A New pH-Dependent Macrocyclic Rhodamine B-Based Fluorescent Probe for Copper Detection in White Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.2.1. NMR Measurements
2.2.2. Mass Spectroscopy
2.2.3. UV-Visible and Fluorescence Measurement
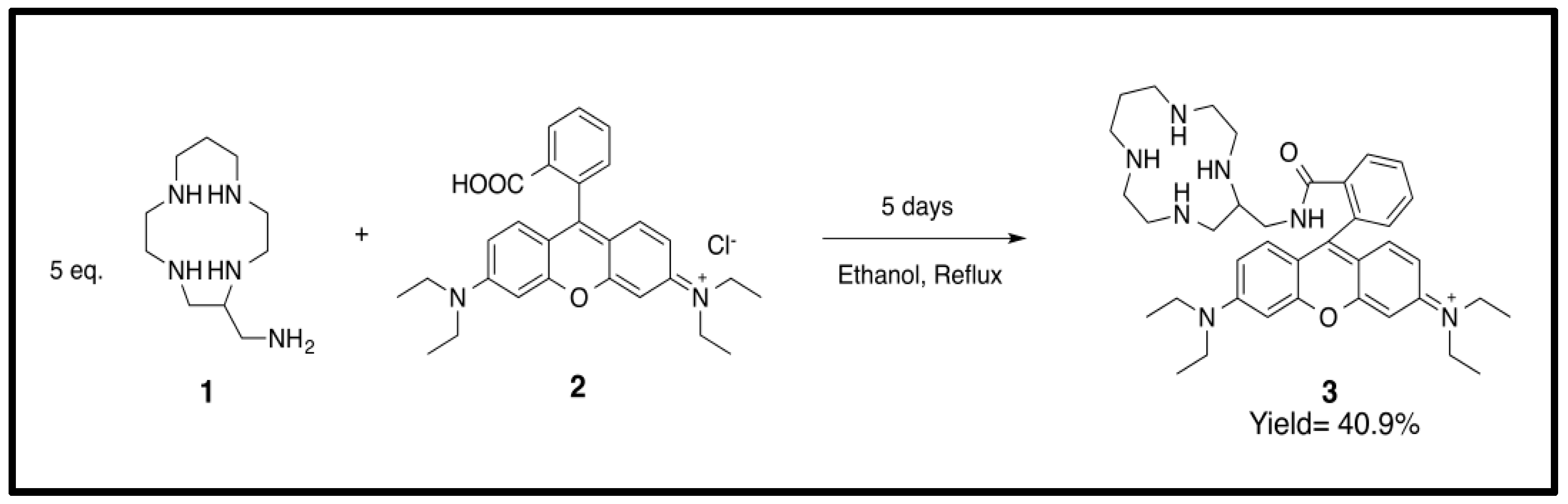
2.3. Synthesis and Characterization of Probe 3 [N-(9-(2-((1,4,7,10-tetraazacyclotridecan-5-yl)methyl)-3-oxoisoindolin-1-yl)-6-(diethylamino)-3H-xanthen-3-ylidene)- N ethylethanamine]
3. Results and Discussion
3.1. Preparation of Probe 3
3.2. General Procedure for the Measurement of Absorption and Fluorescence Analysis
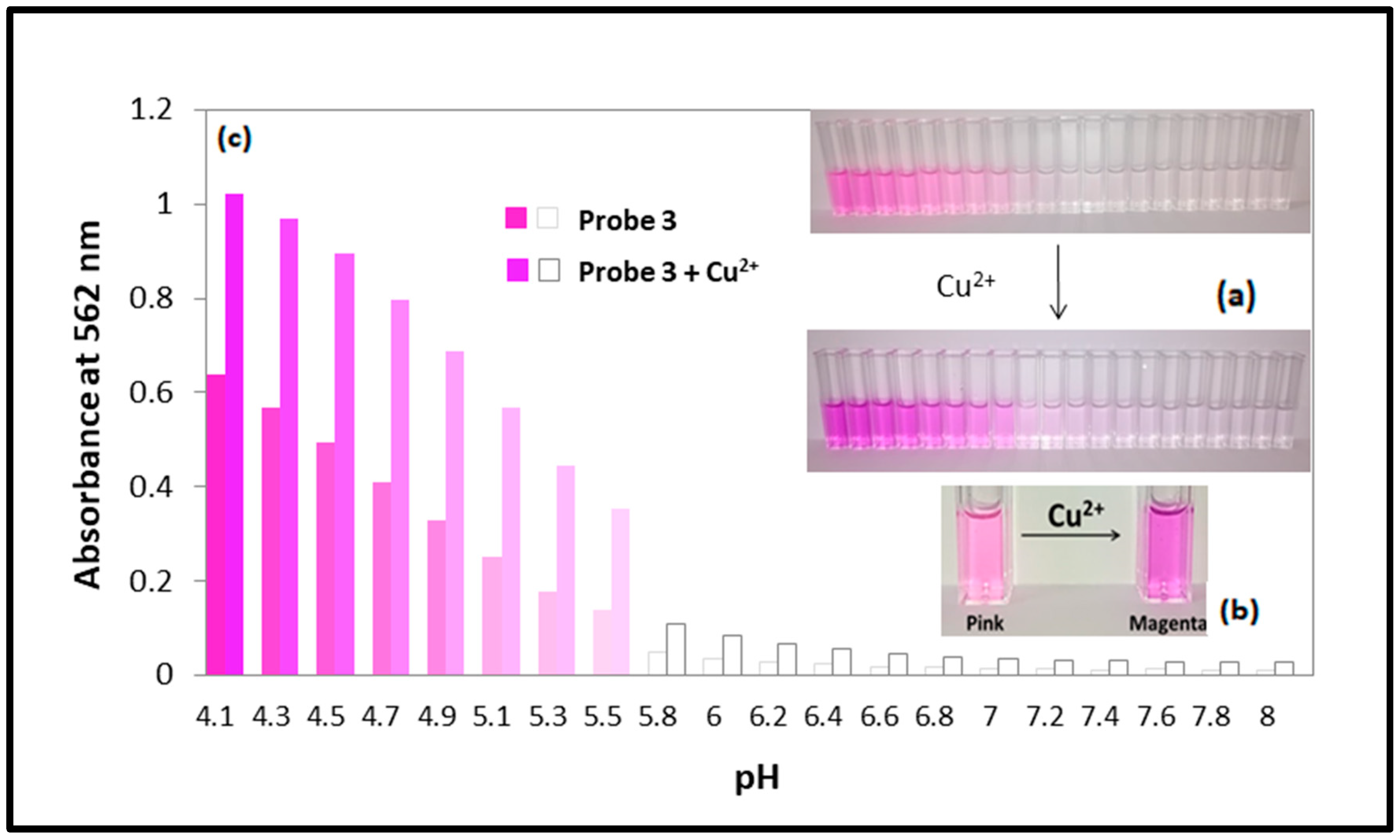
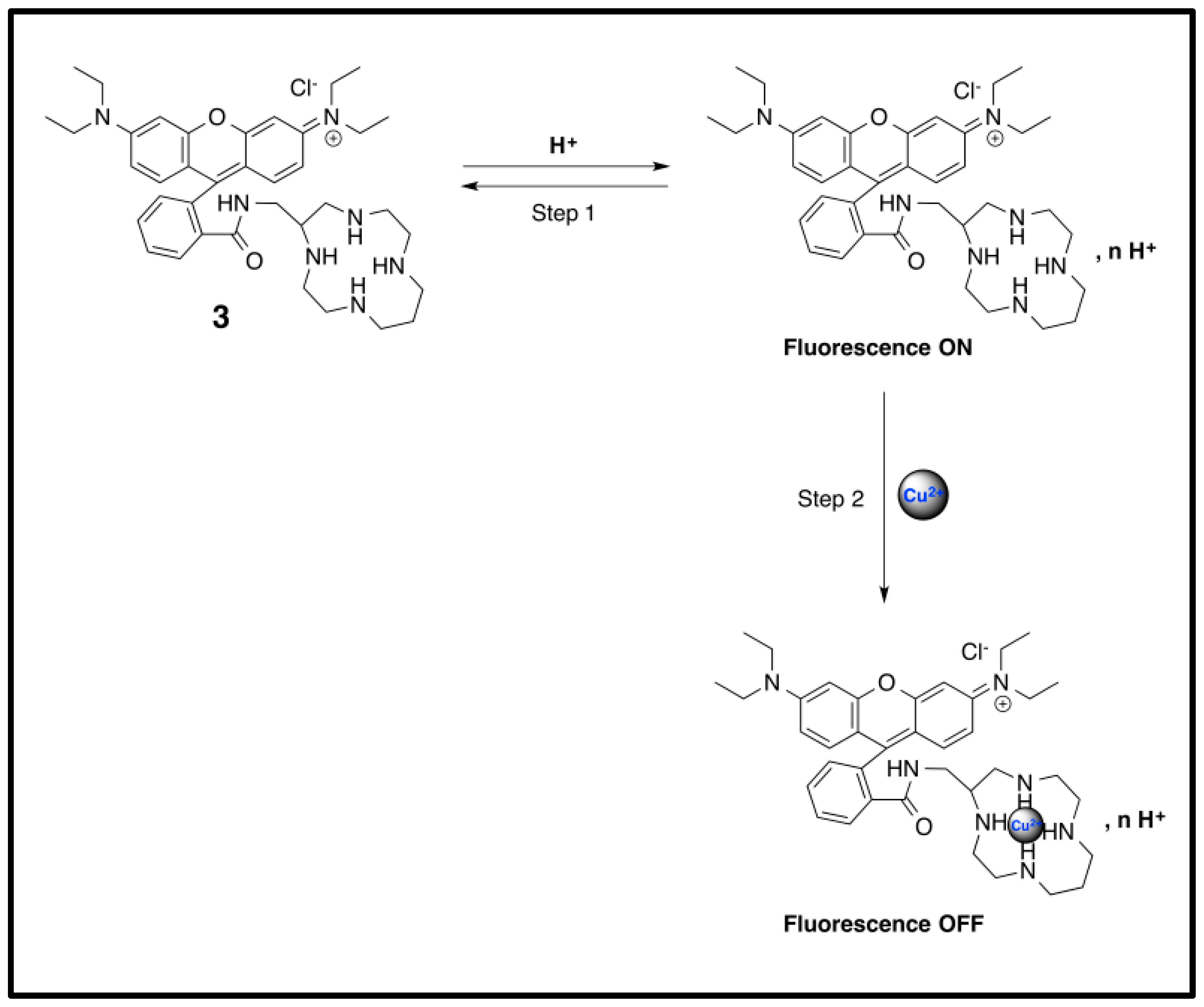
3.3. The pH Dependency of Probe 3 and Its Behavior Towards the Absence or Presence Cu2+ Ions Explained by UV-Vis Measurement
3.4. Fluorescence Measurements of Probe 3 in the Presence of Copper (II) Ions at Different Concentrations
3.5. Limit of Detection (LOD) of Copper (II) Ions by Probe 3
3.6. Selectivity of Probe 3 Toward Cu2+ Ions over Other Metal Ions
3.7. Application Test of Probe 3 in White Wine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, B.; Campbell, P.T.; Gapstur, S.M.; Jacobs, E.J.; Bostick, R.M.; Fedirko, V.; Flanders, W.D.; McCullough, M.L. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: The Cancer Prevention Study II Nutrition Cohort. Am. J. Clin. Nutr. 2016, 103, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Brun, L.; Maillet, J.; Hinsinger, P.; Pépin, M. Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ. Pollut. 2001, 111, 293–302. [Google Scholar] [CrossRef]
- Wain, R.L.; Wilkinson, E.H. Studies upon the Copper Fungicides.: VI. The Solution of Copper from Bordeaux and Burgundy Mixtures. Ann. Appl. Biol. 1943, 30, 379–391. [Google Scholar] [CrossRef]
- Dias, A.A.; Fernandes, J.M.C.; Sousa, R.M.O.F.; Pinto, P.A.; Amaral, C.; Sampaio, A.; Bezerra, R.M.F. Fungal Conversion and Valorization of Winery Wastes. In Mycoremediation and Environmental Sustainability; Springer: Cham, Switzerland, 2018; pp. 239–252. [Google Scholar]
- Tromp, A.; de Klerk, C.A. Effect of Copperoxychloride on the Fermentation of Must and on Wine Quality. S. Afr. J. Enol. Vitic. 1988, 9. [Google Scholar] [CrossRef][Green Version]
- Clark, A.C.; Scollary, G.R. Determination of total copper in white wine by stripping potentiometry utilising medium exchange. Anal. Chim. Acta 2000, 413, 25–32. [Google Scholar] [CrossRef]
- Abe, A.; Yamashita, S.; Noma, A. Sensitive, Direct Colorimetric Assay for Copper in Serum. Clin. Chem. 1989, 35, 552–554. [Google Scholar]
- Chereddy, N.R.; Suman, K.; Korrapati, P.S.; Thennarasu, S.; Mandal, A.B. Design and Synthesis of Rhodamine Based Chemosensors for the Detection of Fe3+ Ions. Dyes Pigm. 2012, 95, 606–613. [Google Scholar] [CrossRef]
- Hayes, F.J.; Halsall, H.B.; Heineman, W.R. Simultaneous Immunoassay Using Electrochemical Detection of Metal Ion Labels. Anal. Chem. 1994, 66, 1860–1865. [Google Scholar] [CrossRef]
- Lévêque, N.; Robin, S.; Makki, S.; Muret, P.; Mary, S.; Berthelot, A.; Humbert, P. Iron Concentrations in Human Dermis Assessed by Microdialysis Associated with Atomic Absorption Spectrometry. Boil. Pharm. Bull. 2001, 24, 10–13. [Google Scholar] [CrossRef][Green Version]
- Mergu, N.; Singh, A.; Gupta, V. Highly Sensitive and Selective Colorimetric and Off-On Fluorescent Reversible Chemosensors for Al3+ Based on the Rhodamine Fluorophore. Sensors 2015, 15, 9097–9111. [Google Scholar] [CrossRef]
- Montalti, M.; Prodi, L.; Zaccheroni, N. Fluorescence quenching amplification in silica nanosensors for metal ions. J. Mater. Chem. 2005, 15, 2810. [Google Scholar] [CrossRef]
- Cotruvo, J.A.; Aron, A.T.; Ramos-Torres, K.M.; Chang, C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015, 44, 4400–4414. [Google Scholar] [CrossRef] [PubMed]
- Huo, F.J.; Su, J.; Sun, Y.Q.; Yin, C.X.; Tong, H.B.; Nie, Z.X. A rhodamine-based dual chemosensor for the visual detection of copper and the ratiometric fluorescent detection of vanadium. Dyes Pigment. 2010, 86, 50–55. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, N.; Bhalla, V.; Sharma, P.R.; Kaur, T. Highly Selective Fluorescence Turn-on Chemodosimeter Based on Rhodamine for Nanomolar Detection of Copper Ions. Org. Lett. 2012, 14, 406–409. [Google Scholar] [CrossRef]
- Mei, L.; Xiang, Y.; Li, N.; Tong, A. A new fluorescent probe of rhodamine B derivative for the detection of copper ion. Talanta 2007, 72, 1717–1722. [Google Scholar] [CrossRef]
- Yang, Z.; She, M.; Zhang, J.; Chen, X.; Huang, Y.; Zhu, H.; Liu, P.; Li, J.; Shi, Z. Highly Sensitive and Selective Rhodamine Schiff Base “off-on” Chemosensors for Cu2+ Imaging in Living Cells. Sens. Actuators B 2013, 176, 482–487. [Google Scholar] [CrossRef]
- Tanthanuch, S.; Kukiattrakoon, B.; Peerasukprasert, T.; Chanmanee, N.; Chaisomboonphun, P.; Rodklai, A. The effect of red and white wine on color changes of nanofilled and nanohybrid resin composites. Restor. Dent. Endod. 2016, 41, 130–136. [Google Scholar] [CrossRef]
- Na Kim, H.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: Application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465. [Google Scholar] [CrossRef]
- Xiang, Y.; Tong, A. A New Rhodamine-Based Chemosensor Exhibiting Selective FeIII-Amplified Fluorescence. Org. Lett. 2006, 8, 1549–1552. [Google Scholar] [CrossRef]
- Yang, Z.; She, M.; Yin, B.; Cui, J.; Zhang, Y.; Sun, W.; Li, J.; Shi, Z. Three Rhodamine-Based “Off–On” Chemosensors with High Selectivity and Sensitivity for Fe3+ Imaging in Living Cells. J. Org. Chem. 2012, 77, 1143–1147. [Google Scholar] [CrossRef]
- Zhang, X.; Shiraishi, Y.; Hirai, T. A new rhodamine-based fluorescent chemosensor for transition metal cations synthesized by one-step facile condensation. Tetrahedron Lett. 2007, 48, 5455–5459. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, X.-F.; He, S.; Wang, L. A rhodamine-based chromogenic and fluorescent chemosensor for copper ion in aqueous media. Sens. Actuators B Chem. 2009, 135, 625–631. [Google Scholar] [CrossRef]
- Jung, H.S.; Kwon, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-Derived Cu2+ -Selective Fluorescence Sensor: Synthesis, Mechanisms, and Applications in Living Cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, C.; Li, B.; Xu, L.; Duan, L. A Rhodamine-Based Colorimetric and Reversible Fluorescent Chemosensor for Selectively Detection of Cu2+ and Hg2+ Ions. Sens. Actuators B 2014, 199, 121–126. [Google Scholar] [CrossRef]
- Denat, F.; Diaz-Fernandez, Y.A.; Pallavicini, P.; Pasotti, L.; Rousselin, Y.; Sok, N. The Cu(II) complex of a C-lipophilized 13aneN4 macrocycle with an additional protonable amino group as micellar anion receptor. Dalton Trans. 2009, 6751–6758. [Google Scholar] [CrossRef]
- Guan, X.; Lin, W.; Huang, W. Development of a New Rhodamine-Based FRET Platform and Its Application as a Cu2+ Probe. Org. Biomol. Chem. 2014, 12, 3944. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Kukhtik, V.I.; Alekseeva, V.I. Ionization and Tautomerism of Fluorescein, Rhodamine B, N, N-Diethylrhodol and Related Dyes in Mixed and Nonaqueous Solvents. Dyes Pigm. 1994, 24, 11–35. [Google Scholar] [CrossRef]
- Rieth, T.; Sasamoto, K. Detection of nitric oxide and nitrite by using a Rhodamine-type fluorescent indicator. Anal. Commun. 1998, 35, 195–198. [Google Scholar] [CrossRef]
- Rousselin, Y.; Sok, N.; Boschetti, F.; Guilard, R.; Denat, F. Efficient Synthesis of NewC-Functionalized Macrocyclic Polyamines. Eur. J. Org. Chem. 2010, 2010, 1688–1693. [Google Scholar] [CrossRef]
- Vogel, M.; Rettig, W.; Sens, R.; Drexhage, K.H. Structural relaxation of rhodamine dyes with different N-substitution patterns: A study of fluorescence decay times and quantum yields. Chem. Phys. Lett. 1988, 147, 452–460. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.-J.; Zhu, Z.-J. A reversible Hg(ii)-selective fluorescent chemosensor based on a thioether linked bis-rhodamine. RSC Adv. 2013, 3, 24891. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Dacarro, G.; Denat, F.; Boschetti, F.; Nikolantonaki, M.; Gougeon, R.; D’Alessio, G.; Viaux, A.-S.; Bertheau, L.; et al. An Off-On-Off Fluorescent Sensor for PH Windows Based on the 13aneN4-Zn2+ System. Eur. J. Inorg. Chem. 2016, 2016, 5106–5113. [Google Scholar] [CrossRef]
- Kodama, M.; Kimura, E. Equilibria and Kinetics of Complex Formation between Zinc (II), Lead (II), and Cadmium (II), and 12-, 13-, 14-, and 15-Membered Macrocyclic Tetra-Amines. Dalton Trans. 1977, 2269–2276. [Google Scholar] [CrossRef]
- ANSES_CIQUAL. Copper (mg/100g) content in French food. Available online: https://ciqual.anses.fr/#/constituants/10290/copper-(mg-100g) (accessed on 8 March 2018).
- Heijerick, D.G.; Van Sprang, P.A.; Van Hyfte, A.D. Ambient copper concentrations in agricultural and natural European soils: An overview. Environ. Toxicol. Chem. 2006, 25, 858–864. [Google Scholar] [CrossRef]
- Hu, X.-X.; Zheng, X.-L.; Fan, X.X.; Su, Y.-T.; Zhan, X.Q.; Zheng, H. Semicarbazide-based naphthalimide as a highly selective and sensitive colorimetric and “turn-on” fluorescent chemodosimeter for Cu2+. Sens. Actuators B Chem. 2016, 227, 191–197. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, X.; Yang, H.; Zhang, T.; Han, A.; Zang, L. A Cu2+ Selective Probe Based on Phenanthro-Imidazole Derivative. Sensors 2017, 17, 35. [Google Scholar] [CrossRef]
- Xiang, Y.; Tong, A. Ratiometric and selective fluorescent chemodosimeter for Cu(II) by Cu(II)-induced oxidation. J. Lumin. 2008, 23, 28–31. [Google Scholar] [CrossRef]
- Ding, J.; Yuan, L.; Gao, L.; Chen, J. Fluorescence quenching of a rhodamine derivative: Selectively sensing Cu2+ in acidic aqueous media. J. Lumin. 2012, 132, 1987–1993. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Ying, S.; Cui, S.; Wang, W.; Feng, J.; Chen, J. Simple and sensitive detection method for diprophylline using glutathione-capped CdTe quantum dots as fluorescence probes. J. Lumin. 2014, 145, 575–581. [Google Scholar] [CrossRef]
- Iannone, R.; Miranda, S.; Riemma, S.; De Marco, I. Improving environmental performances in wine production by a life cycle assessment analysis. J. Clean. Prod. 2016, 111, 172–180. [Google Scholar] [CrossRef]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Diéval, J.-B.; Aagaard, O.; Waters, E.J. Evolution of 3-Mercaptohexanol, Hydrogen Sulfide, and Methyl Mercaptan during Bottle Storage of Sauvignon blanc Wines. Effect of Glutathione, Copper, Oxygen Exposure, and Closure-Derived Oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Viviers, M.Z.; Smith, M.E.; Wilkes, E.; Smith, P. Effects of Five Metals on the Evolution of Hydrogen Sulfide, Methanethiol, and Dimethyl Sulfide during Anaerobic Storage of Chardonnay and Shiraz Wines. J. Agric. Food Chem. 2013, 61, 12385–12396. [Google Scholar] [CrossRef] [PubMed]


| Sample | WS1 | WS2 | WS3 | WS4 | WS5 | WS6 | Controls |
|---|---|---|---|---|---|---|---|
| Cu2+ content (mg/L) 1 | 0.03 ± 0.01 | 0.15 ± 0.01 | 0.38 ± 0.01 | 0.44 ± 0.02 | 0.68 ± 0.00 | 0.76 ± 0.00 | 0.00 ± 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doumani, N.; Bou-Maroun, E.; Maalouly, J.; Tueni, M.; Dubois, A.; Bernhard, C.; Denat, F.; Cayot, P.; Sok, N. A New pH-Dependent Macrocyclic Rhodamine B-Based Fluorescent Probe for Copper Detection in White Wine. Sensors 2019, 19, 4514. https://doi.org/10.3390/s19204514
Doumani N, Bou-Maroun E, Maalouly J, Tueni M, Dubois A, Bernhard C, Denat F, Cayot P, Sok N. A New pH-Dependent Macrocyclic Rhodamine B-Based Fluorescent Probe for Copper Detection in White Wine. Sensors. 2019; 19(20):4514. https://doi.org/10.3390/s19204514
Chicago/Turabian StyleDoumani, Nour, Elias Bou-Maroun, Jacqueline Maalouly, Maya Tueni, Adrien Dubois, Claire Bernhard, Franck Denat, Philippe Cayot, and Nicolas Sok. 2019. "A New pH-Dependent Macrocyclic Rhodamine B-Based Fluorescent Probe for Copper Detection in White Wine" Sensors 19, no. 20: 4514. https://doi.org/10.3390/s19204514
APA StyleDoumani, N., Bou-Maroun, E., Maalouly, J., Tueni, M., Dubois, A., Bernhard, C., Denat, F., Cayot, P., & Sok, N. (2019). A New pH-Dependent Macrocyclic Rhodamine B-Based Fluorescent Probe for Copper Detection in White Wine. Sensors, 19(20), 4514. https://doi.org/10.3390/s19204514





