An Automatic Head Surface Temperature Extraction Method for Top-View Thermal Image with Individual Broiler
Abstract
1. Introduction
2. Materials and Methods
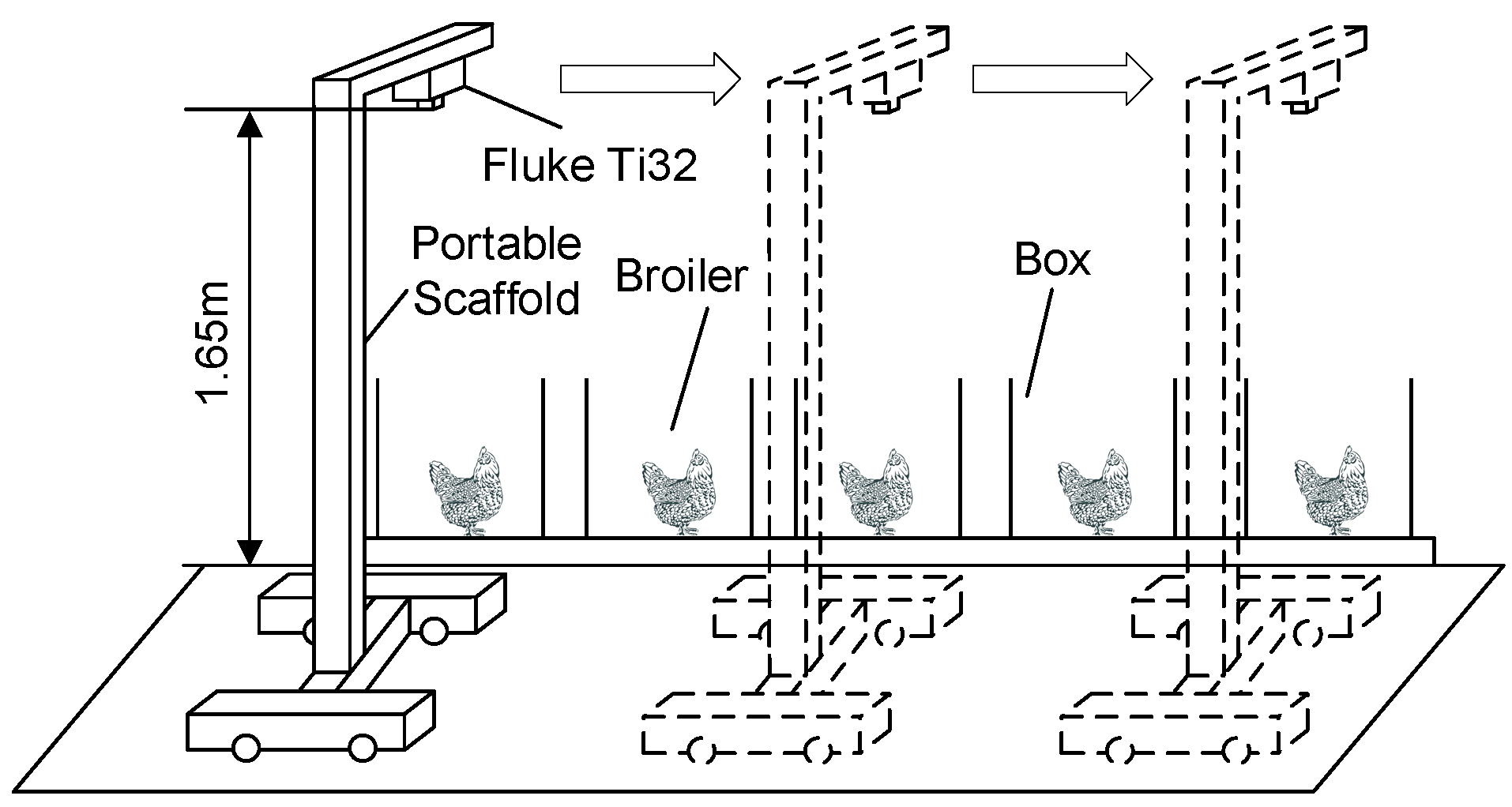
2.1. Image System
2.2. Broilers and Thermal Images Acquisition
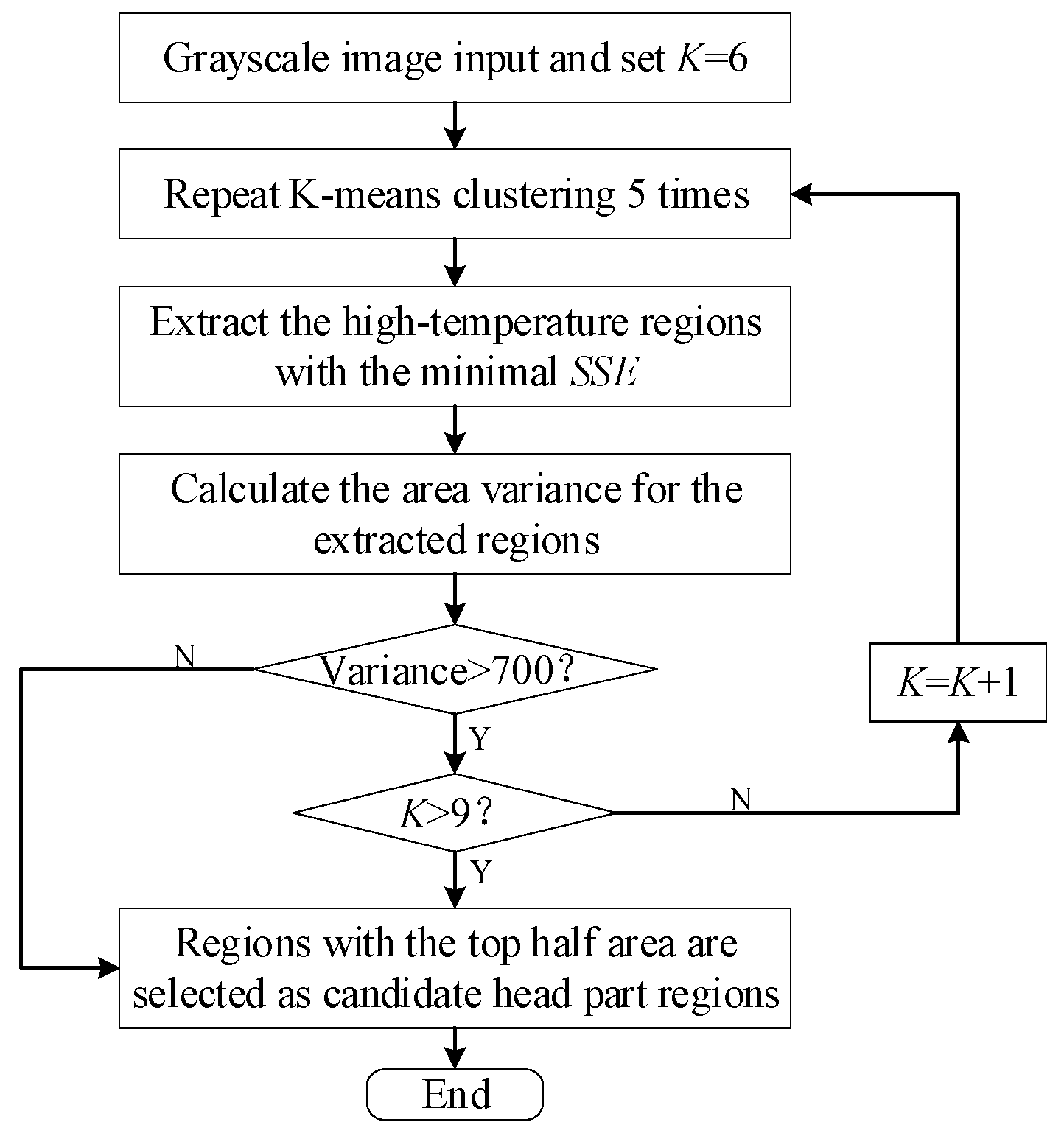
2.3. Head Surface Temperature Extraction
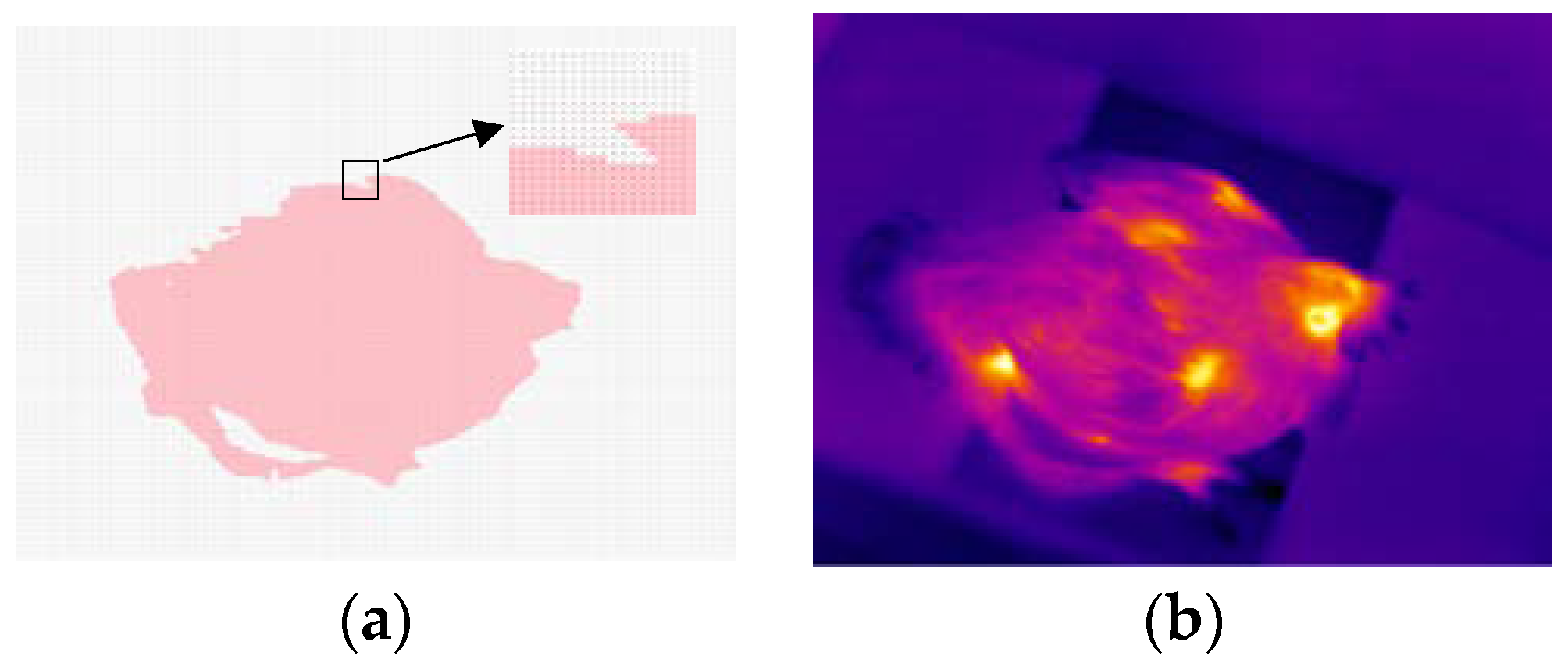
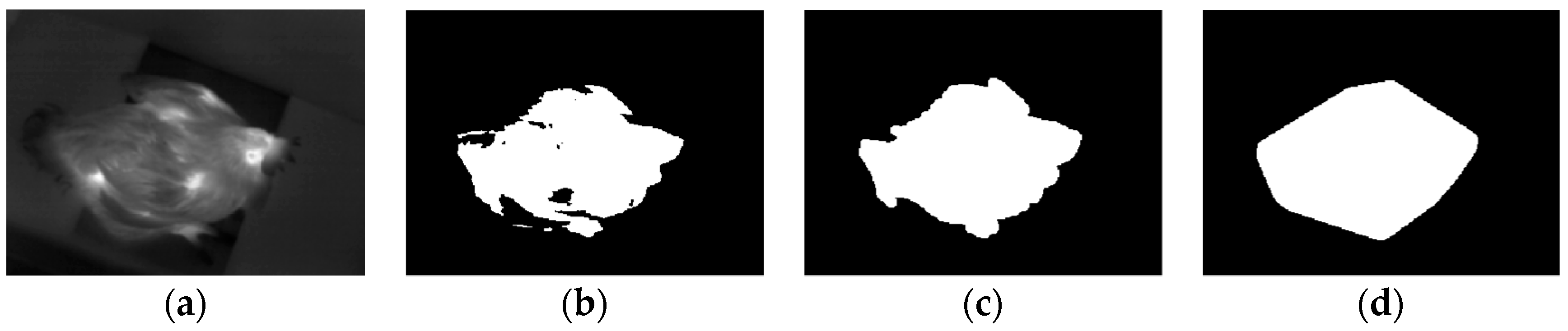
2.3.1. Thermal Image Pre-Processing
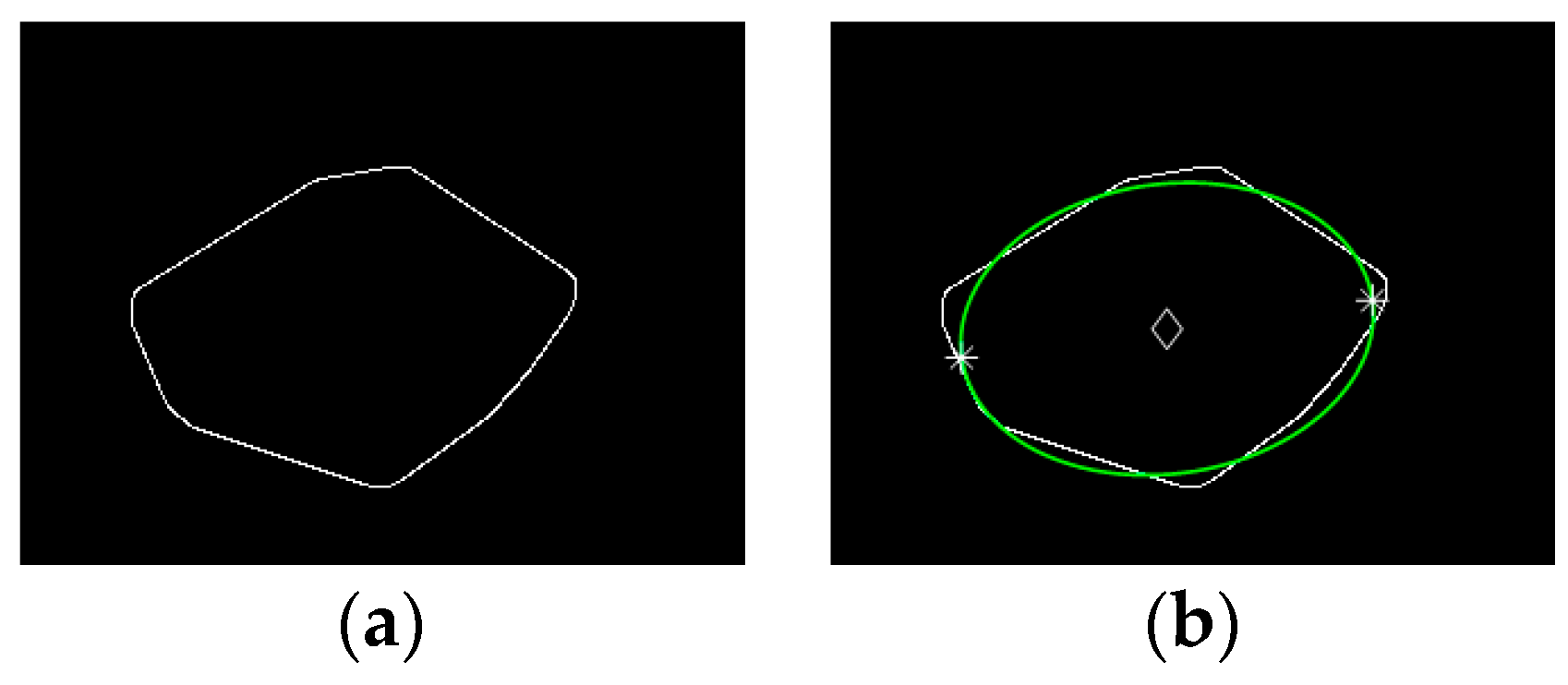
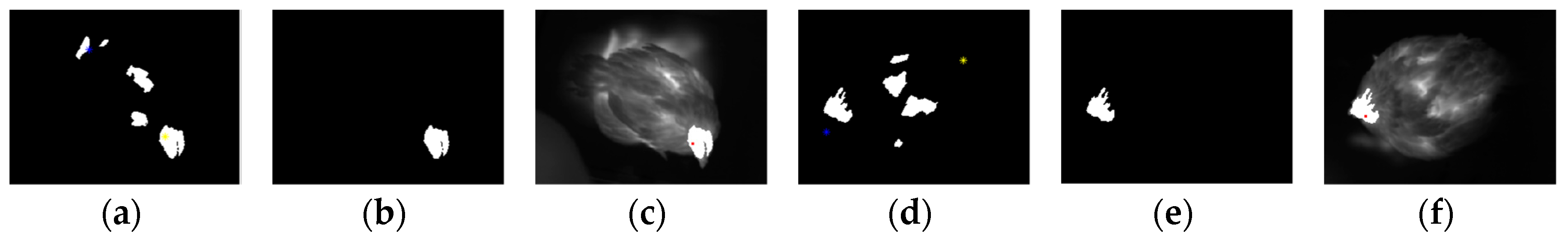
2.3.2. Head Region Locating
2.3.3. Construction of RHT Time Series
3. Results
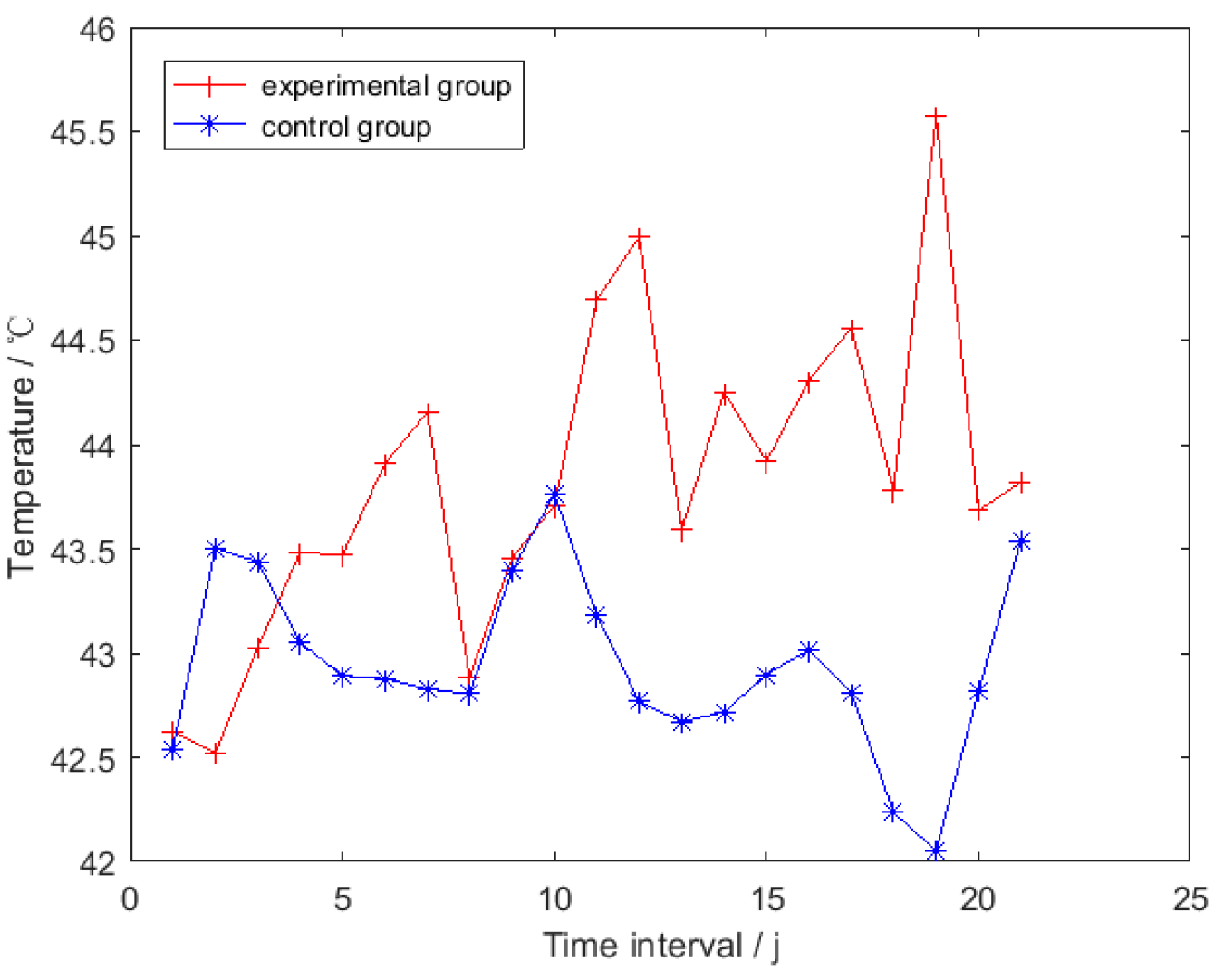
3.1. Testing of HSTE
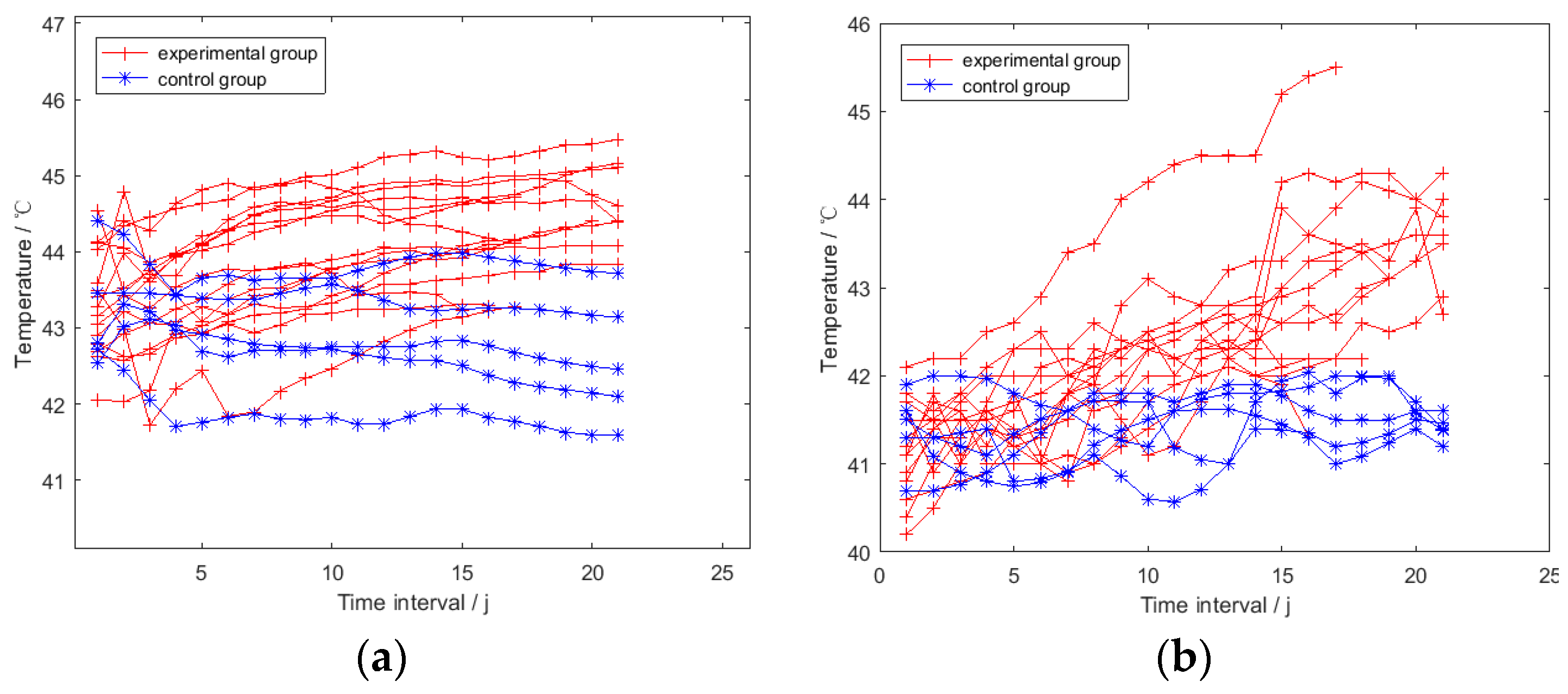
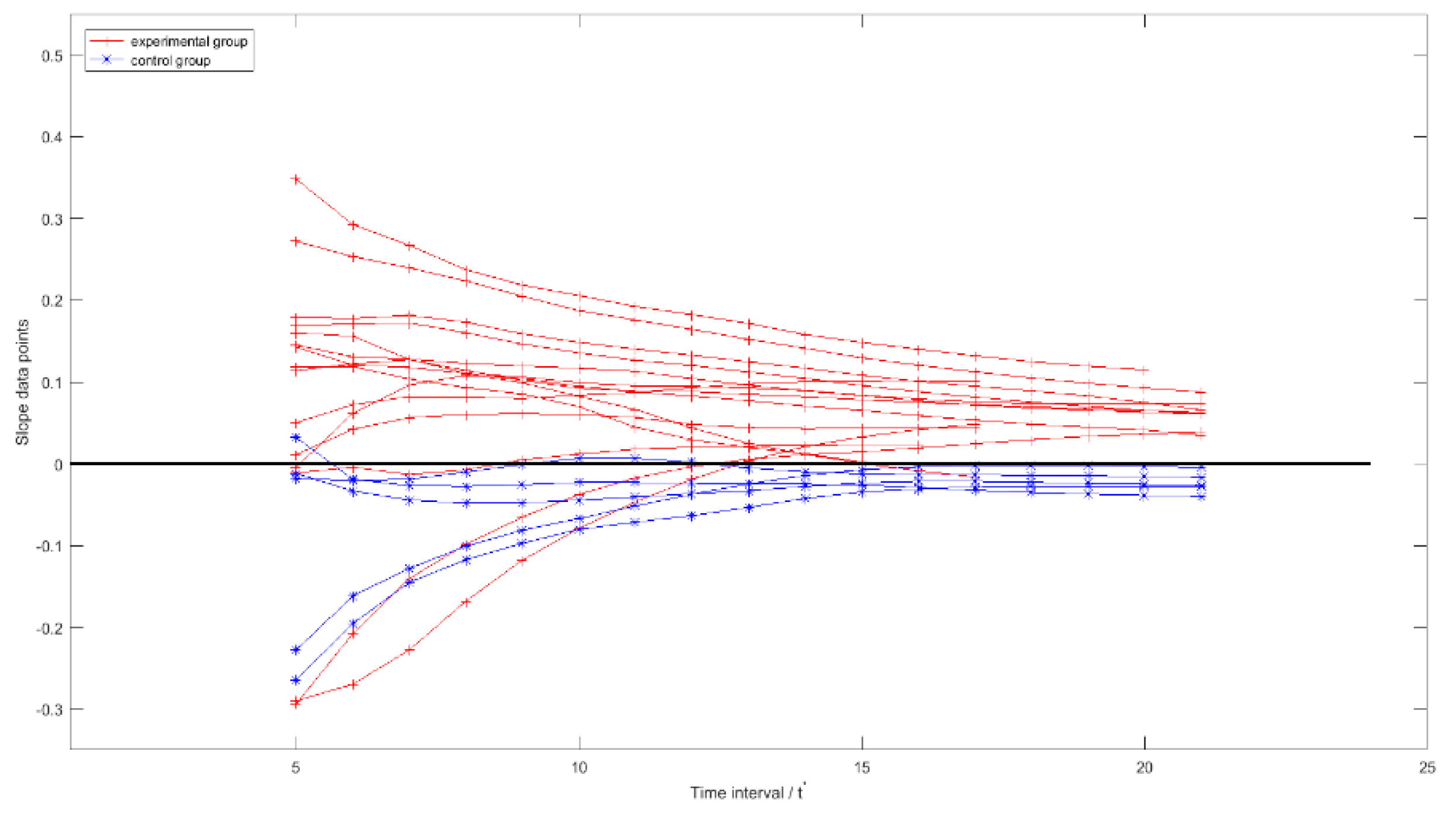
3.2. Overall Trend Analysis for the Smoothed TSRHT
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giloh, M.; Shinder, D.; Yahav, S. Skin surface temperature of broiler chickens is correlated to body core temperature and is indicative of their thermoregulatory status. Poult. Sci. 2012, 91, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.P. Body temperature and fever in a free-living bird. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 156, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Washburn, K.W. The relationships of body temperature to weight gain, feed consumption, and feed utilization in broilers under heat stress. Poult. Sci. 1998, 77, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, W.; Ishida, S.; Kondo, D.; Ito, Y.; Tateno, J.; Tomioka, M. Monitoring of the core body temperature of cows using implantable wireless thermometers. Comp. Electron. Agricult. 2019, 163, 104849. [Google Scholar] [CrossRef]
- Nogami, H.; Arai, S.; Okada, H.; Zhan, L.; Itoh, T. Minimized bolus-type wireless sensor node with a built-in three-axis acceleration meter for monitoring a Cow’s Rumen conditions. Sensors 2017, 17, 687. [Google Scholar] [CrossRef]
- Andersson, L.M.; Okada, H.; Miura, R.; Zhang, Y.; Yoshioka, K.; Aso, H.; Itoh, T. Wearable wireless estrus detection sensor for cows. Comp. Electron. Agricult. 2016, 127, 101–108. [Google Scholar] [CrossRef]
- Reid, E.; Fried, K.; Velasco, J.; Dahl, G. Correlation of rectal temperature and peripheral temperature from implantable radio-frequency microchips in Holstein steers challenged with lipopolysaccharide under thermoneutral and high ambient temperatures. J. Anim. Science 2012, 90, 4788–4794. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; Yu, H.; Huang, R.; Huo, L. Dynamic monitoring device of hens temperature based on wireless transmission. Trans. Chin. Soc. Agricult. Mach. 2013, 6, 242–245. [Google Scholar]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First evaluation of infrared thermography as a tool for the monitoring of udder health status in farms of dairy cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef]
- Alsaaod, M.; Schaefer, A.; Büscher, W.; Steiner, A. The role of infrared thermography as a non-invasive tool for the detection of lameness in cattle. Sensors 2015, 15, 14513–14525. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Veterinaria Scandinavica 2015, 57, 5. [Google Scholar] [CrossRef] [PubMed]
- LokeshBabu, D.S.; Jeyakumar, S.; Vasant, P.J.; Sathiyabarathi, M.; Manimaran, A.; Kumaresan, A.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Kataktalware, M.A. Siddaramanna, Monitoring foot surface temperature using infrared thermal imaging for assessment of hoof health status in cattle: A review. J. Therm. Biol. 2018, 78, 10–21. [Google Scholar]
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Liu, X.; Cao, C.; Lu, H.; Wang, F.; Ge, Y.; Lu, Y. Feasibility study for the use of surface-temperature discriminant poultry health. In 2017 ASABE Annual International Meeting; ASABE: St. Joseph, MI, USA, 2017; p. 1. [Google Scholar]
- Naeaes, I.d.A.; Bites Romanini, C.E.; Neves, D.P.; do Nascimento, G.R.; Vercellino, R.d.A. Broiler surface temperature distribution of 42 day old chickens. Scientia Agricola 2010, 67, 497–502. [Google Scholar] [CrossRef]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.; Stumpf, M.T.; Kolling, G.J.; Dias, E. Infrared thermography in animal production: An overview. Comp. Electron. Agricult. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybernet. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Vincent, L. Morphological area openings and closings for grey-scale images. In Shape in Picture; Springer: Cham, Switzerland, 1994; pp. 197–208. [Google Scholar]
- Barber, C.B.; Dobkin, D.P.; Huhdanpaa, H. The Quickhull algorithm for convex hulls. ACM Trans. Math. Software 1996, 22, 469–483. [Google Scholar] [CrossRef]
- Halir, R.; Flusser, J. Numerically Stable Direct Least Squares Fitting of Ellipses; University of West Bohemia Press: Plzen, Czech Republic, 1998; pp. 125–132. [Google Scholar]
- Lu, M.; Xiong, Y.; Li, K.; Liu, L.; Yan, L.; Ding, Y.; Lin, X.; Yang, X.; Shen, M. An automatic splitting method for the adhesive piglets’ gray scale image based on the ellipse shape feature. Comp. Electron. Agricult. 2016, 120, 53–62. [Google Scholar] [CrossRef]
- Canny, J. A computational approach to edge detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, 8, 679–698. [Google Scholar] [CrossRef]
- Arthur, D.; Vassilvitskii, S. Siam/Acm, k-Means Plus Plus: The Advantages of Careful Seeding; IEEE: Piscataway, NJ, USA, 2007; pp. 1027–1035. [Google Scholar]
- Leisch, F. A toolbox for -centroids cluster analysis. Comput. Stat. Data Anal. 2006, 51, 526–544. [Google Scholar] [CrossRef]
- Meltzer, A. The effect of body temperature on the growth rate of broilers. Br. Poult. Sci. 1983, 24, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Donkoh, A. Ambient-temperature—A factor affecting performance and physiological-response of broiler-chickens. Int. J. Biometeorol. 1989, 33, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Nasirahmadi, A.; Richter, U.; Hensel, O.; Edwards, S.; Sturm, B. Using machine vision for investigation of changes in pig group lying patterns. Comp. Electron. Agricult. 2015, 119, 184–190. [Google Scholar] [CrossRef]
- Psota, E.T.; Mittek, M.; Pérez, L.C.; Schmidt, T.; Mote, B. Multi-pig part detection and association with a fully-convolutional network. Sensors 2019, 19, 852. [Google Scholar] [CrossRef]

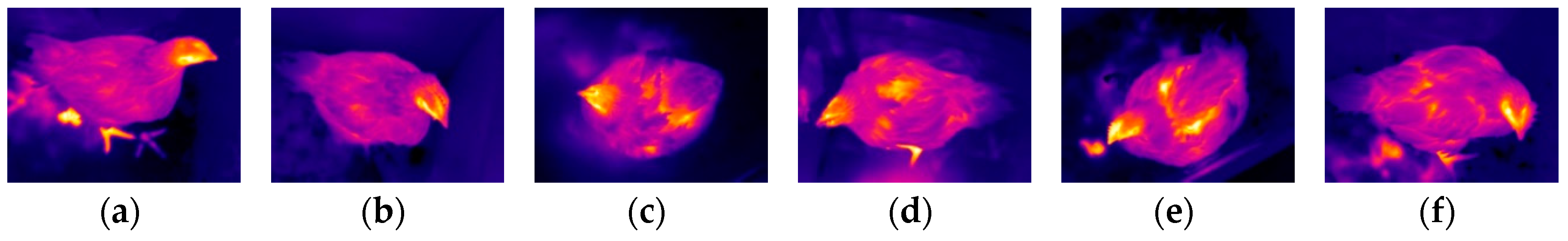
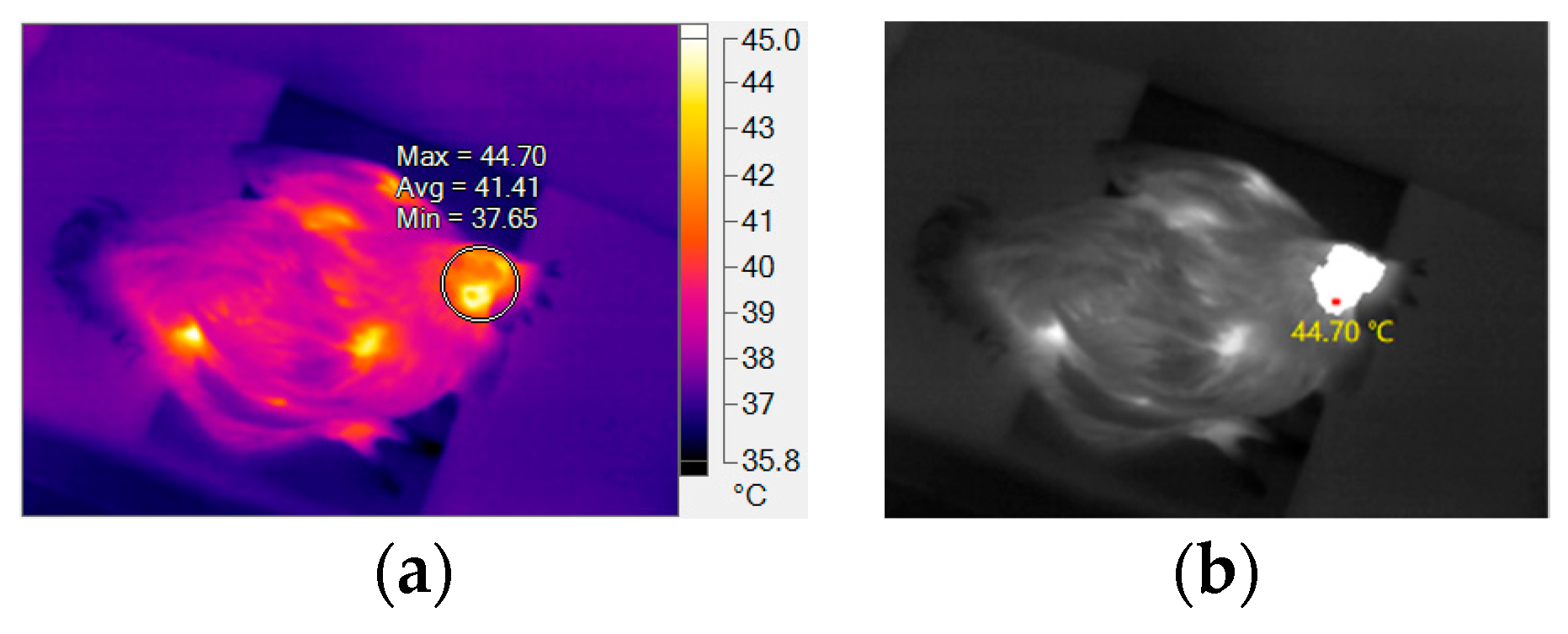

| Category | (i) | (ii) | (iii) | (iv) | (v) | (vi) |
|---|---|---|---|---|---|---|
| Number of images | 41 | 785 | 849 | 295 | 139 | 76 |
| Number of correct locating | 32 | 745 | 806 | 270 | 118 | 56 |
| Ratio of correct locating | 78.05% | 94.90% | 94.94% | 91.53% | 84.89% | 73.68% |
| t′ | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E(%) | 73 | 80 | 80 | 80 | 86.7 | 86.7 | 86.7 | 86.7 | 100 | 100 | 100 | 92.9 | 92.3 | 100 | 100 | 100 | 100 |
| C(%) | 80 | 100 | 100 | 100 | 100 | 80 | 80 | 80 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Lu, M.; Yang, W.; Duan, G.; Yuan, Q.; Shen, M.; Norton, T.; Berckmans, D. An Automatic Head Surface Temperature Extraction Method for Top-View Thermal Image with Individual Broiler. Sensors 2019, 19, 5286. https://doi.org/10.3390/s19235286
Xiong X, Lu M, Yang W, Duan G, Yuan Q, Shen M, Norton T, Berckmans D. An Automatic Head Surface Temperature Extraction Method for Top-View Thermal Image with Individual Broiler. Sensors. 2019; 19(23):5286. https://doi.org/10.3390/s19235286
Chicago/Turabian StyleXiong, Xingguo, Mingzhou Lu, Weizhong Yang, Guanghui Duan, Qingyan Yuan, Mingxia Shen, Tomas Norton, and Daniel Berckmans. 2019. "An Automatic Head Surface Temperature Extraction Method for Top-View Thermal Image with Individual Broiler" Sensors 19, no. 23: 5286. https://doi.org/10.3390/s19235286
APA StyleXiong, X., Lu, M., Yang, W., Duan, G., Yuan, Q., Shen, M., Norton, T., & Berckmans, D. (2019). An Automatic Head Surface Temperature Extraction Method for Top-View Thermal Image with Individual Broiler. Sensors, 19(23), 5286. https://doi.org/10.3390/s19235286






