Ambulatory Evaluation of ECG Signals Obtained Using Washable Textile-Based Electrodes Made with Chemically Modified PEDOT:PSS
Abstract
1. Introduction
2. Materials and Methods
2.1. PEDOT:PSS Solutions
2.2. Viscosity Measurements
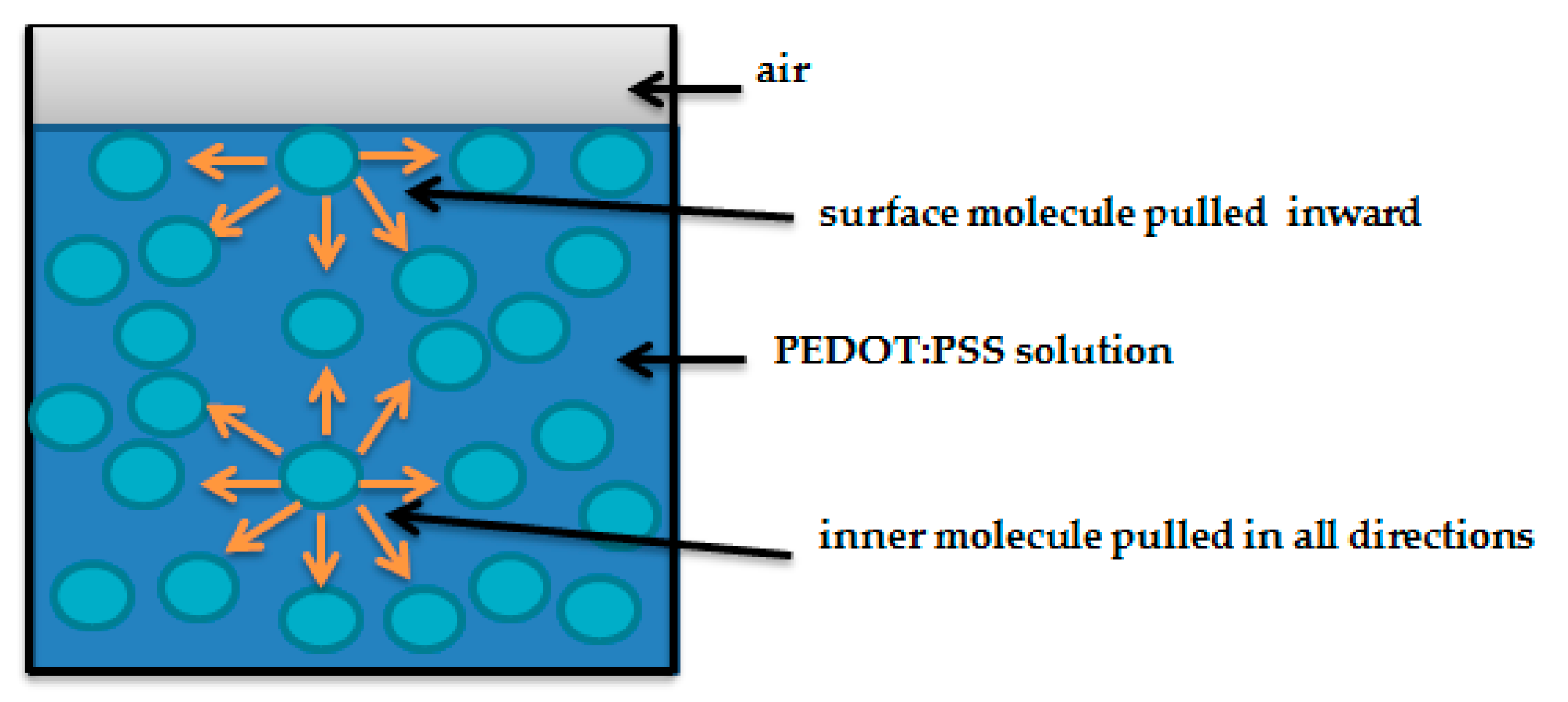
2.3. Surface Tension Measurements of PEDOT:PSS Solution
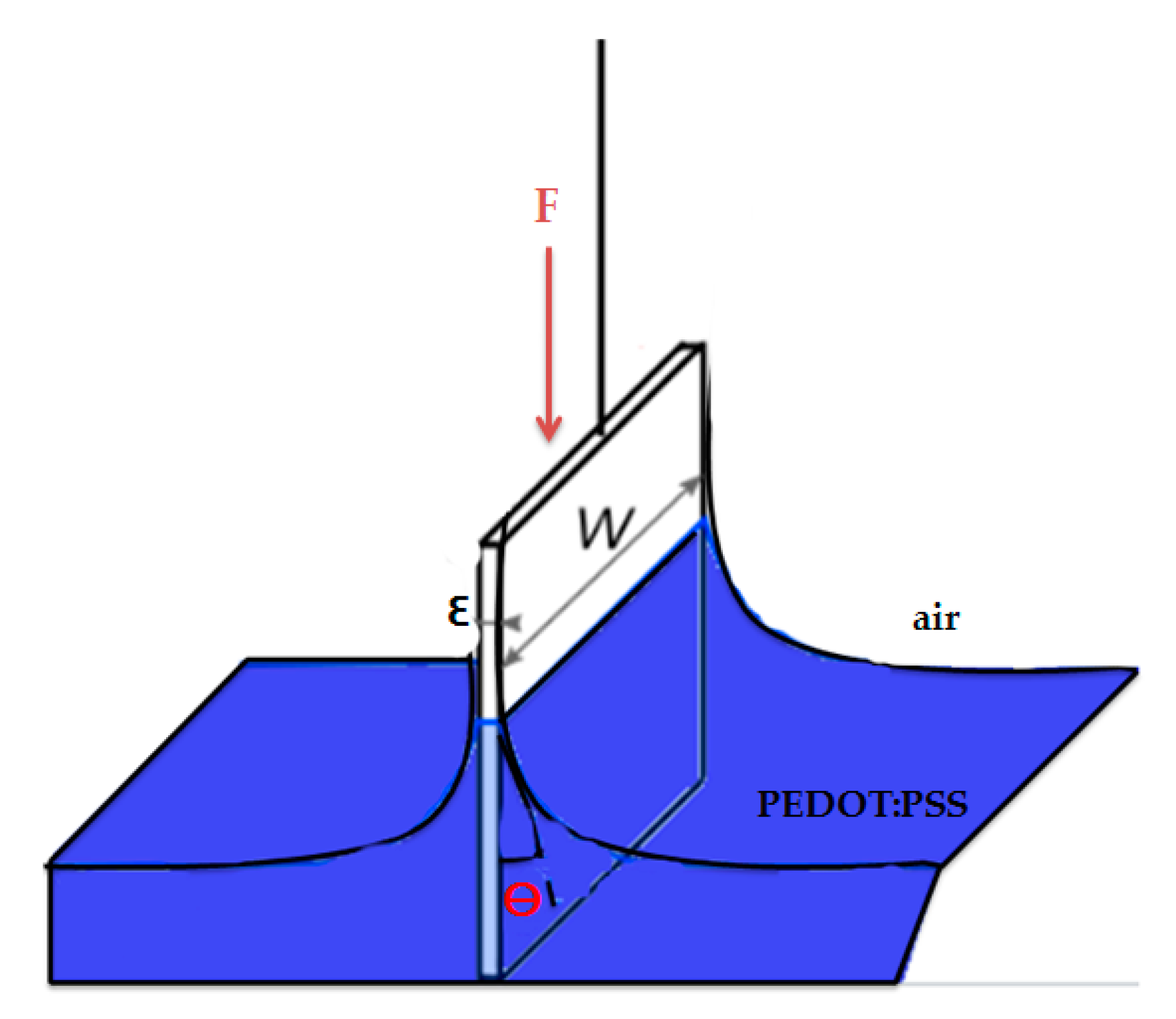
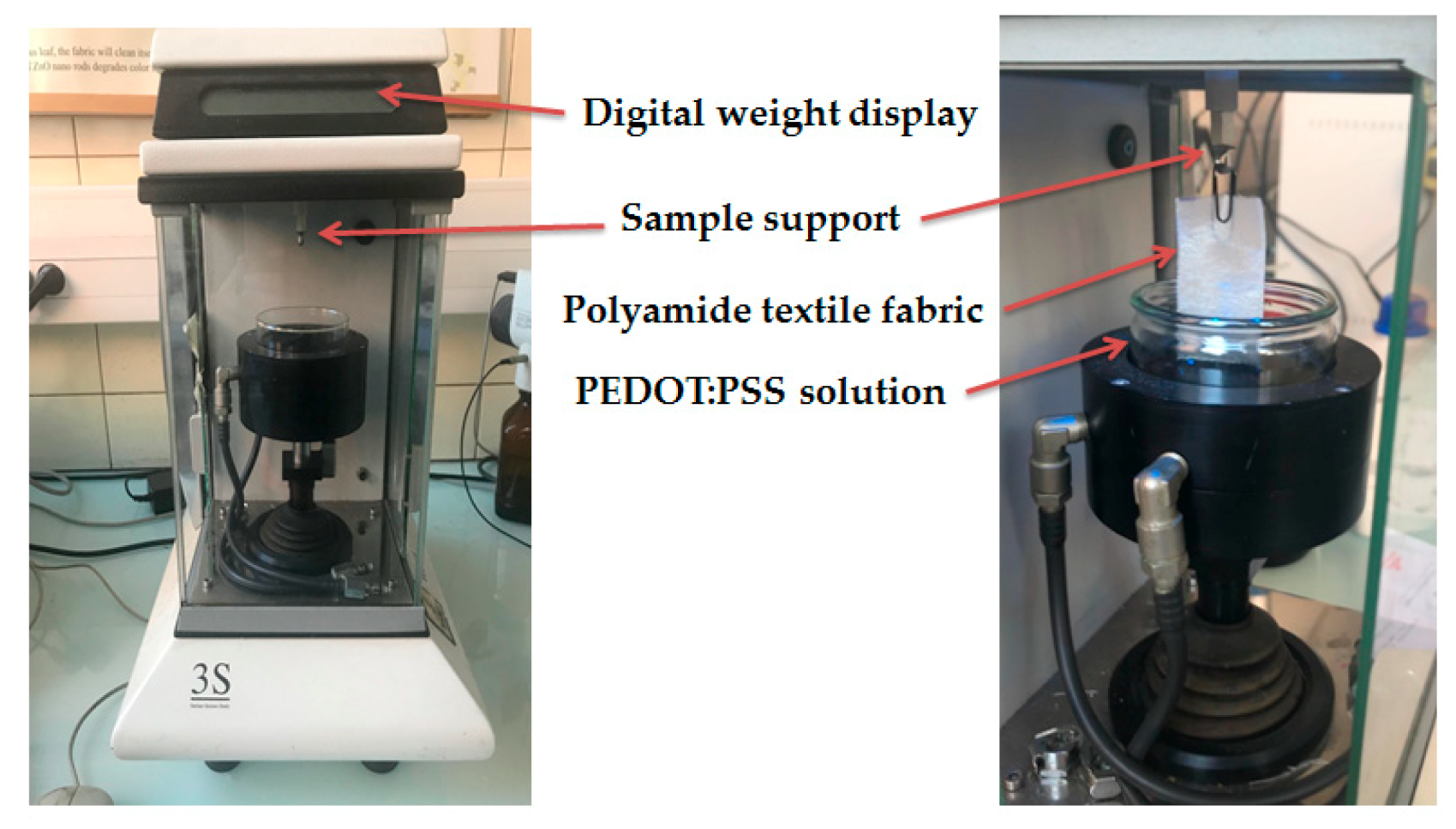
2.4. Contact Angle, Textile Thickness, and PEDOT:PSS Absorption Measurements
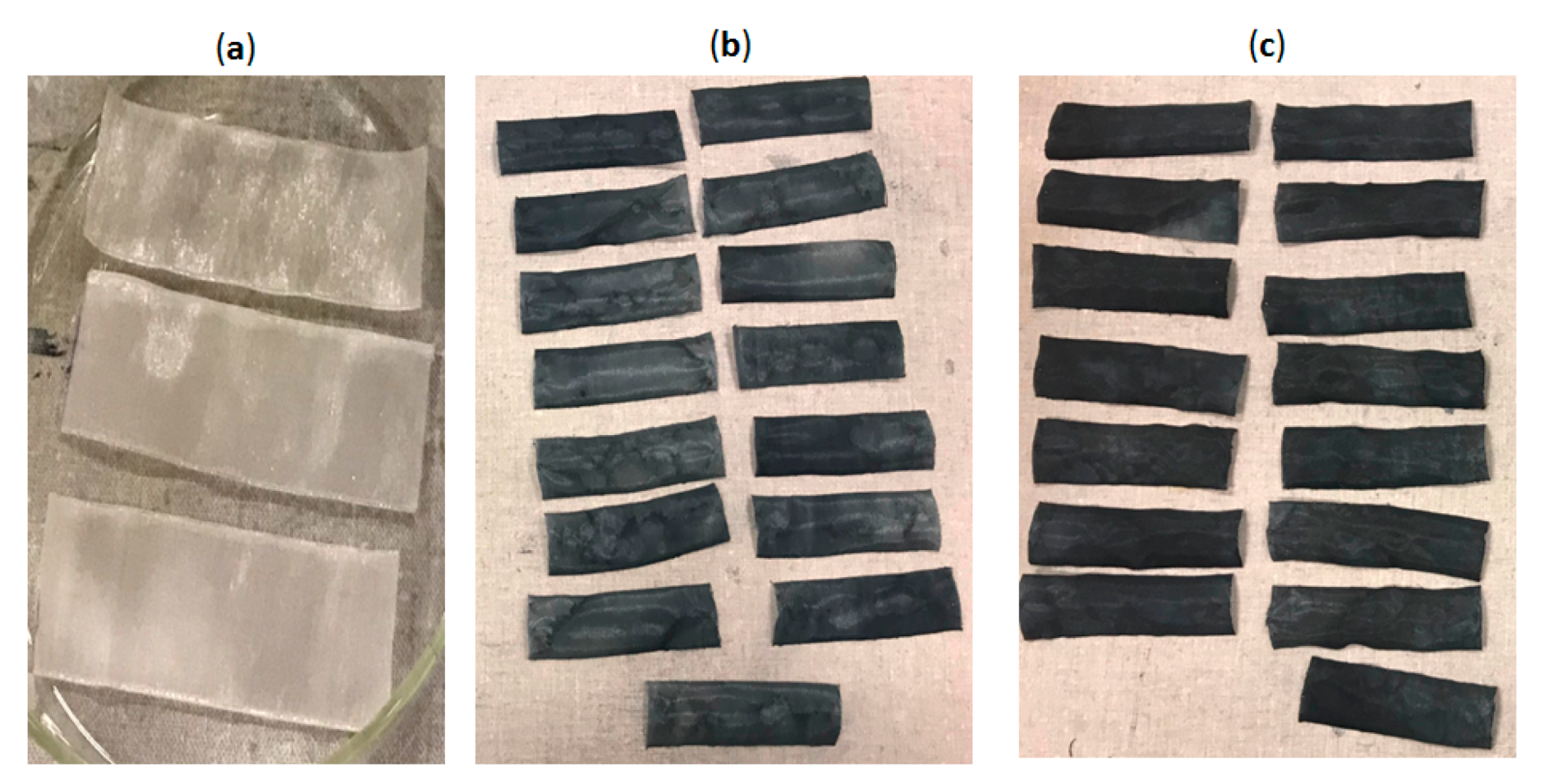
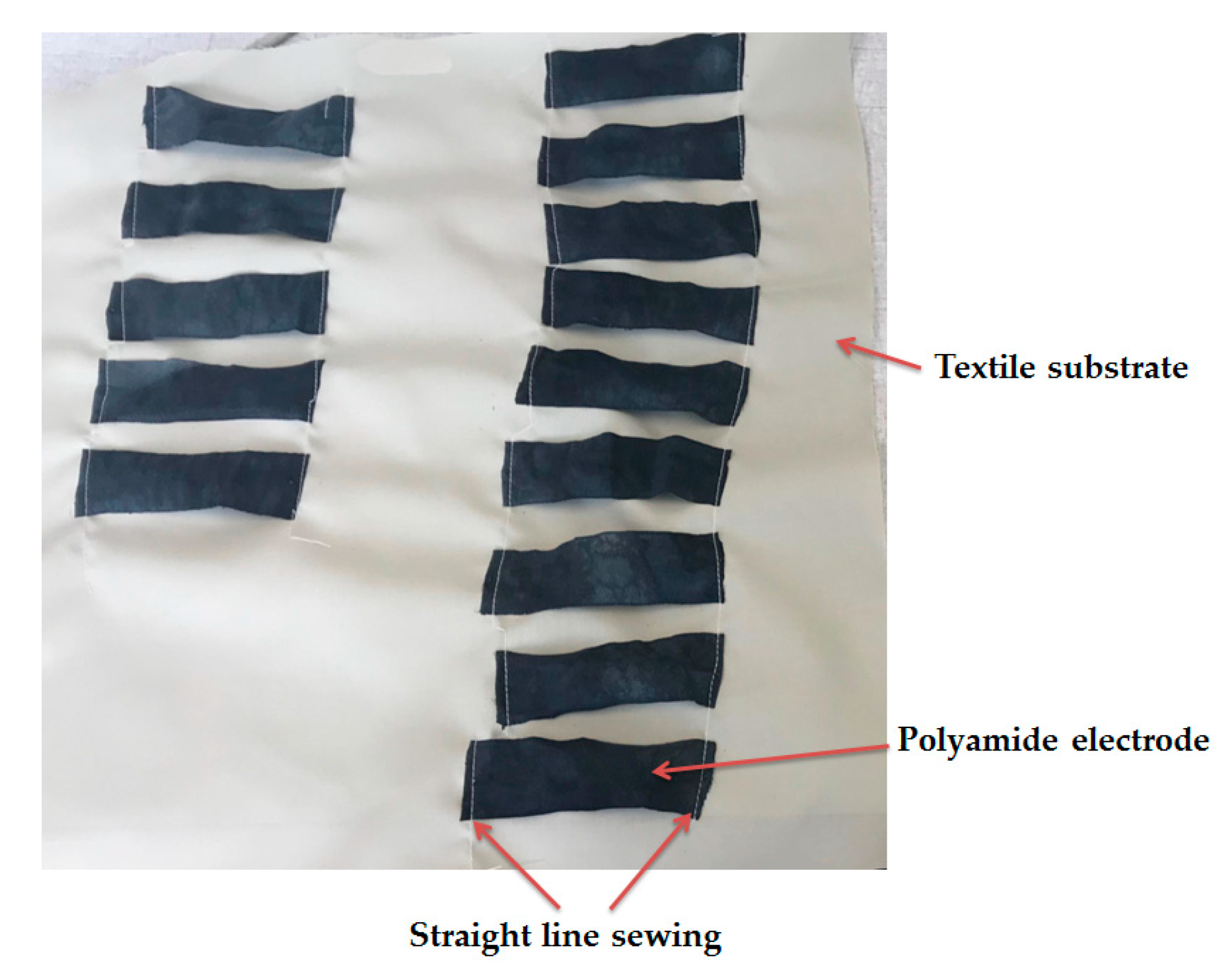
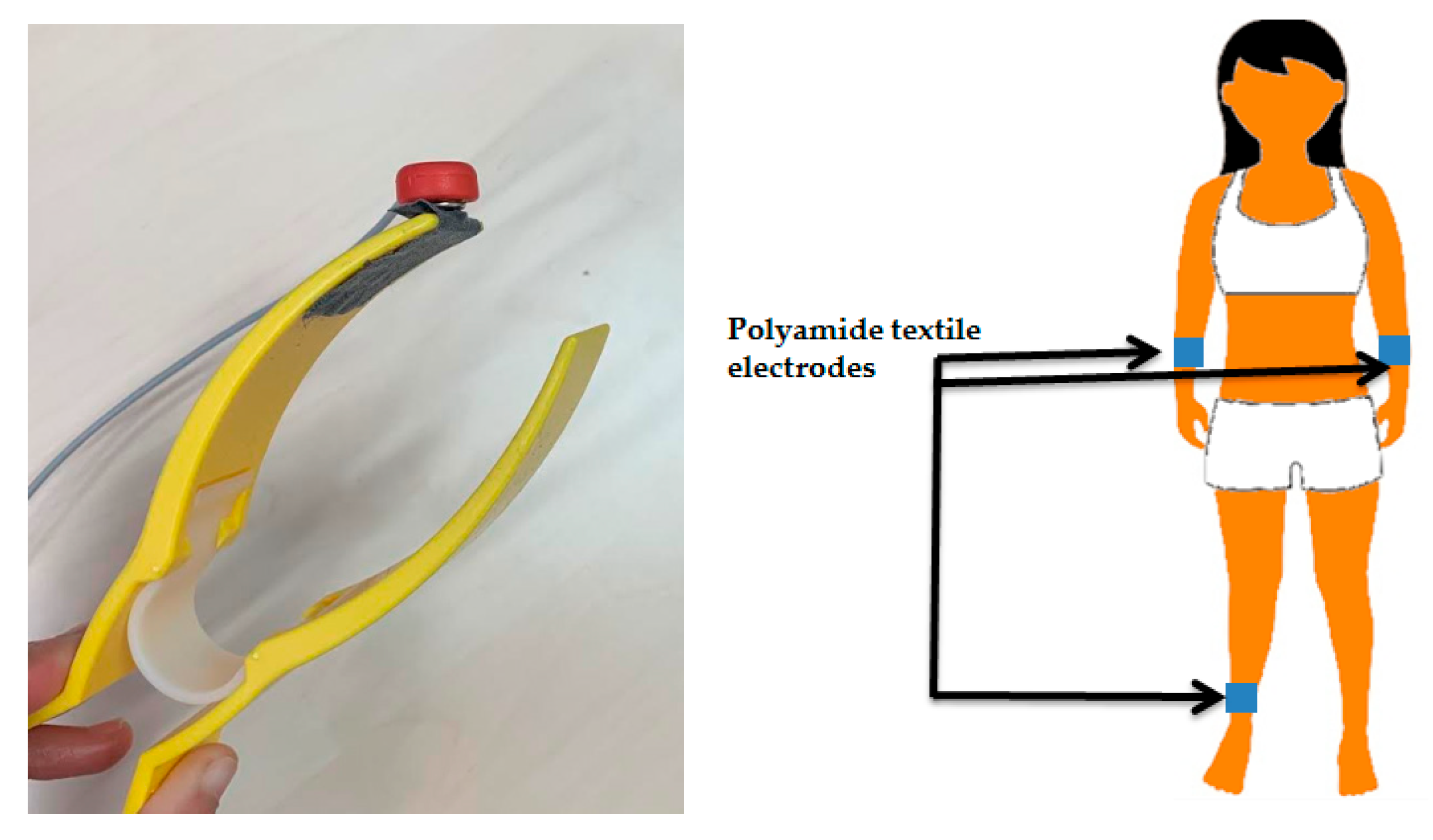
2.5. Textile Electrode Fabrication
2.6. Washing Tests
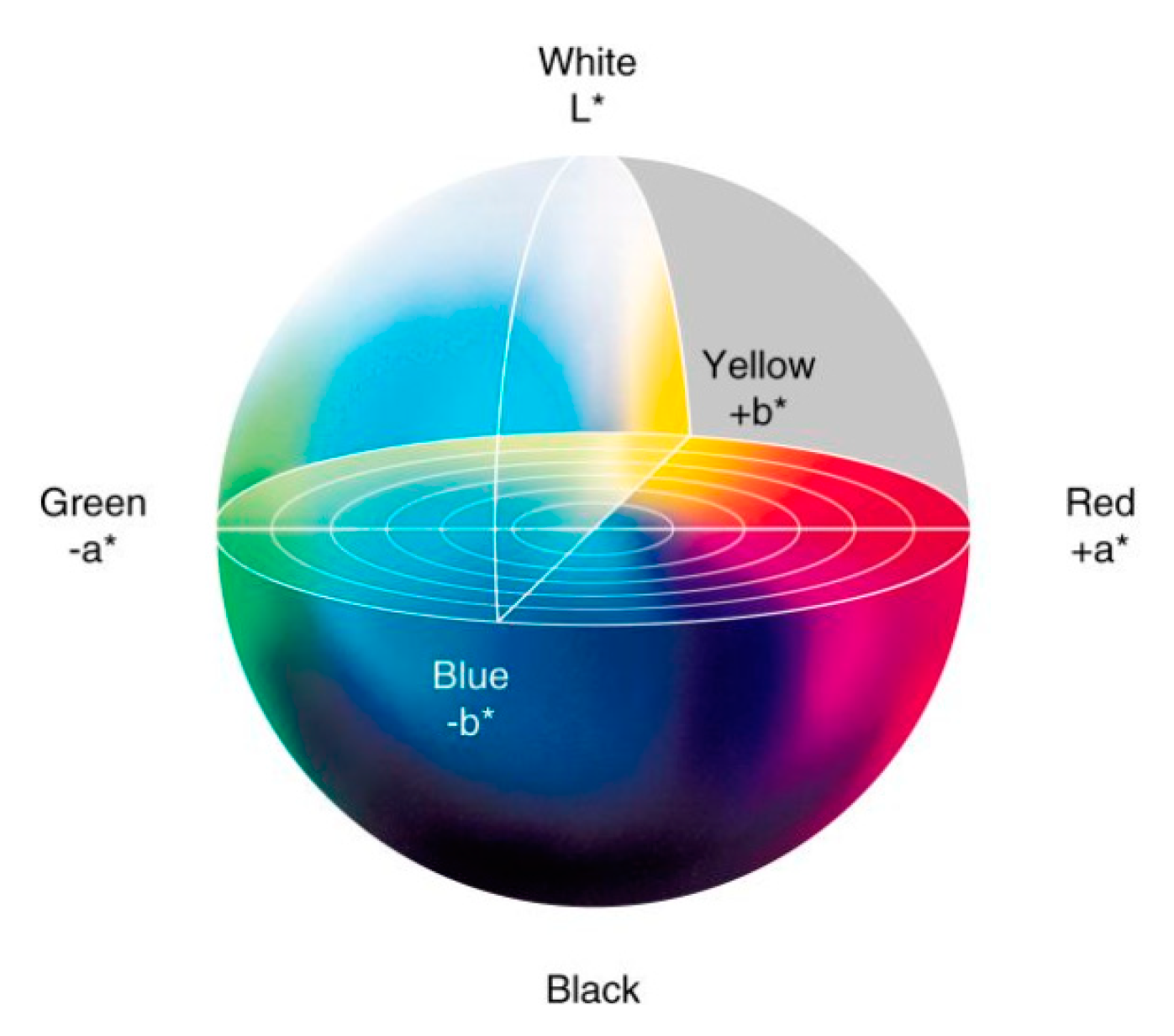
2.7. Electrodes Color Evaluation
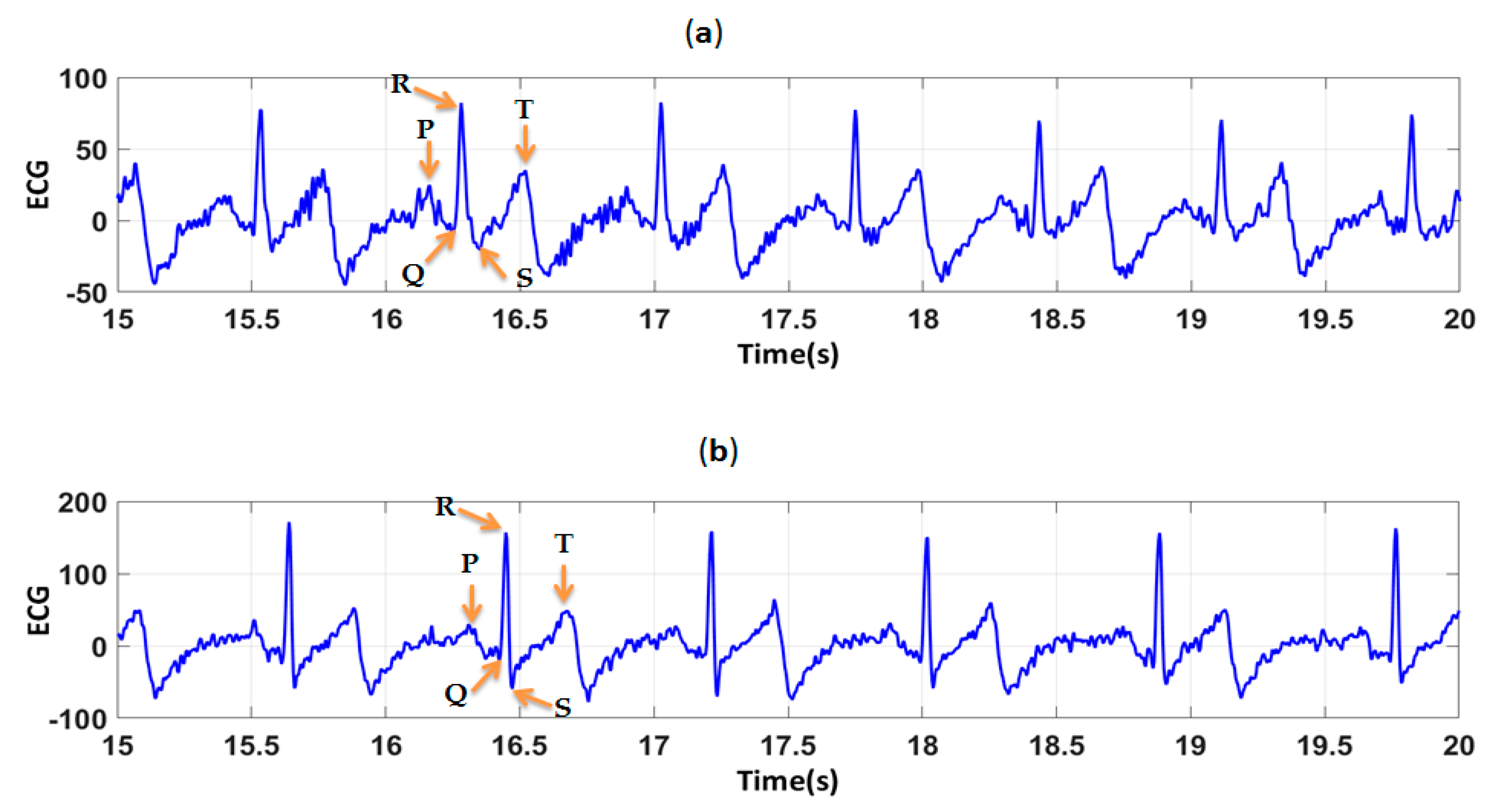
2.8. ECG Analysis
3. Results and Discussion
3.1. Physical Properties of PEDOT:PSS Solutions and Wetting Tests
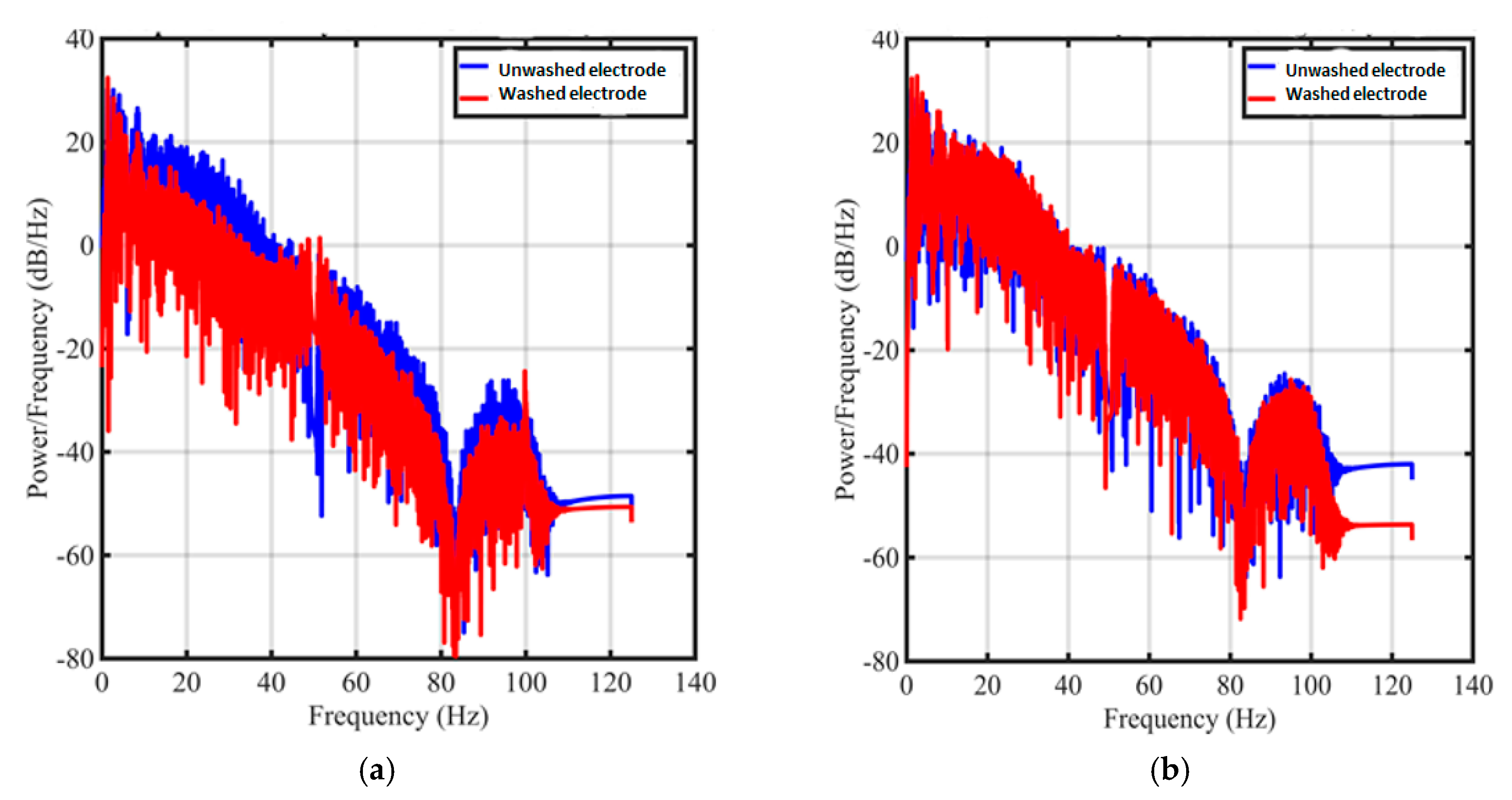
3.2. Evaluation of Textile Electrodes in ECG Monitoring Before and After Washing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, S.; Laing, R. Wearable Technology: Present and Future. Available online: https://www.researchgate.net/publication/327542210_Wearable_Technology_Present_and_Future (accessed on 18 January 2019).
- Ankhili, A.; Tao, X.; Cochrane, C.; Coulon, D.; Koncar, V. Washable and Reliable Textile Electrodes Embedded into Underwear Fabric for Electrocardiography (ECG) Monitoring. Materials 2018, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Activation Cardiaque ECG. Available online: https://book.cardio-fr.com/fr/01-Notions-Elementaires/04-Activation-Cardiaque.html (accessed on 3 October 2018).
- DeSilva, R. Heart Disease; Greenwood: Santa Barbara, CA, USA, 2013; ISBN 978-0-313-37607-8. [Google Scholar]
- Baroldi, G.; Silver, M.D.; Baroldi, G. The Etiopathogenesis of Coronary Heart Disease: A Heretical Theory Based on Morphology; Landes Bioscience; Eurekah.com; CRC Press: Georgetown/Austin, TX, USA, 2004; ISBN 978-1-4175-2343-6. [Google Scholar]
- Livshitz, L.M.; Mizrahi, J.; Einziger, P.D. Interaction of array of finite electrodes with layered biological tissue: Effect of electrode size and configuration. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; de Beeck, M.; Vanderheyden, L.; Carrette, E.; Mihajlović, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; Van Hoof, C. Soft, Comfortable Polymer Dry Electrodes for High Quality ECG and EEG Recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Richardson, P.C. Capacitive Electrocardiographic and Bioelectric Electrodes. IEEE Trans. Biomed. Eng. 1969, BME-16. [Google Scholar] [CrossRef]
- David, R.M.; Portnoy, W.M. Insulated electrocardiogram electrodes. Med. Biol. Eng. 1972, 10, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Balan, A. Wearable sensors for ECG measurement: A review. Sens. Rev. 2018, 38, 412–419. [Google Scholar] [CrossRef]
- Ankhili, A. Study on the Measurement Method of Skin Textile Electrodes Contact Impedance. Available online: https://www.scitechnol.com/peer-review/study-on-the-measurement-method-of-skin-textile-electrodes-contact-impedance-Loks.pdf (accessed on 18 January 2019).
- Lou, C.; Li, R.; Li, Z.; Liang, T.; Wei, Z.; Run, M.; Yan, X.; Liu, X. Flexible Graphene Electrodes for Prolonged Dynamic ECG Monitoring. Sensors 2016, 16, 1833. [Google Scholar] [CrossRef] [PubMed]
- Chlaihawi, A.A.; Narakathu, B.B.; Emamian, S.; Bazuin, B.J.; Atashbar, M.Z. Development of printed and flexible dry ECG electrodes. Sens. Bio-Sens. Res. 2018, 20, 9–15. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, Y. Highly Conductive and Stretchable Silver Nanowire Conductors. Adv. Mater. 2012, 24, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Kaappa, E.S.; Joutsen, A.; Cömert, A.; Vanhala, J. The electrical impedance measurements of dry electrode materials for the ECG measuring after repeated washing. Res. J. Text. Appar. 2017, 21, 59–71. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, Q.; Chen, K.; Chen, Z.; Pan, C.; Jiang, L. Fabrication of a Micro-Needle Array Electrode by Thermal Drawing for Bio-Signals Monitoring. Sensors 2016, 16, 908. [Google Scholar] [CrossRef] [PubMed]
- Chitosan-Based Biosorbents: Modification and Application for Biosorption of Heavy Metals and Radionuclides—Science Direct. Available online: https://www.sciencedirect.com/science/article/pii/S0960852413019500 (accessed on 5 December 2018).
- Myers, A.C.; Huang, H.; Zhu, Y. Wearable silver nanowire dry electrodes for electrophysiological sensing. RSC Adv. 2015, 5, 11627–11632. [Google Scholar] [CrossRef]
- Weder, M.; Hegemann, D.; Amberg, M.; Hess, M.; Boesel, L.; Abächerli, R.; Meyer, V.; Rossi, R. Embroidered Electrode with Silver/Titanium Coating for Long-Term ECG Monitoring. Sensors 2015, 15, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Isaia, C.; McNally, D.S.; McMaster, S.A.; Branson, D.T. Effect of mechanical preconditioning on the electrical properties of knitted conductive textiles during cyclic loading. Text. Res. J. 2018. [Google Scholar] [CrossRef]
- An, X.; Stylios, G.K. A Hybrid Textile Electrode for Electrocardiogram (ECG) Measurement and Motion Tracking. Materials 2018, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Cao, L.; Zhang, C.; Tang, C.; Li, E.; Feng, X.; Liang, H. Textile-based ECG acquisition system with capacitively coupled electrodes. Trans. Inst. Meas. Control. 2017, 39, 141–148. [Google Scholar] [CrossRef]
- Lidón-Roger, J.; Prats-Boluda, G.; Ye-Lin, Y.; Garcia-Casado, J.; Garcia-Breijo, E. Textile Concentric Ring Electrodes for ECG Recording Based on Screen-Printing Technology. Sensors 2018, 18, 300. [Google Scholar] [CrossRef] [PubMed]
- Ankhili, A.; Tao, X.; Cochrane, C.; Koncar, V.; Coulon, D.; Tarlet, J.-M. Comparative Study on Conductive Knitted Fabric Electrodes for Long-Term Electrocardiography Monitoring: Silver-Plated and PEDOT:PSS Coated Fabrics. Sensors 2018, 18, 3890. [Google Scholar] [CrossRef]
- Yapici, M.K.; Alkhidir, T.E. Intelligent Medical Garments with Graphene-Functionalized Smart-Cloth ECG Sensors. Sensors 2017, 17, 875. [Google Scholar] [CrossRef]
- Qin, H.; Li, J.; He, B.; Sun, J.; Li, L.; Qian, L. Novel Wearable Electrodes Based on Conductive Chitosan Fabrics and Their Application in Smart Garments. Materials 2018, 11, 370. [Google Scholar] [CrossRef]
- Chandrasekhar, P. Conduction Models and Electronic Structure of CNTs. In Conducting Polymers, Fundamentals and Applications: Including Carbon Nanotubes and Graphene; Chandrasekhar, P., Ed.; Springer International Publishing: Gewerbestrasse, Cham, 2018; pp. 11–16. ISBN 978-3-319-69378-1. [Google Scholar]
- Grancarić, A.M.; Jerković, I.; Koncar, V.; Cochrane, C.; Kelly, F.M.; Soulat, D.; Legrand, X. Conductive polymers for smart textile applications. J. Ind. Text. 2018, 48, 612–642. [Google Scholar] [CrossRef]
- Lai, J.H. Polymers for Electronic Applications; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-09280-7. [Google Scholar]
- Liao, C.; Zhang, M.; Yao, M.Y.; Hua, T.; Li, L.; Yan, F. Flexible Organic Electronics in Biology: Materials and Devices. Adv. Mater. 2015, 27, 7493–7527. [Google Scholar] [CrossRef] [PubMed]
- Pani, D.; Achilli, A.; Bonfiglio, A. Survey on Textile Electrode Technologies for Electrocardiographic (ECG) Monitoring, from Metal Wires to Polymers. Adv. Mater. Technol. 2018. [Google Scholar] [CrossRef]
- Tao, X.; Huang, T.-H.; Shen, C.-L.; Ko, Y.-C.; Jou, G.-T.; Koncar, V. Bluetooth Low Energy-Based Washable Wearable Activity Motion and Electrocardiogram Textronic Monitoring and Communicating System. Adv. Mater. Technol. 2018. [Google Scholar] [CrossRef]
- Tao, X.; Koncar, V.; Huang, T.-H.; Shen, C.-L.; Ko, Y.-C.; Jou, G.-T. How to Make Reliable, Washable, and Wearable Textronic Devices. Sensors 2017, 17, 673. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Azuaje, F.; McSharry, P. Engineering in medicine & biology. In Advanced Methods and Tools for ECG Data Analysis; Norwood, M.A., Ed.; Artech House: Boston, MA, USA, 2006; ISBN 978-1-58053-966-1. [Google Scholar]



| PEDOT:PSS Solution 1 (S1) | PEDOT:PSS Solution 2 (S2) | |
|---|---|---|
| Viscosity (mPa.s) | 68.2 ± 2.0 | 120.4 ± 1.1 |
| Surface Tension (mN/m) | 27.08 ± 0.02 | 38.88 ± 0.01 |
| Contact angle (polyamide-PEDOT:PSS) (deg) | 25.6 ± 0.32 | 56.4 ± 0.51 |
| S1 | S2 | |
|---|---|---|
| SNR (dB) before washing | 25.3 | 27.8 |
| SNR (dB) after 50 washing cycles | 10.3 | 19.8 |
| Polyamide Fabric before Coating | Before Washing | After 50 Washes | |||
|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | ||
| L* | 79.3 | 29.54 | 25.67 | 40.14 | 33.47 |
| a* | −0.19 | −1.56 | −1.24 | −0.75 | −1.11 |
| b* | −0.04 | −6.43 | −4.74 | −4.86 | −4.01 |
| S1 | S2 | |
|---|---|---|
| ΔECMC(l:c) | 13.43 | 10.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ankhili, A.; Tao, X.; Cochrane, C.; Koncar, V.; Coulon, D.; Tarlet, J.-M. Ambulatory Evaluation of ECG Signals Obtained Using Washable Textile-Based Electrodes Made with Chemically Modified PEDOT:PSS. Sensors 2019, 19, 416. https://doi.org/10.3390/s19020416
Ankhili A, Tao X, Cochrane C, Koncar V, Coulon D, Tarlet J-M. Ambulatory Evaluation of ECG Signals Obtained Using Washable Textile-Based Electrodes Made with Chemically Modified PEDOT:PSS. Sensors. 2019; 19(2):416. https://doi.org/10.3390/s19020416
Chicago/Turabian StyleAnkhili, Amale, Xuyuan Tao, Cédric Cochrane, Vladan Koncar, David Coulon, and Jean-Michel Tarlet. 2019. "Ambulatory Evaluation of ECG Signals Obtained Using Washable Textile-Based Electrodes Made with Chemically Modified PEDOT:PSS" Sensors 19, no. 2: 416. https://doi.org/10.3390/s19020416
APA StyleAnkhili, A., Tao, X., Cochrane, C., Koncar, V., Coulon, D., & Tarlet, J.-M. (2019). Ambulatory Evaluation of ECG Signals Obtained Using Washable Textile-Based Electrodes Made with Chemically Modified PEDOT:PSS. Sensors, 19(2), 416. https://doi.org/10.3390/s19020416








