Switchable Piezoresistive SmS Thin Films on Large Area
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
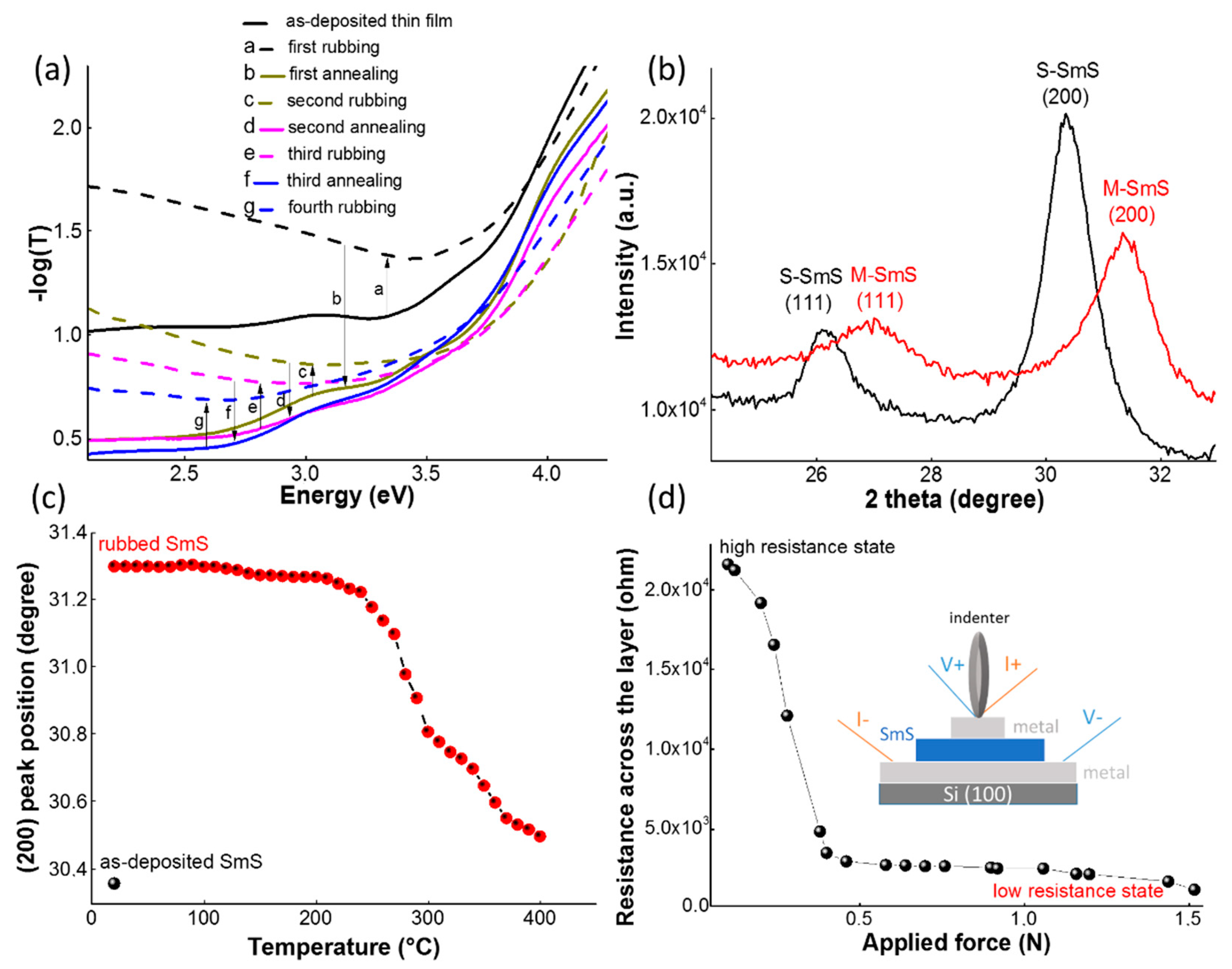
3.1. Basic Properties of SmS Thin Films
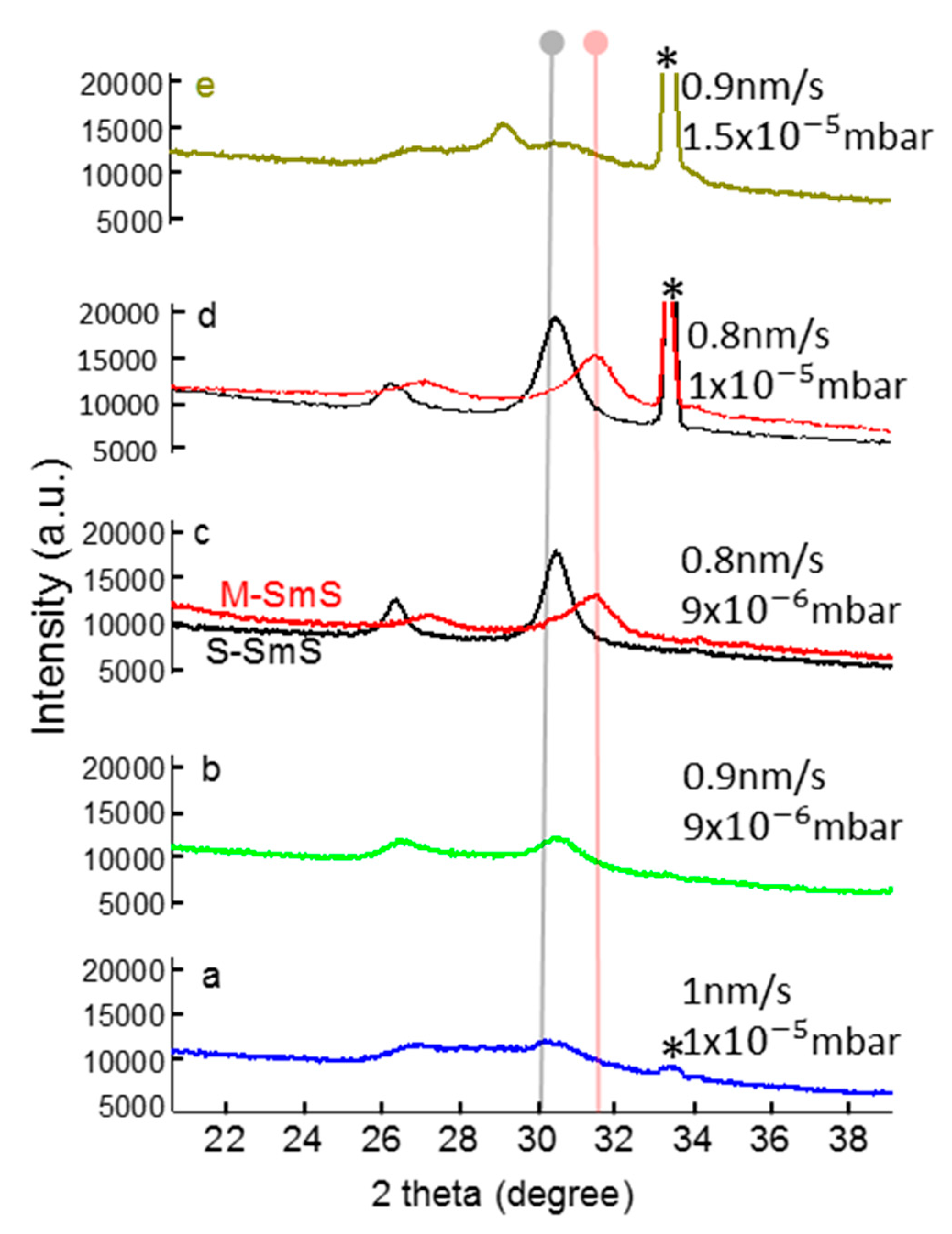
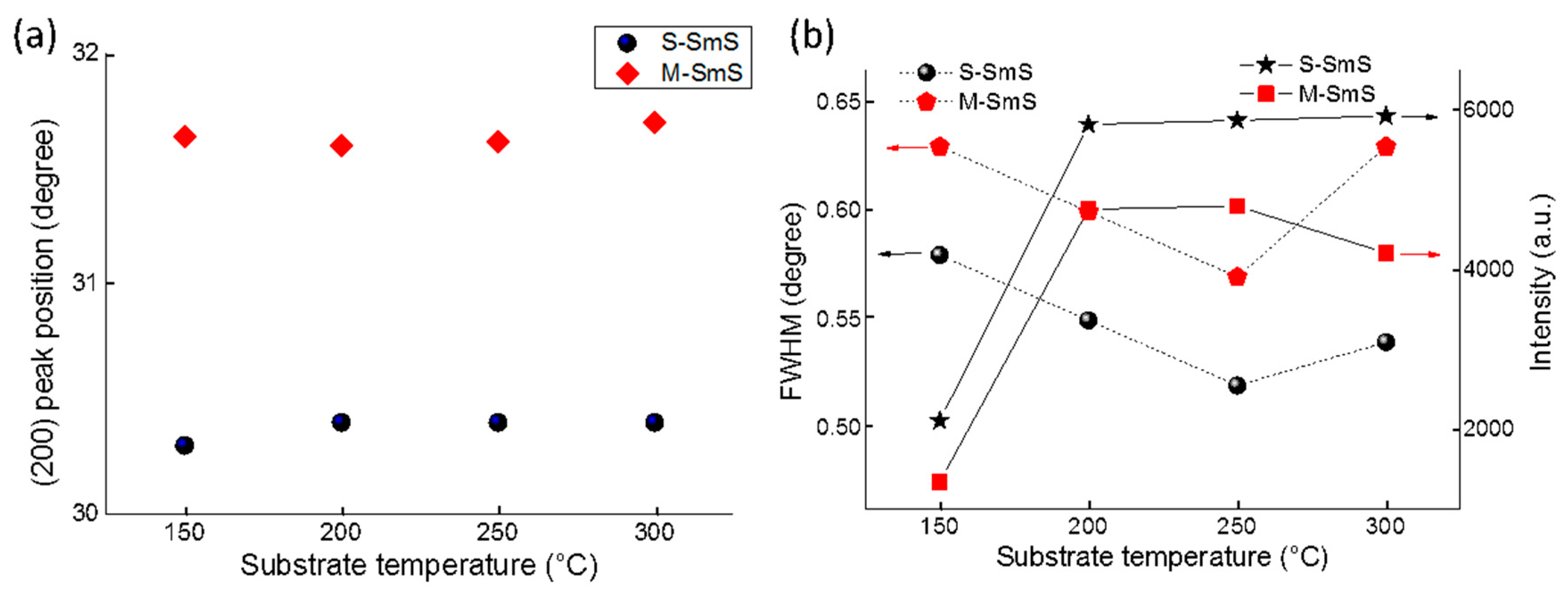
3.2. Optimizing Synthesis Conditions
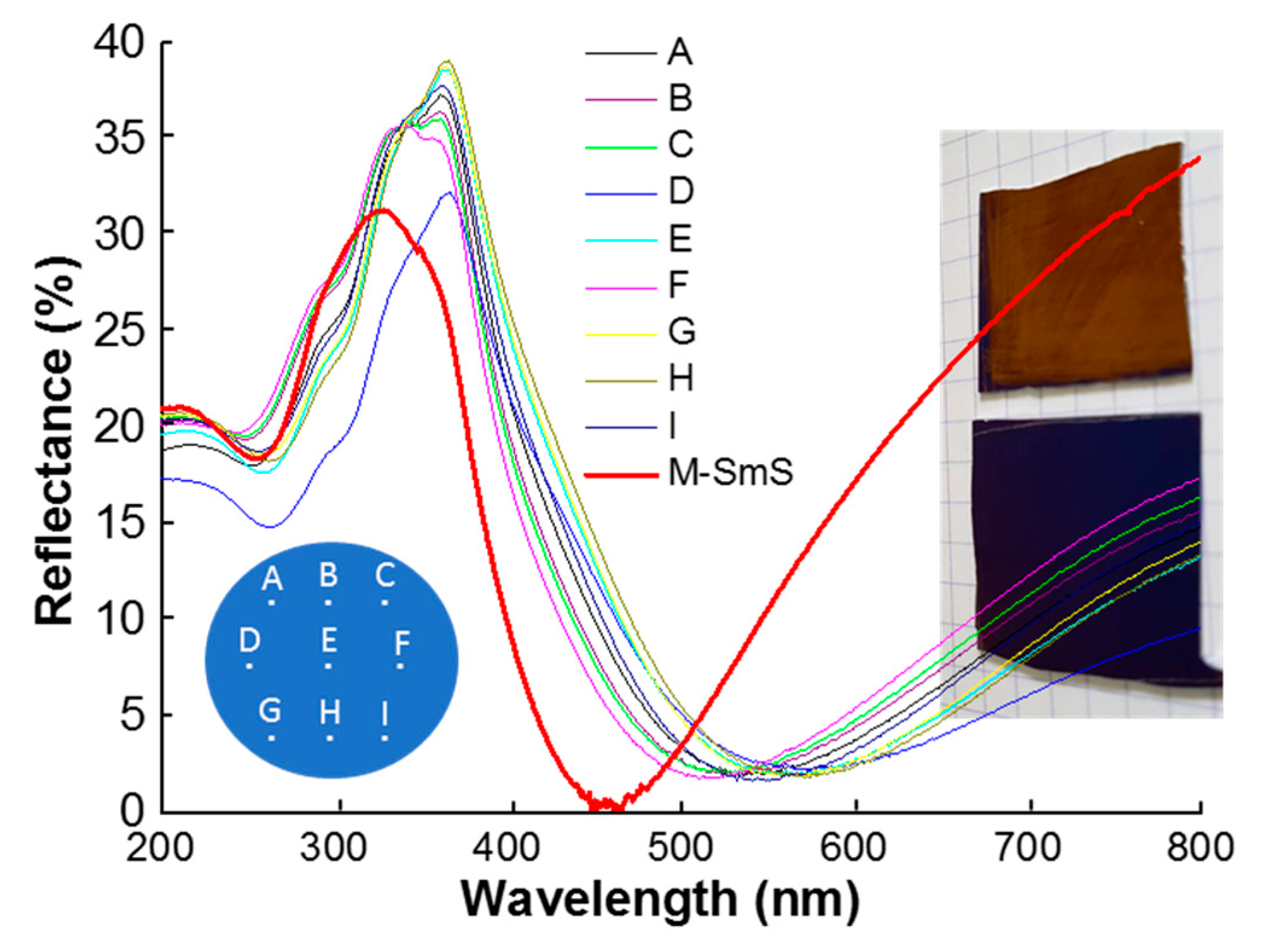
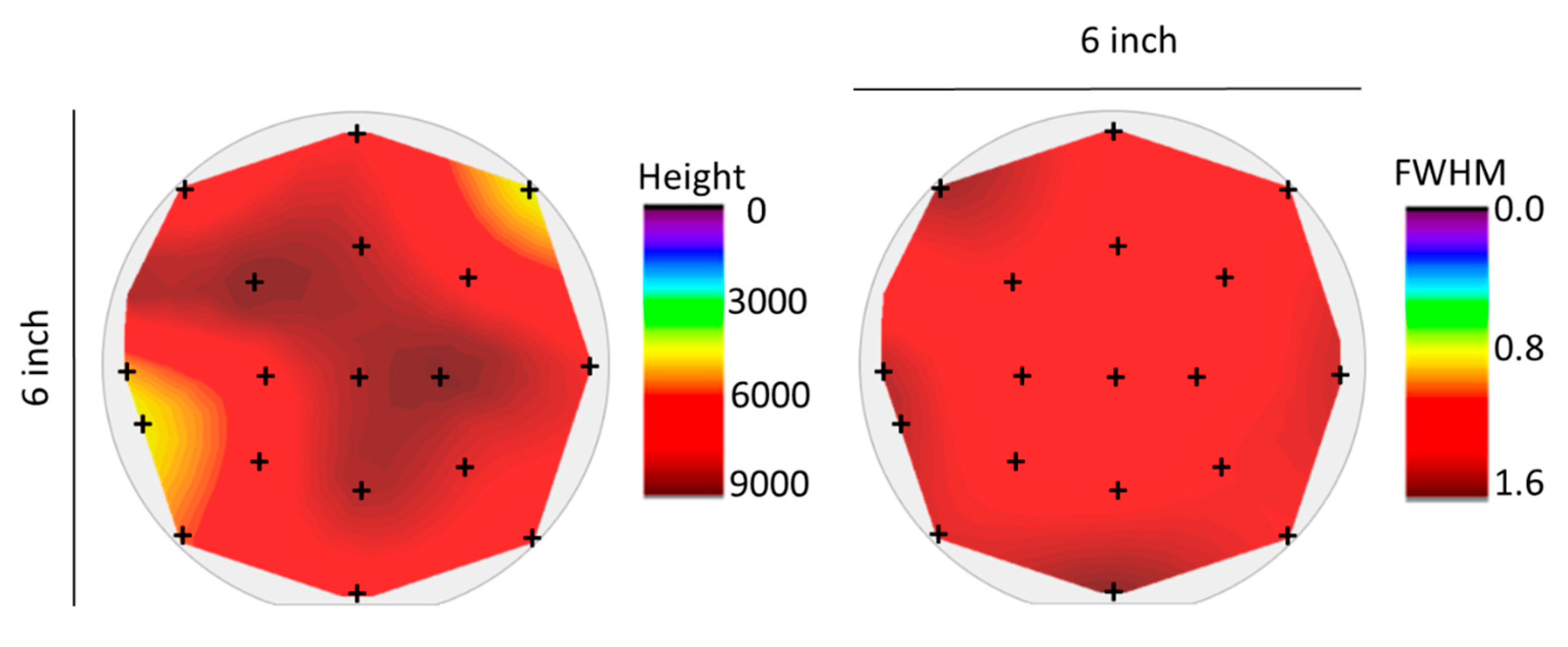
3.3. Homogeneous, Switchable SmS Thin Films on 150 mm Diameter Wafers
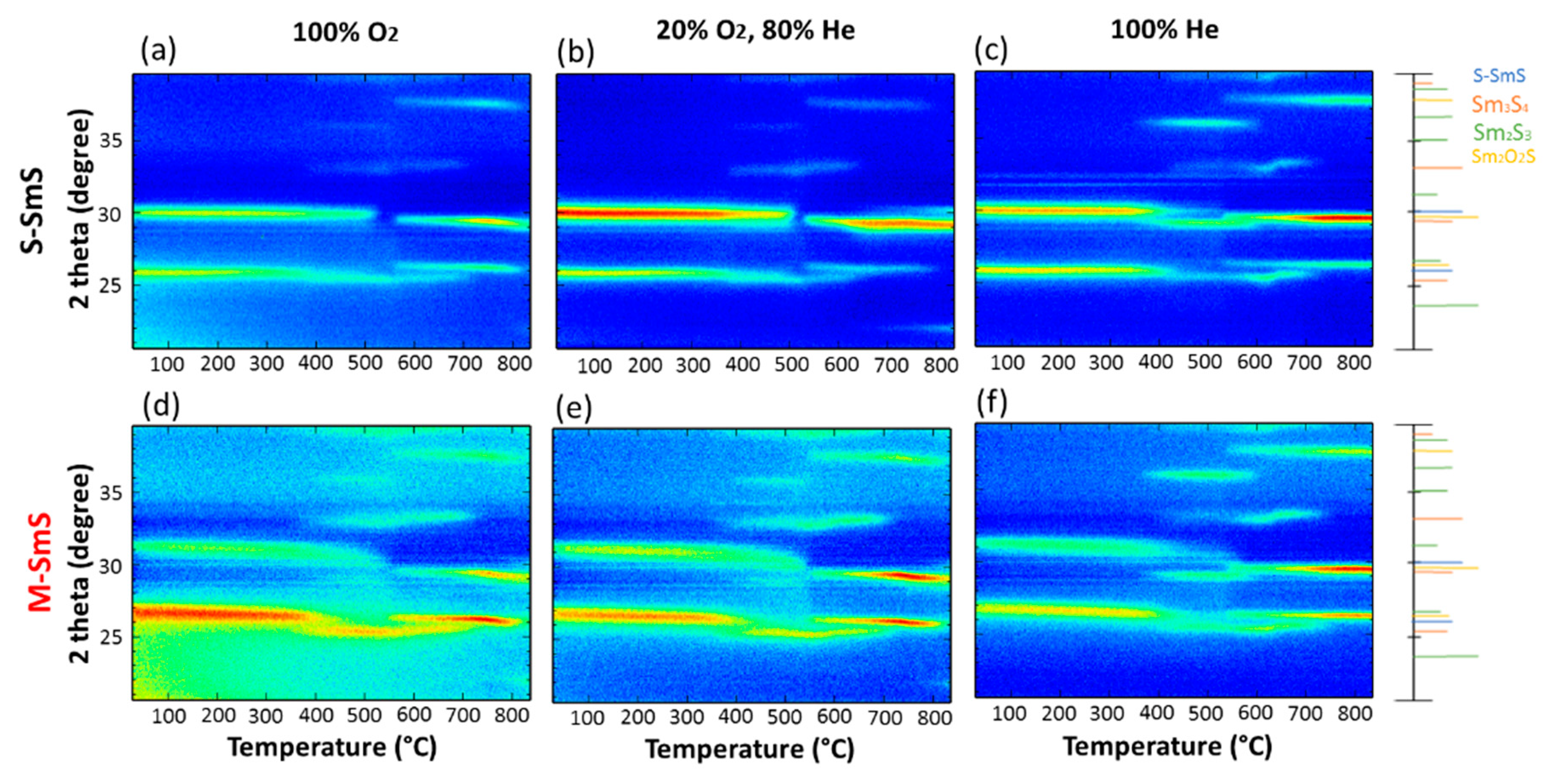
3.4. Thermal Stability of SmS
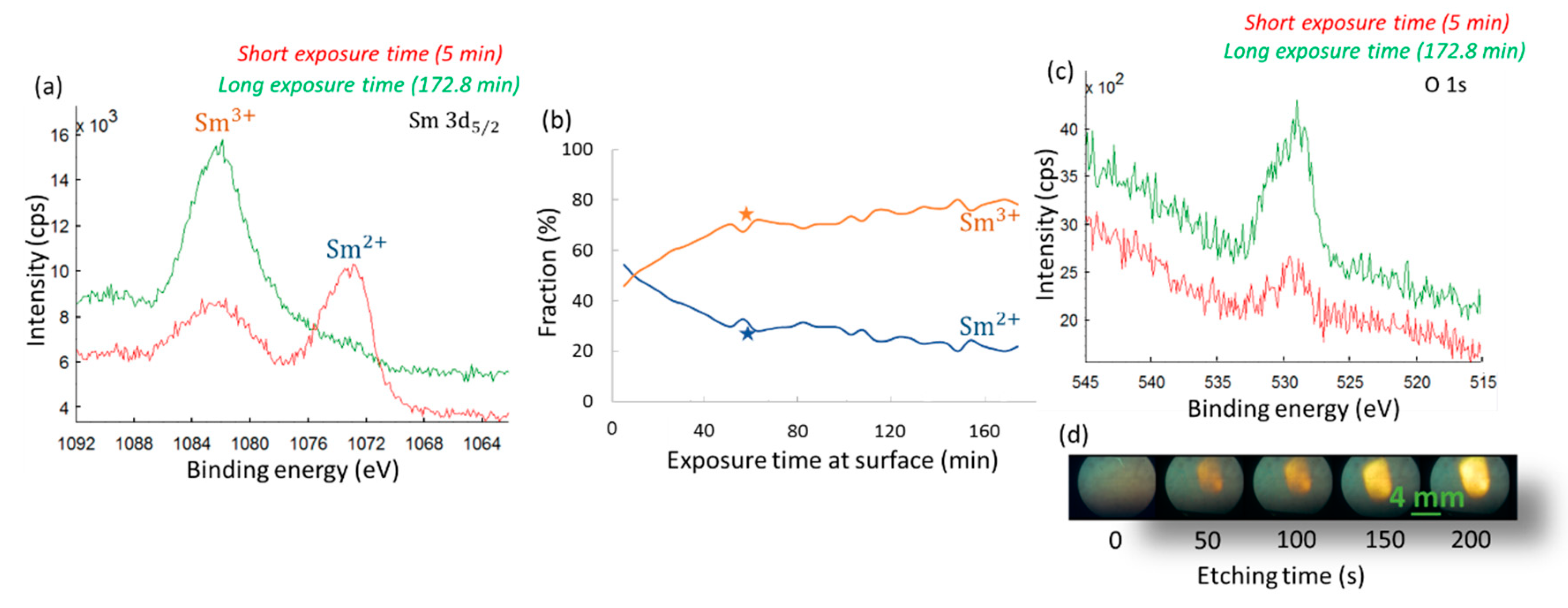
3.5. Using XPS to Probe the Valence State of Sm
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsubayashi, K.; Imura, K.; Suzuki, H.S.; Mizuno, T.; Kimura, S.; Nishioka, T.; Kodama, K.; Sato, N.K. Effect of nominal composition on transport, optical, magnetic, and thermodynamic properties of SmS single crystals. J. Phys. Soc. Jpn. 2007, 76, 064601. [Google Scholar] [CrossRef]
- Sousanis, A.; Smet, P.F.; Detavernier, C.; Poelman, D. Stability of switchable SmS for piezoresistive applications. In Proceedings of the IEEE Nanotechnology Materials and Devices Conference (NMDC), Toulouse, France, 9–12 October 2016. [Google Scholar] [CrossRef]
- Corder, S.N.G.; Chen, X.; Zhang, S.; Hu, F.; Zhang, J.; Luan, Y.; Logan, J.A.; Ciavatti, T.; Bechtel, H.A.; Martin, M.C.; et al. Near-field spectroscopic investigation of dual-band heavy fermion metamaterials. Nat. Commun. 2017, 8, 2262. [Google Scholar] [CrossRef] [PubMed]
- Sousanis, A.; Smet, P.F.; Poelman, D. Samarium Monosulfide (SmS): Reviewing Properties and Applications. Materials 2017, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.; Guntherodt, G.; Holzapfel, W.B.; Dietrich, M.; Holtzberg, F. Effect of pressure on lattice-parameter, magnetic susceptibility and reflectivity of Sms. Solid State Commun. 1979, 29, 753–758. [Google Scholar] [CrossRef]
- Imura, K.; Matsubayashi, K.; Suzuki, H.S.; Kabeya, N.; Deguchi, K.; Sato, N.K. Pressure-temperature phase diagram of golden SmS. J. Phys. Soc. Jpn. 2009, 78, 104602. [Google Scholar] [CrossRef]
- de Tomasi, F.; Perrone, M.R.; Protopapa, M.L.; Leo, G. Laser irradiation effects on the resistance of SmS films. Thin Solid Films 2002, 413, 171–176. [Google Scholar] [CrossRef]
- Barla, A.; Sanchez, J.P.; Derr, J.; Salce, B.; Lapertot, G.; Flouquet, J.; Doyle, B.P.; Leupold, O.; Ruffer, R.; Abd-Elmeguid, M.M.; et al. Valence and magnetic instabilities in Sm compounds at high pressures. J. Phys. Condens. Mater. 2005, 17, 837–848. [Google Scholar] [CrossRef]
- Peter, A.P.; Martens, K.; Rampelberg, G.; Toeller, M.; Ablett, J.M.; Meersschaut, J.; Cuypers, D.; Franquet, A.; Detavernier, C.; Rueff, J.P.; et al. Metal-insulator transition in ALD VO2 ultrathin films and nanoparticles: Morphological control. Adv. Funct. Mater. 2015, 25, 679–686. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Bin, H.; Wang, L.; Hu, K.; Dong, Y.; Xu, H.; Huang, H.; Zhao, J.; Chen, H.; et al. Suppression of structural phase transition in VO2 by epitaxial strain in vicinity of metal-insulator transition. Sci. Rep. 2016, 6, 23119. [Google Scholar] [CrossRef]
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef]
- Welnic, W.; Kalb, J.A.; Wamwangi, D.; Steimer, C.; Wuttig, M. Phase change materials: From structures to kinetics. J. Mater. Res. 2007, 22, 2368–2375. [Google Scholar] [CrossRef]
- Rogers, E.; Smet, P.F.; Dorenbos, P.; Poelman, D.; van der Kolk, E. The thermally induced metal-semiconducting phase transition of samarium monosulfide (SmS) thin films. J. Phys. Condens. Mat. 2010, 22, 015005. [Google Scholar] [CrossRef]
- Takenaka, K.; Asai, D.; Kaizu, R.; Mizuno, Y.; Yokoyama, Y.; Okamoto, Y.; Katayama, N.; Suzuki, H.S.; Imanaka, Y. Giant isotropic negative thermal expansion in Y-doped samarium monosulfides by intra-atomic charge transfer. Sci. Rep. 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Asai, D.; Mizuno, Y.; Hasegawa, H.; Yokoyama, Y.; Okamoto, Y.; Katayama, N.; Suzuki, H.S.; Imanaka, Y.; Takenaka, K. Valence fluctuations and giant isotropic negative thermal expansion in Sm1–xRxS (R = Y, La, Ce, Pr, Nd). Appl. Phys. Lett. 2019, 114, 141902. [Google Scholar] [CrossRef]
- Mizuno, T.; Iizuka, T.; Kimura, S.; Matsubayashi, K.; Imura, K.; Suzuki, H.S.; Sato, N.K. Excitonic instability in the transition from the black phase to the golden phase of SmS under pressure investigated by infrared spectroscopy. J. Phys. Soc. Jpn. 2008, 77, 113704. [Google Scholar] [CrossRef]
- Hickey, C.F. Optical, Chemical, and Structural Properties of Thin Films of Samarium-Sulfide and Zinc-Sulfide. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1987. [Google Scholar]
- Maple, M.B.; Wohlleben, D. Nonmagnetic 4f Shell in the High-Pressure Phase of SmS. Phys. Rev. Lett. 1971, 27, 511–515. [Google Scholar] [CrossRef]
- Barla, A.; Sanchez, J.P.; Haga, Y.; Lapertot, G.; Doyle, B.P.; Leupold, O.; Ruffer, R.; Abd-Elmeguid, M.M.; Lengsdorf, R.; Flouquet, J. Pressure-induced magnetic order in golden SmS. Phys. Rev. Lett. 2004, 92, 066401. [Google Scholar] [CrossRef]
- Matsubayashi, K.; Imura, K.; Suzuki, H.S.; Ban, S.; Chen, G.F.; Deguchi, K.; Sato, N.K. Magnetic properties of golden SmS. J. Magn. Magn. Mater. 2007, 310, 408–410. [Google Scholar] [CrossRef]
- Kang, C.J.; Choi, H.C.; Kim, K.; Min, B.I. Topological properties and the dynamical crossover from mixed-valence to Kondo lattice behavior in the golden phase of SmS. Phys. Rev. Lett. 2015, 114, 166404. [Google Scholar] [CrossRef]
- Zenkevich, A.V.; Parfenov, O.E.; Storchak, V.G.; Teterin, P.E.; Lebedinskii, Y.Y. Highly oriented metallic SmS films on Si(100) grown by pulsed laser deposition. Thin Solid Films 2011, 519, 6323–6325. [Google Scholar] [CrossRef]
- Volodin, N.M.; Zavyalova, L.V.; Kirillov, A.I.; Svechnikov, S.V.; Prokopenko, I.V.; Khanova, A.V. Investigation of growth conditions, crystal structure and surface morphology of SmS films fabricated by MOCVD technique. Semicond. Phys. Quantum Electron. Optoelectron. 1999, 2, 78–83. [Google Scholar]
- Tanemura, S.; Koide, S.; Senzaki, Y.; Miao, L.; Hirai, H.; Mori, Y.; Jin, P.; Kaneko, K.; Terai, A.; Nabatova-Gabain, N. Fabrication and characterization of metal and semiconductor SmS thin films by rf/dc dual magnetron sputtering. Appl. Surf. Sci. 2003, 212, 279–286. [Google Scholar] [CrossRef]
- Rogers, E. Engineering the Electronic Structure of Lanthanide Based Materials. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2012. [Google Scholar]
- Knaepen, W.; Gaudet, S.; Detavernier, C.; Meirhaeghe, R.L.V.; Sweet, J.J.; Lavoie, C. In Situ X-ray diffraction study of metal induced crystallization of amorphous germanium. J. Appl. Phys. 2009, 105, 083532. [Google Scholar] [CrossRef]
- Braeckman, B. Sputter Deposition of Complex Alloy Thin Films. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2016. [Google Scholar]
- Baek, S.H.; Park, J.; Kim, D.M.; Aksyuk, V.A.; Das, R.R.; Bu, S.D.; Felker, D.A.; Lettieri, J.; Vaithyanathan, V.; Bharadwaja, S.S.N.; et al. Giant Piezoelectricity on Si for Hyperactive MEMS. Science 2011, 334, 958–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraman, A.; Maines, R.G. Study of the valence transition in Eu-, Yb-, and Ca-substituted SmS under high pressure and some comments on other substitutions. Phys. Rev. B 1979, 19, 4154–4161. [Google Scholar] [CrossRef]
- Imura, K.; Matsubayashi, K.; Suzuki, H.S.; Deguchi, K.; Sato, N.K. Thermodynamic and transport properties of SmS under high pressure. Phys. B Condens. Matter 2009, 404, 3028–3031. [Google Scholar] [CrossRef]
- Jayarama, A.; Narayana, V.; Bucher, E.; Maines, R.G. Continuous and discontinuous semiconductor-metal transition in samarium monochalcogenides under pressure. Phys. Rev. Lett. 1970, 25, 1430. [Google Scholar] [CrossRef]
- Bzhalava, T.L.; Shulman, S.G.; Dedegkayev, T.T.; Zhukova, T.B.; Smirnov, I.A. Metal modification of SmS in vacuum-deposited films. Phys. Lett. A 1975, 55, 161–162. [Google Scholar] [CrossRef]
- Andreev, O.V.; Ivanov, V.V.; Gorshkov, A.V.; Miodushevskiy, P.V.; Andreev, P.O. Chemistry and Technology of Samarium Monosulfide. Eurasian Chem.-Technol. J. 2016, 18, 55–65. [Google Scholar] [CrossRef]
- Copel, M.; Kuroda, M.A.; Gordon, M.S.; Liu, X.H.; Mahajan, S.S.; Martyna, G.J.; Moumen, N.; Armstrong, C.; Rossnagel, S.M.; Shaw, T.M.; et al. Giant piezoresistive On/Off ratios in rare-earth chalcogenide thin films enabling nanomechanical switching. Nano Lett. 2013, 13, 4650–4653. [Google Scholar] [CrossRef]
- Bamburov, V.G.; Andreev, O.V.; Ivanov, V.V.; Voropai, A.N.; Gorshkov, A.V.; Polkovnikov, A.A.; Bobylev, A.N. Technical ceramics from samarium monosulfide for the thermal explosion and magnetron methods of production of SmS films. Dokl. Phys. Chem. 2017, 473, 66–70. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. An XPS study on the surface reduction of V2O5(001) induced by Ar+ ion bombardment. Surf. Sci. 2006, 600, 3512–3517. [Google Scholar] [CrossRef]
- Batlogg, B.; Schleger, A.; Wachter, P. Degree of valence mixing in the metallic phase of SmS and in TmSe. J. Phys. Colloq. 1976, 37, C4-267–C264-270. [Google Scholar] [CrossRef]
- Deen, P.P.; Braithwaite, D.; Kernavanois, N.; Paolasini, L.; Raymond, S.; Barla, A.; Lapertot, G.; Sanchez, J.P. Structural and electronic transitions in the low-temperature, high-pressure phase of SmS. Phys. Rev. B 2005, 71, 245118. [Google Scholar] [CrossRef]
- Mori, Y.; Tanemura, S.; Koide, S.; Senzaki, Y.; Jin, P.; Kaneko, K.; Terai, A.; Nabotova-Gabin, N. Thermo-coloration of SmS thin flims by XPS in situ observation. Appl. Surf. Sci. 2003, 212, 38–42. [Google Scholar] [CrossRef]
| Substrate Temperature (°C) | ||
|---|---|---|
| 150 | 2.9 () × 10−1 | 4.2 () × 10−3 |
| 200 | 3.1 () × 10−1 | 3.0 () × 10−3 |
| 250 | 1.5 () × 10−1 | 5.1 () × 10−3 |
| 300 | 4.9 () × 10−1 | 5.0 () × 10−3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousanis, A.; Poelman, D.; Detavernier, C.; Smet, P.F. Switchable Piezoresistive SmS Thin Films on Large Area. Sensors 2019, 19, 4390. https://doi.org/10.3390/s19204390
Sousanis A, Poelman D, Detavernier C, Smet PF. Switchable Piezoresistive SmS Thin Films on Large Area. Sensors. 2019; 19(20):4390. https://doi.org/10.3390/s19204390
Chicago/Turabian StyleSousanis, Andreas, Dirk Poelman, Christophe Detavernier, and Philippe F. Smet. 2019. "Switchable Piezoresistive SmS Thin Films on Large Area" Sensors 19, no. 20: 4390. https://doi.org/10.3390/s19204390
APA StyleSousanis, A., Poelman, D., Detavernier, C., & Smet, P. F. (2019). Switchable Piezoresistive SmS Thin Films on Large Area. Sensors, 19(20), 4390. https://doi.org/10.3390/s19204390







