Development and Application of Aptamer-Based Surface-Enhanced Raman Spectroscopy Sensors in Quantitative Analysis and Biotherapy
Abstract
:1. Introduction
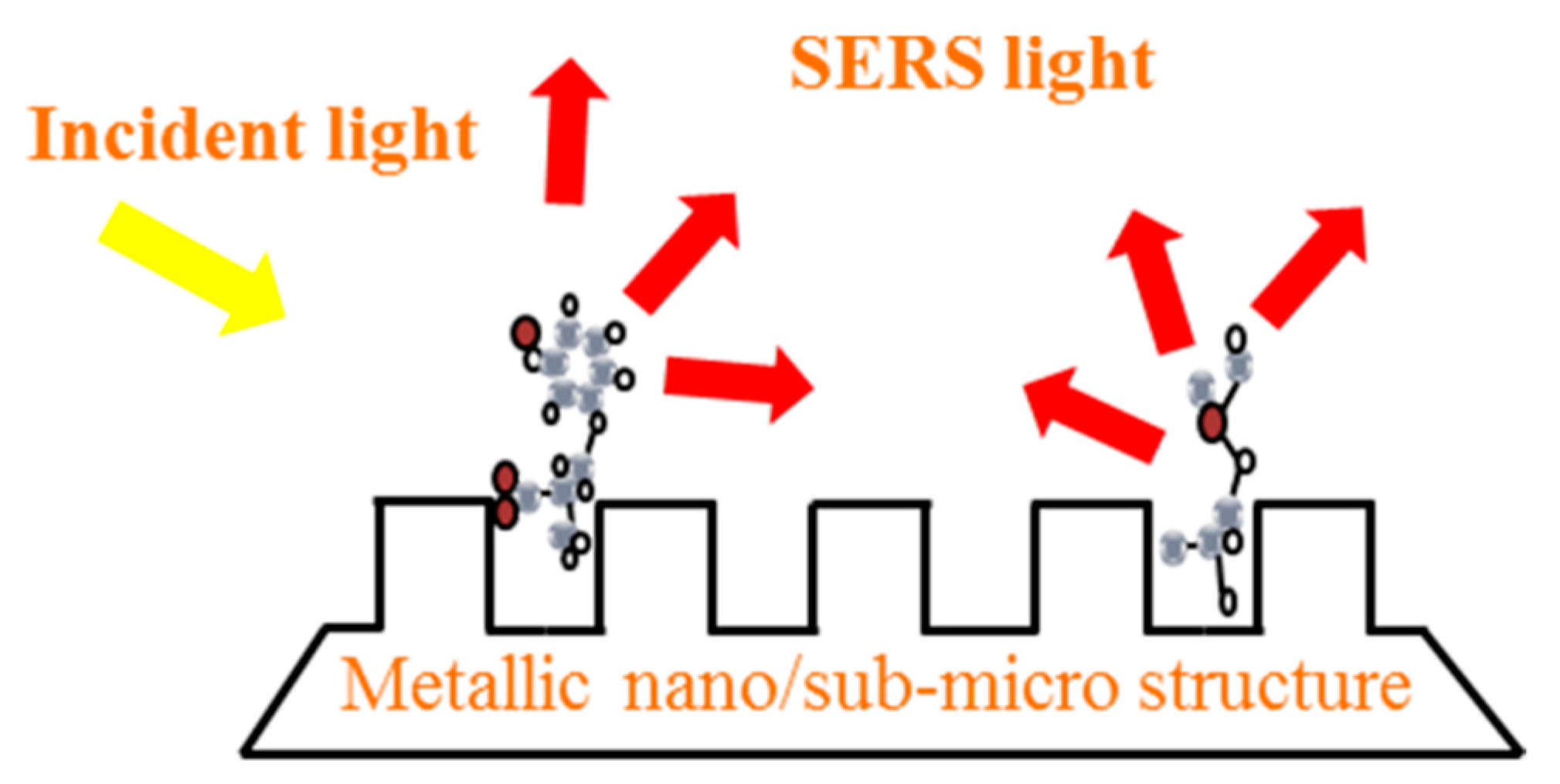
2. Substrates of SERS
2.1. Rough Metal Electrode
2.2. Metal NPs in Suspension
2.3. Nano-composite Structures
3. Aptamer-Based SERS Probes in Quantitative Analysis and Biotherapy
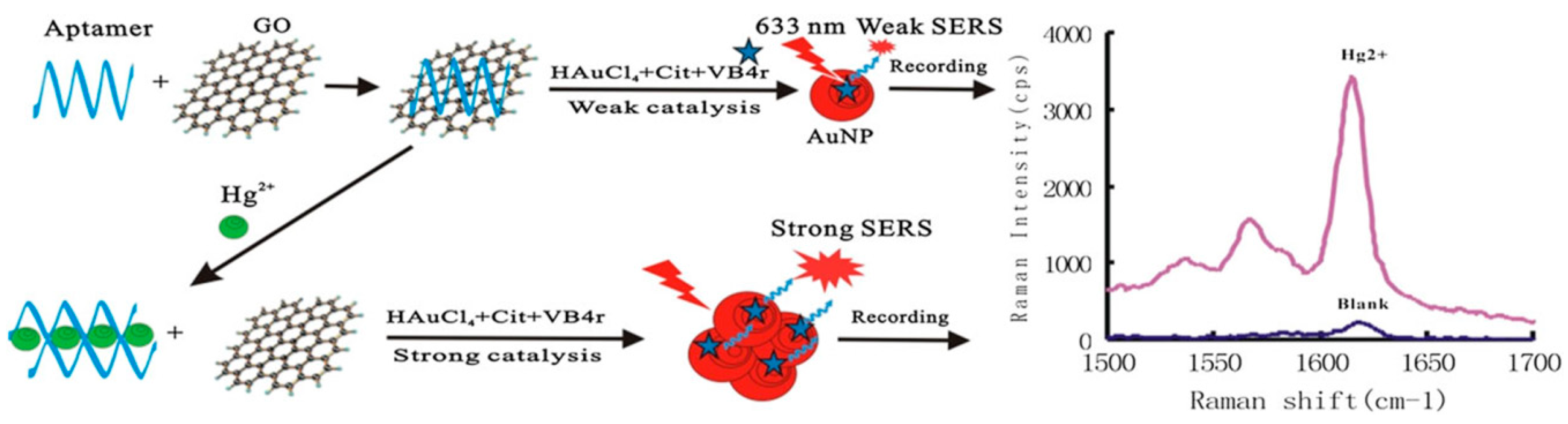
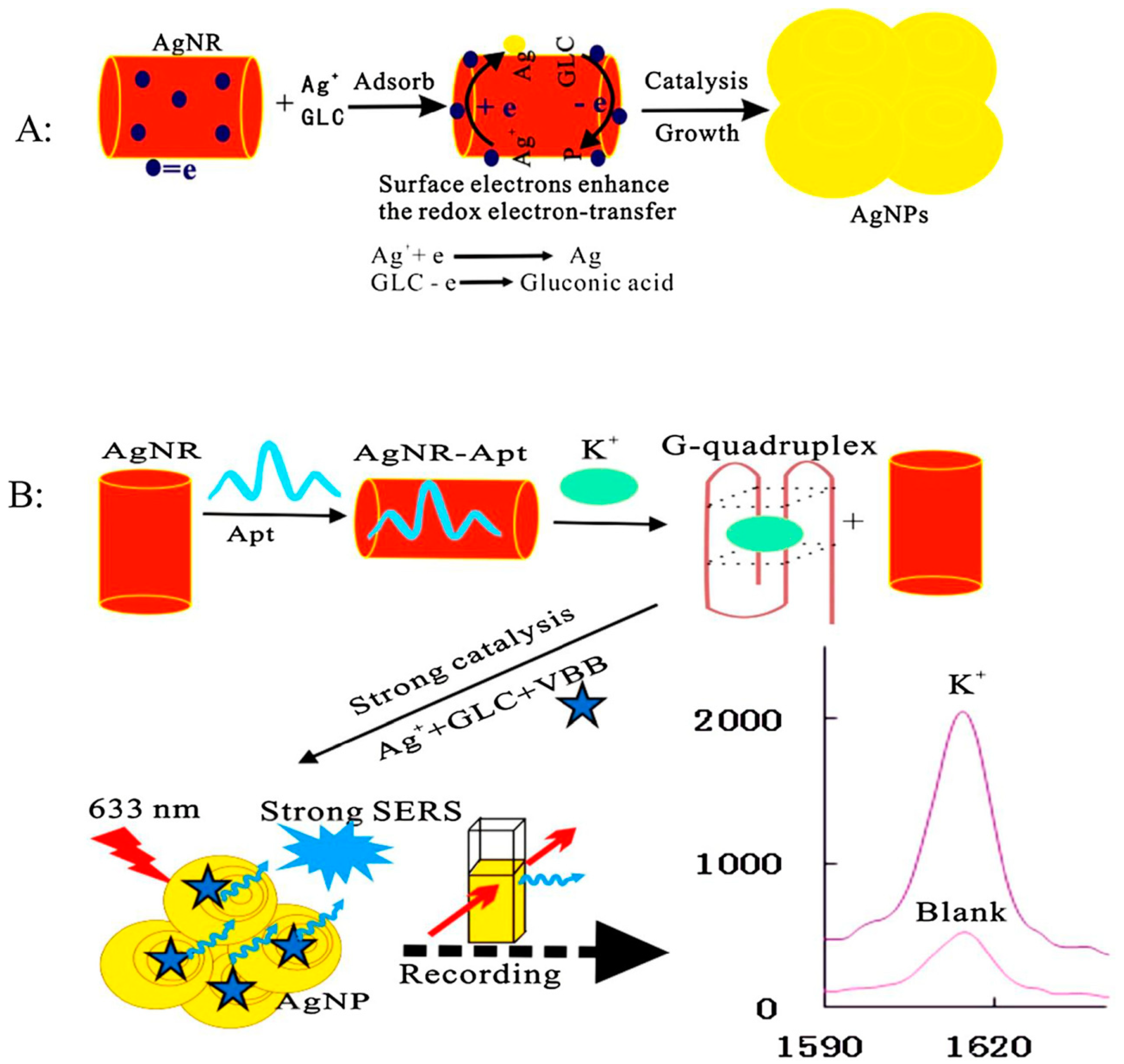
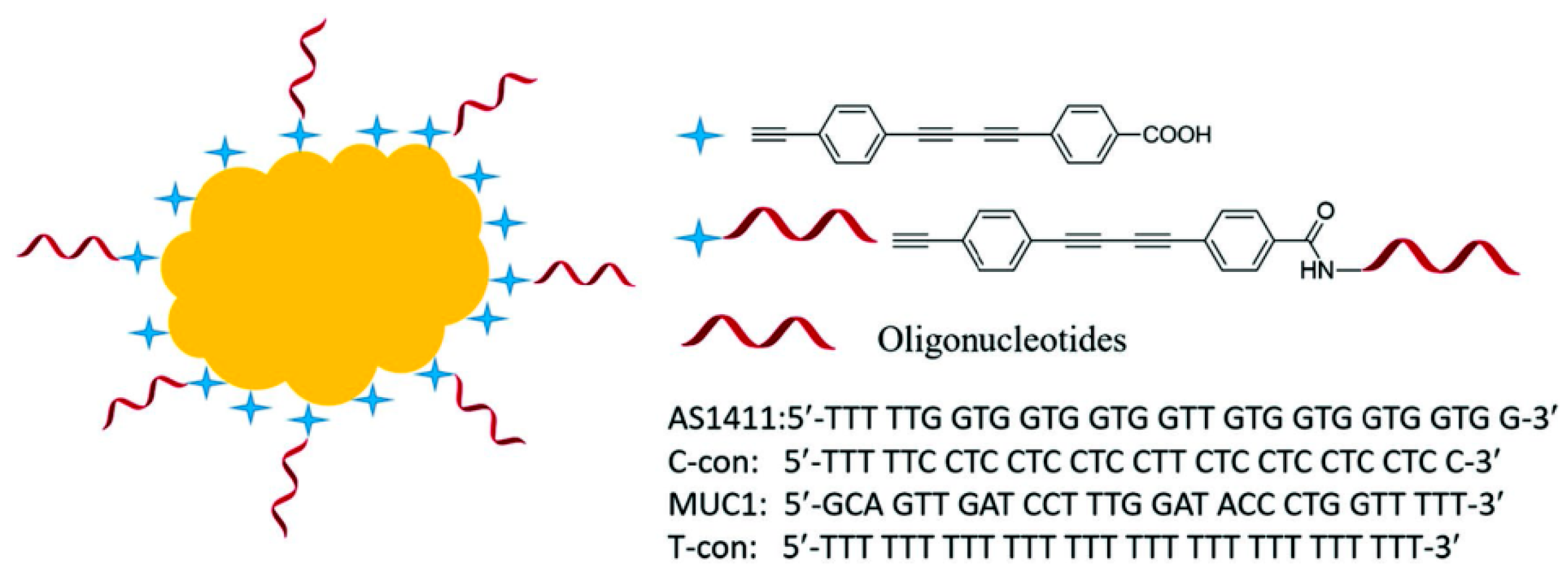
3.1. Determination of Small Molecules and Ions
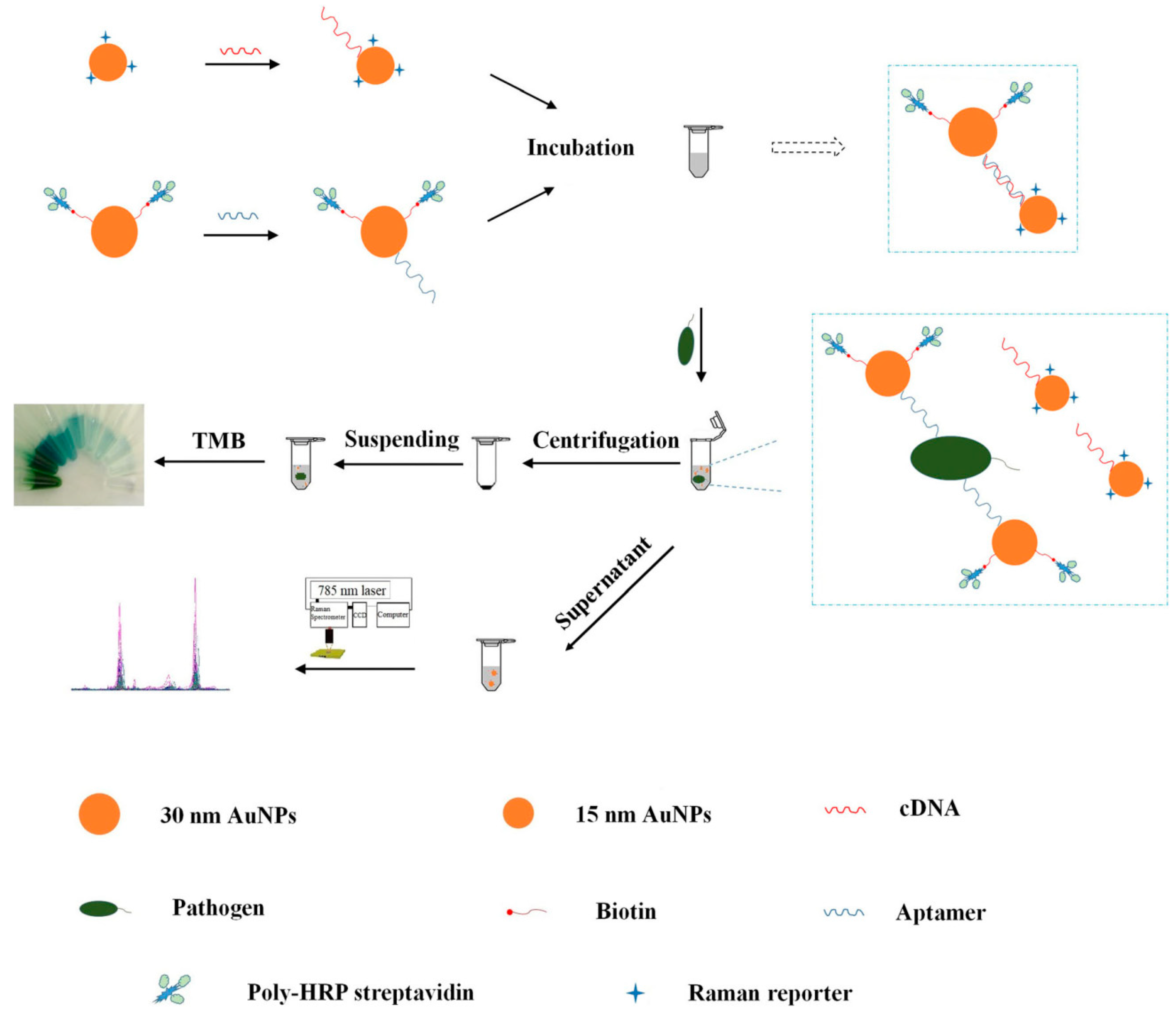
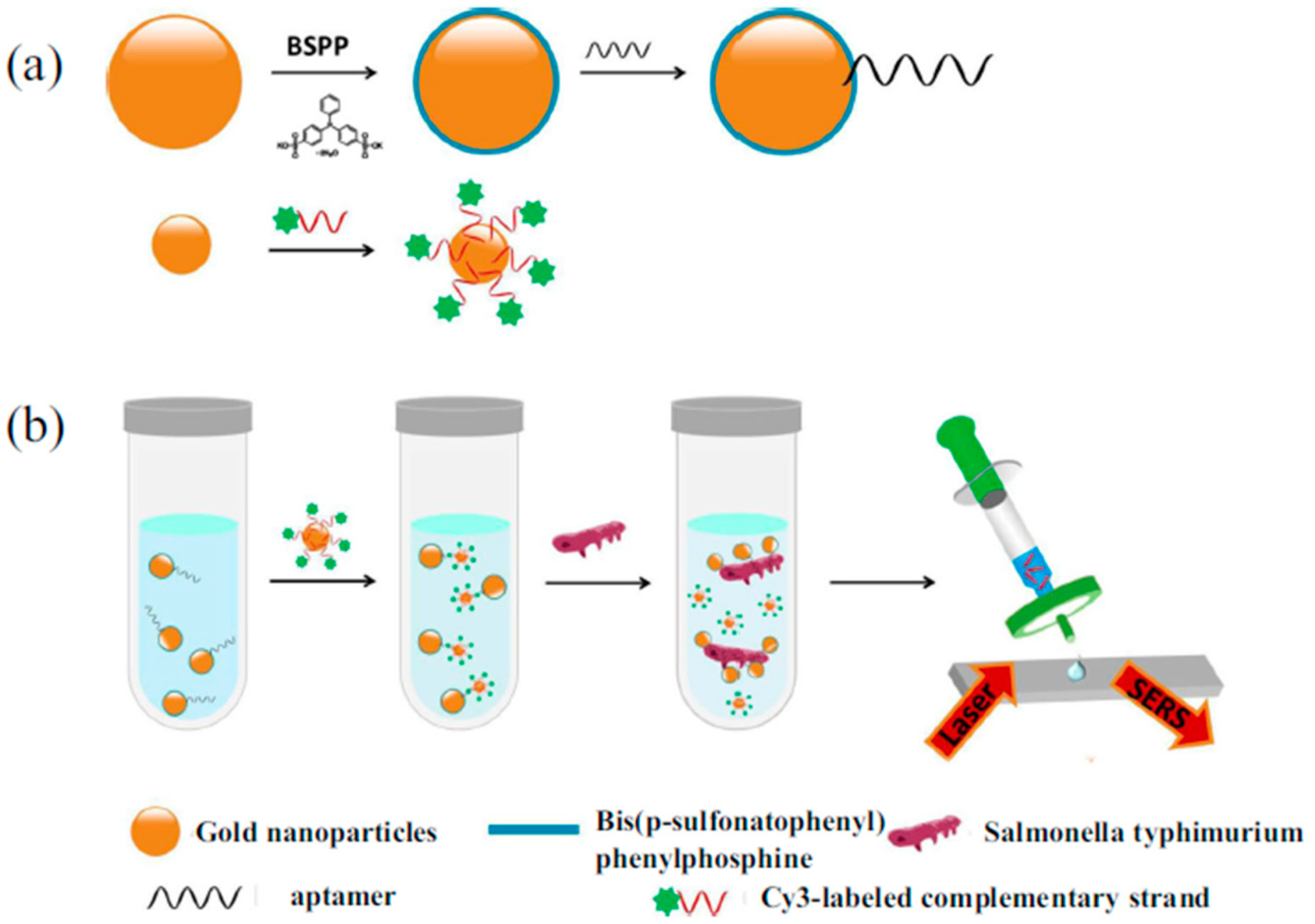
3.2. Determination of Pathogenic Microorganism
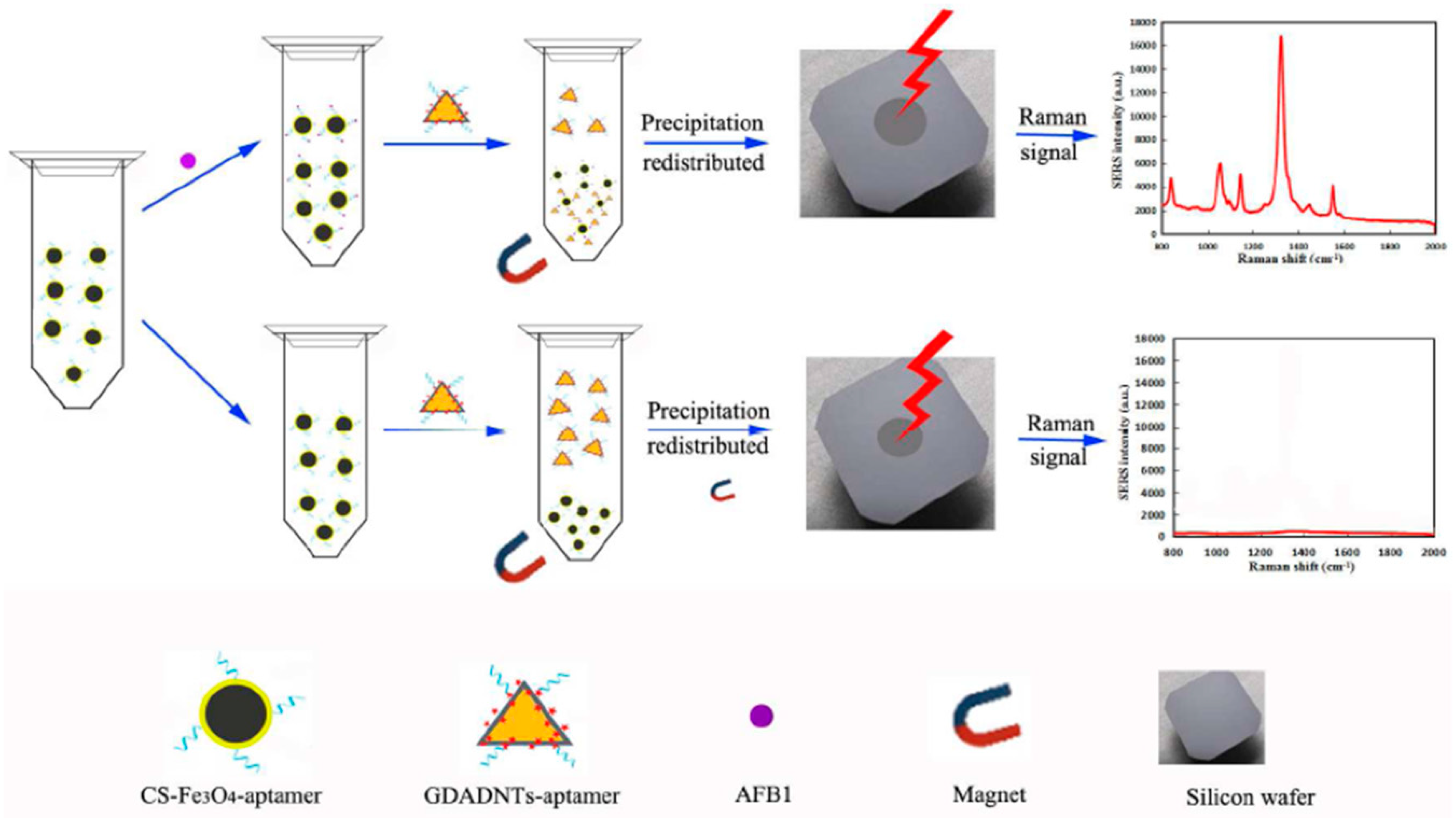
3.3. Determination of Mycotoxins, Microcystin and Pesticide Residues
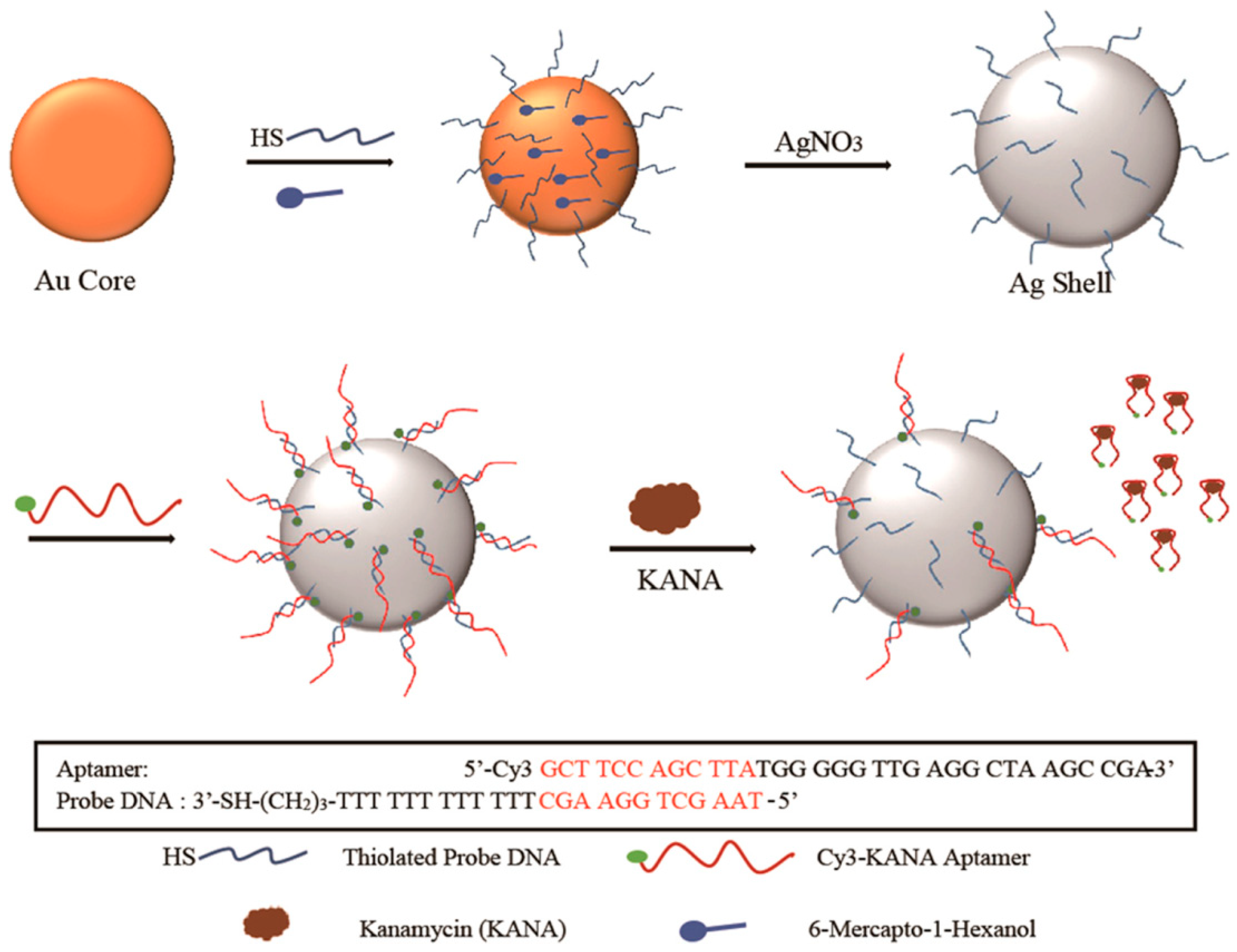
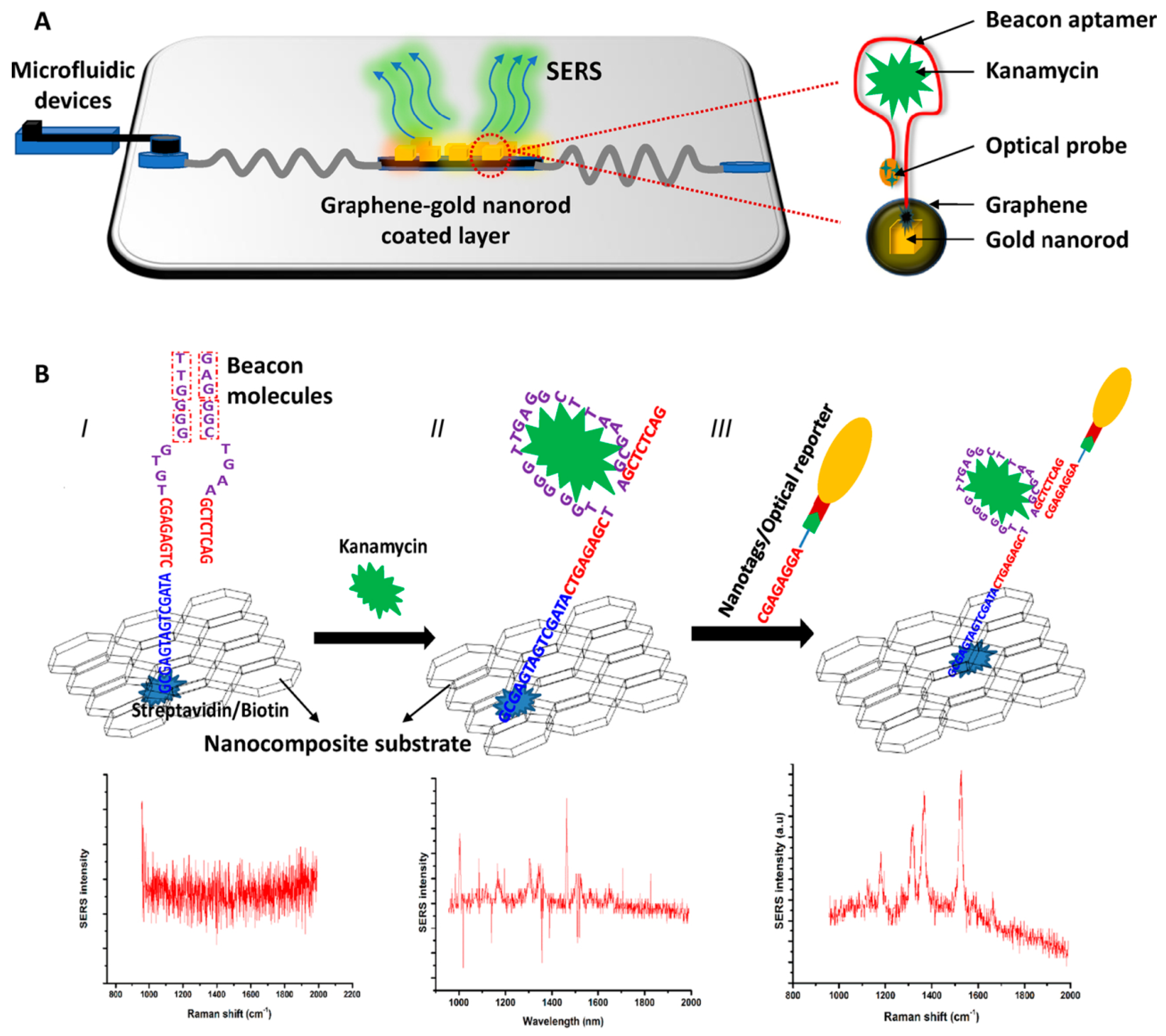
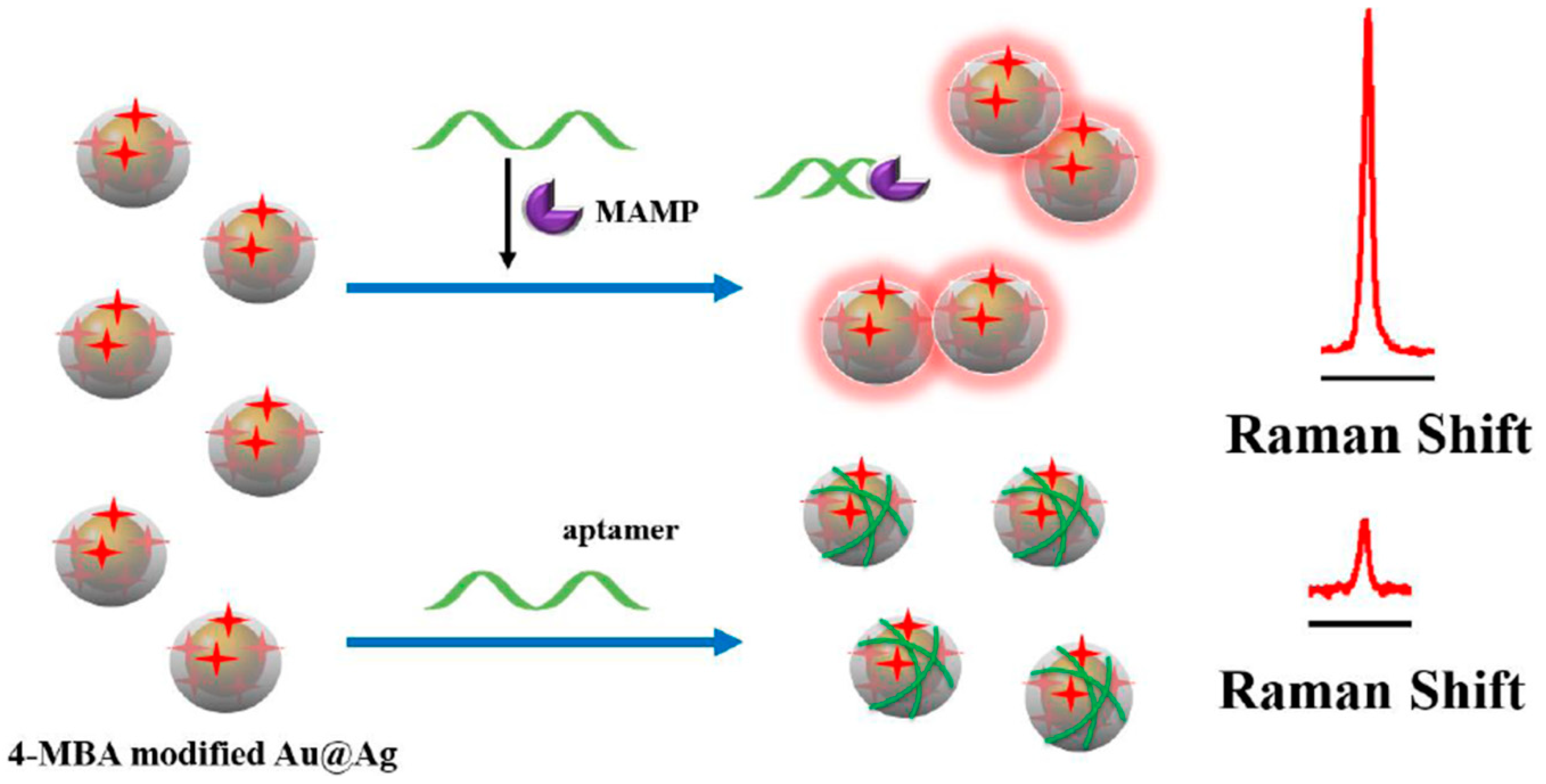
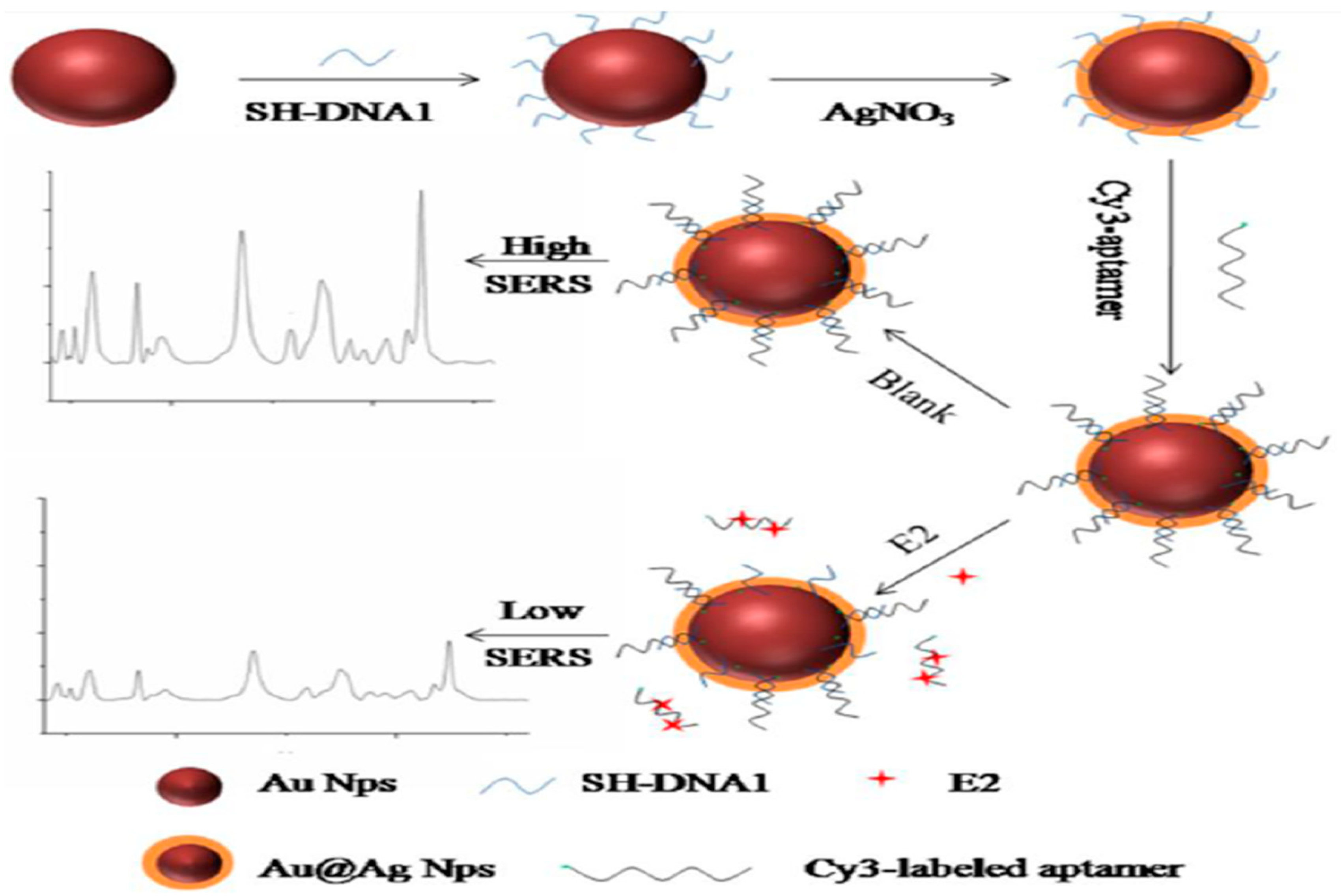
3.4. Determination of Antibiotics, Illicit Drugs, Hormones
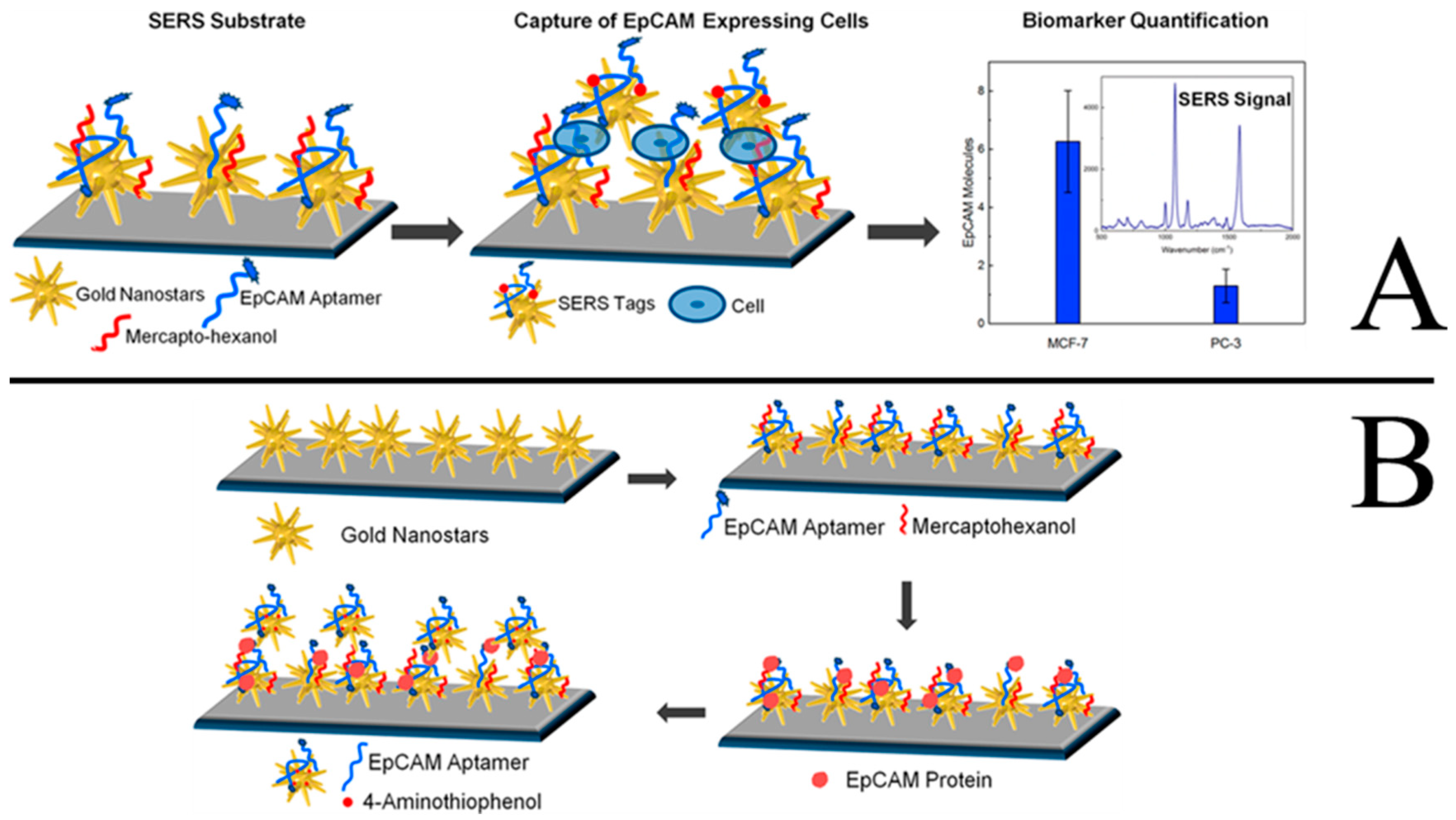
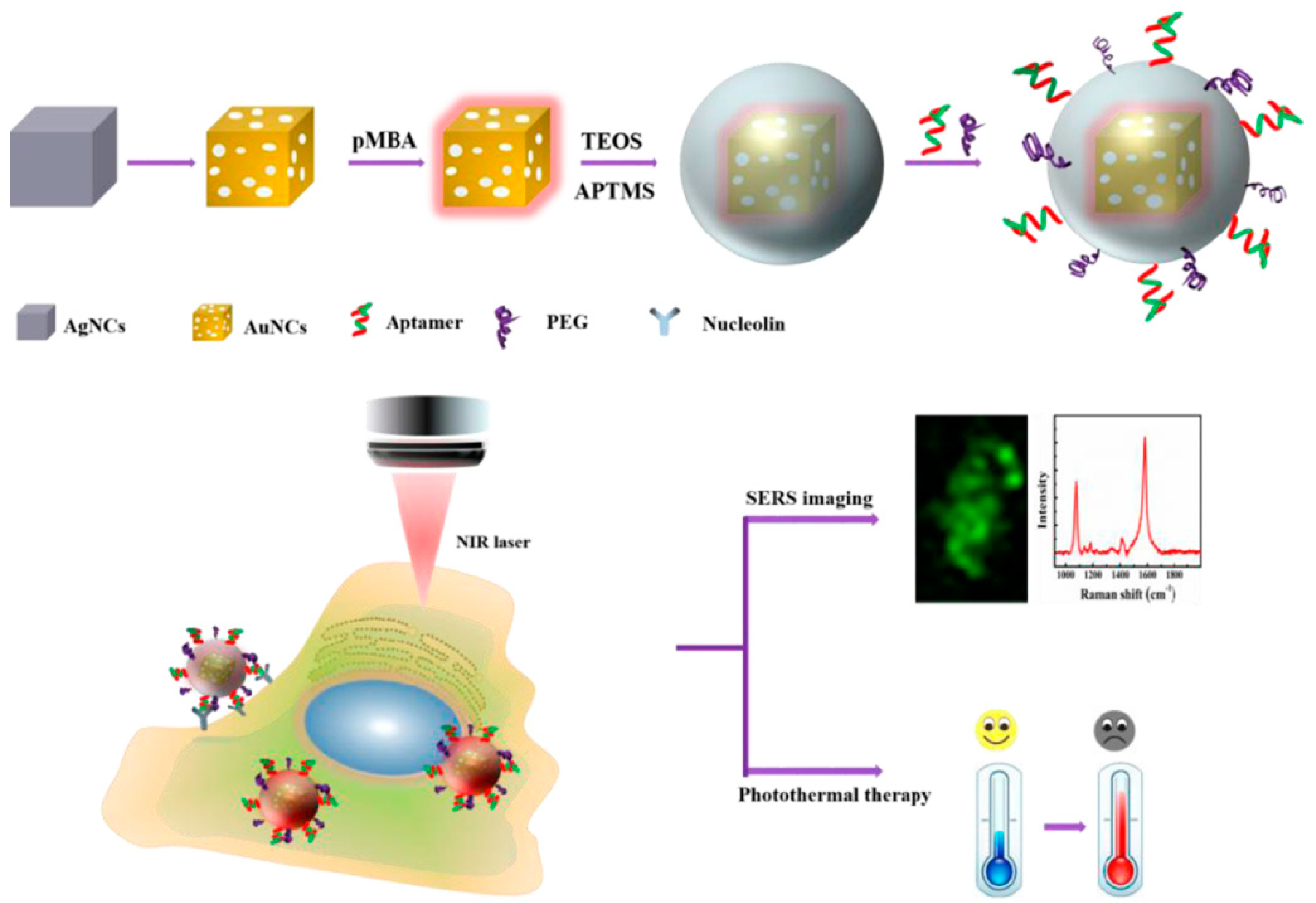
3.5. Tumor Detection and Photothermal Therapy
4. Discussion and Conclusions
5. The Challenges and Outlook of the Aptamer-Based SERS Sensors
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SERS | surface-enhanced Raman scattering |
| NPs | nanoparticles |
| 3D | three-dimensional |
| SELEX | systematic evolution of ligands by exponential enrichment |
| p-MBA | p-Mercapto benzoic acid |
| GO | graphene oxide |
| CNT | carbon nanotube |
| DEHP | bis(2-ethylhexyl)phthalate |
| ELISA | Enzyme linked immunosorbent assay |
| MNPs | magnetic particles |
| DTNB | 5,5′-Dithiobis-(2-nitrobenzoic acid) |
| LOD | limit of detection |
| VB4r | Victoria blue 4R |
| AgNR | Ag nanorods |
| GLC | glucose |
| VBB | Victoria blue B |
| Au-PDMS | Au NPs coated polydimethylsiloxane film |
| 4-MBA | 4-mercaptobenzoic acid |
| cDNA | complementary DNA |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| CS-Fe3O4 | chitosan modified Fe3O4 |
| AFB1 | Aflatoxin B1 |
| (GNTs)/Ag | Ag NPs core-shell nanotriangle |
| OTA | ochratoxin A |
| MC-LR | Microcystin-LR |
| AC | acetamiprid |
| HPLC | high performance liquid chromatography |
| GC/MS | gas chromatography-mass spectrometry |
| Cy3 | Cyanine-3 |
| KANA | kanamycin |
| MAMP | Methylamphetamine |
| HCR | hybridization chain reaction |
| EpCAM | epithelial cell adhesion molecule |
| MCH | mercaptohexanol |
| AuNCs | Au nanocage |
| NMOFs | nanoscale metal organic frameworks |
| DOX | doxorubicin |
References
- Ferraro, J.R.; Nakamoto, K.; Brown, C.W. Introductory Raman spectroscopy, 2nd ed.; Academic Press: Boston, MA, USA, 2003. [Google Scholar]
- Fleischmann, M.; Hendra, P.J.; Mcquillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Duyne, R.P.V. Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Gillibert, R.; Huang, J.Q.; Zhang, Y.; Fu, W.L.; Lamy de la Chapelle, M. Explosive detection by surface enhanced raman scattering. TrAC-Trends Anal. Chem. 2018, 105, 166–172. [Google Scholar] [CrossRef]
- Uskoković-Marković, S.; Kuntić, V.; Bajuk-Bogdanović, D.; Holclajtner-Antunović, I. Surface-enhanced raman scattering (SERS) biochemical applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 383–388. [Google Scholar]
- Kho, K.W.; Dinish, U.S.; Olivo, M. Biomedicine with surface enhanced raman scattering (SERS). Biophotonics Med. Appl. 2015, 5, 101–134. [Google Scholar]
- Guillot, N.; de la Chapelle, M.L. The electromagnetic effect in surface enhanced raman scattering: Enhancement optimization using precisely controlled nanostructures. J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 2321–2333. [Google Scholar] [CrossRef]
- Nie, S.M.; Steven, R.E. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Jiang, J.; Bosnick, K.; Maillard, M.; Brus, L. Single molecule Raman spectroscopy at the junctions of large Ag nanocrystals. J. Phys. Chem. B 2003, 107, 9964–9972. [Google Scholar] [CrossRef]
- Galvan, D.D.; Yu, Q. Surface-enhanced Raman scattering for rapid detection and characterization of antibiotic-resistant bacteria. Adv. Healthc. Mater. 2018, 7, e1701335. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, D.; Li, M.; Yin, D.; Wang, S.; Wang, J. Ag@Au core-shell porous nanocages with outstanding SERS activity for highly sensitive SERS immunoassay. Sensors 2019, 19, 1554. [Google Scholar] [CrossRef]
- Shen, N.; Xu, H.; Zhao, W.; Zhao, Y.; Zhang, X. Highly responsive and ultrasensitive non-enzymatic electrochemical glucose sensor based on Au foam. Sensors 2019, 19, 1203. [Google Scholar] [CrossRef] [PubMed]
- Guselnikova, O.; Svorcik, V.; Lyutakov, O.; Chehimi, M.M.; Postnikov, P.S. Preparation of selective and reproducible SERS sensors of Hg2+ ions via a sunlight-induced thiol(-)yne reaction on gold gratings. Sensors 2019, 19, 2110. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Pu, Y.; Chen, Y.; Liao, M.; Li, M. Novel SERS labels: Rational design, functional integration and biomedical applications. Coordin. Chem. Rev. 2018, 371, 11–37. [Google Scholar] [CrossRef]
- Dasary, S.S.R.; Singh, A.K.; Senapati, D.; Yu, H.; Ray, P.C. Gold nanoparticle based label-free SERS probe for ultrasensitive and selective detection of trinitrotoluene. J. Am. Chem. Soc. 2009, 131, 13806–13812. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Zheng, J.; Yang, D.; Jurado Sánchez, B.; Liu, X.; Guo, X.; Liu, C.; Dina, N.E.; Jian, J.; Bao, Z.; et al. Self-assembly of Au@Ag nanoparticles on mussel shell to form large-scale 3D supercrystals as natural SERS substrates for the detection of pathogenic bacteria. ACS Omega 2018, 3, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; You, T.; Yang, N.; Gao, Y.; Jiang, L.; Yin, P. Hydrophobic paper-based sers platform for direct-droplet quantitative determination of melamine. Food Chem. 2019, 287, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Mo, R.; Wang, L.; Zhou, C.; Hong, P.; Li, C. Surface enhanced Raman spectroscopy detection of sodium thiocyanate in milk based on the aggregation of Ag nanoparticles. Sensors 2019, 19, 1363. [Google Scholar] [CrossRef]
- Weng, S.; Zhu, W.; Dong, R.; Zheng, L.; Wang, F. Rapid detection of pesticide residues in paddy water using surface-enhanced Raman spectroscopy. Sensors 2019, 19, 506. [Google Scholar] [CrossRef]
- Zhu, P.X.; Shelton, D.R.; Li, S.H.; Adams, D.L.; Karns, J.S.; Amstutz, P.; Tang, C.M. Detection of E. coli O157:H7 by immunomagnetic separation coupled with fluorescence immunoassay. Biosens. Bioelectron. 2011, 30, 337–341. [Google Scholar] [CrossRef]
- Wu, W.; Li, J.; Pan, D.; Li, J.; Song, S.; Rong, M.; Li, Z.; Gao, J.; Lu, J. Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of salmonella typhimurium. ACS Appl. Mater. Inter. 2014, 6, 16974–16981. [Google Scholar] [CrossRef]
- Peng, Z.H.; Ling, M.; Ning, Y.; Deng, L. Rapid fluorescent detection of Escherichia coli K88 based on DNA aptamer library as direct and specific reporter combined with immuno-magnetic separation. J. Fluoresc. 2014, 4, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.F.; Dai, Z.W.; Tang, X.; Lin, Z.H.; Lo, P.K.; Meyyappan, M.; Chiu Lai, K.W. Graphene field-effect transistors for the sensitive and selective detection of Escherichia coli using pyrene-tagged DNA aptamer. Adv. Healthcare Mater. 2017, 6, 1700736. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Gu, B.; Liu, Q.Q.; Pang, Y.F.; Xiao, R.; Wang, S.Q. Combined use of vancomycin-modified Ag-coated magnetic nanoparticles and secondary enhanced nanoparticles for rapid surface-enhanced Raman scattering detection of bacteria. Int. J. Nano. Med. 2018, 13, 1159–1178. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Mei, Q.; Guo, X.; Xu, Y.; Yang, D.; Sanchez, B.J.; Sheng, B.; Liu, C.; Hu, Z.; Yu, G.; et al. Antimicrobial peptide based magnetic recognition elements and Au@Ag-GO sers tags with stable internal standards: A three in one biosensor for isolation, discrimination and killing of multiple bacteria in whole blood. Chem. Sci. 2018, 9, 8781–8795. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, B.; Yao, R.; Li, Z.; Wang, X.; Dong, X.; Qu, H.; Li, Q.; Li, N.; Chi, H.; et al. Intuitive label-free sers detection of bacteria using aptamer-based in situ silver nanoparticles synthesis. Anal. Chem. 2017, 89, 9836–9842. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, Y.; Cheng, K.; Pan, D.; Xu, J.; Chen, W. Determination of 17 beta-estradiol by surface-enhanced Raman spectroscopy merged with hybridization chain reaction amplification on Au@Ag core-shell nanoparticles. Mikrochim. Acta 2019, 186, 52. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Xie, Y.; Ren, B.; Yan, J.W.; Mao, B.W.; Tian, Z.Q. Surface enhanced Raman scattering from bare cobalt electrode surfaces. Phys. Chem. Comm. 2001, 18, 1–3. [Google Scholar]
- Tian, Z.Q.; Ren, B.; Mao, B.W. Extending surface Raman spectroscopy to transition metal surfaces for practical applications. 1. vibrational properties of thiocyanate and carbon monoxide adsorbed on electrochemically activated platinum surfaces. J. Phys. Chem. B 1997, 101, 1338–1346. [Google Scholar] [CrossRef]
- Ren, B.; Lin, X.F.; Yan, J.W.; Mao, B.W.; Tian, Z.Q. Electrochemically roughened rhodium electrode as a substrate for surface-enhanced Raman spectroscopy. J. Phys. Chem. B 2003, 107, 899–902. [Google Scholar] [CrossRef]
- Ding, S.Y.; Yi, J.; Li, J.F.; Ren, B.; Wu, D.Y.; Panneerselvam, R.; Tian, Z.Q. Nanostructure-based plasmonenhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. 2016, 1. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Moran, C.; Zeng, J.; Chen, J.; Wen, L.P.; Xia, Y. Seed-mediated synthesis of Ag nanocubes with controllable edge lengths in the range of 30–200 nm and comparison of their optical properties. J. Am. Chem. Soc. 2010, 132, 11372–11378. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; El-Sayed, M.A. Dependence of the enhanced optical scattering efficiency relative to that of absorption for gold metal nanorods on aspect ratio, size, end-cap shape, and medium refractive index. J. Phys. Chem. B 2005, 109, 20331–20338. [Google Scholar] [CrossRef] [PubMed]
- Christopher, G.K.; Tuan, V.D. Gold nanostars for surface-enhanced Raman scattering: synthesis, characterization and optimization. J. Phys. Chem. C 2008, 2008, 18849–18859. [Google Scholar]
- Lu, X.M.; Rycenga, M.; Skrabalak, S.E.; Wiley, B.; Xia, Y.N. Chemical synthesis of novel plasmonic nanoparticles. Annu. Rev. Phys. Chem. 2009, 60, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, S.; Agrawal, A.; Rodriguez-Lorenzo, L.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Kornowski, A.; Weller, H.; Liz-Marzan, L.M. Tuning size and sensing properties in colloidal gold nanostars. Langmuir 2010, 26, 14943–14950. [Google Scholar] [CrossRef]
- Nalbant Esenturk, E.; Hight Walker, A.R. Surface-enhanced Raman scattering spectroscopy via gold nanostars. J. Raman Spectrosc. 2009, 40, 86–91. [Google Scholar] [CrossRef]
- Xu, D.; Gu, J.; Wang, W.; Yu, X.; Xi, K.; Jia, X. Development of chitosan-coated gold nanoflowers as sers-active probes. Nanotechnology 2010, 21, 375101. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.; Wu, Y.; Ying, Y.; Wen, Y.; Guo, X.; Yang, H. Heterostructured cube au-ag composites for rapid Raman detection of antibiotic ciprofloxacin. J. Raman Spectrosc. 2017, 48, 525–529. [Google Scholar] [CrossRef]
- Lu, L.; Sun, G.; Zhang, H.; Wang, H.; Xi, S.; Hu, J.; Tian, Z.; Chen, R. Fabrication of core-shell Au-Pt nanoparticle film and its potential application as catalysis and sers substrate. J. Mater. Chem. 2004, 14, 1005–1009. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Ma, X.; Park, H.G.; Sim, S.J. Low-blinking sers substrate for switchable detection of kanamycin. Sens. Actuators B Chem. 2019, 282, 765–773. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, A.; Zhou, T.; Wang, D. Synthesis of caron nanobutes@SiO2@Ag nanocomposites for surface-enhanced Raman scattering. Chin. J. Org. Chem. 2014, 30, 543–549. [Google Scholar]
- Xiang, Y.; Yang, H.; Guo, X.; Wu, Y.; Ying, Y.; Wen, Y.; Yang, H. Surface enhanced Raman detection of the colon cancer biomarker cytidine by using magnetized nanoparticles of the type Fe3O4/Au/Ag. Mikrochim. Acta 2018, 185, 195. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.S.; Jahna, I.J.; Weber, K.; Cialla-May, D.; Popp, J. label-free SERS in biological and biomedical applications-recent progress, current challenges and opportunities. Spectrochim. Acta A 2018, 197, 56–77. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dong, T.; Nie, P.; Qu, F.; He, Y.; Chu, B.; Xiao, S. Rapid determination of thiabendazole pesticides in rape by surface enhanced Raman spectroscopy. Sensors 2018, 18, 1082. [Google Scholar] [CrossRef] [PubMed]
- Sukesan, R.; Chen, Y.T.; Shahim, S.; Wang, S.L.; Sarangadharan, I.; Wang, Y.L. Instant mercury ion detection in industrial waste water with a microchip using extended gate field-effect transistors and a portable device. Sensors 2019, 19, 2209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Kasani, S.; Shi, X.; Boryczka, A.E.; Yang, F.; Tang, H.; Li, M.; Zheng, W.; Elswick, D.E.; Wu, N. Detection of nitrite with a surface-enhanced Raman scattering sensor based on silver nanopyramid array. Anal. Chim. Acta 2018, 1040, 158–165. [Google Scholar] [CrossRef] [PubMed]
- González-Sálamo, J.; Socas-Rodríguez, B.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Determination of phthalic acid esters in water samples using core-shell poly(dopamine) magnetic nanoparticles and gas chromatography tandem mass spectrometry. J. Chromatogr. A 2017, 1530, 35–44. [Google Scholar]
- Tahmasebi, E.; Masoomi, M.Y.; Yamini, Y.; Morsali, A. Application of a Zn(II) based metal-organic framework as an efficient solid-phase extraction sorbent for preconcentration of plasticizer compounds. RSC Adv. 2016, 6, 40211–40218. [Google Scholar] [CrossRef]
- Makkliang, F.; Kanatharana, P.; Thavarungkul, P.; Thammakhet-Buranachai, C. A polypyrrole-chitosan cryogel stir-bead micro-solid phase extractor for the determination of phthalate esters in contact lenses storage solutions and in artificial saliva in contact with baby teethers. Anal. Chim. Acta 2017, 985, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N. Ionic liquids for mass spectrometry: Matrices, separation and microextraction. TrAC-Trends Anal. Chem. 2016, 77, 122–138. [Google Scholar] [CrossRef]
- Kissner, R.; Koppenol, W.H. Qualitative and quantitative determination of nitrite and nitrate with ion chromatography. Method Enzymol. 2005, 396, 61–68. [Google Scholar]
- Fang, H.Q.; Wang, J.; Lynch, R.A. Migration of di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DBP) from polypropylene food containers. Food Control 2017, 73, 1298–1302. [Google Scholar] [CrossRef]
- Sun, R.Y.; Zhuang, H.S. A sensitive heterogeneous biotin–streptavidin enzyme-linked immunosorbent assay for the determination of di-(2-ethylhexyl)phthalate (DEHP) in beverages using a specific polyclonal antibody. Anal. Methods 2014, 6, 9807–9815. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Wang, H.X.; Jia, G.C.; Li, Z. Application of aptamer-based biosensor for rapid detection of pathogenic escherichia coli. Sensors 2018, 18, 2518. [Google Scholar] [CrossRef]
- Kaneko, N.; Horii, K.; Akitomi, J.; Kato, S.; Shiratori, I.; Waga, I. An aptamer-based biosensor for direct, label-free detection of melamine in raw milk. Sensors 2018, 18, 3227. [Google Scholar] [CrossRef]
- Tu, D.; Garza, J.T.; Coté, G.L. A sers aptasensor for sensitive and selective detection of bis(2-ethylhexyl)phthalate. RSC Adv. 2019, 9, 2618–2625. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Liang, A.; Luo, Y.; Wen, G.; Jiang, Z. A simple gold nanoplasmonic sers method for trace Hg2+ based on aptamer-regulating graphene oxide catalysis. Luminescence 2018, 33, 1113–1121. [Google Scholar] [CrossRef]
- Li, C.; Peng, Y.; Wang, H.; Liang, A.; Jiang, Z. A nanosol sers method for quantitative analysis of trace potassium based on aptamer recognition and silver nanorod catalysis of Ag(I)-glucose reaction. Sens. Actuators B Chem. 2019, 281, 53–59. [Google Scholar] [CrossRef]
- Guo, Q.; Han, J.J.; Shan, S.; Liu, D.F.; Wu, S.S.; Xiong, Y.H.; Lai, W.H. DNA-based hybridization chain reaction and biotin-streptavidin signal amplification for sensitive detection of Escherichiacoli O157:H7 through ELISA. Biosens. Bioelectron. 2016, 86, 990–995. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, H.W.; Xiao, P.F.; Singh, R.; He, N.Y.; Liu, B.; Li, Z.Y. Highly selective, sensitive and rapid detection of Escherichia coli O157:H7 using duplex PCR and magnetic nanoparticle-based chemiluminescence assay. J. Biomed. Nanotechnol. 2017, 13, 1243–1252. [Google Scholar] [CrossRef]
- Shen, M.; Duan, N.; Wu, S.; Zou, Y.; Wang, Z. Polydimethylsiloxane gold nanoparticle composite film as structure for aptamer-based detection of vibrio parahaemolyticus by surface-enhanced Raman spectroscopy. Food Anal. Method 2018, 12, 595–603. [Google Scholar] [CrossRef]
- Porstmann, T.; Kiessig, S.T. Enzyme immunoassay techniques. J. lmmunological Methods 1992, 150, 5–21. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Cui, B.; Jin, Z. A bimodal (sers and colorimetric) aptasensor for the detection of pseudomonas aeruginosa. Mikrochim. Acta 2018, 185, 528. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ma, X.; Wang, H.; Wang, Z. Aptamer based sers detection of salmonella typhimurium using DNA-assembled gold nanodimers. Mikrochim. Acta 2018, 185, 325. [Google Scholar] [CrossRef]
- Karapetis, S.; Nikolelis, D.; Hianik, T. Label-free and redox markers-based electrochemical aptasensors for aflatoxin M1 detection. Sensors 2018, 18, 4218. [Google Scholar] [CrossRef]
- Albero, B.; Sanchez-Brunete, C.; Miguel, E.; Tadeo, J.L. Application of matrix solid-phase dispersion followed by GC-MS/MS to the analysis of emerging contaminants in vegetables. Food Chem. 2017, 217, 660–667. [Google Scholar] [CrossRef]
- Meulenberg, E.P. Immunochemical methods for ochratoxin a detection: A review. Toxins 2012, 4, 244–266. [Google Scholar] [CrossRef]
- Yang, M.; Liu, G.; Mehedi, H.M.; Ouyang, Q.; Chen, Q. A universal sers aptasensor based on DTNB labeled GNTs/Ag core-shell nanotriangle and CS-Fe3O4 magnetic-bead trace detection of Aflatoxin B1. Anal. Chim. Acta 2017, 986, 122–130. [Google Scholar] [CrossRef]
- Song, D.; Yang, R.; Fang, S.; Liu, Y.; Long, F.; Zhu, A. Sers based aptasensor for ochratoxin a by combining Fe3O4@Au magnetic nanoparticles and Au-DTNB@Ag nanoprobes with multiple signal enhancement. Mikrochim. Acta 2018, 185, 491. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Ma, X.; Zhao, S.; Lv, Y.; Hun, X.; Wang, H.; Wang, Z. Nanogapped Au(core) @Au-Ag(shell) structures coupled with Fe3O4 magnetic nanoparticles for the detection of Ochratoxin A. Anal. Chim. Acta 2018, 1033, 165–172. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, Z.; Cui, B.; Jin, Z. A novel sers-based aptasensor for ultrasensitive sensing of microcystin-LR. Food Chem. 2019, 278, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, W.; Hassan, M.M.; Zhang, Z.; Chen, Q. A facile and sensitive sers-based biosensor for colormetric detection of acetamiprid in green tea based on unmodified gold nanoparticles. J. Food Meas. Charact. 2018, 13, 259–268. [Google Scholar] [CrossRef]
- Subedi, B.; Kannan, K. Mass loading and removal of select illicit drugs in two wastewater treatment plants in New York State and estimation of illicit drug usage in communities through wastewater analysis. Environ. Sci. Technol. 2014, 48, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Shi, Y.; Pan, Y.; Yue, Z.; Zhang, H.; Yi, C. Colorimetric and bare eye determination of urinary methylamphetamine based on the use of aptamers and the salt-induced aggregation of unmodified gold nanoparticles. Microchim. Acta 2015, 182, 505–511. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.W.; Pu, H.; Wei, Q. Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 2019, 197, 151–158. [Google Scholar] [CrossRef]
- Mao, K.; Zhou, Z.; Han, S.; Zhou, X.; Hu, J.; Li, X.; Yang, Z. A novel biosensor based on Au@Ag core-shell nanoparticles for sensitive detection of methylamphetamine with surface enhanced Raman scattering. Talanta 2018, 190, 263–268. [Google Scholar] [CrossRef]
- Hao, J.; Xiong, B.; Cheng, X.; He, Y.; Yeung, E.S. High-throughput sulfide sensing with colorimetric analysis of single Au-Ag core-shell nanoparticles. Anal. Chem. 2014, 86, 4663–4667. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, G.; Shi, H.; Liu, M.; Wang, Y.; Ke, H. A femtomolar level and highly selective 17 beta-estradiol photoelectrochemical aptasensor applied in environmental water samples analysis. Environ. Sci. Technol. 2014, 48, 5754–5761. [Google Scholar] [CrossRef]
- Janssens, G.; Mangelinckx, S.; Courtheyn, D.; Prevost, S.; De Poorter, G.; De Kimpe, N.; Le Bizec, B. Application of gas chromatography-mass spectrometry/combustion/isotope ratio mass spectrometry (GC-MS/C/IRMS) to detect the abuse of 17 beta-estradiol in cattle. J. Agr. Food Chem. 2013, 61, 7242–7249. [Google Scholar] [CrossRef] [PubMed]
- Caron, E.; Sheedy, C.; Farenhorst, A. Development of competitive ELISAs for 17 beta-estradiol and 17 beta-estradiol + estrone + estriol using rabbit polyclonal antibodies. J. Environ. Sci. Heal. B 2010, 5, 145–151. [Google Scholar] [CrossRef]
- Pu, H.; Xie, X.; Sun, D.W.; Wei, Q.; Jiang, Y. Double strand DNA functionalized Au@Ag NPs for ultrasensitive detection of 17 beta-estradiol using surface-enhanced Raman spectroscopy. Talanta 2019, 195, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Bhamidipati, M.; Cho, H.Y.; Lee, K.B.; Fabris, L. Sers-based quantification of biomarker expression at the single cell level enabled by gold nanostars and truncated aptamers. Bioconjugate Chem. 2018, 29, 2970–2981. [Google Scholar] [CrossRef]
- Liang, D.; Jin, Q.; Yan, N.; Feng, J.; Wang, J.; Tang, X. Sers nanoprobes in biologically raman silent region for tumor cell imaging and in vivo tumor spectral detection in mice. Adv. Biosyst. 2018, 2, 1800100. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. Int. Ed. 2013, 52, 13958–13964. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Galarreta, B.C. Noble metal-based plasmonic nanoparticles for SERS imaging and photothermal therapy. Nanomater. Magn. Opt. Hyperth. Appl. 2019. [Google Scholar] [CrossRef]
- Khoee, S.; Khezrian, S. Applications of aptamers for the diagnosis and therapy of different disases. Nanostruct. Novel Ther. 2017, 591–619. [Google Scholar] [CrossRef]
- Wen, S.; Miao, X.; Fan, G.C.; Xu, T.; Jiang, L.P.; Wu, P.; Cai, C.; Zhu, J.J. Aptamer-conjugated Au nanocage/SiO2 core-shell bifunctional nanoprobes with high stability and biocompatibility for cellular sers imaging and near-infrared photothermal therapy. ACS Sensors 2019, 4, 308. [Google Scholar] [CrossRef]
- He, J.; Dong, J.; Hu, Y.; Li, G.; Hu, Y. Design of raman tag-bridged core-shell Au@Cu3(BTC)2 nanoparticles for Raman imaging and synergistic chemo-photothermal therapy. Nanoscale 2019, 11, 6089–6100. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, T.T.; Tian, Y. Functionalized h-BN nanosheets as a novel theranostic platform for SERS real-time monitoring of microRNA and photodynamic therapy. Angew. Chem. Int. Ed. 2019, 131, 7839–7843. [Google Scholar] [CrossRef]
- Zhao, L.L.; Choi, J.; Lu, Y.; Kim, S.Y. Targeted photodynamic therapy activities of surface-enhanced Raman scattering-active theranostic system based on folate/hyaluronic acid-functionalized gold nanochains. J. Biomed. Nanotechnol. 2019, 15, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Ouyang, W.W.; Zhang, X.R.; Xue, J.; Lou, X.R.; Fan, R.R.; Zhao, X.N.; Shan, L.Q.; Jiang, T.T. Bacteria-induced aggregation of bioorthogonal gold nanoparticles for SERS imaging and enhanced photothermal ablation of Gram-positive bacteria. J. Mater. Chem. B 2019, 7, 4630–4637. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.C.; Hu, Y.L.; Li, G.K. Biocompatible Au@Ag nanorod@ZIF-8 core-shell nanoparticles for surface-enhanced Raman scattering imaging and drug delivery. Talanta 2019. [Google Scholar] [CrossRef] [PubMed]







| Target Analytes | Actual Samples | Linear Range | LOD | Ref. | |
|---|---|---|---|---|---|
| Small molecules and ions | DEHP | tap water, bottled water, and a carbonate beverage | 0.008–182 nM | 8 pM | [60] |
| Hg2+ | laboratory water, rainwater, and pond water | 0.25–10 nM | 0.08 nM | [61] | |
| K+ | rice samples | 50–3000 nM | 25 nM | [62] | |
| Pathogenic microorganism | Vibrio parahaemolyticus | spiked prawn samples | 1.2 × 102 and 1.2 × 106 CFU·mL−1 | -- | [65] |
| Pseudomonas aeruginosa | spiked tap water and chicken meat | 102 and 106 CFU·mL−1 | 20 CFU·mL−1 for SERS mode and 50 CFU·mL−1 for color mode | [67] | |
| Salmonella typhimurium | spiked milk | 102 to 107 CFU·mL−1 | 35 CFU·mL−1 | [68] | |
| Mycotoxins, microcystin and pesticide residues | AFB1 | peanut oil | 0.001 to 10 ng/mL | 0.54 pg/mL | [72] |
| OTA | red wine and coffee | 1.20 pg·mL−1 to 3.31 μg·mL−1 | 0.48 pg·mL−1 | [73] | |
| MC-LR | tap water | 0.01 to 200 ng/mL | 0.002 ng/mL | [75] | |
| AC | green tea and adulterated tea | 3.0 × 10−8 to 4.0 × 10−6 M | 1.76 × 10−8 M | [76] | |
| Antibiotics, illicit drugs, hormones | KANA | liquid whole milk | 10 μg/mL to 100 ng/mL | 0.90 pg/mL | [79] |
| KANA | milk, orange juice, tape water, and drinking water | 1 nM to 100 nM | 0.75 nM | [44] | |
| MAMP | human urine sample | 0.5 ppb to 40 ppb | 0.16 ppb | [80] | |
| 17 β-estradiol | human urine sample | 1 pM to 10 nM | 0.1 pM | [28] | |
| 17 β-estradiol | aquaculture water, lake water and tap water | 1.0 × 10−13 to 1.0 × 10−9 M | 2.75 fM | [85] | |
| Tumor | EpCAM | MCF-7 cells | 500 nM to 10 pM for EpCAM protein, 10 and 5000 cells for MCF-7 cells | 10 pM for EpCAM protein, the single cell level for MCF-7 cells | [86] |
| MCF-7 cells | live mice | 5–100 cell/mL | -- | [87] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-X.; Zhao, Y.-W.; Li, Z.; Liu, B.-S.; Zhang, D. Development and Application of Aptamer-Based Surface-Enhanced Raman Spectroscopy Sensors in Quantitative Analysis and Biotherapy. Sensors 2019, 19, 3806. https://doi.org/10.3390/s19173806
Wang H-X, Zhao Y-W, Li Z, Liu B-S, Zhang D. Development and Application of Aptamer-Based Surface-Enhanced Raman Spectroscopy Sensors in Quantitative Analysis and Biotherapy. Sensors. 2019; 19(17):3806. https://doi.org/10.3390/s19173806
Chicago/Turabian StyleWang, Hai-Xia, Yu-Wen Zhao, Zheng Li, Bo-Shi Liu, and Di Zhang. 2019. "Development and Application of Aptamer-Based Surface-Enhanced Raman Spectroscopy Sensors in Quantitative Analysis and Biotherapy" Sensors 19, no. 17: 3806. https://doi.org/10.3390/s19173806
APA StyleWang, H.-X., Zhao, Y.-W., Li, Z., Liu, B.-S., & Zhang, D. (2019). Development and Application of Aptamer-Based Surface-Enhanced Raman Spectroscopy Sensors in Quantitative Analysis and Biotherapy. Sensors, 19(17), 3806. https://doi.org/10.3390/s19173806





