrossRef]
- Reitz, E.; Jia, W.; Gentile, M.; Wang, Y.; Lei, Y. CuO Nanospheres Based Nonenzymatic Glucose Sensor. Electroanalysis 2008, 20, 2482–2486. [Google Scholar]
- Li, S.; Zheng, Y.; Qin, G.W.; Ren, Y.; Pei, W.; Zuo, L. Enzyme-free amperometric sensing of hydrogen peroxide and glucose at a hierarchical Cu2O modified electrode. Talanta 2011, 85, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, F.; Yu, Q.; Fan, R.; Yang, M.; Rao, S.; Lan, Q.; Yang, Z.; Yang, Z. Three-dimensional porous Cu@Cu2O aerogels for direct voltammetric sensing of glucose. Microchim. Acta 2019, 186, 192. [Google Scholar] [CrossRef] [PubMed]

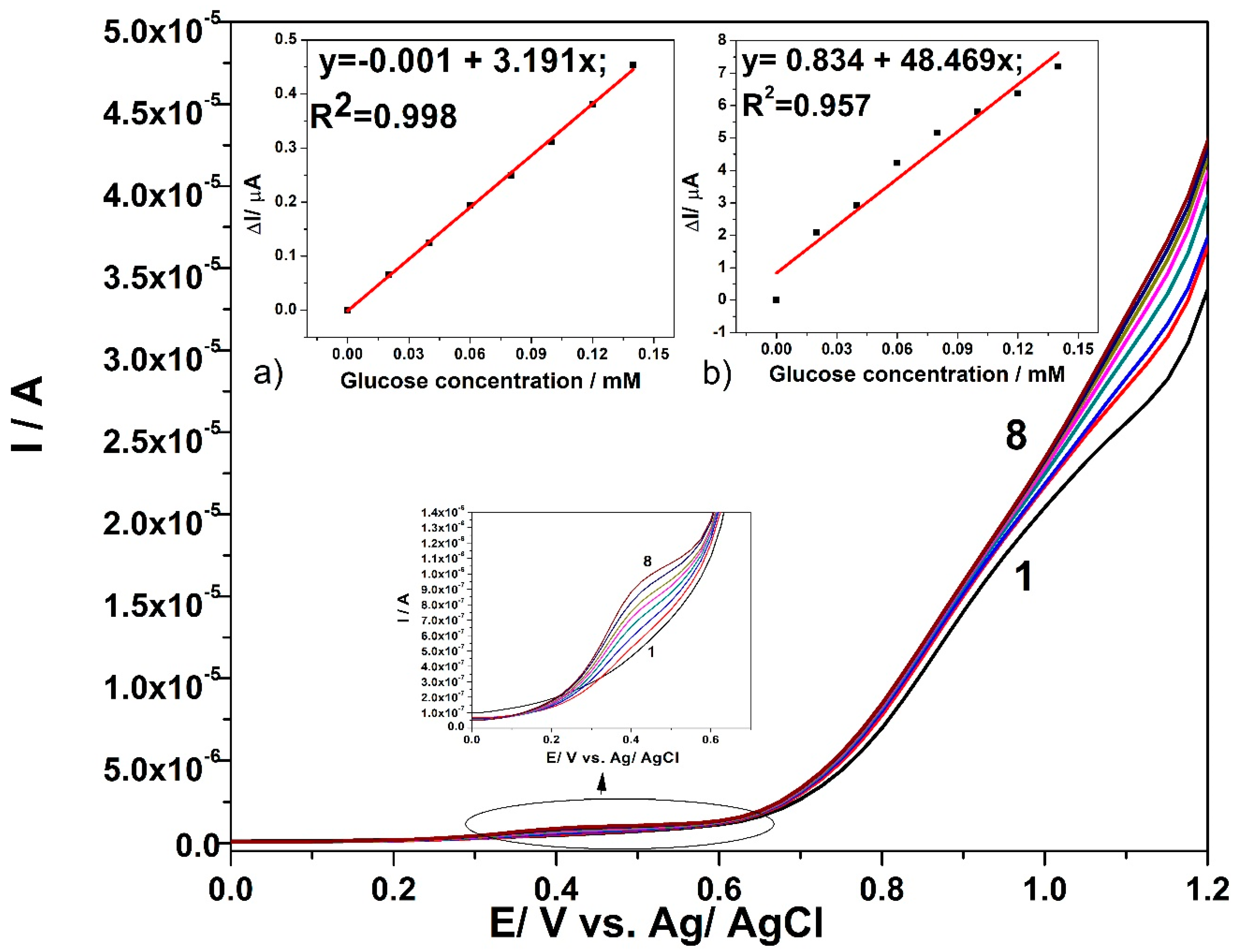
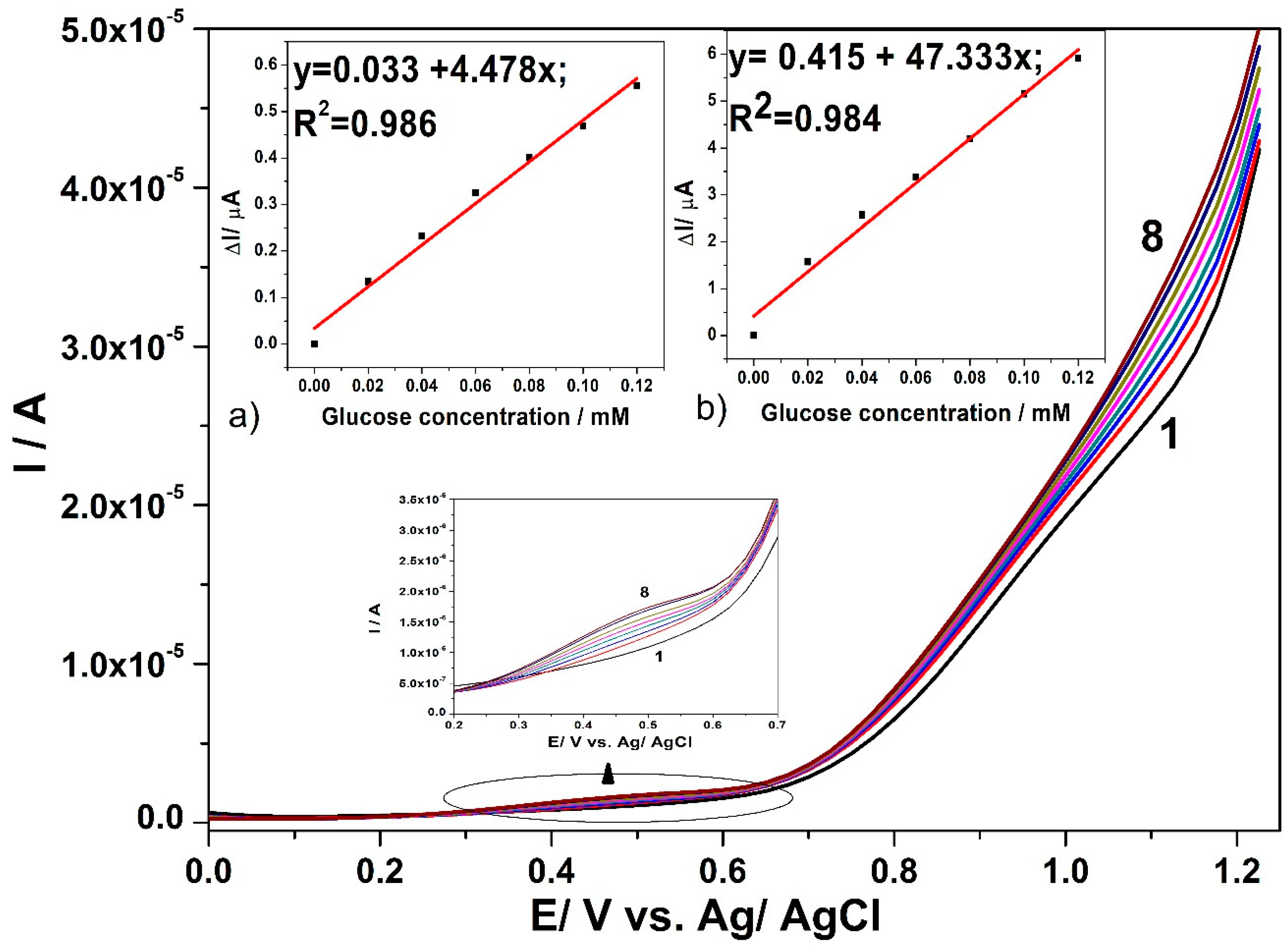
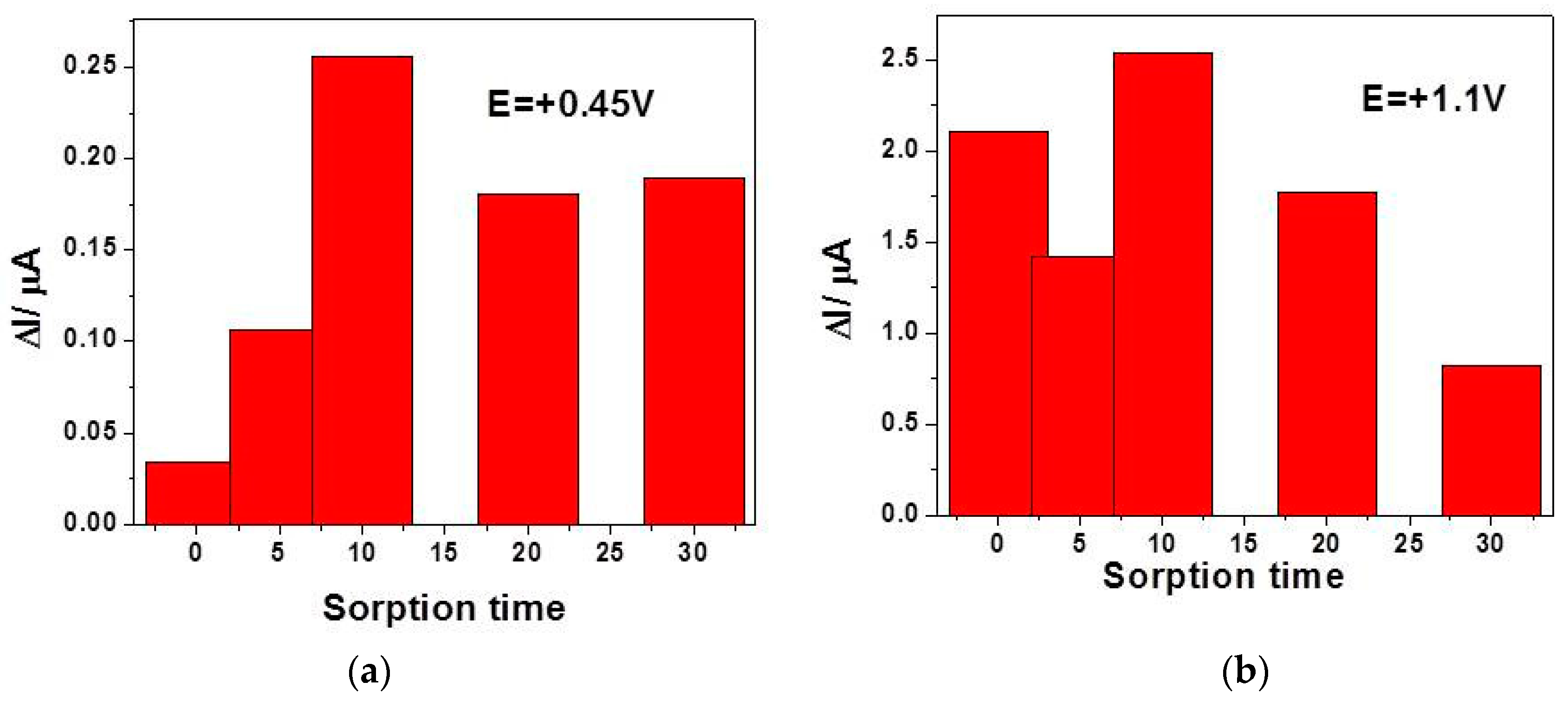
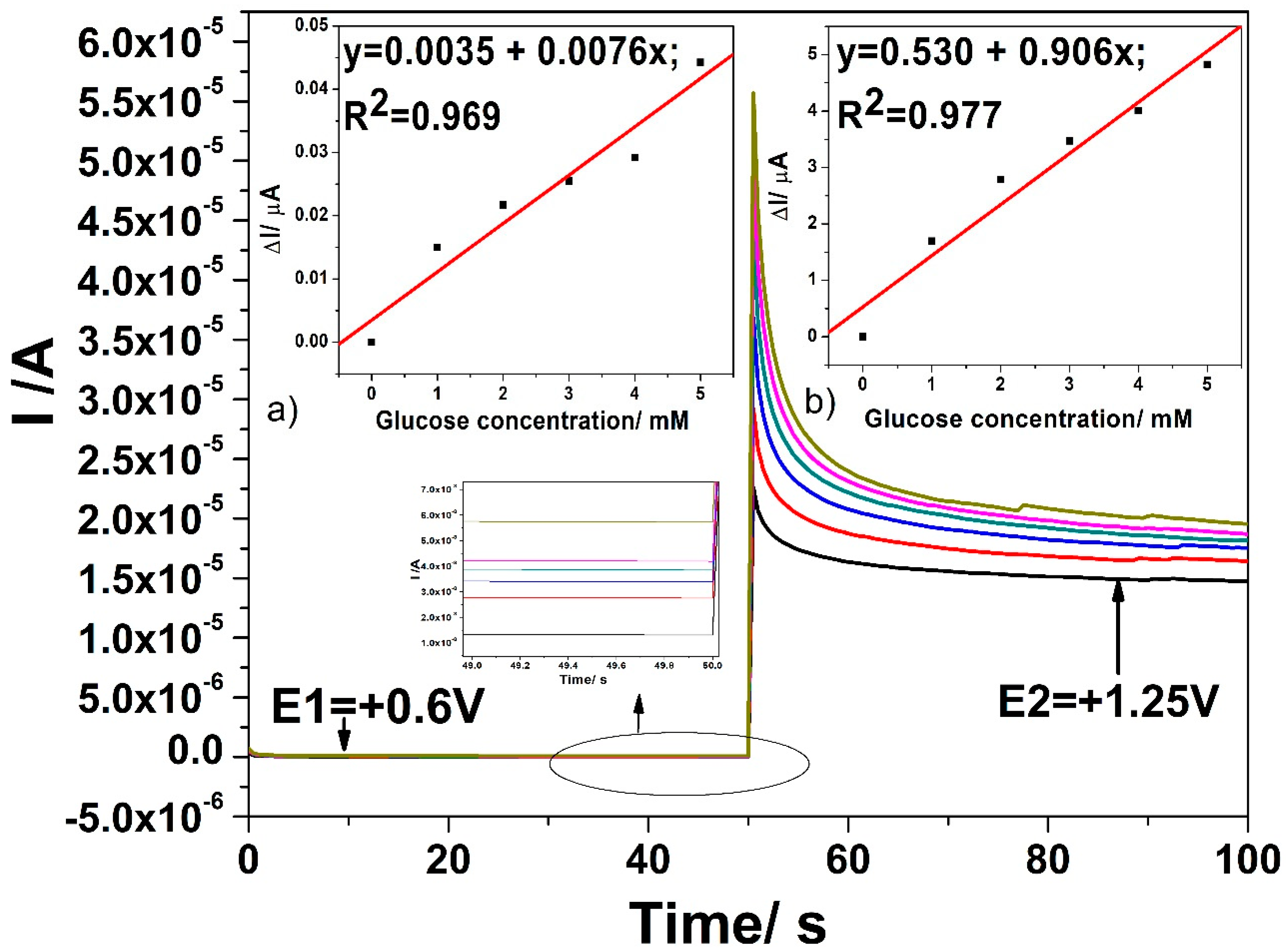
| Tehnique | Potential Detection/ V vs. Ag/AgCl | Sensitivity/ µA·mM−1cm−2 | Correlation Coefficient/R2 | LOD[a]/µM | LQ[a]/µM | RSD[b](%) |
|---|---|---|---|---|---|---|
| CV | +0.6 V | 8.70 | 0.995 | 39 | 130 | 1.7 |
| +1.2 V | 1953.30 | 0.998 | 1.88 | 6 | 1.5 | |
| DPV | +0.4 V | 181.31 | 0.998 | 0.54 | 1.8 | 1.2 |
| +1.1 V | 2753.92 | 0.957 | 0.07 | 0.23 | 0.5 | |
| SWV | +0.45 V | 254.43 | 0.986 | 0.38 | 1.2 | 0.5 |
| +1.1 V | 2689.38 | 0.984 | 0.07 | 0.24 | 1 | |
| Preconc./SWV | +0.45 V | 451.25 | 0.979 | 0.43 | 1.45 | 1.6 |
| +1.1 V | 5419.77 | 0.986 | 0.05 | 0.16 | 0.5 | |
| CA | +0.6 V | 0.43 | 0.969 | 22 | 75 | 0.5 |
| +1.25 V | 51.48 | 0.977 | 3.3 | 11 | 0.5 | |
| MPA | +0.6 V for 0.05 s | 80.63 | 0.976 | 1.2 | 4.06 | 0.5 |
| +1.25 for 0.1 s | 2462.67 | 0.984 | 0.082 | 0.27 | 0.5 |
| Electrode Material | Linear Range (mM) | Supporting Electrolyte | Technique Used | Sensitivity (µA·mM−1·cm−2) | Detection Limit, µM | Reference |
|---|---|---|---|---|---|---|
| MOF-derived CuO arhitectures | 0.0005–2.8 | 0.1 M NaOH | CA | 934.2 | 0.1 | [48] |
| CuO/CuBi2O4 | 0.1–8 | 0.1 M NaOH | CA | 330 | 0.7 | [49] |
| CuS microflowers | 0.001–5.4 | 0.1 M NaOH | CA | 1007 | n.a. | [50] |
| CuO nanospheres | 0.05–20 | 0.1 M NaOH | CA | 404.53 | 1 | [51] |
| CuO/graphene | 5–14 | Phosphate buffer (pH = 7.4) | CA | 37.63 | 5 | [9] |
| CuO/graphene | 0.00021–12 | 0.1 M NaOH | CV | 408.16 | 0.21 | [9] |
| Cu-Cu2O nanoporous NPs | 0.01–55 | 0.1 M NaOH | CA | 123.8 | 0.05 | [7] |
| Cu-Cu2O hollow microspheres | 0.22–10.89 | 0.1 M NaOH | CA | 33.63 | 0.05 | [8] |
| Cu2O NPs | 0.05–1.1 | 0.1 M NaOH | CA | 53.69 | 47.2 | [52] |
| 3D Cu@Cu2O aerogels | 0.001–17.2 | 0.1 M NaOH | CA | 194.88 | 0.60 | [53] |
| CuOx/CNF | 0.02–0.14 | 0.1 M NaOH | Preconc.SWV/SWV | 5419.7/2754[a] | 0.048/0.07[a] | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoc, S.; Cretu, C.; Costisor, O.; Baciu, A.; Manea, F.; Szerb, E.I. Cu(I) Coordination Complex Precursor for Randomized CuOx Microarray Loaded on Carbon Nanofiber with Excellent Electrocatalytic Performance for Electrochemical Glucose Detection. Sensors 2019, 19, 5353. https://doi.org/10.3390/s19245353
Motoc S, Cretu C, Costisor O, Baciu A, Manea F, Szerb EI. Cu(I) Coordination Complex Precursor for Randomized CuOx Microarray Loaded on Carbon Nanofiber with Excellent Electrocatalytic Performance for Electrochemical Glucose Detection. Sensors. 2019; 19(24):5353. https://doi.org/10.3390/s19245353
Chicago/Turabian StyleMotoc, Sorina, Carmen Cretu, Otilia Costisor, Anamaria Baciu, Florica Manea, and Elisabeta I. Szerb. 2019. "Cu(I) Coordination Complex Precursor for Randomized CuOx Microarray Loaded on Carbon Nanofiber with Excellent Electrocatalytic Performance for Electrochemical Glucose Detection" Sensors 19, no. 24: 5353. https://doi.org/10.3390/s19245353
APA StyleMotoc, S., Cretu, C., Costisor, O., Baciu, A., Manea, F., & Szerb, E. I. (2019). Cu(I) Coordination Complex Precursor for Randomized CuOx Microarray Loaded on Carbon Nanofiber with Excellent Electrocatalytic Performance for Electrochemical Glucose Detection. Sensors, 19(24), 5353. https://doi.org/10.3390/s19245353







