The Growth of Ga2O3 Nanowires on Silicon for Ultraviolet Photodetector
Abstract
1. Introduction
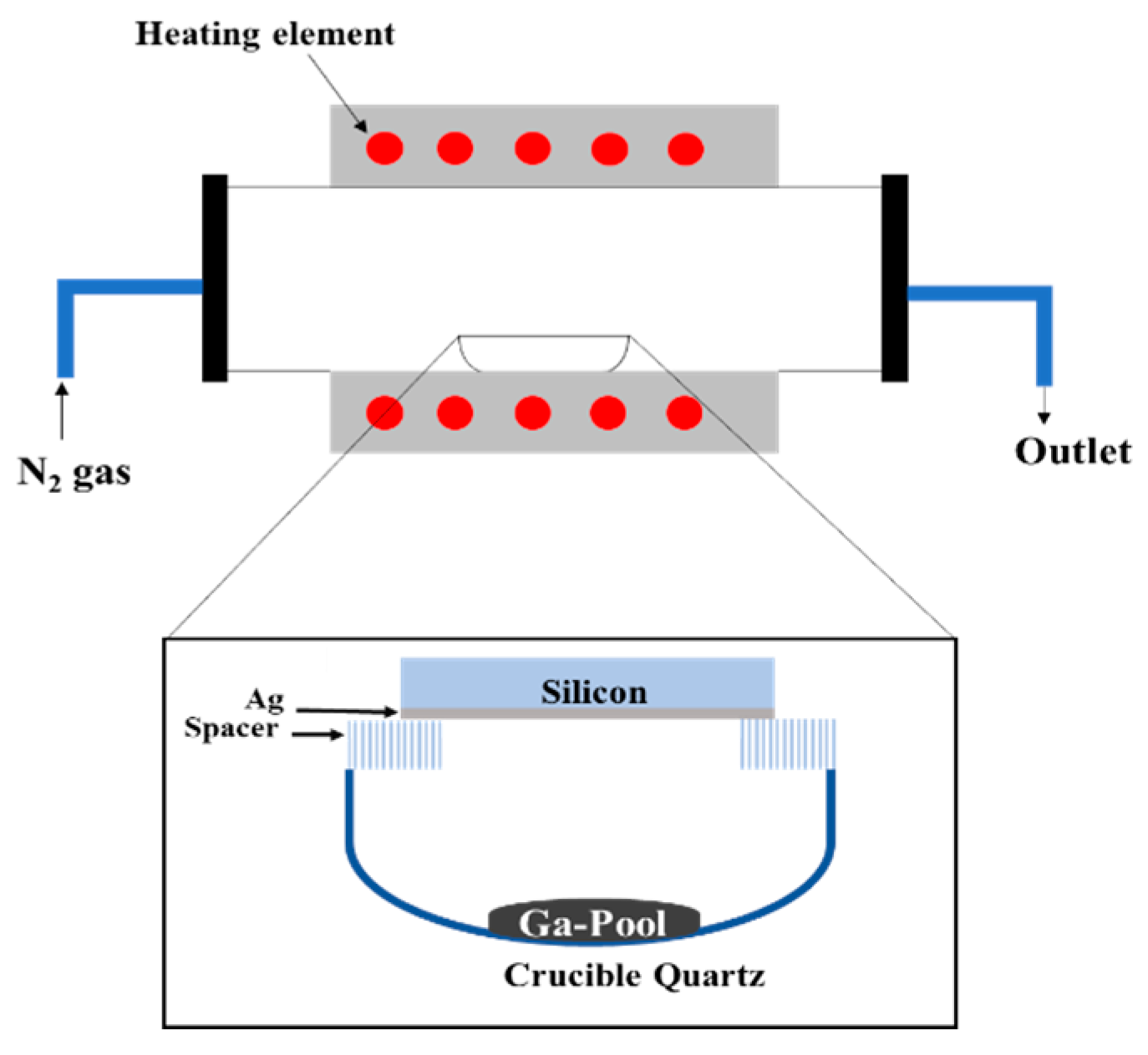
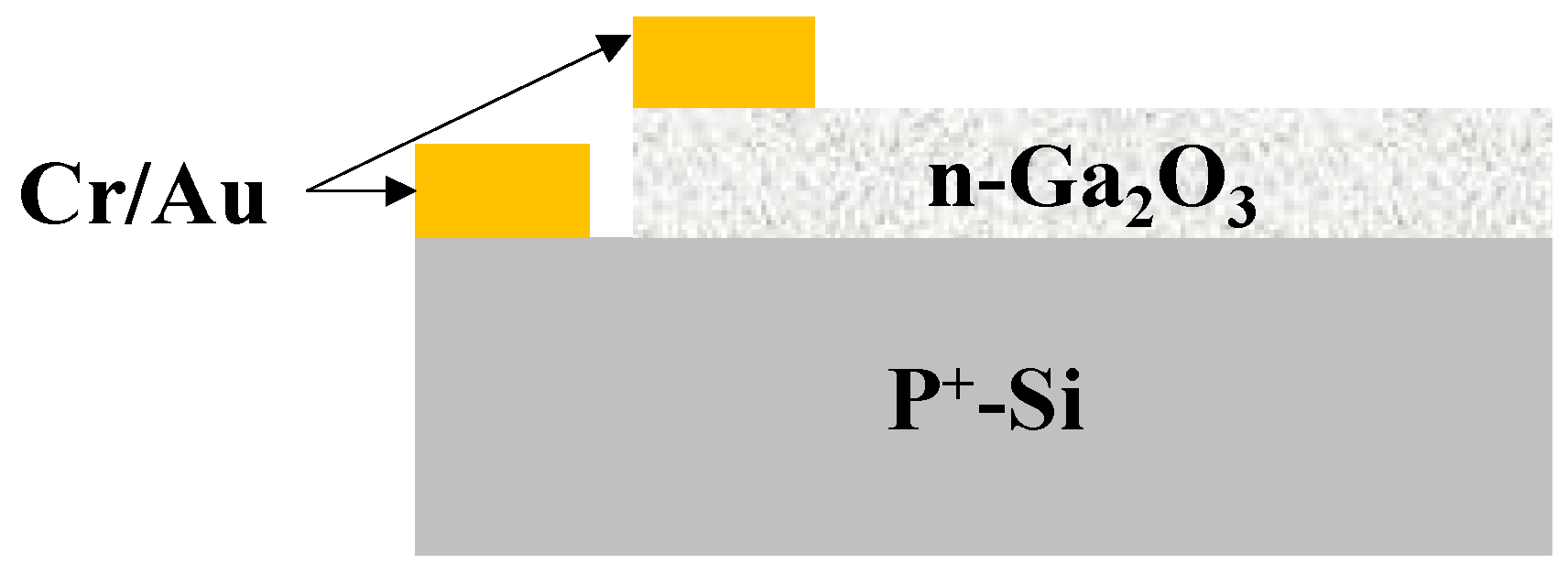
2. Materials and Methods
3. Results and Discussion
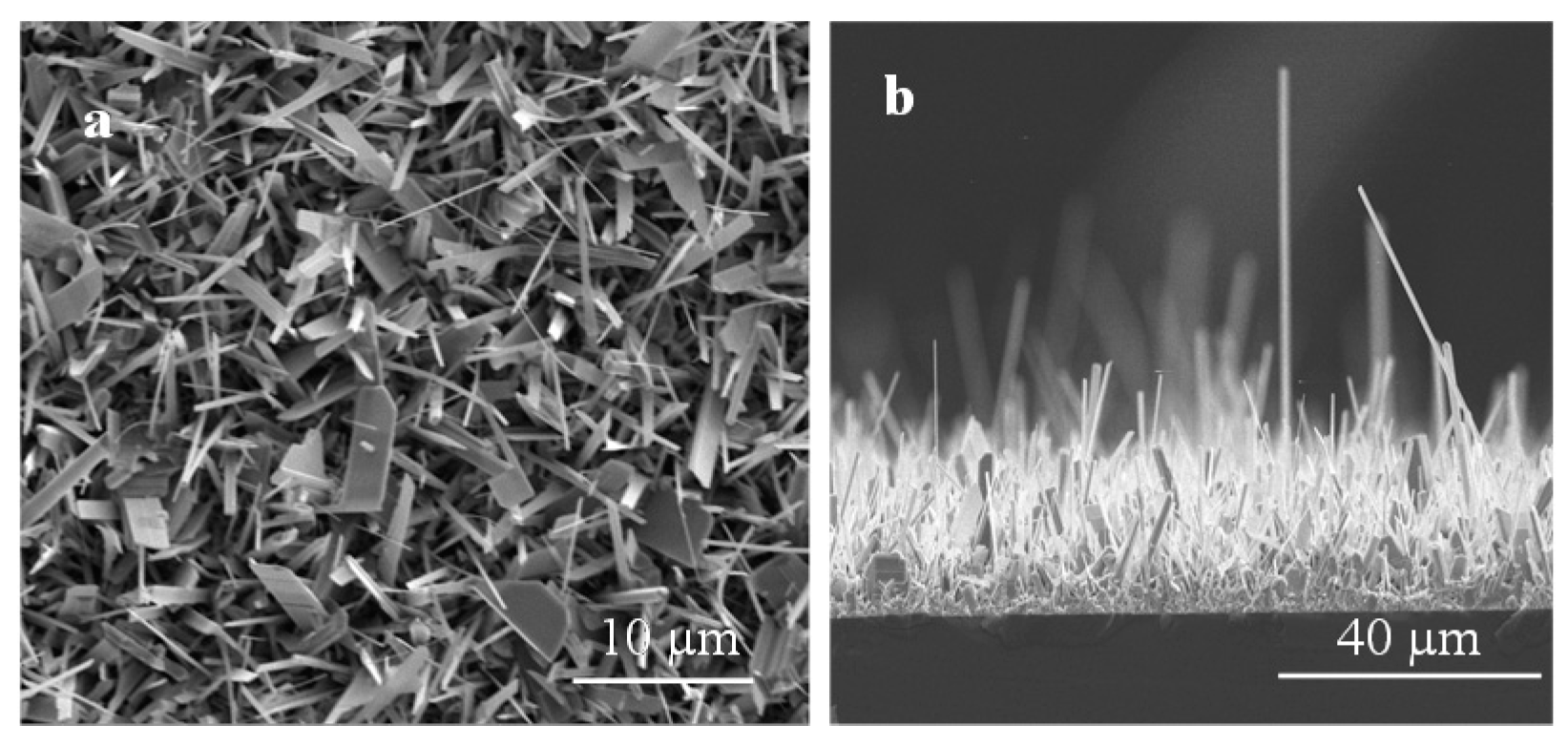
3.1. Surface Morphology
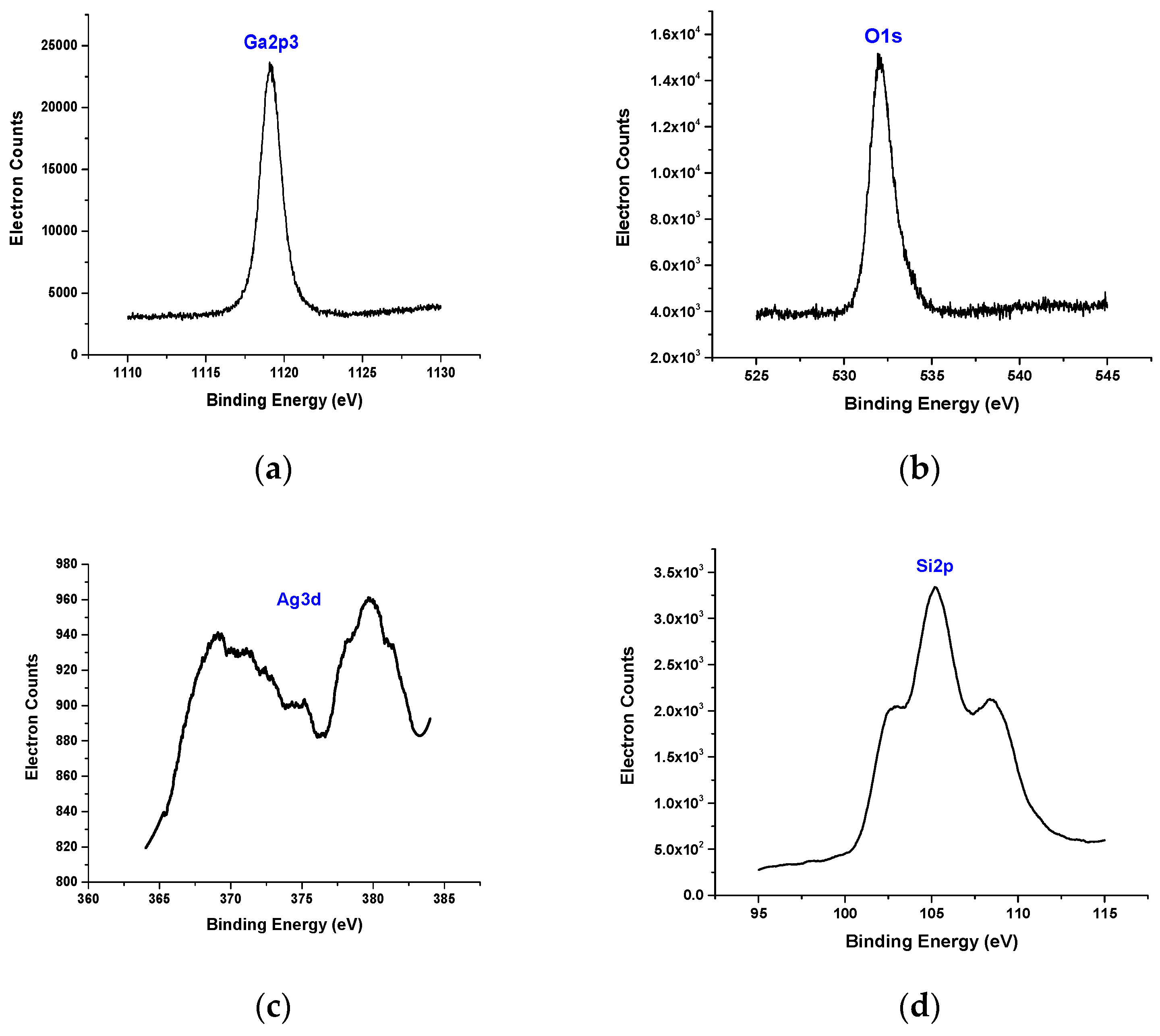
3.2. X-ray Photoelectron Spectroscopy (XPS)
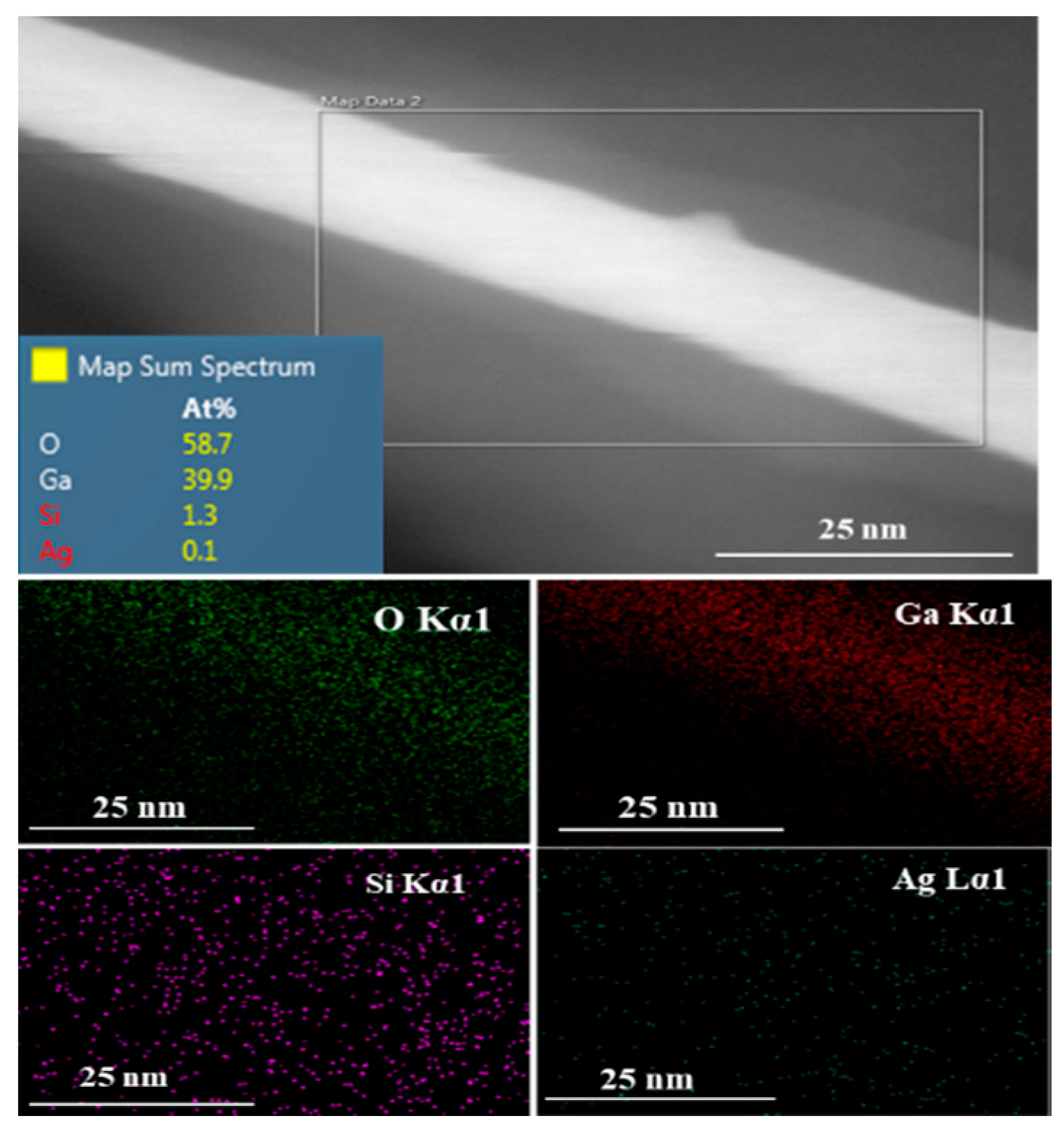
3.3. High-Resolution Transmission Electron Microscopy (HRTEM)/Energy-Dispersive Spectroscopy (EDS)
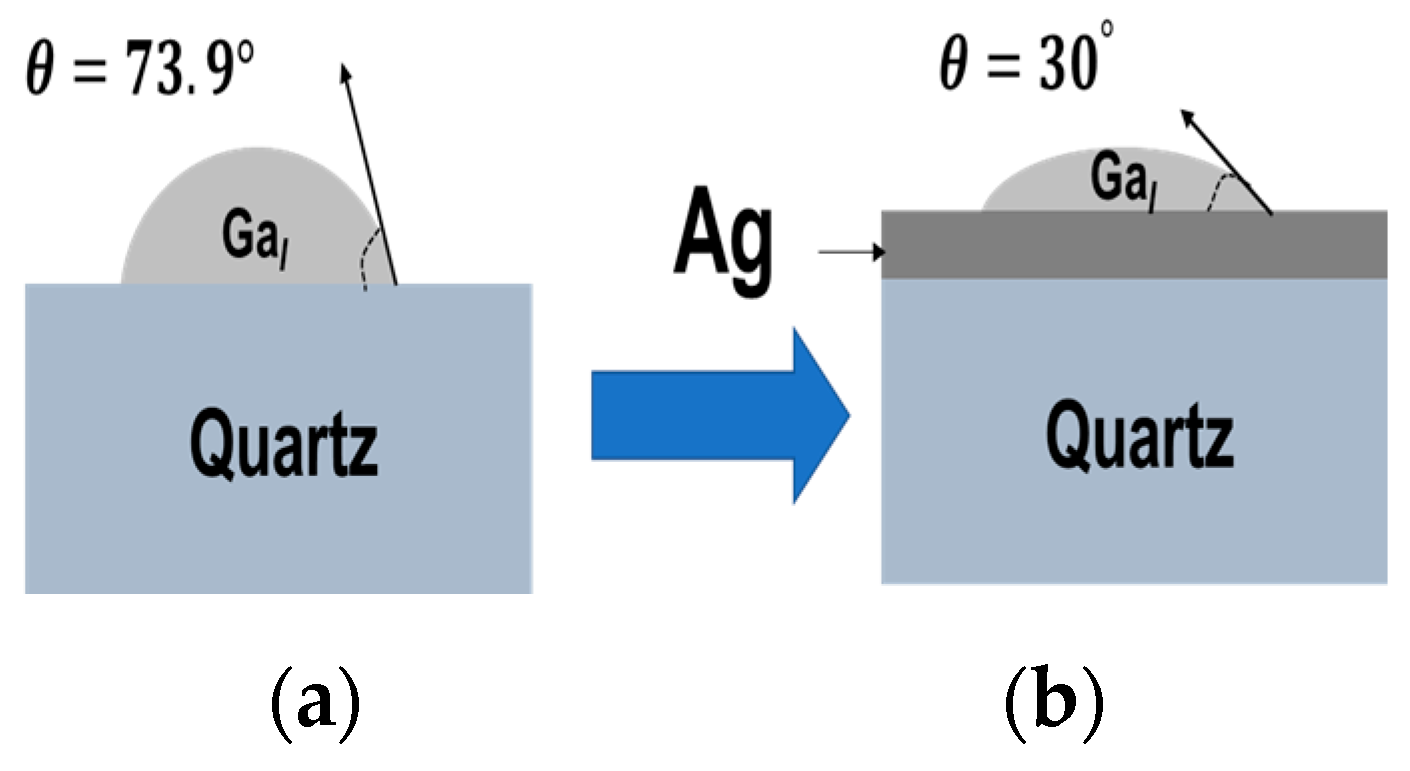
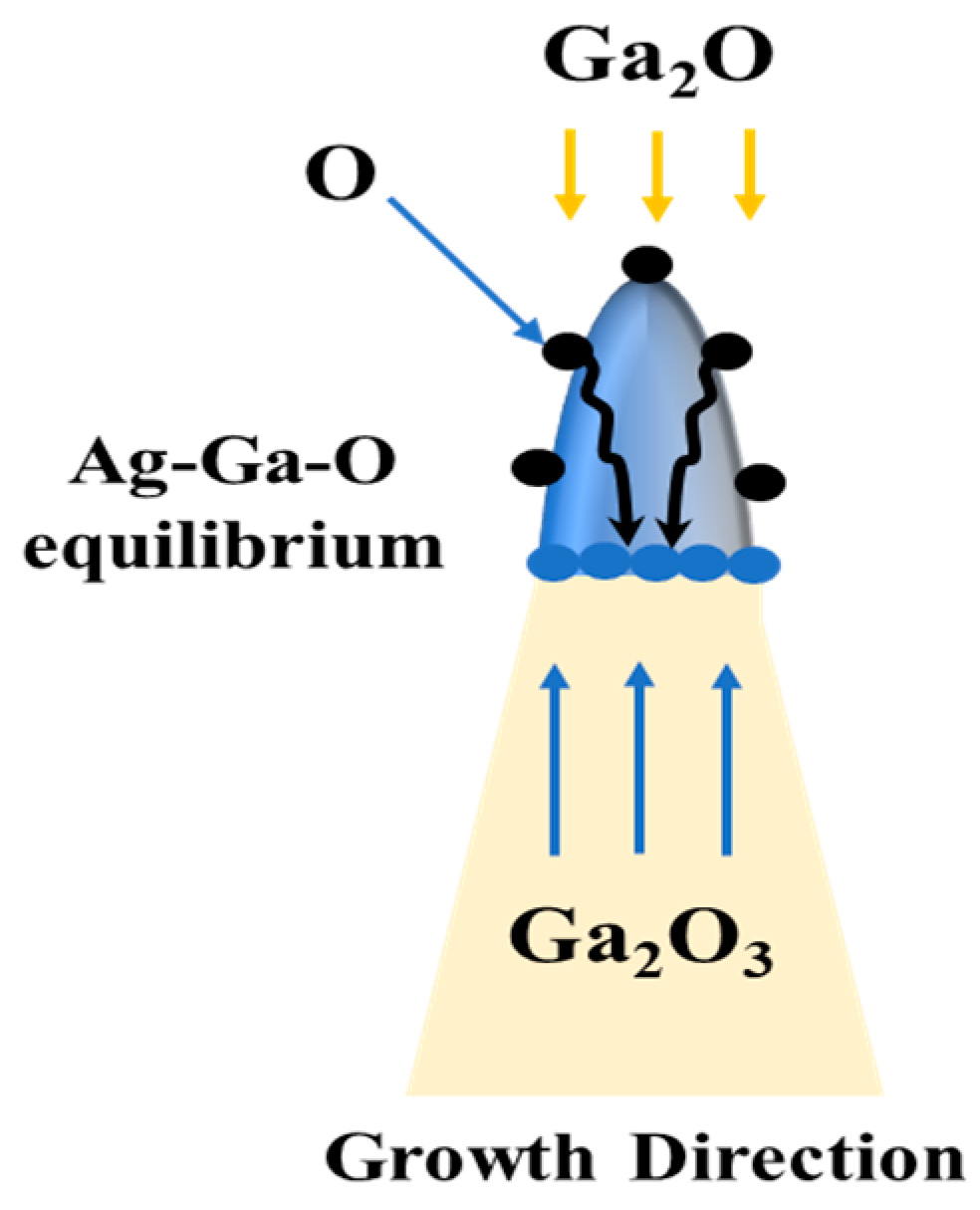
3.4. Growth Mechanism of β-Ga2O3 Nanowires
3.5. Electrical Characterization
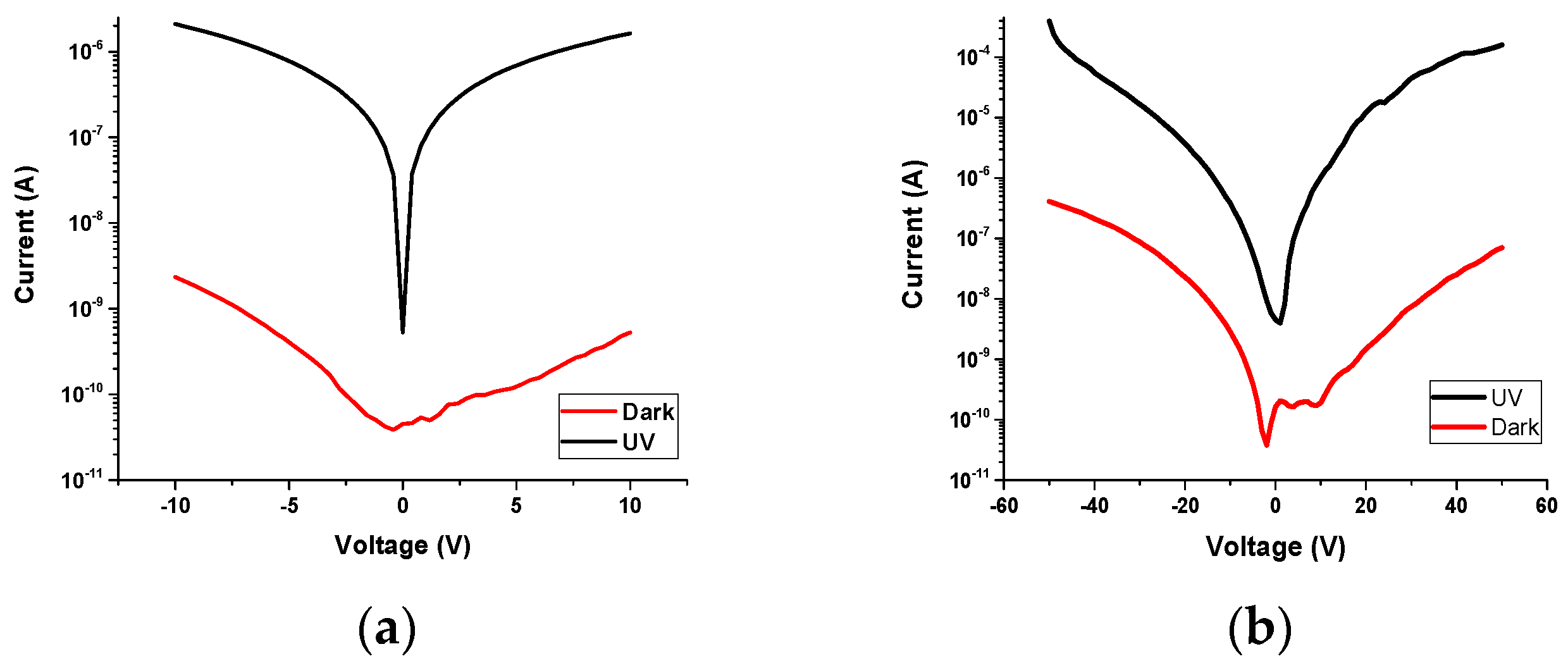
3.5.1. I-V Characterization
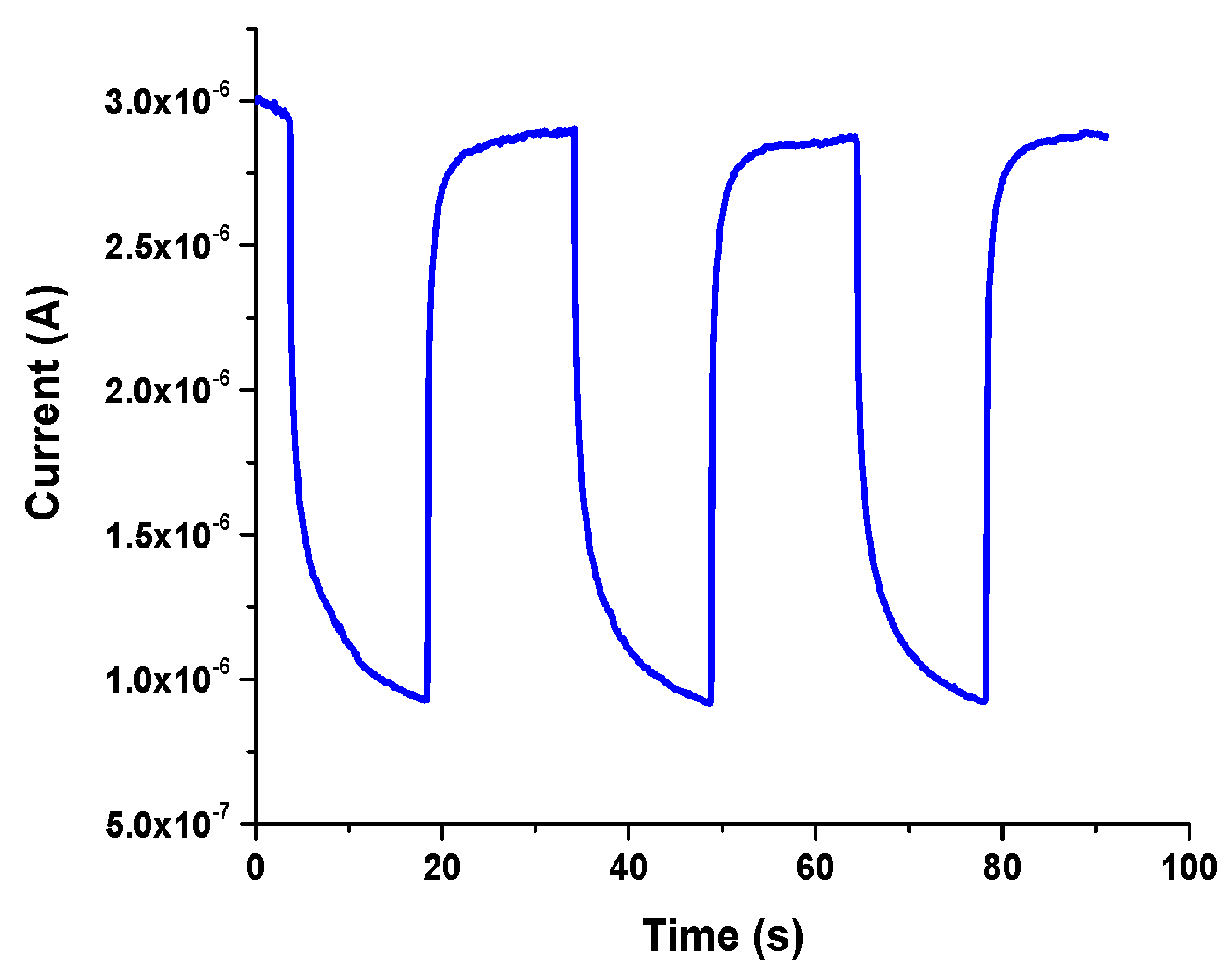
3.5.2. Transient Time
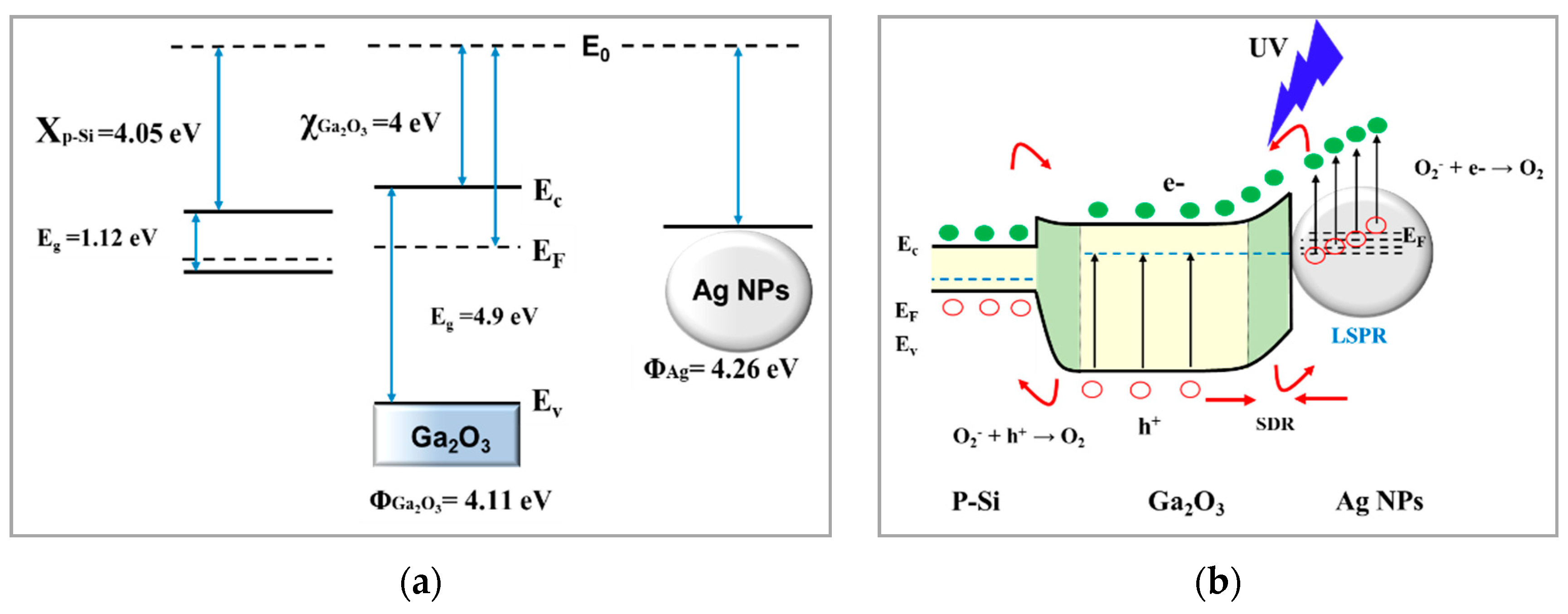
3.5.3. Detection Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González-Posada, R.S.F.; den Hertog, M.; Monroy, E. Room-Temperature Photodetection Dynamics of Single GaN Nanowires. Nano Lett. 2012, 12, 172–176. [Google Scholar] [CrossRef]
- Weng, W.Y.; Hsueh, T.J.; Chang, S.J.; Huang, G.J.; Hsueh, H.T. A beta-Ga2O3 Solar-Blind Photodetector Prepared by Furnace Oxidization of GaN Thin Film. IEEE Sens. J. 2011, 11, 999–1003. [Google Scholar] [CrossRef]
- Mazeina, L.; Perkins, F.K.; Bermudez, V.M.; Arnold, S.P.; Prokes, S.M. Functionalized Ga2O3 Nanowires as Active Material in Room Temperature Capacitance-Based Gas Sensors. Langmuir 2010, 26, 13722–13726. [Google Scholar] [CrossRef]
- Lin, H.J.; Baltrus, J.P.; Gao, H.; Ding, Y.; Nam, C.Y.; Ohodnicki, P.; Gao, P.X. Perovskite Nanoparticle-Sensitized Ga2O3 Nanorod Arrays for CO Detection at High Temperature. ACS Appl. Mater. Interface. 2016, 8, 8880–8887. [Google Scholar] [CrossRef]
- Mao, A.K.H.; Gao, J.; Chopdekar, R.; Takamura, Y.; Chowdhury, S.; Islam, M.S. An Investigation of Electrical and Dielectric Parameters of Sol-Gel Process Enabled beta-Ga2O3 as a Gate Dielectric Material. IEEE Trans. Elect. Devices 2017, 64, 2047–2053. [Google Scholar]
- Pearton, S.J.; Yang, J.; Carey, P.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Kaya, A. β-Ga2O3 films grown via oxidation of GaAs substrates and their device demonstrations. In Proceedings of the Wide Bandgap Power Devices and Applications II SPIE, San Diego, CA, USA, 7–8 August 2017. [Google Scholar]
- Patil-Chaudhari, D.; Ombaba, M.; Oh, J.Y.; Mao, H.; Montgomery, K.; Lange, A.; Mahajan, S.; Woodall, J.M.; Islam, M.S. Solar Blind Photodetectors Enabled by Nanotextured β-Ga2O3 Films Grown via Oxidation of GaAs Substrates. IEEE Photon. J. 2017, 9, 1–7. [Google Scholar] [CrossRef]
- Otsuka, S.; Katayama, I.; Kozuka, Z. Measurements of Diffusivity of Oxygen in Liquid Silver by Potentiostatic Methods Employing Solid Electrolyte. Trans. Jpn. Inst. Met. 1971, 12, 442–447. [Google Scholar] [CrossRef][Green Version]
- Nguyen, T.D.; Kim, E.T.; Dao, K.A. Ag nanoparticle catalyst based on Ga2O3/GaAs semiconductor nanowire growth by VLS method. J. Mater. Sci. Mater. Electron. 2015, 26, 8747–8752. [Google Scholar] [CrossRef]
- Guo, D.; Wu, Z.; Li, P.; Wang, Q.; Lei, M.; Lie, L.; Tang, W. Magnetic anisotropy and deep ultraviolet photoresponse characteristics in Ga2O3: Cr vermicular nanowire thin film nanostructure. RSC Adv. 2015, 5, 12894–12898. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, K.H.; Kang, H.C. Growth mechanism of In-doped beta- Ga2O3 nanowires deposited by radio frequency powder sputtering. Mater. Lett. 2016, 176, 213–218. [Google Scholar] [CrossRef]
- Choi, K.H.; Cho, K.K.; Kim, K.W.; Cho, G.B.; Ahn, H.J.; Nam, T.H. Catalytic Growth and Structural Characterization of Semiconducting beta-Ga2O3 Nanowires. J. Nanosci. Nanotechnol. 2009, 9, 3728–3733. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Sun, G.J.; Lee, C. UV-assisted room temperature-gas sensing of Ga2O3-core/ZnO-shell nanowires. J. Ceram. Process. Res. 2015, 16, 367–371. [Google Scholar]
- Jang, Y.G.; Kim, W.S.; Kim, D.H.; Hong, S.H. Fabrication of Ga2O3/SnO2 core-shell nanowires and their ethanol gas sensing properties. J. Mater. Res. 2011, 26, 2322–2327. [Google Scholar] [CrossRef]
- Ghose, S.; Rahman, M.S.; Arias, A.; Rojas-Ramirez, J.S.; Caro, M.; Nedev, N.; Droopad, R. Structural and optical properties of beta-Ga2O3 thin films grown by plasma-assisted molecular beam epitaxy. J. Vac. Sci. Technol. B 2016, 34. [Google Scholar] [CrossRef]
- Feng, Q.; Li, F.; Dai, B.; Jia, Z.; Xie, W.; Xu, T.; Lu, X.; Tao, X.; Zhang, J.; Hao, Y. The properties of gallium oxide thin film grown by pulsed laser deposition. Appl. Surf. Sci. 2015, 359, 847–852. [Google Scholar] [CrossRef]
- Han, W.Q.; Kohler-Redlich, P.; Ernst, F.; Ruhle, M. Growth and microstructure of Ga2O3 nanorods. Solid State Commun. 2000, 115, 527–529. [Google Scholar] [CrossRef]
- Cao, C.B.; Chen, Z.; An, X.Q.; Zhu, H.S. Growth and field emission properties of cactus-like gallium oxide nanostructures. J. Phys. Chem. C 2008, 112, 95–98. [Google Scholar] [CrossRef]
- Sharma, S.; Sunkara, M.K. Direct synthesis of gallium oxide tubes, nanowires, and nanopaintbrushes. J. Am. Chem. Soc. 2002, 124, 12288–12293. [Google Scholar] [CrossRef]
- Pallister, P.J.; Buttera, S.C.; Barry, S.T. Self-seeding gallium oxide nanowire growth by pulsed chemical vapor deposition. Phys. Status Solidi Appl. Mater. Sci. 2015, 212, 1514–1518. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Frost, R.L.; Yang, J.; Martens, W.N. Size and morphology control of gallium oxide hydroxide GaO(OH), nano- to micro-sized particles by soft-chemistry route without surfactant. J. Phys. Chem. C 2008, 112, 3568–3579. [Google Scholar] [CrossRef]
- Reddy, L.S.; Ko, Y.H.; Yu, J.S. Hydrothermal Synthesis and Photocatalytic Property of beta-Ga2O3 Nanorods. Nanoscale Res. Lett. 2015, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, S.; Wang, Z.; Adamo, G.; Liu, H.; Huang, Y.; Couteau, C.; Soci, C. GaAs/AlGaAs Nanowire Photodetector. Nano Lett. 2014, 14, 2688–2693. [Google Scholar] [CrossRef]
- Alhalaili, B.; Mao, H.; Islam, M.S. Ga2O3 Nanowire Synthesis and Device Applications. In Novel Nanomaterials—Synthesis and Applications; IntechOpen Limited: London, UK, 2017; Volume 2. [Google Scholar]
- Ogita, M.; Higo, K.; Nakanishi, Y.; Hatanaka, Y. Ga2O3 thin film for oxygen sensor at high temperature. Appl. Surf. Sci. 2001, 175–176, 721–725. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, N.H.; Lee, C. Growth of Ga2O3 thin films on Si (100) substrates using a trimethylgallium and oxygen mixture. J. Mater. Sci. 2004, 39, 3461–3463. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chang, S.-J.; Weng, W.-Y.; Liu, C.; Tsai, T.Y.; Hsu, C.-L.; Chen, K.C. Ga2O3 Nanowire Photodetector Prepared on SiO2/Si Template. IEEE Sens. J. 2013, 13, 2368–2373. [Google Scholar] [CrossRef]
- Alhalaili, B.; Bunk, R.; Vidu, R.; Islam, M.S. Dynamics Contributions to the Growth Mechanism of Ga2O3 Thin Film and NWs Enabled by Ag Catalyst. Nanomaterials 2019, 9, 1272. [Google Scholar] [CrossRef]
- Glickman, E.; Levenshtein, M.; Budic, L.; Eliaz, N. Interaction of liquid and solid gallium with thin silver films: Synchronized spreading and penetration. Acta Mater. 2011, 59, 914–926. [Google Scholar] [CrossRef]
- Detz, H.; Kriz, M.; MacFarland, D.; Lancaster, S.; Zederbauer, T.; Capriotti, M.; Andrews, A.M.; Schrenk, W.; Strasser, G. Nucleation of Ga droplets on Si and SiOx surfaces. Nanotechnology 2015, 26. [Google Scholar] [CrossRef]
- Mao, H.; Alhalaili, B.; Kaya, A.; Dryden, D.M.; Woodall, J.M.; Islam, M.S. Oxidation of GaAs substrates to enable β-Ga2O3 films for sensors and optoelectronic devices (SPIE Optical Engineering + Applications). In Proceedings of the Wide Bandgap Power Devices and Applications II, San Diego, CA, USA, 7–8 August 2017. [Google Scholar]
- Song, P.Y.; Wu, Z.Y.; Shen, X.Y.; Kang, J.Y.; Fang, Z.L.; Zhang, T.Y. Self-consistent growth of single-crystalline ((2)over-bar01) beta-Ga2O3 nanowires using a flexible GaN seed nanocrystal. Crystengcomm 2017, 19, 625–631. [Google Scholar] [CrossRef]
- Chun, H.J.; Choi, Y.S.; Bae, S.Y.; Seo, H.W.; Hong, S.J.; Park, J.; Yang, H. Controlled structure of gallium oxide nanowires. J. Phys. Chem. B 2003, 107, 9042–9046. [Google Scholar] [CrossRef]
- Crist, V. Handbook of Monochromatic XPS Spectra: The Elements of Native Oxides; Wiley-VCH: Wenheim, Germany, 2000. [Google Scholar]
- Logofatu, C.; Negrila, C.C.; Ghita, R.V.; Ungureanu, F.; Cotirlan, C.; Manea, C.G.A.S.; Lazarescu, M.F. Study of SiO2/Si Interface by Surface Techniques. Cryst. Silicon Prop. Uses 2011, 23–42. [Google Scholar] [CrossRef]
- Dong, C.Y.; Shang, D.S.; Shi, L.; Sun, J.; Shen, B.G.; Zhuge, F.; Li, R.-W.; Chen, W. Roles of silver oxide in the bipolar resistance switching devices with silver electrode. Appl. Phys. Lett. 2011, 98. [Google Scholar] [CrossRef]
- Salido, I.L. Electronic and Geometric Properties of Silver and Gold Nanoparticles. Ph.D. Thesis, University of Konstanz, Konstanz, Germany, 2007. [Google Scholar]
- Chevalier, P.Y. Thermodynamic Evaluation of the Ag-Si System. Thermochim. Acta 1988, 130, 33–41. [Google Scholar] [CrossRef]
- Varley, J.B.; Weber, J.R.; Janotti, A.; van de Walle, C.G. Oxygen vacancies and donor impurities in beta-Ga2O3. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Gould, A.L.; Kadkhodazadeh, S.; Wagner, J.B.; Catlow, C.R.A.; Logsdail, A.J.; di Vece, M. Understanding the Thermal Stability of Silver Nanoparticles Embedded in a-Si. J. Phys. Chem. C 2015, 119, 23767–23773. [Google Scholar] [CrossRef]
- Zinkevich, M.; Aldinger, F. Thermodynamic assessment of the gallium-oxygen system. J. Am. Ceram. Soc. 2004, 87, 683–691. [Google Scholar] [CrossRef]
- Klinedinst, K.A.; Stevenson, D.A. Oxygen Diffusion in Liquid Gallium and Indium. J. Electrochem. Soc. 1973, 120, 304–308. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Ma, Y.M.; Han, Q.F.; Liu, Y.L. Solubility, permeation, and capturing of impurity oxygen in Au/Ag: A comparative investigation from first-principles. Comput. Mater. Sci. 2016, 114, 79–85. [Google Scholar] [CrossRef]
- Ramanarayanan, T.A.; Rapp, R.A. The Diffusivity and Solubility of Oxygen in Liquid Tin and Solid Silver and the Diffusivity of Oxygen in Solid Nickel. Metall. Trans. 1972, 3, 3239–3246. [Google Scholar] [CrossRef]
- Park, J.H. Measuring Oxygen Diffusivity and Solubility in Solid Silver with a Gas-Tight Electrochemical-Cell. Mater. Lett. 1990, 9, 313–316. [Google Scholar] [CrossRef]
- Kontoulis, I.; Steele, B.C.H. Determination of Oxygen Diffusion in Solid Ag by an Electrochemical Technique. Solid State Ion. 1991, 47, 317–324. [Google Scholar] [CrossRef]
- Heshmatpour, B.; Stevenson, D.A. An Electrochemical Study of the Solubility and Diffusivity of Oxygen in the Respective Liquid-Metals Indium, Gallium, Antimony and Bismuth. J. Electroanal. Chem. 1981, 130, 47–55. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Mastelaro, V.R.; Mangalaraj, D. Catalyst free vapor–solid deposition of morphologically different β-Ga2O3 nanostructure thin films for selective CO gas sensors at low temperature. Anal. Method 2016, 3224–3235. [Google Scholar] [CrossRef]
- Butt, D.P.; Park, Y.; Taylor, T.N. Thermal vaporization and deposition of gallium oxide in hydrogen. J. Nucl. Mater. 1999, 264, 71–77. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R. Nanofunctional gallium oxide (Ga2O3) nanowires/nanostructures and their applications in nanodevices. Phys. Status Solidi Rapid Res. Lett. 2013, 7, 781–792. [Google Scholar] [CrossRef]
- Xu, C.; Chung, S.; Kim, M.; Kim, D.E.; Chon, B.; Hong, S.; Joo, T. Doping of Si into GaN nanowires and optical properties of resulting composites. J. Nanosci. Nanotechnol. 2005, 5, 530–535. [Google Scholar] [CrossRef]
- Walukiewicz, W. Intrinsic limitations to the doping of wide-gap semiconductors. Phys. B 2001, 302, 123–134. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Yanagi, H.; Kamiyab, T. Field-induced current modulation in epitaxial film of deep-ultraviolet transparent oxide semiconductor Ga2O3. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Villora, E.G.; Shimamura, K.; Yoshikawa, Y.; Ujiie, T.; Aoki, K. Electrical conductivity and carrier concentration control in beta-Ga2O3 by Si doping. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Shao, D.L.; Yu, M.P.; Sun, H.T.; Hu, T.; Lian, J.; Sawyer, S. High responsivity, fast ultraviolet photodetector fabricated from ZnO nanoparticle-graphene core-shell structures. Nanoscale 2013, 5, 3664–3667. [Google Scholar] [CrossRef]
- Oh, S.; Mastro, M.A.; Tadjer, M.J.; Kim, J. Solar-Blind Metal-Semiconductor-Metal Photodetectors Based on an Exfoliated beta- Ga2O3 Micro-Flake. ECS J. Solid State Sci. Technol. 2017, 6, Q79–Q83. [Google Scholar] [CrossRef]
- Guo, D.Y.; Wu, Z.; An, Y.H.; Guo, X.; Chu, X.L.; Sun, C.L.; Li, L.; Li, P.G.; Tang, W.H. Oxygen vacancy tuned Ohmic-Schottky conversion for enhanced performance in β-Ga2O3 solar-blind ultraviolet photodetectors. Appl. Phys. Lett. 2014, 105, 023507. [Google Scholar] [CrossRef]
- Manjavacas, A.; Liu, J.; Kulkarni, V.; Nordlander, P. Plasmon-Induced Hot Carriers in Metallic Nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef]
- Oh, J.; Ma, J.; Yoo, G. Simulation study of reduced self-heating in β-Ga2O3 MOSFET on a nano-crystalline diamond substrate. Results Phys. 2019, 13, 102151. [Google Scholar] [CrossRef]
- Kleyn, A.W.; Butler, D.A.; Raukema, A. Dynamics of the interaction of O2 with silver surfaces. Surf. Sci. 1996, 363, 29–41. [Google Scholar] [CrossRef]
- Hou, Y.N.; Mei, Z.X.; Liang, H.L.; Ye, D.Q.; Liang, S.; Gu, C.Z.; Du, X.L. Comparative study of n-MgZnO/p-Si ultraviolet-B photodetector performance with different device structures. Appl. Phys. Lett. 2011, 98, 263501. [Google Scholar] [CrossRef]
- Mohamed, M.; Irmscher, K.; Janowitz, C.; Galazka, Z.; Manzke, R.; Fornari, R. Schottky barrier height of Au on the transparent semiconducting oxide beta-Ga2O3. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Emilio, M.G.; Alarcon, I.; Klas, I.U. Silver Nanoparticle Applications: In the Fabrication and Design of Medical and Biosensing Devices; Springer Berlin Heidelberg: New York, NY, USA, 2015. [Google Scholar]
- Arora, K.; Kumar, V.; Kumar, M. Silver plasmonic density tuned polarity switching and anomalous behaviour of high performance self-powered β-gallium oxide solar blind photodetector. arXiv 2018, arXiv:1809.10724. Available online: https://arxiv.org/pdf/1809.10724 (accessed on 25 October 2019).
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO nanowire UV photodetectors with high internal gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.D.; Hernandez-Ramirez, F.; Jimenez-Diaz, R.; Manzanares, M.; Andreu, T.; Cirera, A.; Romano-Rodriguez, A.; Morante, J.R. The effects of electron-hole separation on the photoconductivity of individual metal oxide nanowires. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Guan, X.; Zhu, X.; Hu, H.; Bozhevolnyi, S.I.; Oxenloewe, L.K.; Jin, K.; Mortensen, N.A.; Xiao, S. Effective electro-optic modulation in low-loss graphene-plasmonic slot waveguides. Nanoscale 2017, 9. [Google Scholar] [CrossRef] [PubMed]
| Metal | Diffusion Coefficient (D)(cm2/s) | Activation Energy (EA) (eV/K) | T (°C) | Year | Ref. |
|---|---|---|---|---|---|
| Ags | 1.79 × 10−3 | 0.58 | 127–977 | 2016 | [44] |
| 4.90 × 10−3 | 0.56 | 740–915 | 1972 | [45] | |
| 3.66 × 10−3 | 0.48 | 1990 | [46] | ||
| 4.98 × 10−3 | 0.63 | 1991 | [47] | ||
| Agl | 20.1 × 10−4 | 0.91 | 980–1130 | 1971 | [9] |
| 4.9 × 10−3 | 0.12 | 763–937 | 1972 | [45] | |
| Gal | 4.1 × 10−3 | 8.9 × 10−5 | 750–950 | 1981 | [43] |
| 2.27 × 10−3 | 8.33 × 10−5 | 750–1000 | 1972 | [48] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhalaili, B.; Vidu, R.; Islam, M.S. The Growth of Ga2O3 Nanowires on Silicon for Ultraviolet Photodetector. Sensors 2019, 19, 5301. https://doi.org/10.3390/s19235301
Alhalaili B, Vidu R, Islam MS. The Growth of Ga2O3 Nanowires on Silicon for Ultraviolet Photodetector. Sensors. 2019; 19(23):5301. https://doi.org/10.3390/s19235301
Chicago/Turabian StyleAlhalaili, Badriyah, Ruxandra Vidu, and M. Saif Islam. 2019. "The Growth of Ga2O3 Nanowires on Silicon for Ultraviolet Photodetector" Sensors 19, no. 23: 5301. https://doi.org/10.3390/s19235301
APA StyleAlhalaili, B., Vidu, R., & Islam, M. S. (2019). The Growth of Ga2O3 Nanowires on Silicon for Ultraviolet Photodetector. Sensors, 19(23), 5301. https://doi.org/10.3390/s19235301





