Real-Time Ozone Sensor Based on Selective Oxidation of Methylene Blue in Mesoporous Silica Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
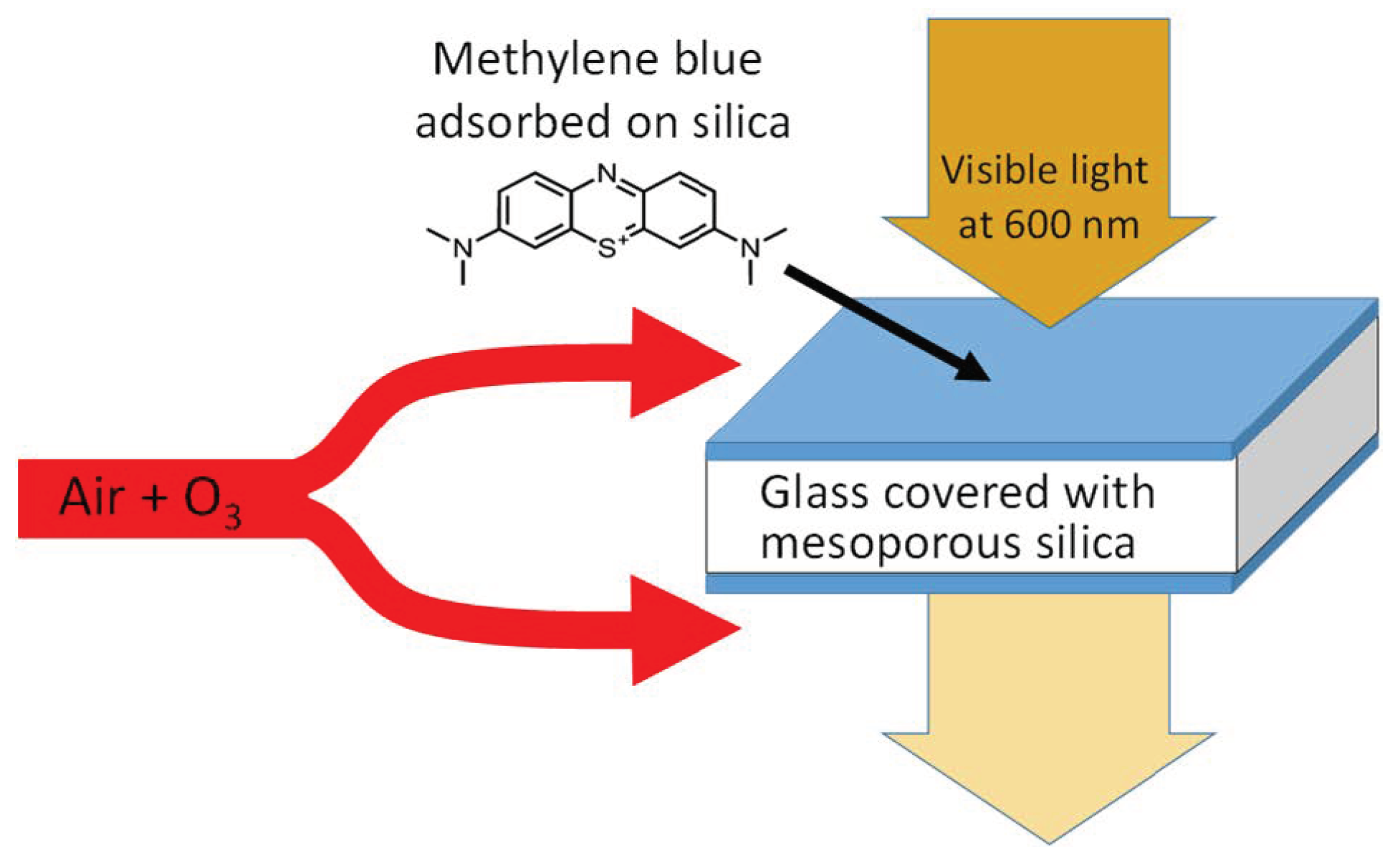
2.2. Sensor Chip Preparation
2.3. Experimental Setup
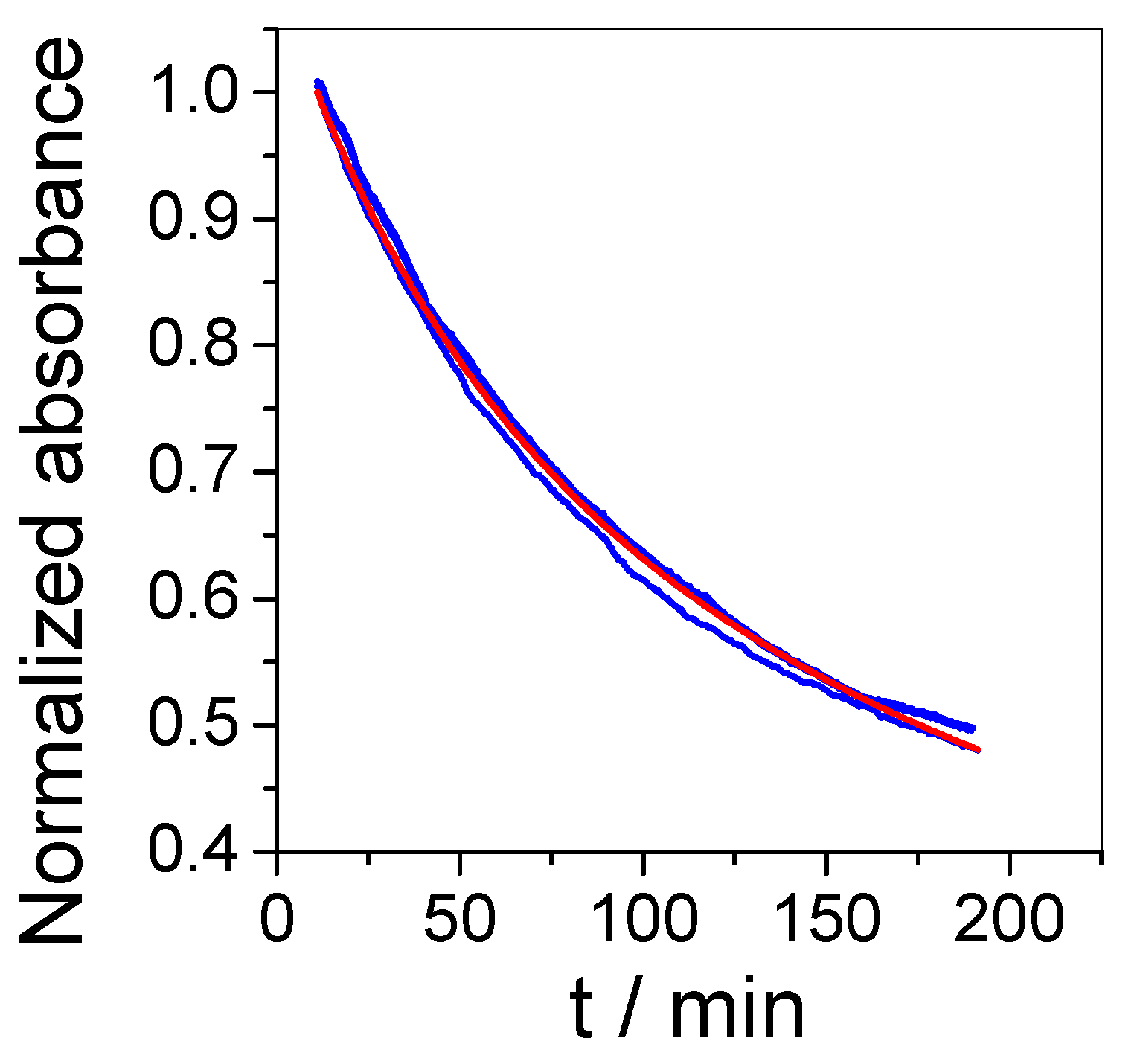
2.4. Mathematical Model
3. Results and Discussion
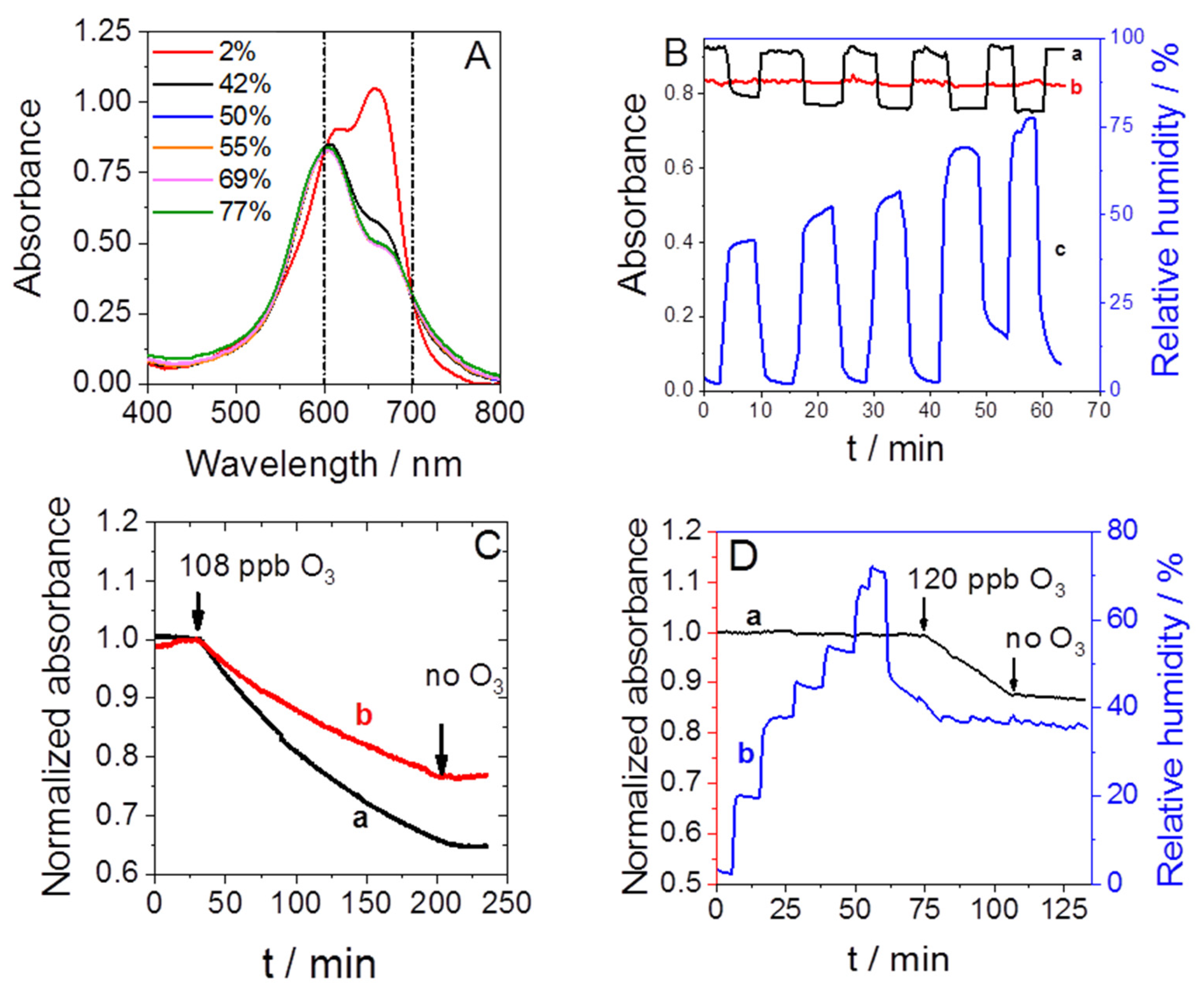
3.1. Preliminary Studies
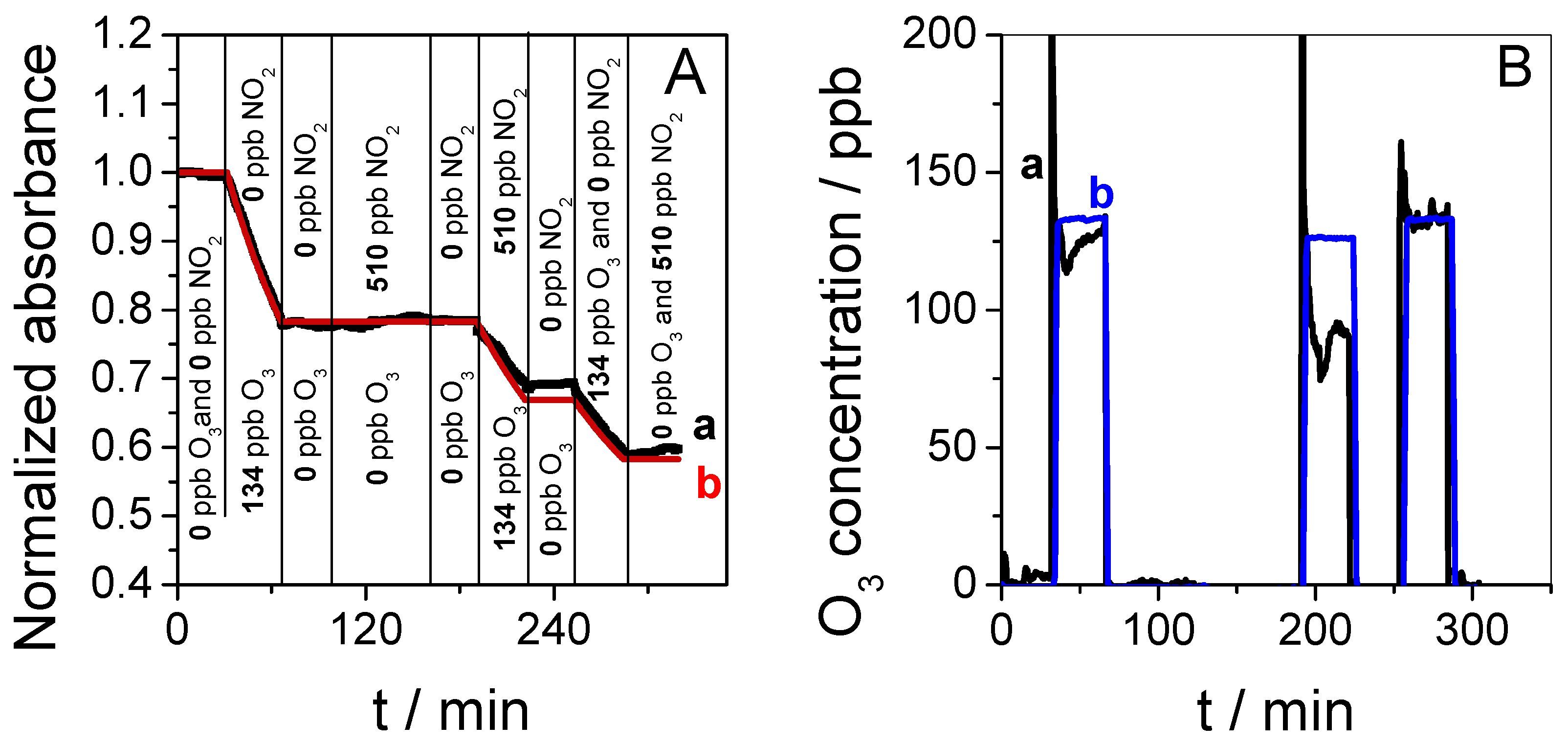
3.2. Sensor Sensitivity and Selectivity
3.3. Application of the Sensor in a Complex Scenario
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singer, B.C.; Coleman, B.K.; Destaillats, H.; Hodgson, A.T.; Lunden, M.M.; Weschler, C.J.; Nazaroff, W.W. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos. Environ. 2006, 40, 6696–6710. [Google Scholar] [CrossRef]
- Wang, H.; He, C.; Morawska, L.; McGarry, P.; Johnson, G. Ozone-initiated particle formation, particle aging, and precursors in a laser printer. Environ. Sci. Technol. 2012, 46, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Inrs Fiche Toxicologique n°43: Ozone. Available online: http://www.inrs.fr/publications/bdd/fichetox/fiche.html?refINRS=FICHETOX_43 (accessed on 9 August 2019).
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Özden, Ö.; Döǧeroǧlu, T.; Gaga, E.O. Personal exposure of primary school children to BTEX, NO2and ozone in Eskişehir, Turkey: Relationship with indoor/outdoor concentrations and risk assessment. Sci. Total Environ. 2014, 473–474, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Liard, R.; Zureik, M.; Le Moullec, Y.; Soussan, D.; Glorian, M.; Grimfeld, A.; Neukirch, F. Use of personal passive samplers for measurement of NO2, NO, and O3 levels in panel studies. Environ. Res. 1999, 81, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Bernard, N.L.; Gerber, M.J.; Astre, C.M.; Saintot, M.J. Ozone measurement with passive samplers: Validation and use for ozone pollution assessment in Montpellier, France. Environ. Sci. Technol. 1999, 33, 217–222. [Google Scholar] [CrossRef]
- Surgi, M.R.; Hodgeson, J.A. 10,10′-Dimethyl-9,9′-biacridylidene Impregnated Film Badge Dosimeters for Passive Ozone Sampling. Anal. Chem. 1985, 57, 1737–1740. [Google Scholar] [CrossRef]
- Geyh, A.S.; Wolfson, J.M.; Koutrakis, P.; Mulik, J.D.; Avol, E.L. Development and evaluation of a small active ozone sampler. Environ. Sci. Technol. 1997, 31, 2326–2330. [Google Scholar] [CrossRef]
- Acuautla, M.; Bernardini, S.; Gallais, L.; Fiorido, T.; Patout, L.; Bendahan, M. Ozone flexible sensors fabricated by photolithography and laser ablation processes based on ZnO nanoparticles. Sens. Actuators B Chem. 2014, 203, 602–611. [Google Scholar] [CrossRef]
- Vallejos, S.; Khatko, V.; Aguir, K.; Ngo, K.A.; Calderer, J.; Gràcia, I.; Cané, C.; Llobet, E.; Correig, X. Ozone monitoring by micro-machined sensors with WO3 sensing films. Sens. Actuators B Chem. 2007, 126, 573–578. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Blinov, I.; Brinzari, V.; Stetter, J.R. Effect of air humidity on gas response of SnO2 thin film ozone sensors. Sens. Actuators B Chem. 2007, 122, 519–526. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cerneavschi, A.; Brinzari, V.; Vasiliev, A.; Ivanov, M.; Cornet, A.; Morante, J.; Cabot, A.; Arbiol, J. In2O3 films deposited by spray pyrolysis as a material for ozone gas sensors. Sens. Actuators B Chem. 2004, 99, 297–303. [Google Scholar] [CrossRef]
- Kang, G.; Zhu, Z.; Tang, B.H.; Wu, C.H.; Wu, R.J. Rapid detection of ozone in the parts per billion range using a novel Ni–Al layered double hydroxide. Sens. Actuators B Chem. 2017, 241, 1203–1209. [Google Scholar] [CrossRef]
- Bejaoui, A.; Guerin, J.; Zapien, J.A.; Aguir, K. Theoretical and experimental study of the response of CuO gas sensor under ozone. Sens. Actuators B Chem. 2014, 190, 8–15. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Mastelaro, V.R.; Escanhoela, C.A. In-Depth Understanding of the Relation between CuAlO 2 Particle Size and Morphology for Ozone Gas Sensor Detection at a Nanoscale Level. ACS Appl. Mater. Interfaces 2014, 6, 21739–21749. [Google Scholar] [CrossRef]
- Sizun, T.; Bouvet, M.; Chen, Y.; Suisse, J.; Barochi, G.; Rossignol, J. Differential study of substituted and unsubstituted cobalt phthalocyanines for gas sensor applications. Sens. Actuators B. Chem. 2011, 159, 163–170. [Google Scholar] [CrossRef]
- Brunet, J.; Pauly, A.; Mazet, L.; Germain, J.P.; Bouvet, M.; Malezieux, B. Improvement in real time detection and selectivity of phthalocyanine gas sensors dedicated to oxidizing pollutants evaluation. Thin Solid Films 2005, 490, 28–35. [Google Scholar] [CrossRef][Green Version]
- Ashley, K. Developments in electrochemical sensors for occupational and environmental health applications. J. Hazard. Mater. 2003, 102, 1–12. [Google Scholar] [CrossRef]
- Pang, X.; Shaw, M.D.; Lewis, A.C.; Carpenter, L.J.; Batchellier, T. Electrochemical ozone sensors: A miniaturised alternative for ozone measurements in laboratory experiments and air-quality monitoring. Sens. Actuators B Chem. 2017, 240, 829–837. [Google Scholar] [CrossRef]
- Knake, R.; Hauser, P.C. Sensitive electrochemical detection of ozone. Anal. Chim. Acta 2002, 459, 199–207. [Google Scholar] [CrossRef]
- Muller, J.B.A.; Smith, C.E.; Newton, I.; Percival, C.J. Evaluation of coated QCM for the detection of atmospheric ozone. Analyst 2011, 136, 2963–2968. [Google Scholar] [CrossRef]
- Black, D.R.; Harley, R.A.; Hering, S.V.; Stolzenburg, M.R. A new, portable, real-time ozone monitor. Environ. Sci. Technol. 2000, 34, 3031–3040. [Google Scholar] [CrossRef]
- Fog, H.M.; Rietz, B. Piezoelectric crystal detector for the monitoring of Ozone in working environments.pdf. Anal. Chem. 1985, 57, 2638–2643. [Google Scholar] [CrossRef]
- 2BTechnologies Ozone Monitor. Available online: https://twobtech.com/docs/manuals/model_202_revH.pdf (accessed on 9 August 2019).
- Fisher, T. UV Photometric O3 Analyzer Model 49i. Available online: https://www.thermofisher.com/order/catalog/product/49i (accessed on 9 August 2019).
- Andersen, P.C.; Williford, C.J.; Birks, J.W. Miniature personal ozone monitor based on UV absorbance. Anal. Chem. 2010, 82, 7924–7928. [Google Scholar] [CrossRef]
- Sagona, J.A.; Weisel, C.P.; Meng, Q. Accuracy and practicality of a portable ozone monitor for personal exposure estimates. Atmos. Environ. 2018, 175, 120–126. [Google Scholar] [CrossRef]
- Grosjean, D.; Harrison, J. Response of Chemiluminescence NO, Analyzers and Ultraviolet Ozone Analyzers to Organic Air Pollutants. Environ. Sci. Technol. 1985, 19, 862–865. [Google Scholar] [CrossRef]
- Warren, G.J.; Babcock, G. Portable ethylene chemiluminescence ozone monitor. Rev. Sci. Instrum. 1970, 41, 280–282. [Google Scholar] [CrossRef]
- Ray, J.D.; Stedman, D.H.; Wendel, G.J. Fast Chemiluminescent Method for Measurement of Ambient Ozone. Anal. Chem. 1986, 58, 598–600. [Google Scholar] [CrossRef]
- Ando, M. Recent advances in optochemical sensors for the detection of H2, O2, O3, CO, CO2 and H2O in air. TrAC Trends Anal. Chem. 2006, 25, 937–948. [Google Scholar] [CrossRef]
- Bergshoeff, G.; Lanting, R.W.; Van Ham, J.; Prop, J.M.G.; Reijnders, H.F.R. Spectrophotometric Determination of Ozone in Air with Indigo Disulphonate. Analyst 1984, 109, 1165–1169. [Google Scholar] [CrossRef]
- Alexy, M.; Voss, G.; Heinze, J. Optochemical sensor for determining ozone based on novel soluble indigo dyes immobilised in a highly permeable polymeric film. Anal. Bioanal. Chem. 2005, 382, 1628–1641. [Google Scholar] [CrossRef]
- Grosjean, D.; Hisham, M.W.M. A passive sampler for atmospheric ozone. J. Air Waste Manag. Assoc. 1992, 42, 169–173. [Google Scholar] [CrossRef]
- Maruo, Y.Y. Measurement of ambient ozone using newly developed porous glass sensor. Sens. Actuators B 2007, 126, 485–491. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Sohrabnezhad, S. Evaluation of methylene blue incorporated in zeolite for construction of an optical humidity sensor. Sens. Actuators B Chem. 2005, 105, 502–507. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Ordóñez, Y.F.; Capitán-Vallvey, L.F.; De Vargas-Sansalvador, I.M.P.; Ballesta-Claver, J. Optical humidity sensor using methylene blue immobilized on a hydrophilic polymer. Sens. Actuators B Chem. 2015, 220, 528–533. [Google Scholar] [CrossRef]
- Ishizaki, R.; Katoh, R. Fast-response humidity-sensing films based on methylene blue aggregates formed on nanoporous semiconductor films. Chem. Phys. Lett. 2016, 652, 36–39. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, K.H.; Cui, L.; Jeong, T. Degradation of methylene blue in aqueous solution by ozone-based processes. J. Ind. Eng. Chem. 2009, 15, 185–189. [Google Scholar] [CrossRef]
- Al jibouri, A.K.H.; Wu, J.; Upreti, S.R. Continuous ozonation of methylene blue in water. J. Water Process Eng. 2015, 8, 142–150. [Google Scholar] [CrossRef]
- Iwamori, S.; Nishiyama, N.; Oya, K. A colorimetric indicator for detection of hydroxyl radicals in atmosphere using a methylene blue dye based on nafion film. Polym. Degrad. Stab. 2016, 123, 131–136. [Google Scholar] [CrossRef]
- Etienne, M.; Quach, A.; Grosso, D.; Nicole, L.; Sanchez, C.; Walcarius, A. Molecular Transport into Mesostructured Silica Thin Films: Electrochemical Monitoring and Comparison between p 6 m, P6 3/mmc, and Pm 3 n Structures. Chem. Mater. 2007, 19, 844–856. [Google Scholar] [CrossRef]
- Hamdi, K.; Hébrant, M.; Martin, P.; Galland, B.; Etienne, M. Mesoporous silica nanoparticle film as sorbent for in situ and real-time monitoring of volatile BTX (benzene, toluene and xylenes). Sens. Actuators B Chem. 2016, 223, 904–913. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef]
- Mills, A.; Hawthorne, D.; Burns, L.; Hazafy, D. Novel temperature-activated humidity-sensitive optical sensor. Sens. Actuators B Chem. 2017, 240, 1009–1015. [Google Scholar] [CrossRef]
- Staehelln, J.; Hoigne, J. Decomposition of Ozone in Water in the Presence of Organic Solutes Acting as Promoters and Inhibitors of Radical Chain Reactions. Environ. Sci. Technol. 1985, 19, 1206–1213. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Wang, H.; Yan, Z. Analysis of the degradation mechanism of methylene blue by atmospheric pressure dielectric barrier discharge plasma. Chem. Eng. J. 2010, 162, 250–256. [Google Scholar] [CrossRef]
- Boissiere, C.; Grosso, D.; Lepoutre, S.; Nicole, L.; Bruneau, A.B.; Sanchez, C. Porosity and mechanical properties of mesoporous thin films assessed by environmental ellipsometric porosimetry. Langmuir 2005, 21, 12362–12371. [Google Scholar] [CrossRef]
- Izumi, K.; Utiyama, M.; Maruo, Y.Y. A porous glass-based ozone sensing chip impregnated with potassium iodide and α-cyclodextrin. Sens. Actuators B Chem. 2017, 241, 116–122. [Google Scholar] [CrossRef]
- Aleixandre, M.; Del Carmen Horrillo, M.; Gerboles, M.; Spinelle, L.; Bonavitacola, F. Comparison of Tin Dioxide Ozone Sensor Operated in On/Off Switching Temperature Cycles and at Constant Temperature. Chem. Eng. Trans. 2016, 54, 49–54. [Google Scholar]
- European Commission (EC). Recommendation from the Scientific Committee on Occupational Exposure Limits (SCOEL) for Nitrogen Dioxide. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=4&ved=2ahUKEwjH8bz2nvfjAhXbxosBHQ2cAzAQFjADegQIAxAC&url=http%3A%2F%2Fec.europa.eu%2Fsocial%2FBlobServlet%3FdocId%3D12432%26langId%3Den&usg=AOvVaw14WVsmj6Vff1fsg_aWeZv2 (accessed on 9 August 2019).
- Wu, C.H.; Morris, E.D.; Niki, H. The reaction of nitrogen dioxide with ozone. J. Phys. Chem. 1973, 77, 2507–2511. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, Z.; Sun, C.; Zhao, N.; Liu, Y.; Wu, Z. Numerical evaluation of the effectiveness of NO2 and N2O5 generation during the NO ozonation process. J. Environ. Sci. 2016, 41, 51–58. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazaly, C.; Hébrant, M.; Langlois, E.; Castel, B.; Guillemot, M.; Etienne, M. Real-Time Ozone Sensor Based on Selective Oxidation of Methylene Blue in Mesoporous Silica Films. Sensors 2019, 19, 3508. https://doi.org/10.3390/s19163508
Ghazaly C, Hébrant M, Langlois E, Castel B, Guillemot M, Etienne M. Real-Time Ozone Sensor Based on Selective Oxidation of Methylene Blue in Mesoporous Silica Films. Sensors. 2019; 19(16):3508. https://doi.org/10.3390/s19163508
Chicago/Turabian StyleGhazaly, Christelle, Marc Hébrant, Eddy Langlois, Blandine Castel, Marianne Guillemot, and Mathieu Etienne. 2019. "Real-Time Ozone Sensor Based on Selective Oxidation of Methylene Blue in Mesoporous Silica Films" Sensors 19, no. 16: 3508. https://doi.org/10.3390/s19163508
APA StyleGhazaly, C., Hébrant, M., Langlois, E., Castel, B., Guillemot, M., & Etienne, M. (2019). Real-Time Ozone Sensor Based on Selective Oxidation of Methylene Blue in Mesoporous Silica Films. Sensors, 19(16), 3508. https://doi.org/10.3390/s19163508





