1. Introduction
Prostate cancer (PCa) is a malignant tumor of the prostate, whose etiology and pathogenesis are still understudied. PCa is among the most common types of malignant tumors (according to the World Health Organization (WHO)). According to the statistics, prostate cancer has been ranked second after lung cancer among other malignant tumors in men across Russia over the past decade. The incidence rate of prostate cancer in Russia has tripled from 19.01 to 57.22 cases per 100,000 people over the past 15 years from 2001 to 2015 (according to the National Medical Research Radiological Center of the Ministry of Health of the Russian Federation).
Such a difficult situation is mainly associated with late diagnosis of the disease [
1]. Currently available methods of instrumental examination and associated diagnostic procedures are far from perfect. The main screening methods include clinical examination and biopsy. The application of magnetic resonance imaging (MRI) is becoming more popular [
2]. The biopsy method allows the determination of the types of cells that are susceptible to proliferation, detecting histological features, genetic damage and other problems associated with oncology [
3]. Biopsy study, on the other hand, is limited to an indeterminate interpretation of the results associated with various individual structural features of the mammary gland; in addition, it is an invasive method that causes patient discomfort and remains ineffective at the early stage of the disease [
1]. Therefore, the development of novel methods for PCa diagnosis at an early stage is an important field of research.
Potential next-generation methods include those based on nanowire detection, which allows for the registration of protein markers in biological fluid at low concentrations (<10
−13 M) when the pathological process is at an early stage of development [
4]. In addition to high sensitivity, this type of detection allows for label-free real-time analysis.
The SOI-NW biosensor is a biosensor based on silicon-on-insulator (SOI) structures. SOI nanowire structures are formed with the use of complementary metal-oxide-semiconductor (CMOS)-compatible technology by gas phase reduction and lithography [
5]. This biosensor has been found to be an efficient tool for the detection of proteins and nucleic acids with extremely high sensitivity at levels from 10
−17 to 10
−14 M [
6,
7,
8].
MicroRNAs have recently been used as biomolecular markers of early stages of cancer [
9,
10,
11]. Gao A. et al., demonstrated the possibility for the specific revelation of lung cancer by NW-biosensor detection of marker miRNA-126 at a concentration of 10
−16 M [
12]. The literature contains discussions on the following miRNAs as potential markers: miR1246, miR-4634, miR1307-3p, miR-6875-5p, miR-6861-5p, and (miR)-10b [
13,
14].
Our present research has demonstrated the possibility to detect DNA oligonucleotides (oDNAs)—the synthetic analogues of PCa-associated miRNAs—in buffer solution, using SOI-NW biosensor chips. The surface of the sensor structures was sensitized with immobilized molecular probes in the form of DNA oligonucleotides complimentary to the target oDNAs. It has been shown that the SOI-NW biosensor can be used to effectively detect DNA oligonucleotides in buffer solution with a high concentration sensitivity of ~10−16 M.
The effect of storage time on the characteristics of SOI-NW biosensor chips made with the use of CMOS-compatible technology was also studied. The findings have shown that a two-year storage time leads to a significant change in their characteristics, resulting in an “inversed” sensitivity towards registration of negatively charged oDNA probes. Taking into account that it is the change in the surface layer that leads to inverse conductance during a two-year storage of the chip, it is concluded that the surface layer of the chip makes a significant contribution to the modulation of its conductance and its sensitivity toward miRNA. The results obtained herein indicate that the detection of target nucleic acid molecules can be carried out with high sensitivity using sensor chips after long-term storage, but that changes in their surface properties leading to inversed detection signal must be taken into account. The possibility of detecting microRNAs isolated from plasma of prostate cancer patients is also demonstrated herein. Thus, our results must be taken into account in the development of highly sensitive nanowire-based diagnostic systems for the revelation of PCa-associated microRNAs in human plasma.
2. Materials and Methods
2.1. Chemicals and Oligonucleotides
Chemicals
The cross-linker, 3,3′-dithiobis (sulfosuccinimidyl propionate) (DTSSP) was purchased from Pierce (USA); potassium phosphate monobasic (KH2PO4), dimethyl sulfoxide (DMSO), and 3-aminopropyltriethoxysilane (APTES) were purchased from Sigma Aldrich (St. Louis, MA, USA). Methanol (CH3OH) was from Sigma (St. Louis, MA, USA). Hydrofluoric acid (HF) were from Reakhim (Moscow, Russia). Deionized water was obtained in a Simplicity UV purification system (Millipore, Molsheim, France).
Oligonucleotides
The following oDNA probes purchased from Evrogen, Russia were used for modification of the SOI-NW surface (
Table 1).
In accordance with their numbering, these oDNA probes were complimentary to DNA oligonucleotides, which were used as target molecules in the model system analysis as synthetic analogues of the sequences published in [
15]. The sequences of oDNA and miRNA are shown below (
Table 2). The DNA oligonucleotide, used as the control probe, was non-complimentary to both cs_1 nor and cs_2 and had the following sequence: (NH
2)-(T)
10-GAGGCTGAGATGTTGCTACTGCTATGAGAAGATATGTCAAGCCAGAGTAT («control»).
2.2. SOI-NW Sensors Fabrication
The design and characteristics of the SOI-NW structures used as sensor elements are described in [
5,
6,
8]. SOI-NW structures with n-type conductance were used. These structures were freshly prepared and stored for either one or two years in clean room conditions. The thickness of the silicon cut-off layer was 32 nm, and the buried oxide (BOX) thickness was 300 nm. In the experiments, the width of the sensor elements was 3 nm, the thickness was 32 nm, the length was 10 nm, and the number of SOI-NWs on the chip was 12. The SOI-NW biosensor scheme is described in [
5,
8].
This study design used a 500-mL measuring cell, the bottom of which was the chip sensitive area with the SOI-NW structures. The diameter of the sensitive area was about 2 mm. The solution stirring was carried out with a stirrer at 3000 rpm.
2.3. Sensor Surface Modification
The surfaces of chips (either freshly prepared or stored for one or two years) were treated according to the standard procedure using isopropanol, water, and the solution of HF in CH
3OH to remove organic contaminants by sequential double treatment [
5,
6,
8]. The freshly prepared chips were then additionally placed into the ozonizer to form hydroxyl groups on the surface of the SOI-NW structures [
5]. The chips’ surfaces were silanized in APTES vapors according to the protocol described elsewhere [
5,
8].
In cases of intensive oxide layer removal from the surfaces of chips stored for more than two years, it was necessary to remove structures formed during long-term storage, and chip surfaces were treated with isopropanol, water, and a five-time treatment of 1:50 v/v HF in CH3OH.
2.4. Covalent Immobilization of Oligonucleotide Probes
The oDNA probes (probe_1, probe_3, «control») were covalently immobilized on the modified surface of the SOI-NW with the use of the DTSSP cross-linker [
6]. For this purpose, solutions (V = 3 nL) containing one of the types of oligonucleotide probe (1 μM) in potassium phosphate buffer (50 mM, pH 7.4) were precisely applied onto the surface of each individual SOI-NW. Solutions were applied using the Piezorray non-contact dispensing system (PerkinElmer, Inc., Waltham, MA, USA). Solutions were incubated for 30 min at 15 °C and 80% humidity on the surface of the SOI-NW structures. After that, the sensor chip area was washed in deionized water.
2.5. Preparation of Solutions of TARGEt oDNAs in Buffer
Target oDNA solutions with concentrations ranging from 3.3 × 10−12 M to 3.3 × 10−18 M were prepared from the initial solution (100 μM in 50 mM potassium phosphate buffer, pH 7.4) with the use of 10-fold serial dilution in a working buffer solution (1 mM potassium phosphate buffer, pH 7.4). The solution was kept in a shaker for 30 min at 10 °C at each stage of dilution. All the solutions were prepared immediately before measurements.
2.6. Electrical Measurements
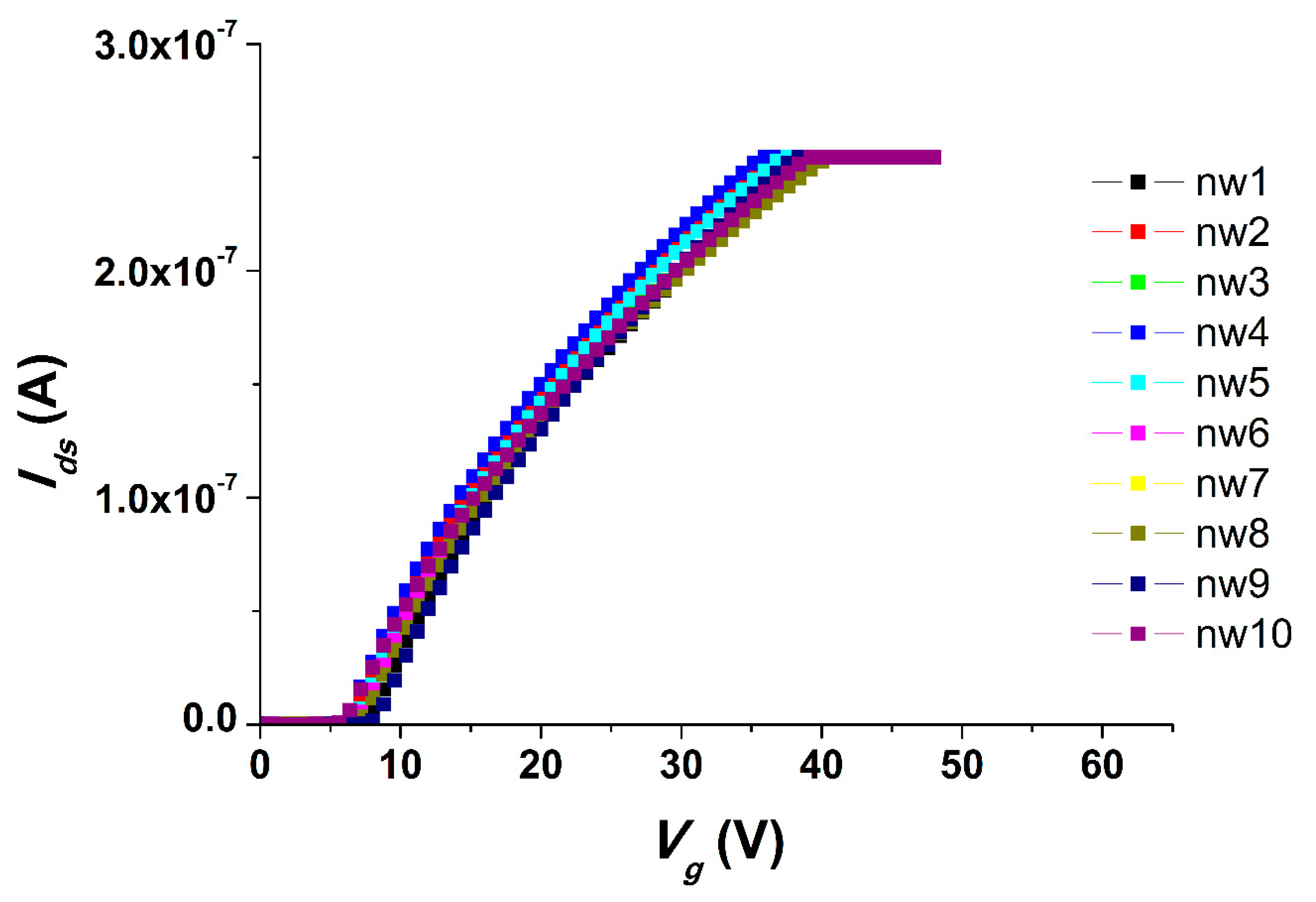
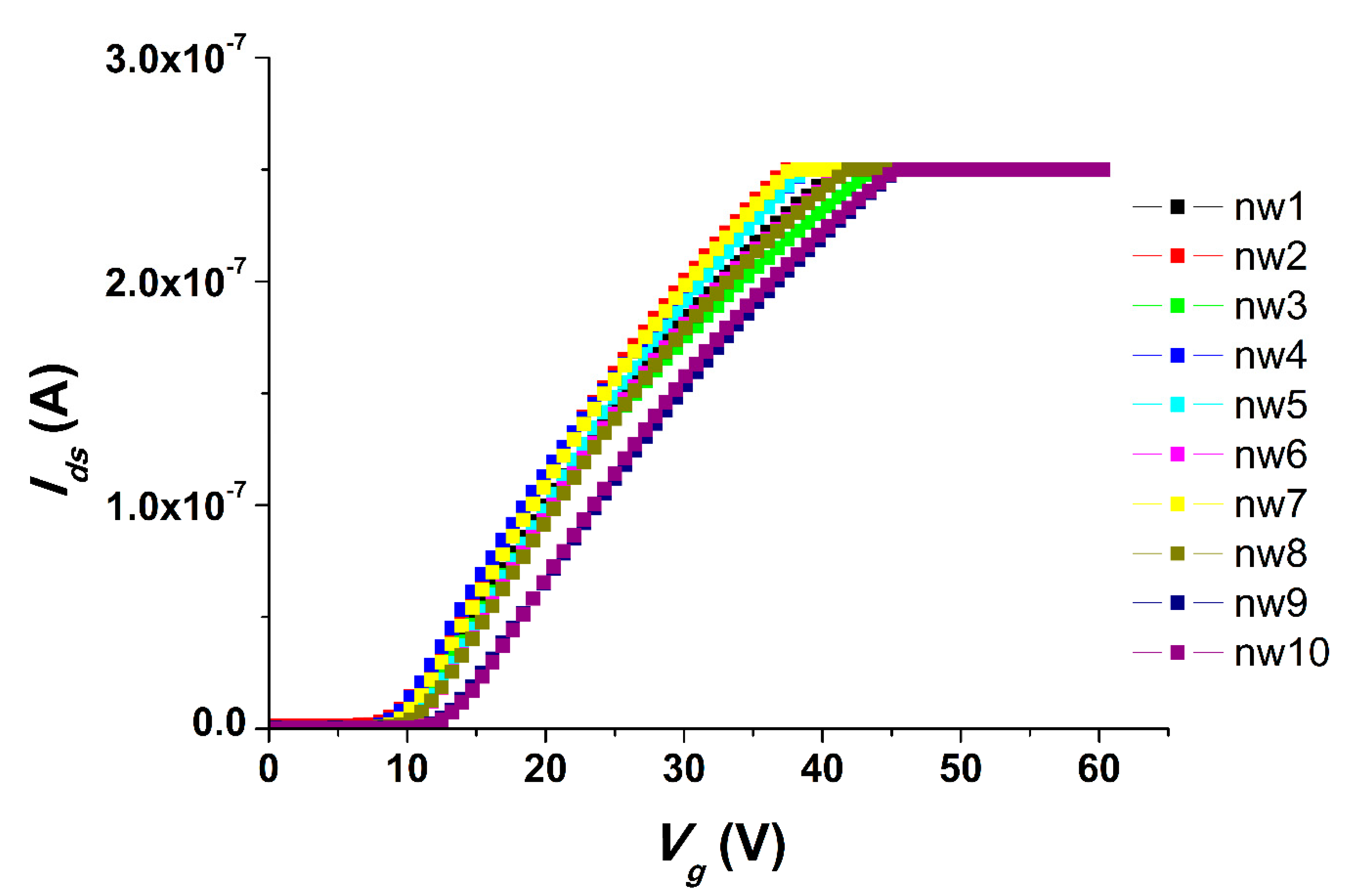
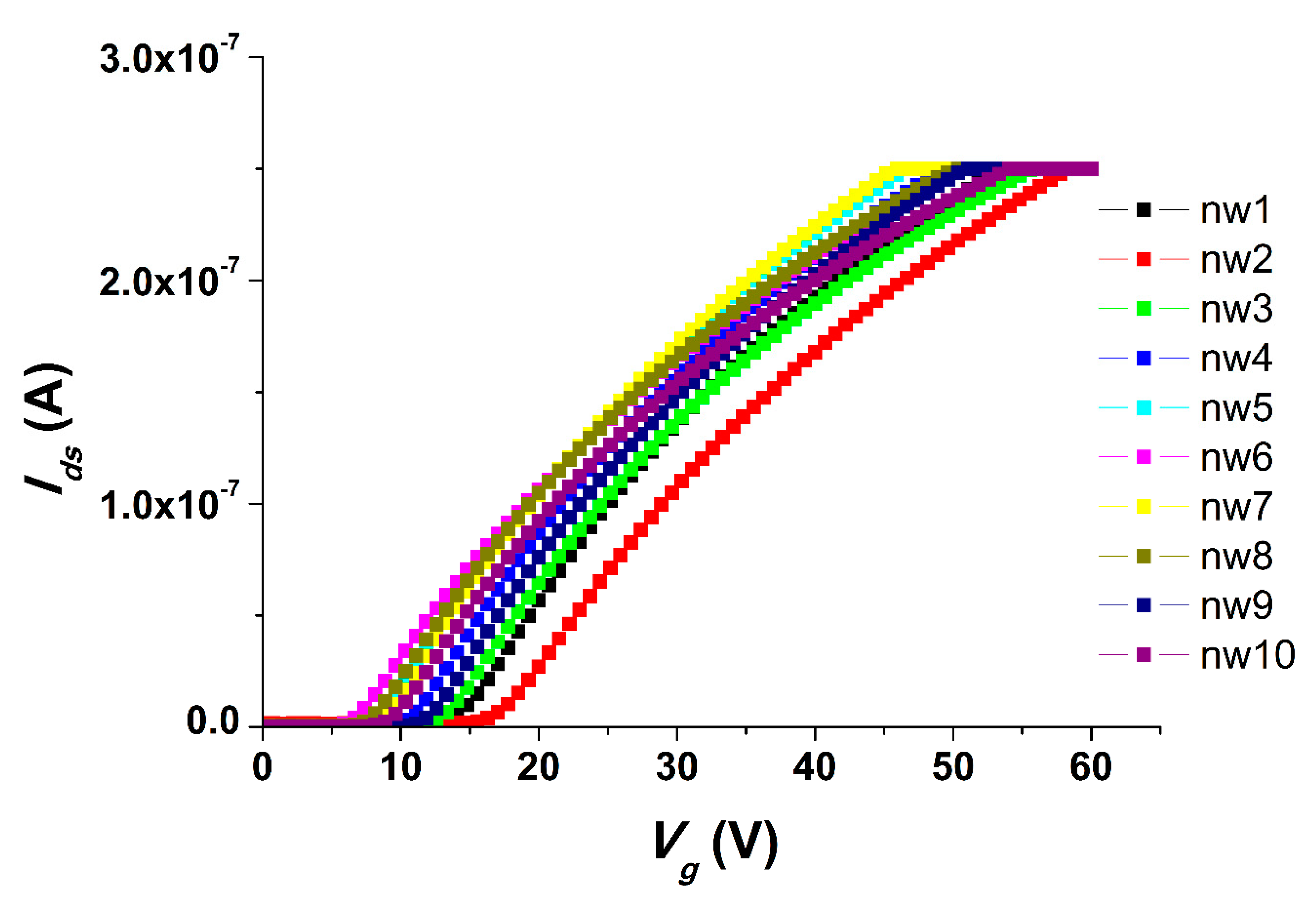
We carried out all the electrical measurements with the use of an analog-to-digital converter, while the support of the SOI structures was used as a control electrode (transistor gate). The registration of the drain-source Ids dependence on the value of the voltage supplied to the gate Vg so as the volt-ampere characteristics were obtained at Vg from 0 to 60 V and Vds = 0.1 V. The registration of the drain-source current Ids dependencies on time (t) were carried out at gate voltage Vg = 55 V and at Vds = 0.1 V.
2.7. NW Biosensor Measurements
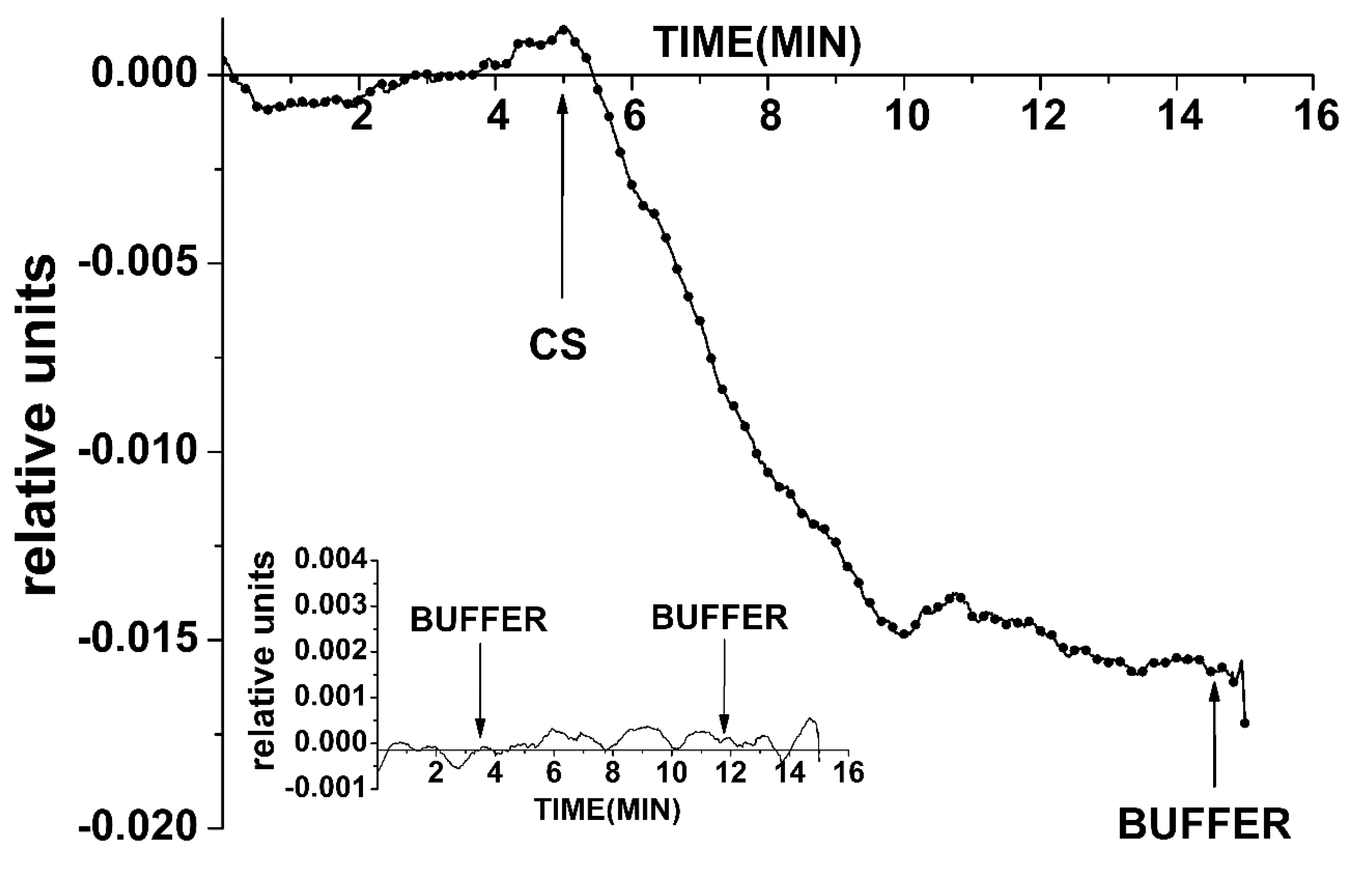
The analyzed solution (150 μL, in 1 mM potassium phosphate buffer) containing one of the types of oDNA (“cs”) at a concentrations from 3.3 × 10−12 M to 3.3 × 10−18 M was added into the measuring cell containing 300 μL of buffer solution (1 mM potassium phosphate buffer). Thus, the initial concentration of the solution decreased threefold. Control experiments were performed under similar conditions, but the oDNA-free buffer was added.
SOI-NW biosensor signals were recorded in real time. To account for the nonspecific sorption of the target objects, chips containing working sensors also contained a pair of control sensors, the surfaces of which were sensitized by the “control” probe. The obtained results are presented in the form of Δ
Ids(t) dependencies reflecting the differential signal between the working (with immobilized probe_1 or probe_3 oligonucleotides) and the control SOI-NW sensor. The detection of DNA oligonucleotides and miRNA were performed in buffer with a low salt concentration (1 mM potassium phosphate buffer) in order to exclude the effects of Debye screening [
16].
Our experiments concerned the effects of storage time and clean room conditions on the performance of SOI-NW sensor chips under the following conditions: (1) freshly prepared chips; (2) chips stored for one year after fabrication; (3) chips stored for two years after fabrication; and (4) treating the chip that was stored for two years after fabrication five times with HF to intensively remove the surface layer.
2.8. Plasma Samples
In our research, plasma samples were obtained from patients diagnosed with prostate cancer. The patients were examined in Institute of Urology and Reproductive Health (Sechenov University) and in the National Medical Research Radiological Center of the Ministry of Health of the Russian Federation (Moscow, Russia). In control experiments, we used plasma samples from a healthy volunteer. Plasma samples nos. 2, 36, and 44 (see
Table 3) were tested. All experiments using serum were performed in compliance with order no. 1177n (Ministry of Health of Russian Federation, 20 December 2012). Plasma samples were collected from patients according to the patient examination protocol. This study was approved by independent ethical committees organized on the basis of the organizations that provided the samples. Written informed consent was obtained from the patients and from healthy volunteer authorizing their participation in the study and the use of the biological material. All samples were deactivated prior to their use in the study to provide biological safety.
The blood samples were taken before treatment on an empty stomach using the cubital vein vacutainers with a 3.8% Na citrate anticoagulant (S-Monovett, SARSTEDT, Nümbrecht, Germany) then centrifuged at 3000 rpm for 6 min at RT. Each plasma sample (500 μL) was collected in two dry test tubes, frozen, and stored at −70 °C prior to the analysis.
To extract miRNAs from the blood plasma samples, miRCURY RNA Isolation Kit Biofluids were used.
4. Biospecific Detection of miRNA that Has Been Isolated from Plasma Using Three Types of SOI-NW Chips
Below, we describe the results obtained upon the detection of miRNA that has been isolated from plasma samples of prostate cancer patients and one healthy volunteer. These results have been obtained using three types of sensor chips (see
Section 2): (1) a freshly prepared one; (2) a chip stored for two years; and (3) a chip treated five times with HF to intensively remove the surface layer.
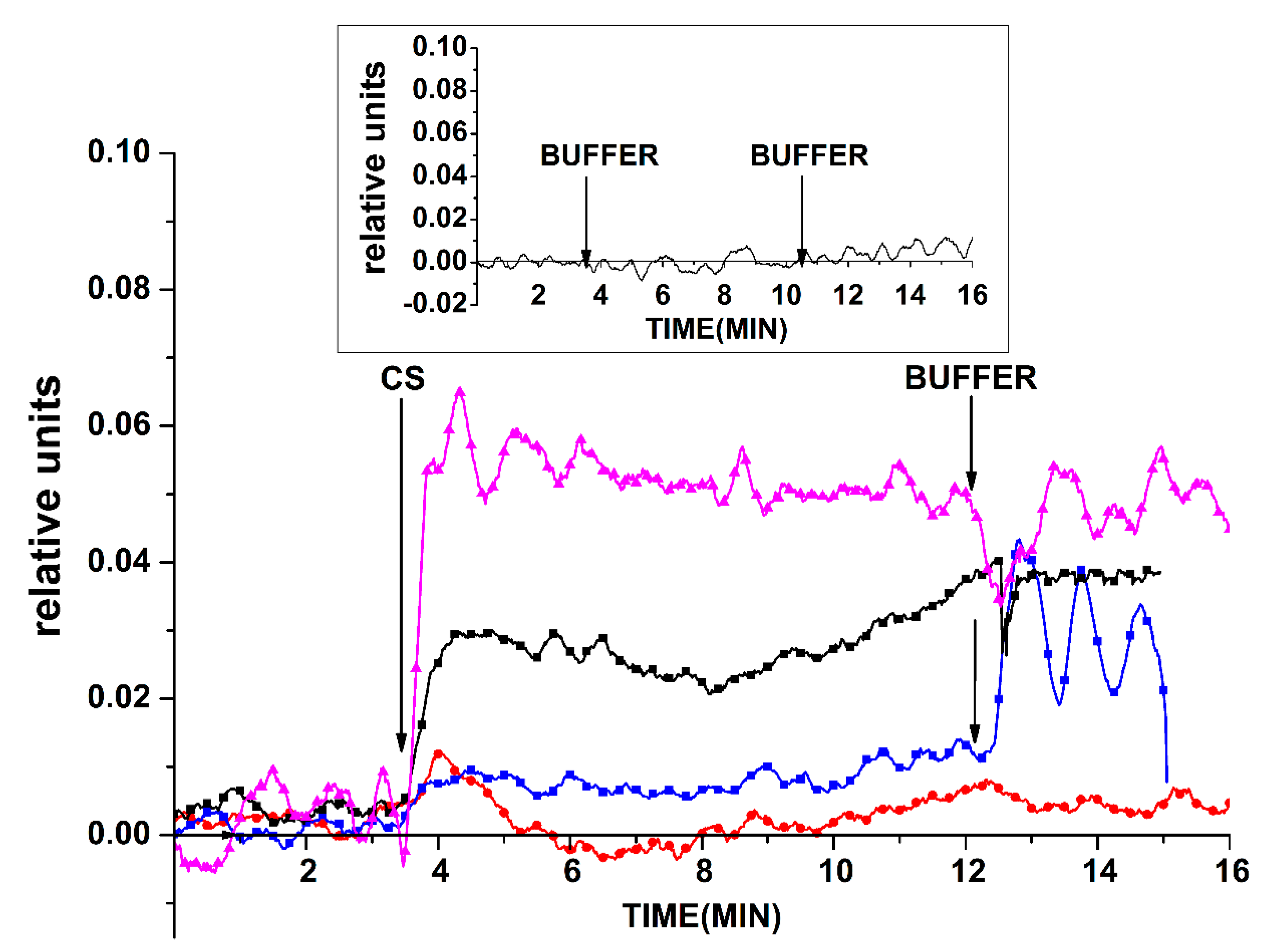
Figure 10 displays the
Ids(t) dependencies obtained for these chips upon analysis of the plasma of several patients. As seen in
Figure 10, a decrease in the biosensor signal was observed upon using the freshly prepared chip for the analysis of a sample containing a pool of miRNAs that was isolated from plasma sample #44 (of a prostate cancer patient). Such a change in the signal has also been observed upon the detection of cs_1 oDNA (described in the
Section 3.1.2) when a decrease in SOI-NW conductance has also been observed. Upon the detection of miRNA that was isolated from the plasma of a healthy volunteer (#36), the signal fluctuated near the zero line, and its level had a slight tendency to increase.
For the chip stored for two years, a significant increase in the biosensor signal (in absolute value) was noted upon addition of sample #44 into the measuring cell. This increase was similar to that obtained in the case of the freshly prepared chip, unlike the decrease in conductance in the latter case. Such a behavior—exhibited by the chip stored for two years upon addition of the sample from a cancer patient—is similar to that observed upon the addition of a complimentary oDNA (as described in
Section 3.3.2). In
Figure 10, the response of a chip that has been intensively treated with HF to remove its surface layer is shown in an analysis of sample #2. As one can see, the addition of this sample led to a decrease in the biosensor signal, which is similar to the case with complimentary oDNA (as described in
Section 3.3.3).
5. Discussion
A nanowire biosensor is a very promising device for a highly sensitive bimolecular detection, since it allows for the registration of a signal from a single biomolecule adsorbed on a sensor element [
18]. Protein markers such as PSA (prostate specific antigen), which are used in clinical practice, must be detected in blood with a sensitivity of 10
−17 M in order to detect prostate cancer at an early stage [
19]. We can conclude that a nanowire biosensor is suitable for this purpose, as was illustrated before. At the same time, it is known that protein markers of prostate cancer are not products of tumor cells, but are associated with the inflammatory process. We can also observe an increase in scientific publications related to the use of miRNAs for diagnosis as a result of the unique microRNA expression profiles of tumors at the very early stages of carcinogenesis [
6,
7,
20,
21].
We have investigated the possibility of biospecific detection of model DNA oligonucleotides complimentary to a probe for PCa-associated miRNAs. It was shown that oDNAs, which are synthetic analogues of PCa-associated miRNAs, can be effectively detected in a buffer solution with the use of a nanowire biosensor with high sensitivity (~3.3 × 10−16 M) in real time without the use of additional labels.
One of the most important characteristics of chips for the SOI-NW biosensor is their change in properties during long-term storage. We have studied the influence of chip storage times on their properties, and it has been demonstrated that, in the case of a one-year storage of SOI chips, no significant changes in the properties of the chips could be observed. At the same time, when the chips were stored for two years, one can see an interesting change in the chips’ properties that results in an inversed signal upon the detection of oDNA molecules. The reason for the change in characteristics during long-term storage (i.e., two years) may be the influence of atmospheric oxygen on natural oxides, which are formed on the outer surfaces of nanowire sensor elements. Bohling has shown that the properties of an oxide can change dramatically upon contact with the environment [
22], and the mechanism causing the difference in response from the freshly prepared chip with that from the chip stored for two years could be explained in the following way. On the surface of a freshly prepared chip, the molecules of covalently immobilized oDNA probes can have an arcuate shape (reaching to the surface of the chip), as the negatively charged bases are attracted to the positively charged nanowire surface, whose positive net charge is caused by application of positive voltage to the gate.
Over time (after two years of storage in our present study), islands with excessive negative charges are formed on the chip’s surface. The accumulation of a negative charge on the chip’s surface can be caused by the following factors: (1) the presence of negative ions in the environment; (2) a negatively charged layer formed during oxidation; or (3) the presence of a negative layer in silicon dioxide due to excessive interstitial oxygen. Accordingly, the surface of a chip stored for two years bears an additional (with respect to the charge of the freshly prepared chip) negative charge due to the negative defects, and this charge compensates the positive charge of the gate, which causes the oDNA probes—immobilized on the surface of the old chip—to be less attracted to the nanowire surface, as compared with a freshly prepared chip.
In the case of a freshly prepared chip, upon the addition of oDNA into the cell the oDNA base sequence hybridizes to the complementary base sequence of the immobilized oDNA probes, while the positively charged amino group NH2+ of the oDNA moves up from the substrate due to the repulsion caused by the positive potential of the gate. Taking into account that the oDNA sequence contains 84 to 110 base pairs, its linear length makes up 29 to 37 nm, and these groups can leave the zone of Debye length. Because of this, negatively charged bases of the oDNA, hybridizing on the surface, cause a decrease in the chip’s conductance. In the case of the chip stored for two years, the oDNA base sequence hybridizes with the sequence of complementary bases of immobilized oDNA probes, but the excess negative charge of the chip surface, which neutralizes the positive charge on the nanowire surface, acts in such a way that the positively charged NH2+ group of the oDNA is attracted very close to the surface. This is what leads to the “opening” of the chip transistor and, as a result, to the observed inversion mode.
In the case of plasma containing a pool of microRNAs, these microRNAs most likely present in the form of complexes with positively charged proteins, which can cause an excessive positive charge on the microRNA-protein structure. The differently directed response to hybridization of plasma microRNAs on the surface of new and old chips will be similar to that given above for the case of experiments with synthetic oDNA structures.
We have also demonstrated that the use of a procedure for intensive removal of the surface layer from the chip can allow one to convert the inversed signal from the chip to the expected direct one, as tested upon the addition of negatively charged oDNA. An interesting effect of the chip’s inverted conductance upon modification of its surface layer during long-term storage is that it is the surface (not the whole cross-section of the conductor) that forms the response to the biosensor on nucleic acids such as oDNA and miRNA.
In our study, the capability of SOI-NW chips to detect miRNAs associated with prostate cancer in plasma samples from patients has been demonstrated. In the case of a freshly prepared n-type chip, an anticipated decrease in conductance was observed upon addition of a sample containing negatively charged miRNA. Inverse sensitivity of the chip after two years of storage was observed not only in the case of oDNA, but also in the case of corresponding miRNA. After intensive removal of the oxide surface layer of the chip that was stored for two years using five treatments with HF, chip conductivity returned from the inverse to the direct mode of detection for both the oDNA and miRNA in the blood plasma of a cancer patient.
The results obtained should be taken into account in the development of highly sensitive nanowire-based diagnostic systems for the revelation of (prostate) cancer-associated microRNAs in human plasma.