Development of Ratiometric Fluorescence Sensors Based on CdSe/ZnS Quantum Dots for the Detection of Hydrogen Peroxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CdSe/ZnS QDs
2.3. Synthesis of Aminofluorescein (AF)-Encapsulated Melamine-formaldehyde (MF) Particles
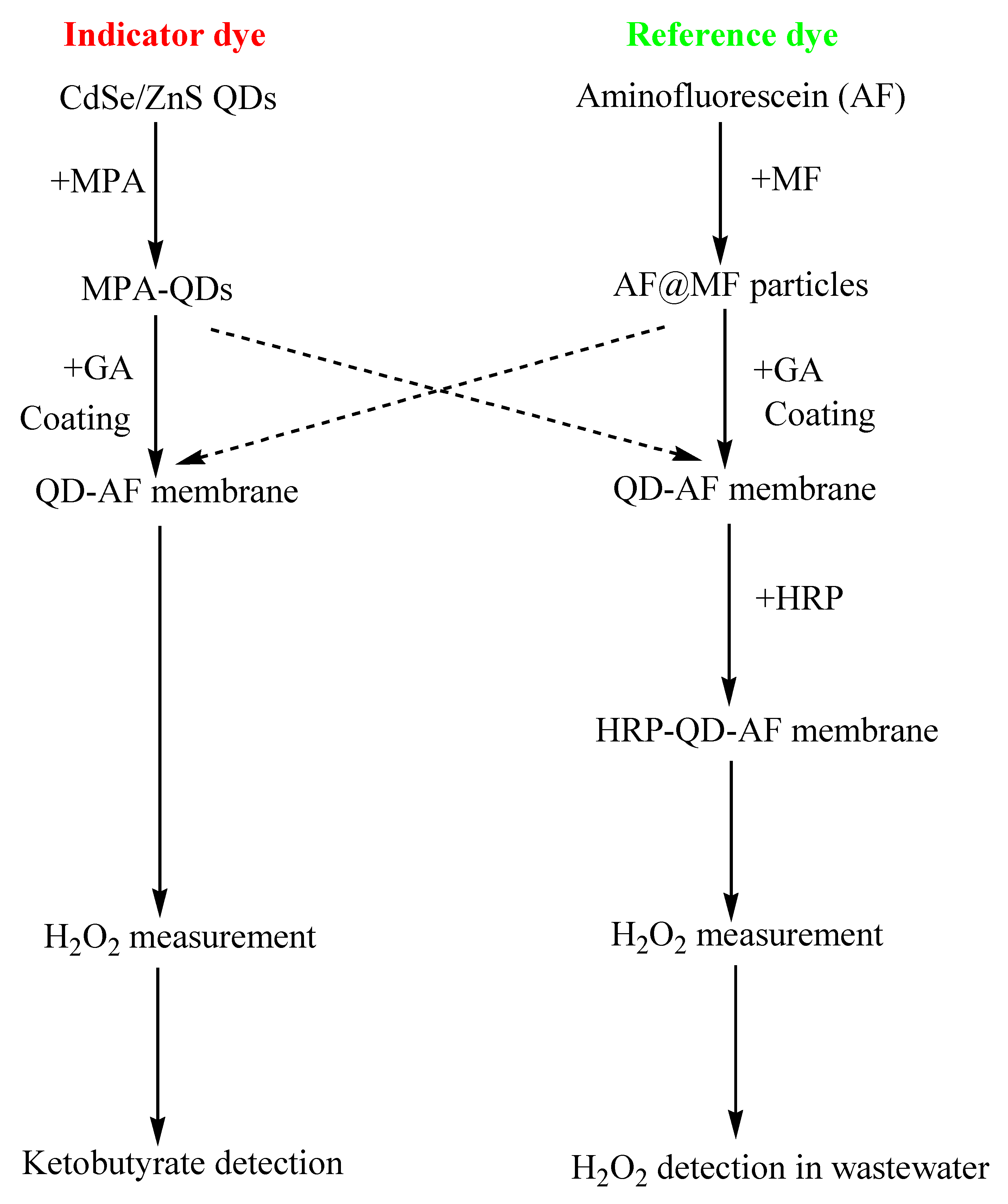
2.4. Preparation of H2O2-sensing Membranes
2.4.1. QD–AF Membrane
2.4.2. HRP–QD–AF Membrane
2.5. Fluorescence Measurements
2.6. Ratiometric Method
2.7. Data Analysis
3. Results and Discussion
3.1. Properties of CdSe/ZnS QDs and AF@MF Particles
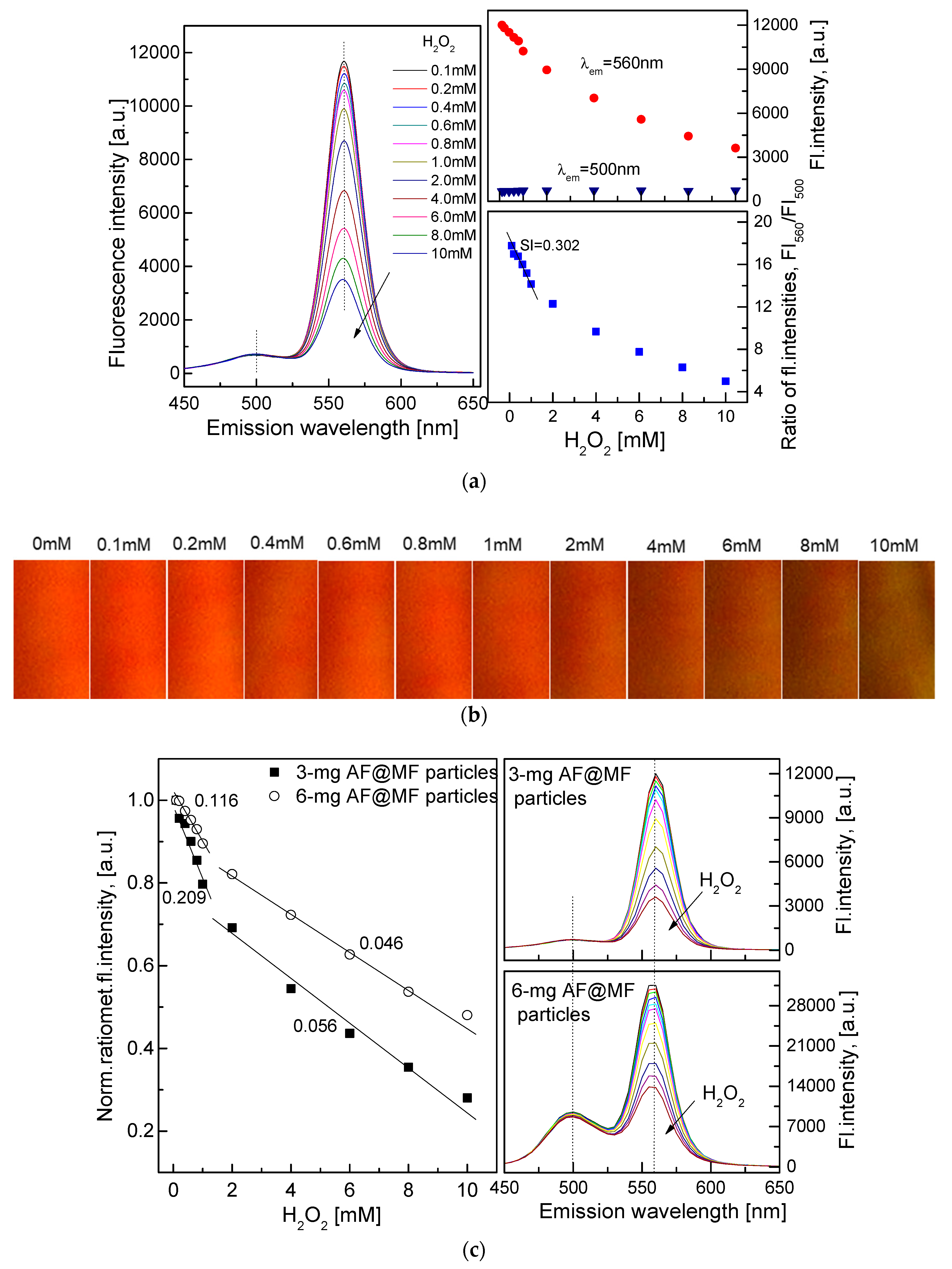
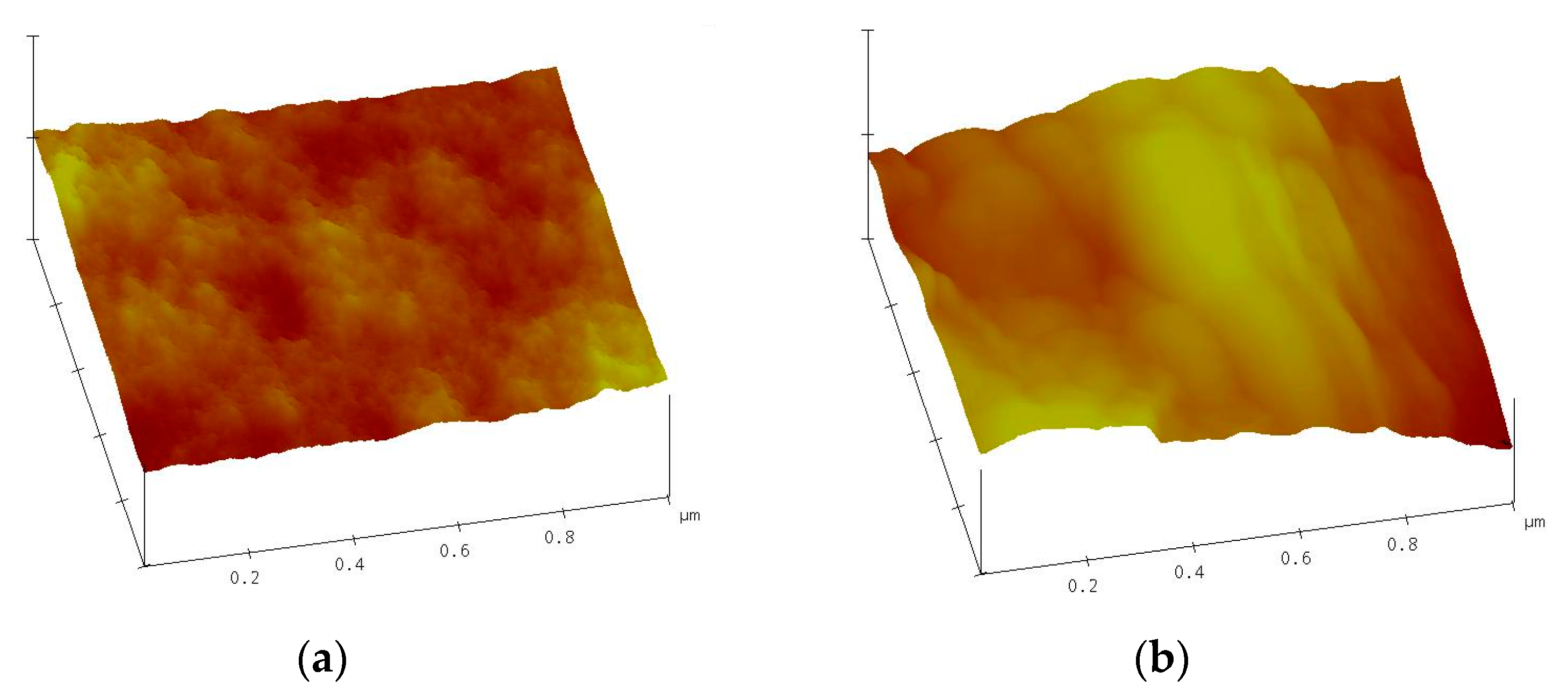
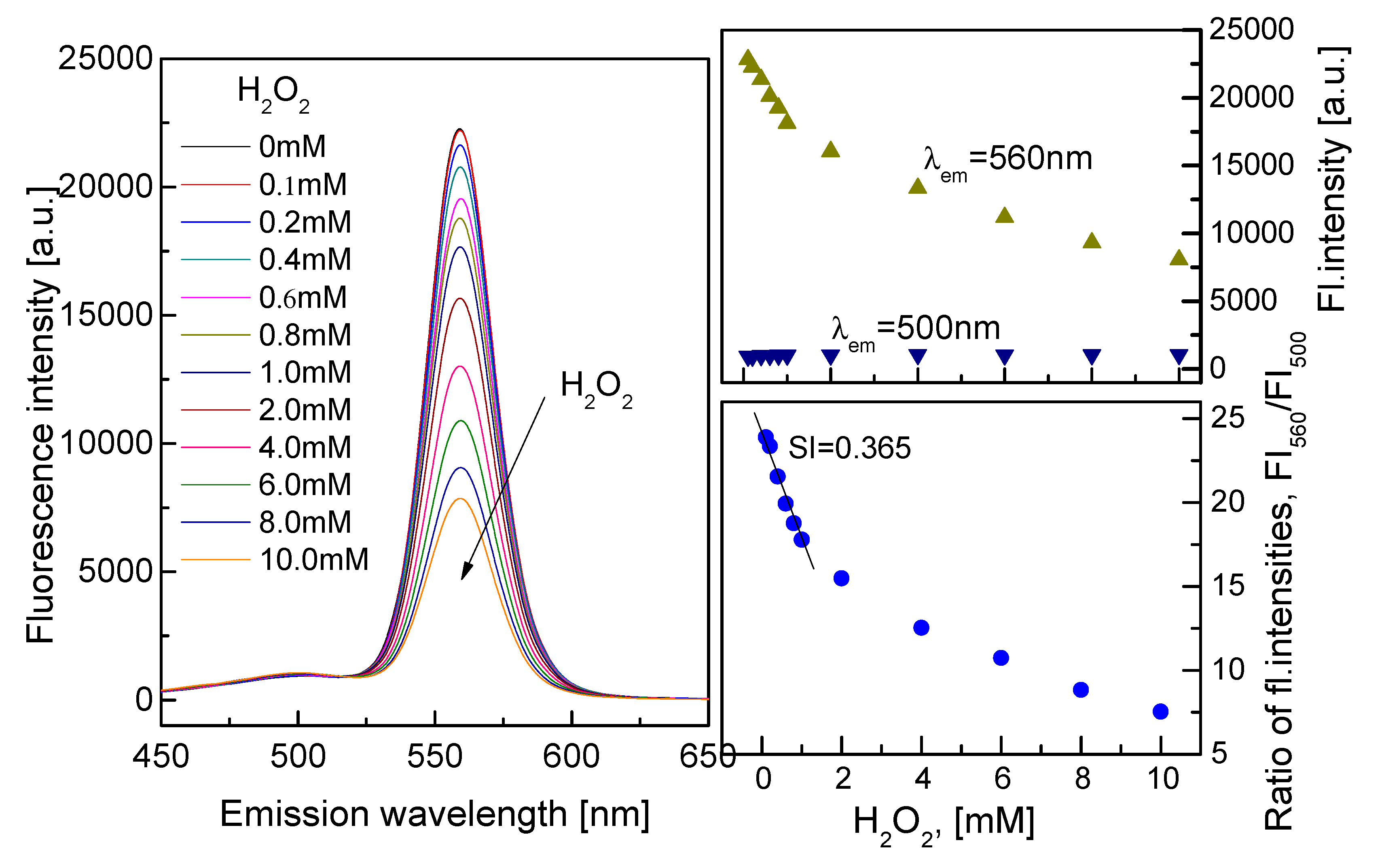
3.2. Characterization of the QD–AF Membrane
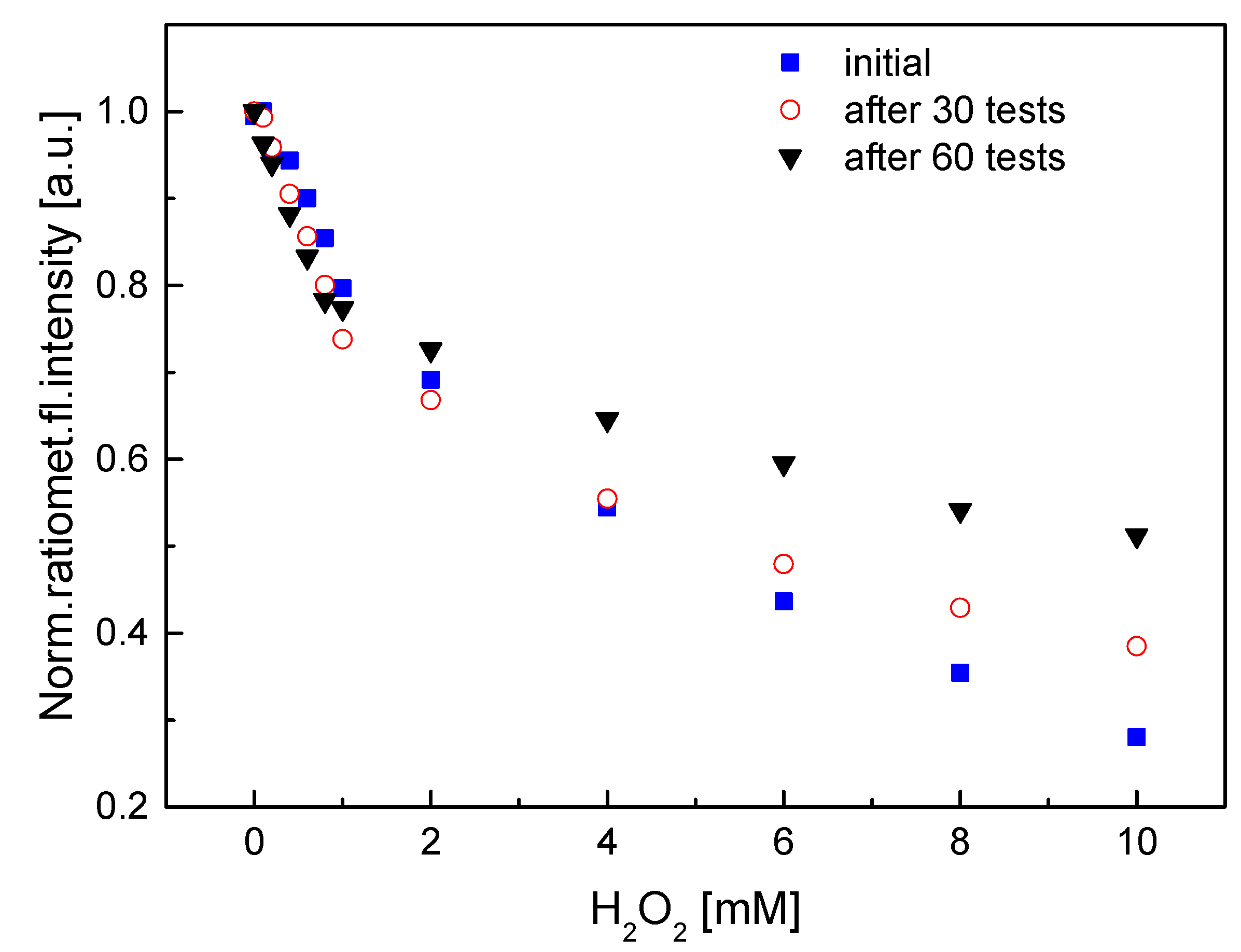
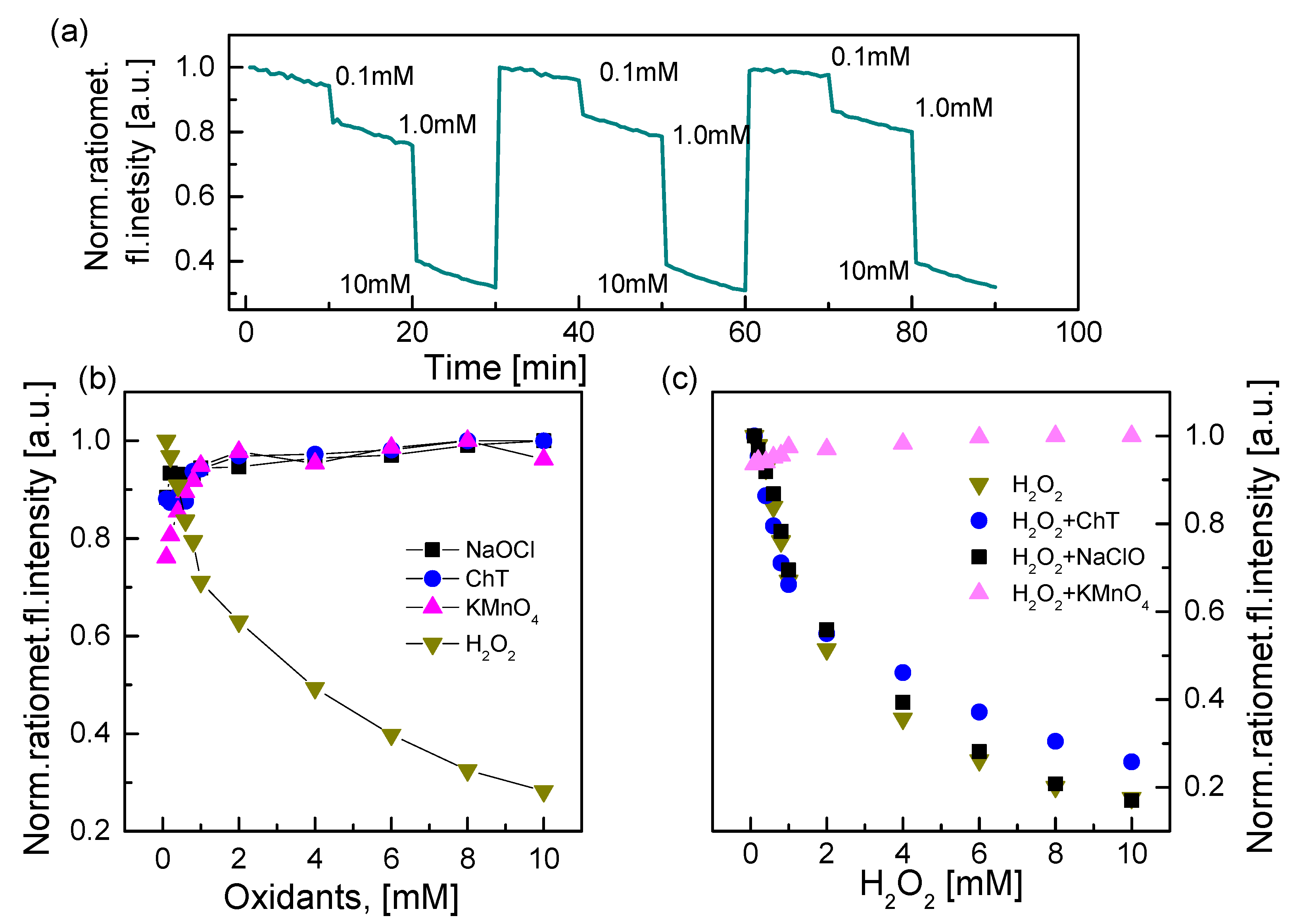
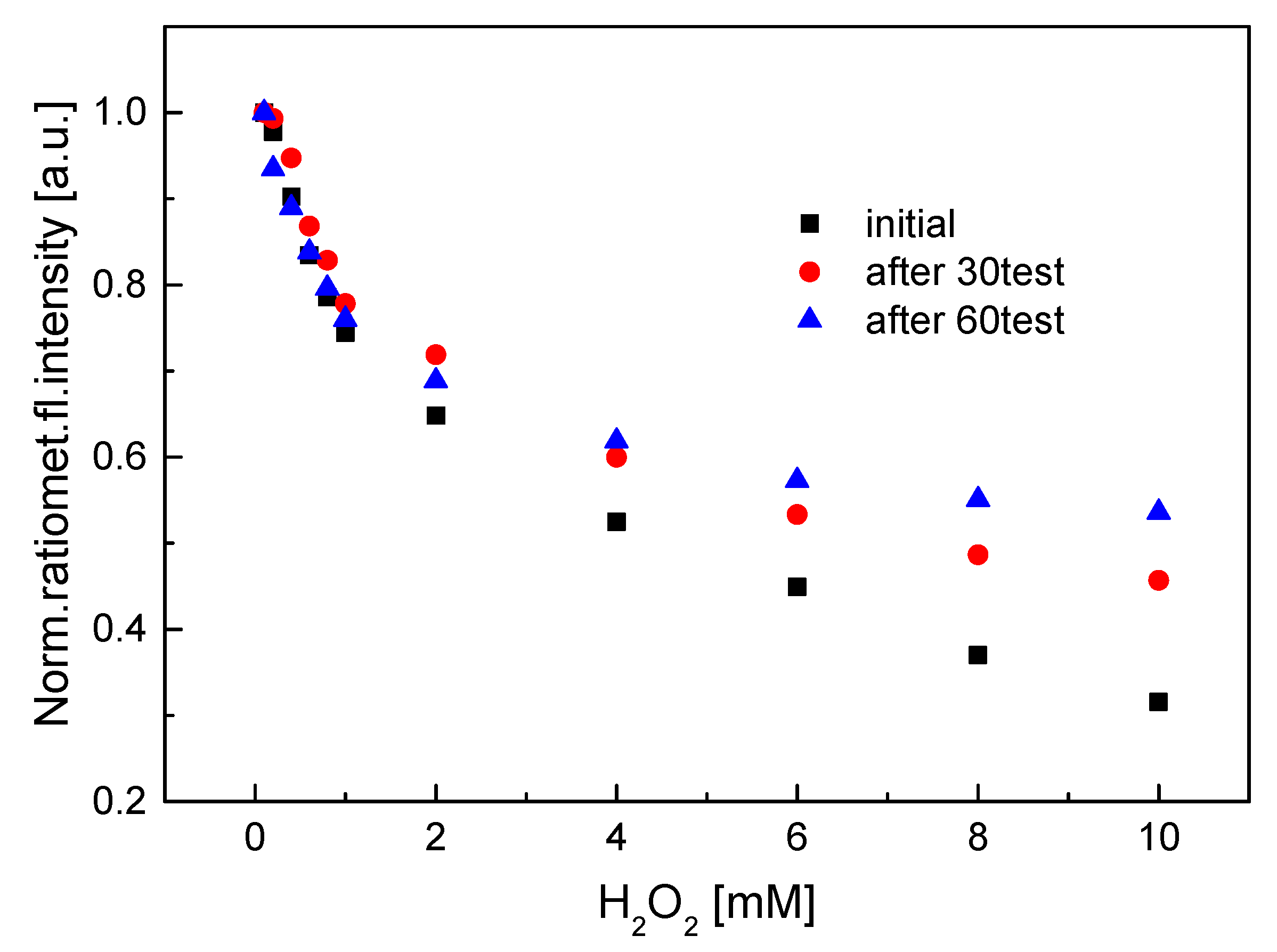
3.3. Characterization of the HRP–QD–AF Membrane
3.4. Applications of QD–AF and HRP–QD–AF Membranes
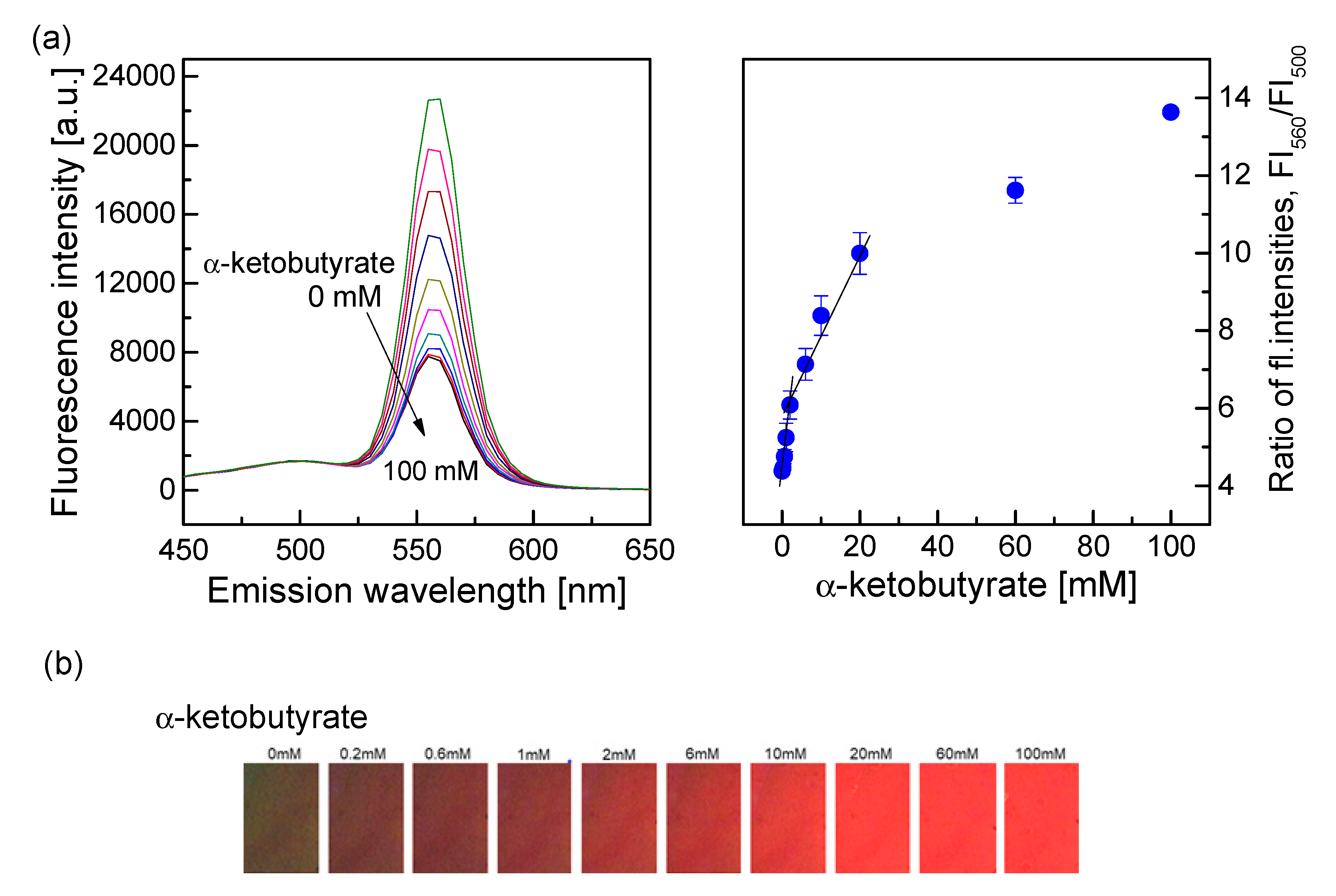
3.4.1. Detection of α-ketobutyrate Using the QD–AF Membrane
3.4.2. Determination of H2O2 in Artificial Wastewater Using the HRP–QD–AF Membrane
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burmistrova, N.A.; Kolontaeva, O.A.; Duerkop, A. New nanomaterials and luminescent optical sensors for detection of hydrogen peroxide. Chemosensors 2015, 3, 253–273. [Google Scholar] [CrossRef]
- Gill, R.; Bahshi, L.; Freeman, R.; Willner, I. Optical detection of glucose and acetylcholine esterase inhibitors by H2O2-sensitive CdSe/ZnS quantum dots. Angew. Chem. Int. Ed. 2008, 47, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Tiana, J.; Lua, H.T.; Weng, L.X.; Wanga, L.H. H2O2-sensitive quantum dots for the label-free detection of glucose. Talanta 2010, 82, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Zhang, J. H2O2-and pH-sensitive CdTe quantum dots as fluorescence probes for the detection of glucose. Luminescence 2013, 28, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Hage, R.; Lienke, A. Applications of transition-metal catalysts to textile and wood-pulp bleaching. Angew. Chem. Int. Ed. 2006, 45, 206–222. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Garcia-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection and sterilization in health care facilities: What clinicians need to know? Clin. Infect. Dis. 2004, 39, 702–709. [Google Scholar] [CrossRef]
- Capizzi, R.; Landi, F.; Milani, M.; Amerio, P. Skin tolerability and efficacy of combination therapy with hydrogen peroxide stabilized cream and adapalene gel in comparison with benzoyl peroxide cream and adapalene gel in common acne. A randomized, investigator-masked, controlled trial. Br. J. Dermatol. 2004, 151, 481–484. [Google Scholar] [CrossRef]
- Feng, C.; Wang, F.; Dang, Y.; Xu, Z.; Yu, H.; Zhang, W. A self-assembled ratiometric polymeric nanoprobe for highly selective fluorescence detection of hydrogen peroxide. Langmuir 2017, 33, 3287–3295. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, X.; Lu, Z.; Wang, X.; Wang, Z. A fluorescent probe for hydrogen peroxide in vivo based on the modulation of intramolecular charge transfer. Anal. Chem. 2017, 89, 5278–5284. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, H.; Yang, L.; Su, Y.; Guo, M.; Song, X. A sensitive and selective fluorescent probe for the detection of hydrogen peroxide with a red emission and a large Stokes shift. Sens. Actuator B Chem. 2018, 25, 1160–1165. [Google Scholar] [CrossRef]
- Żamojć, K.; Zdrowowicz, M.; Rudnicki-Velasquez, P.B.; Krzymiński, K.; Zaborowski, B.; Niedziałkowski, P.; Jacewicz, D.; Chmurzyński, L. The development of 1,3-diphenylisobenzofuran as a highly selective probe for the detection and quantitative determination of hydrogen peroxide. Free Radic. Res. 2017, 51, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Purdey, M.S.; McLennan, H.J.; Sutton-McDowall, M.L.; Drumm, D.W.; Zhang, X.; Capon, P.K.; Heng, S.; Thompson, J.G.; Abell, A.D. Biological hydrogen peroxide detection with aryl boronate and benzyl BODIPY-based fluorescent probes. Sens. Actuator B Chem. 2018, 262, 750–757. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yang, Q.; Zhang, Z.; Zhang, Q.; Zheng, G.; Zhan, F. A highly selective fluorescent probe for hydrogen peroxide and its applications in living cells. J. Photochem. Photobiol. A Chem. 2017, 344, 8–14. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Wang, H.Q.; Yang, Q.; Yu, J.H.; Zhao, Y.D. Hydrogen peroxide biosensor based on direct electron transfer of horseradish peroxidase with vapor deposited quantum dots. Sens. Actuator B Chem. 2009, 138, 278–282. [Google Scholar] [CrossRef]
- Jina, D.; Sakthivel, K.; Gandhi, S.; Huy, B.T.; Lee, Y.I. An improved non-enzymatic hydrogen peroxide sensor based on europium functionalized inorganic hybrid material—evaluation of optical and electrochemical properties. Sens. Actuator B Chem. 2016, 237, 81–89. [Google Scholar] [CrossRef]
- Gan, Z.; Gui, Q.; Shan, Y.; Pan, P.; Zhang, N.; Zhang, L. Photoluminescence of MoS2 quantum dots quenched by hydrogen peroxide: A fluorescent sensor for hydrogen peroxide. J. Appl. Phys. 2016, 120, 104503. [Google Scholar] [CrossRef]
- Soh, N.; Sakawaki, O.; Makihara, K.; Odo, Y.; Fukaminato, T.; Kawai, T.; Irie, M.; Imato, T. Design and development of a fluorescent probe for monitoring hydrogen peroxide using photoinduced electron transfer. Bioorg. Med. Chem. 2005, 13, 1131–1139. [Google Scholar] [CrossRef]
- Shiang, Y.C.; Huang, C.C.; Chang, H.T. Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose. Chem. Commun. 2009, 3437–3439. [Google Scholar] [CrossRef]
- Cao, L.; Ye, J.; Tong, L.; Tang, B. A new route to the considerable enhancement of glucose oxidase (GOx) activity: The simple assembly of a complex from CdTe quantum dots and GOx, and its glucose sensing. Chem. Eur. J. 2008, 14, 9633–9640. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.D.; Rhee, J.I. Use of CdSe/ZnS core-shell quantum dots as energy transfer donors in sensing glucose. Talanta 2007, 73, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Voraberger, H.S.; Trettnak, W.; Ribitsch, V. Optochemical hydrogen peroxide sensor based on oxygen detection. Sens. Actuator B Chem. 2003, 90, 324–331. [Google Scholar] [CrossRef]
- Mills, A.; Tommons, C.; Bailey, R.T.; Tedford, M.C.; Crilly, P.J. Reversible, fluorescence-based optical sensor for hydrogen peroxide. Analyst 2007, 132, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; de Marcos, S.; Galban, J. Fluorometric enzymatic autoindicating biosensor for H2O2 determination based on modified catalase. Biosens. Bioelectron. 2013, 41, 150–156. [Google Scholar] [CrossRef]
- Burmistrova, N.A.; Meier, R.J.; Schreml, S.; Duerkop, A. Reusable optical sensing microplate for hydrogen peroxide using a fluorescent photoinduced electron transfer probe (HP Green). Sens. Actuator B Chem. 2014, 193, 799–805. [Google Scholar] [CrossRef]
- Wolfbeis, O.S.; Schaferling, M.; Durkop, A. Reversible optical sensor membrane for hydrogen peroxide using an immobilized fluorescent probe, and its application to a glucose biosensor. Microchim. Acta 2003, 143, 221–227. [Google Scholar] [CrossRef]
- Qu, L.; Peng, X. Control of photoluminescence properties of CdSe nanocrystals in growth. J. Am. Chem. Soc. 2002, 124, 2049–2055. [Google Scholar] [CrossRef]
- Gaunt, J.A.; Knight, A.E.; Windsor, S.A.; Chechik, V. Stability and quantum yield effects of small molecule additives on solutions of semiconductor nanoparticles. J. Colloid Interface Sci. 2005, 290, 437–443. [Google Scholar] [CrossRef]
- Grabolle, M.; Spieles, M.; Lesnyak, V.; Gaponik, N.; Eychmuller, A.U. Resch-Genger. Determination of the fluorescence quantum yield of quantum dots: Suitable procedures and achievable uncertainties. Anal. Chem. 2009, 81, 6285–6294. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- Chen, C.C.; Yet, C.P.; Wang, H.N.; Chao, C.Y. Self-assembly of monolayers of cadmium selenide nanocrystals with dual color emission. Langmuir 1999, 15, 6845–6850. [Google Scholar] [CrossRef]
- Aldana, J.; Wang, Y.A.; Peng, X. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc. 2001, 123, 8844–8850. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Xu, J.; Wu, D. Incorporating fluorescent dyes into monodisperse melamine–formaldehyde resin microspheres via an organic sol–gel process: A pre-polymer doping strategy. J. Mater. Chem. B 2014, 2, 5837–5846. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. Use of CdSe/ZnS luminescent quantum dots incorporated within sol–gel matrix for urea detection. Anal. Chim. Acta 2008, 626, 53–61. [Google Scholar] [CrossRef]
- Duong, H.D.; Sohn, O.J.; Lam, H.T.; Rhee, J.I. An optical pH sensor with extended detection range based on fluoresceinamine covalently bound to sol–gel support. Microchem. J. 2006, 84, 50–55. [Google Scholar] [CrossRef]
- Prasad, P.N. Nanophotonics; John Wiley & Sonc, Inc.: Hoboken, NJ, USA, 2004; p. 35. [Google Scholar]
- Blaudeck, T.; Zenkevich, E.I.; Cichos, F. C von Borczyskowski, Probing wave functions at semiconductor quantum-dot surfaces by non-FRET photoluminescence quenching. J. Phys. Chem. C 2008, 112, 20251–20257. [Google Scholar] [CrossRef]
- Nirmal, M.; Brus, L. Luminescence photophysics in semiconductor nanocrystals. Acc. Chem. Res. 1999, 32, 407–414. [Google Scholar] [CrossRef]
- Wosnick, J.H. New Sensor Applications of Poly(Phenylene Ethynylene)s. Ph.D. Thesis, MIT, Cambridge, MA, USA, 2004. [Google Scholar]
- Duong, H.D.; Rhee, J.I. Development of a ratiometric fluorescent urea biosensor based on the urease immobilized onto the oxazine 170 perchlorate-ethyl cellulose membrane. Talanta 2015, 134, 333–339. [Google Scholar] [CrossRef]
- Duong, H.D.; Rhee, J.I. Enhancement of the sensitivity of a quantum dot-based fiber optic temperature sensor using the sol–gel technique. Sens. Actuator B Chem. 2008, 134, 423–426. [Google Scholar] [CrossRef]
- Jacewicz, D.; Siedlecka-Kroplewska, K.; Drzeżdżon, J.; Piotrowska, A.; Wyrzykowski, D.; Tesmar, A.; Żamojć, K.; Chmurzyński, L. Method for detection of hydrogen peroxide in HT22 cells. Sci. Rep. 2017, 7, 45673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, H.; Cho, K.M.; Liao, J.C. Expanding metabolism for total biosynthesis of the nonnatural amino acid L-homoalanine. Proc. Natl. Acad. Sci. USA 2010, 107, 6234–6239. [Google Scholar] [CrossRef] [PubMed]
- Zurbriggen, B.D.; Rekhif, N.; Mehlmann-De-Campos, M.; Lerch, K. Production of α-Ketobutyrate. U.S. Patent 7,144,715,B2, 5 December 2006. [Google Scholar]
- Hidalgo, F.J.; Delgado, R.M.; Zamora, R. Intermediate role of α-keto acids in the formation of Strecker aldehydes. Food Chem. 2013, 141, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Guichard, E.; Schlich, P.; Charpentier, C. Optimal conditions for the formation of Sotolon from α-ketobutyric acid in the French “Vin Jaune”. J. Agric. Food Chem. 1995, 43, 2616–2619. [Google Scholar]
- Zhang, C.; Qi, J.; Li, Y.; Fan, X.; Xu, Q.; Chen, N.; Xie, X. Production of α-ketobutyrate using engineered Escherichia coli via temperature shift. Biotechnol. Bioeng. 2016, 113, 2054–2059. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, C.; Che, B.; Ma, C.; Zheng, Z.; Qin, T.; Xu, P. Efficient bioconversion of l-threonine to 2-oxobutyrate using whole cells of Pseudomonas stutzeri SDM. Bioresour. Technol. 2012, 110, 719–722. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Cirone, J.; Chen, A. Fluorescent Fe3O4 quantum dots for H2O2 detectio. ACS Appl. Nano Mater. 2019, 2, 2076–2085. [Google Scholar] [CrossRef]
- Ge, J.J.; Ren, X.L.; Qiu, X.Z.; Shi, H.T.; Meng, X.W.; Tang, F.Q. Fast synthesis of fluorescent SiO2@CdTe nanoparticles with reusability in detection of H2O2. J. Mater. Chem. B 2015, 3, 6385–6390. [Google Scholar] [CrossRef]
- Lin, L.; Wen, Y.Q.; Liang, Y.X.; Zhang, N.; Xiao, D. Aqueous synthesis of Ag+ doped CdS quantum dots and its application in H2O2 sensing. Anal. Methods 2013, 5, 457–464. [Google Scholar] [CrossRef]
- Chu, S.C.; Hsieh, W.M.; Su, R.Z. Hydrogen Peroxide Sensing Based on Carbon Quantum dots. In Proceedings of the MATEC Web of Conferences, Hongkong, China, 12–14 March 2016. [Google Scholar]
- Wang, Y.; Qian, J.; Chen, Z.; Wang, C.; Liu, C.; Yang, Y.; Wu, Z.; Song, Y. CeO2 quantum dots modified electrode for detecting hydrogen peroxide. Inorg. Chem. Commun. 2019, 101, 62–68. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Xu, H.; Li, D.; Wang, Y. Para-hydroxy thiophenol-coated CdSe/ZnS quantum dots as a turn-on fluorescence probe for H2O2 detection in aqueous media. Aust. J. Chem. 2018, 71, 971–977. [Google Scholar] [CrossRef]





| Sensing Element and Method | Supporting Matrix | Linear Detection Range | Limit of Detection (LOD) | Reference |
|---|---|---|---|---|
| Fe3O4 QDs and fluorescence | Particles | 10 nM–10 mM | 3.87 nM | [50] |
| CdTe QDs and fluorescence | Particles | 5 uM–200 uM | 10 nM | [51] |
| CdS/Ag2S QDs and fluorescence | Particles | 1–10 mM | 30 uM | [52] |
| MoS2 QDs and fluorescence | Particles | 2–20 uM | n.d | [18] |
| CQDs and fluorescence | Particles | 0–88.2 mM | n.d | [53] |
| CeO2 QDs and electrode | Membrane (glassy carbon) | 2–1000 ppm | 900 ppb | [54] |
| CdSe/ZnS QDs and fluorescence | Particles | 0.309–4.9 mM | 0.135 mM | [55] |
| HRP + CdSe/ZnS QDs and electrode | Membrane (glassy carbon) | 5–100 uM | 28.4 uM | [16] |
| HRP + CdSe/ZnS QDs and fluorescence | Membrane (GA sol–gel/ethyl cellulose) | 0–1 mM & 1–10 mM | 0.016 and 0.058 mM, 0.011 and 0.068 mM (HRP) | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, H.D.; Rhee, J.I. Development of Ratiometric Fluorescence Sensors Based on CdSe/ZnS Quantum Dots for the Detection of Hydrogen Peroxide. Sensors 2019, 19, 4977. https://doi.org/10.3390/s19224977
Duong HD, Rhee JI. Development of Ratiometric Fluorescence Sensors Based on CdSe/ZnS Quantum Dots for the Detection of Hydrogen Peroxide. Sensors. 2019; 19(22):4977. https://doi.org/10.3390/s19224977
Chicago/Turabian StyleDuong, Hong Dinh, and Jong Il Rhee. 2019. "Development of Ratiometric Fluorescence Sensors Based on CdSe/ZnS Quantum Dots for the Detection of Hydrogen Peroxide" Sensors 19, no. 22: 4977. https://doi.org/10.3390/s19224977
APA StyleDuong, H. D., & Rhee, J. I. (2019). Development of Ratiometric Fluorescence Sensors Based on CdSe/ZnS Quantum Dots for the Detection of Hydrogen Peroxide. Sensors, 19(22), 4977. https://doi.org/10.3390/s19224977






