Structural and Functional Sensing of Bio-Tissues Based on Compressive Sensing Spectral Domain Optical Coherence Tomography
Abstract
:1. Introduction
2. Methods
2.1. Structural and Functional Sensing Based on SD-OCT
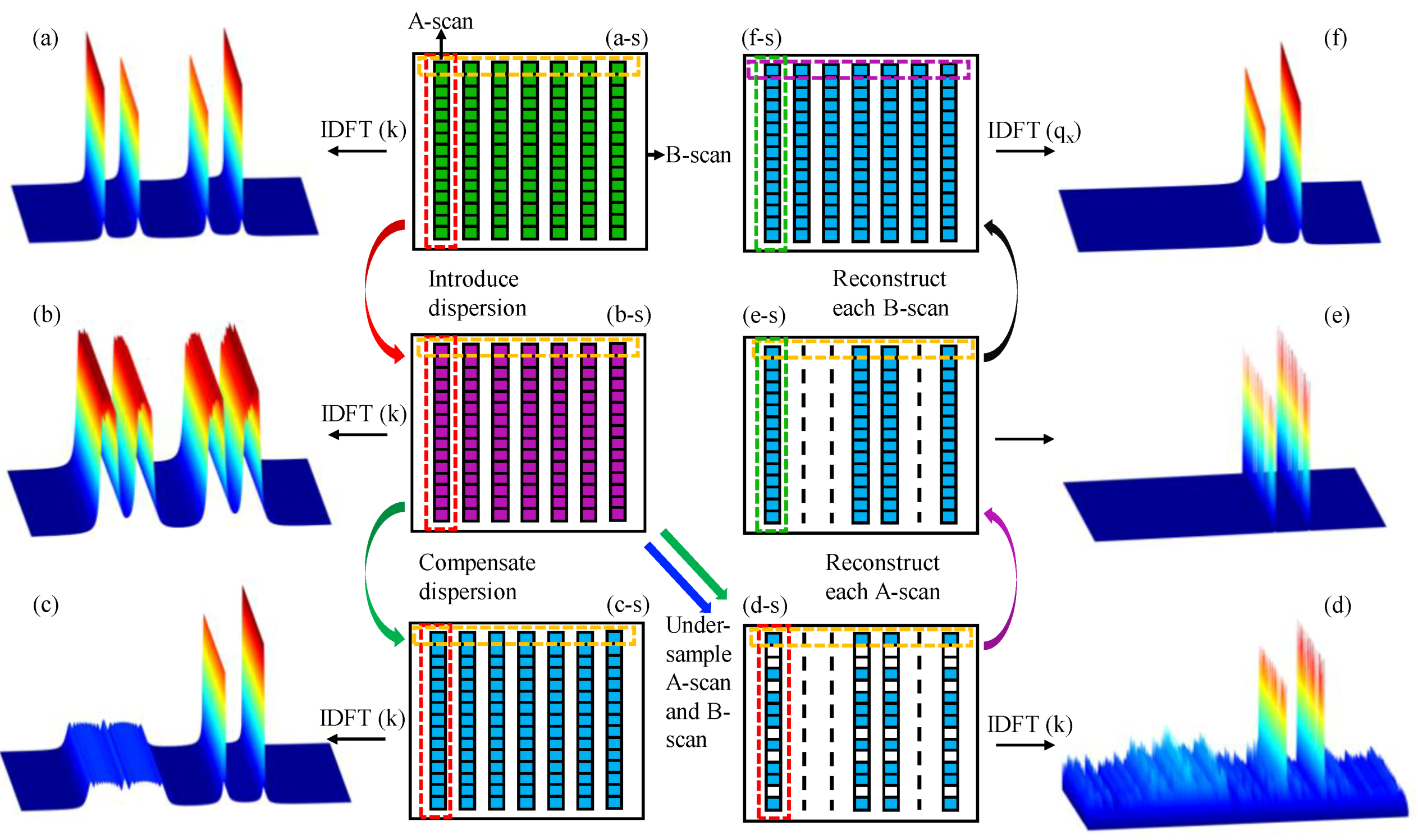
2.2. Full-Depth 2D CS-SDOCT
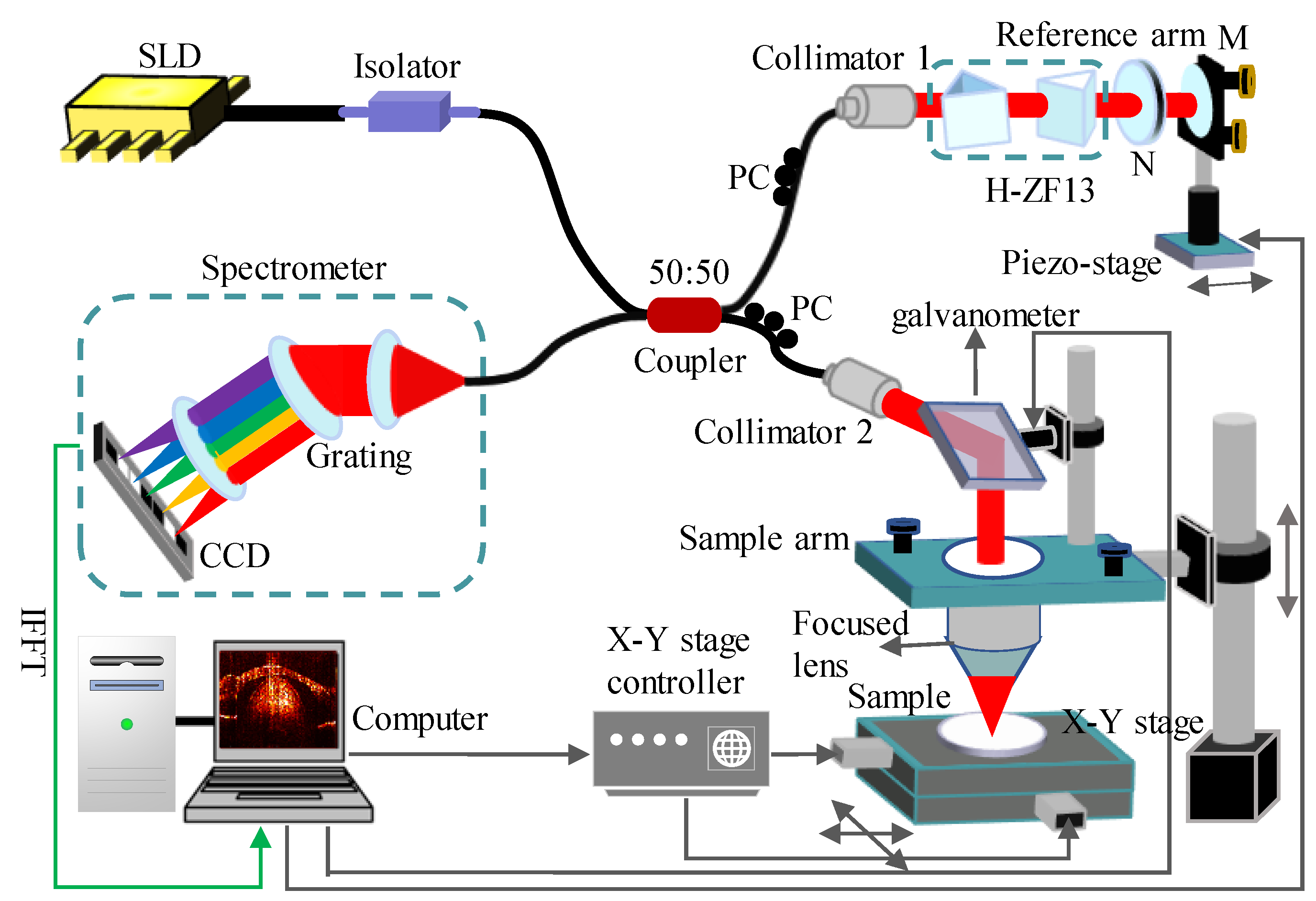
2.3. Hardware and Software Setup
3. Structural Imaging Results
4. Functional Sensing Results
4.1. Simulation Results
4.2. Experimental Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178. [Google Scholar] [CrossRef] [PubMed]
- Fercher, A.F.; Hitzenberger, C.K.; Kamp, G.; El-Zaiat, S.Y. Measurement of intraocular distances by backscattering spectral interferometry. Opt. Commun. 1995, 117, 43–48. [Google Scholar] [CrossRef]
- Leitgeb, R.; Hitzenberger, C.; Adolf, F. Performance of Fourier domain vs. time domain optical coherence tomography. Opt. Express 2003, 11, 889–894. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.F.; Leitgeb, R.; Wojtkowski, M. Twenty-five years of optical coherence tomography: The paradigm shift in sensitivity and speed provided by Fourier domain OCT. Biomed. Opt. Express 2017, 8, 3248. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Harsan, L.A.; Bienert, T.; Kirch, R.D.; Von, E.D.; Hofmann, U.G. Qualitative and quantitative evaluation of in vivo SD-OCT measurement of rat brain. Biomed. Opt. Express 2017, 8, 593. [Google Scholar] [CrossRef]
- Chong, S.P.; Merkle, C.W.; Leahy, C.; Radhakrishnan, H.; Srinivasan, V.J. Quantitative microvascular hemoglobin mapping using visible light spectroscopic Optical Coherence Tomography. Biomed. Opt. Express 2015, 6, 1429. [Google Scholar] [CrossRef]
- Ji, Y.; Vadim, B. Imaging a full set of optical scattering properties of biological tissue by inverse spectroscopic optical coherence tomography. Opt. Lett. 2012, 37, 4443–4445. [Google Scholar] [Green Version]
- Ireneusz, G.; Michalina, G.; Maciej, S.; Iwona, G.; Daniel, S.; Susana, M.; Andrzej, K.; Maciej, W. Anterior segment imaging with Spectral OCT system using a high-speed CMOS camera. Opt. Express 2009, 17, 4842–4858. [Google Scholar] [Green Version]
- Xuan, L.; Kang, J.U. Compressive SD-OCT: The application of compressed sensing in spectral domain optical coherence tomography. Opt. Express 2010, 18, 22010–22019. [Google Scholar]
- Daguang, X.; Namrata, V.; Yong, H.; Kang, J.U. Modified compressive sensing optical coherence tomography with noise reduction. Opt. Lett. 2012, 37, 4209–4211. [Google Scholar]
- Luo, S.; Qiang, G.; Hui, Z.; Xin, A.; Li, H. Noise Reduction of Swept Source Optical Coherence Tomography via Compressed Sensing. IEEE Photonics J. 2017, 10, 3800109. [Google Scholar] [CrossRef]
- Daguang, X.; Yong, H.; Kang, J.U. GPU-accelerated non-uniform fast Fourier transform-based compressive sensing spectral domain optical coherence tomography. Opt. Express 2014, 22, 14871. [Google Scholar]
- Fang, L.; Li, S.; Nie, Q.; Izatt, J.A.; Toth, C.A.; Farsiu, S. Sparsity based denoising of spectral domain optical coherence tomography images. Biomed. Opt. Express 2012, 3, 927–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Huang, Y.; Kang, J.U. Volumetric (3D) compressive sensing spectral domain optical coherence tomography. Biomed. Opt. Express 2014, 5, 3921. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, Y.; Kang, J.U. Two-dimensional compressive sensing in spectral domain optical coherence tomography. SPIE BIOS 2015, 93301A. [Google Scholar] [CrossRef]
- Wright, S.J.; Nowak, R.D.; Figueiredo, M.A.T. Sparse reconstruction by separable approximation. IEEE Trans. Signal Process. 2009, 57, 2479–2493. [Google Scholar] [CrossRef]
- Wojtkowski, M.; Kowalczyk, A.; Leitgeb, R.; Fercher, A.F. Full range complex spectral optical coherence tomography technique in eye imaging. Opt. Lett. 2002, 27, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Bo, E.; Chen, S.; Cui, D.; Chen, S.; Yu, X.; Luo, Y.; Liu, L. Single-camera full-range high-resolution spectral domain optical coherence tomography. Appl. Opt. 2017, 56, 470. [Google Scholar] [CrossRef]
- Hofer, B.; Považay, B.; Unterhuber, A.; Wang, L.; Hermann, B.; Rey, S.; Matz, G.; Drexler, W. Fast dispersion encoded full range optical coherence tomography for retinal imaging at 800 nm and 1060 nm. Opt. Express 2010, 18, 4898–4919. [Google Scholar] [CrossRef] [Green Version]
- Witte, S.; Baclayon, M.; Peterman, E.J.; Toonen, R.F.; Mansvelder, H.D.; Groot, M.L. Single-shot two-dimensional full-range optical coherence tomography achieved by dispersion control. Opt. Express 2009, 17, 11335–11349. [Google Scholar] [CrossRef]
- Kãttig, F.; Cimalla, P.; Gã¤Rtner, M.; Koch, E. An advanced algorithm for dispersion encoded full range frequency domain optical coherence tomography. Opt. Express 2013, 20, 24925–24948. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Sun, L.; Ming, X.; Zou, M. Full-depth spectral domain optical coherence tomography technology insensitive to phase disturbance. Biomed. Opt. Express 2018, 9, 5071–5083. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Chi, T.T.; Kiang, Y.W.; Yang, C.C. Computation time-saving mirror image suppression method in Fourier-domain optical coherence tomography. Opt. Express 2012, 20, 8270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, L.; Yu, P. Spatial convolution for mirror image suppression in Fourier domain optical coherence tomography. Opt. Lett. 2017, 42, 506. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, Y.; Makita, S.; Endo, T.; Aoki, G.; Itoh, M.; Yatagai, T. Simultaneous B-M-mode scanning method for real-time full-range Fourier domain optical coherence tomography. Appl. Opt. 2006, 45, 1861–1865. [Google Scholar] [CrossRef]
- Yi, L.; Sun, L. Full-depth compressive sensing spectral-domain optical coherence tomography based on a compressive dispersion encoding method. Appl. Opt. 2018, 57, 9316–9321. [Google Scholar] [CrossRef]
- Yi, J.; Wei, Q.; Liu, W.; Backman, V.; Zhang, H.F. Visible-light optical coherence tomography for retinal oximetry. Opt. Lett. 2013, 38, 1796. [Google Scholar] [CrossRef]
- Lemij, H.G.; Schoot, J.V.D.; Boer, J.F.D.; Vermeer, K.A. Automated segmentation by pixel classification of retinal layers in ophthalmic OCT images. Biomed. Opt. Express 2011, 2, 743–1756. [Google Scholar]
- Nienke, B.; Van Leeuwen, T.G.; Aalders, M.C.G.; Faber, D.J. Quantitative comparison of analysis methods for spectroscopic optical coherence tomography. Biomed. Opt. Express 2013, 4, 2570–2584. [Google Scholar] [Green Version]
- Vermeer, K.A.; Mo, J.; Weda, J.J.A.; Lemij, H.G.; Boer, J.F.D. Depth-resolved model-based reconstruction of attenuation coefficients in optical coherence tomography. Biomed. Opt. Express 2013, 5, 322–337. [Google Scholar] [CrossRef]
- Bosschaart, N.; Edelman, G.J.; Aalders, M.C.G.; Leeuwen, T.G.V.; Faber, D.J. A literature review and novel theoretical approach on the optical properties of whole blood. Lasers Med. Sci. 2014, 29, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Israelsen, N.M.; Maria, M.; Feuchter, T.; Podoleanu, A.; Bang, O. All-depth dispersion cancellation in spectral domain optical coherence tomography using numerical intensity correlations. Sci. Rep. 2018, 8, 9170. [Google Scholar] [CrossRef] [PubMed]





| System Parameters | Specification |
|---|---|
| Lateral resolution | 7.54 μm |
| Axial resolution (air/tissue) | 6.88 μm/4.74 μm |
| Optical power of light source | 24 mW |
| Maximum imaging depth (air/tissue) | 3.12 × 2 mm/2.15 × 2 mm |
| Maximum imaging width | 21.8 mm |
| Working distance of sample arm | 22mm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, L.; Guo, X.; Sun, L.; Hou, B. Structural and Functional Sensing of Bio-Tissues Based on Compressive Sensing Spectral Domain Optical Coherence Tomography. Sensors 2019, 19, 4208. https://doi.org/10.3390/s19194208
Yi L, Guo X, Sun L, Hou B. Structural and Functional Sensing of Bio-Tissues Based on Compressive Sensing Spectral Domain Optical Coherence Tomography. Sensors. 2019; 19(19):4208. https://doi.org/10.3390/s19194208
Chicago/Turabian StyleYi, Luying, Xiangyu Guo, Liqun Sun, and Bo Hou. 2019. "Structural and Functional Sensing of Bio-Tissues Based on Compressive Sensing Spectral Domain Optical Coherence Tomography" Sensors 19, no. 19: 4208. https://doi.org/10.3390/s19194208
APA StyleYi, L., Guo, X., Sun, L., & Hou, B. (2019). Structural and Functional Sensing of Bio-Tissues Based on Compressive Sensing Spectral Domain Optical Coherence Tomography. Sensors, 19(19), 4208. https://doi.org/10.3390/s19194208





