Multispectral Fluorescence Imaging Technique for On-Line Inspection of Fecal Residues on Poultry Carcasses
Abstract
1. Introduction
2. Materials and Methods
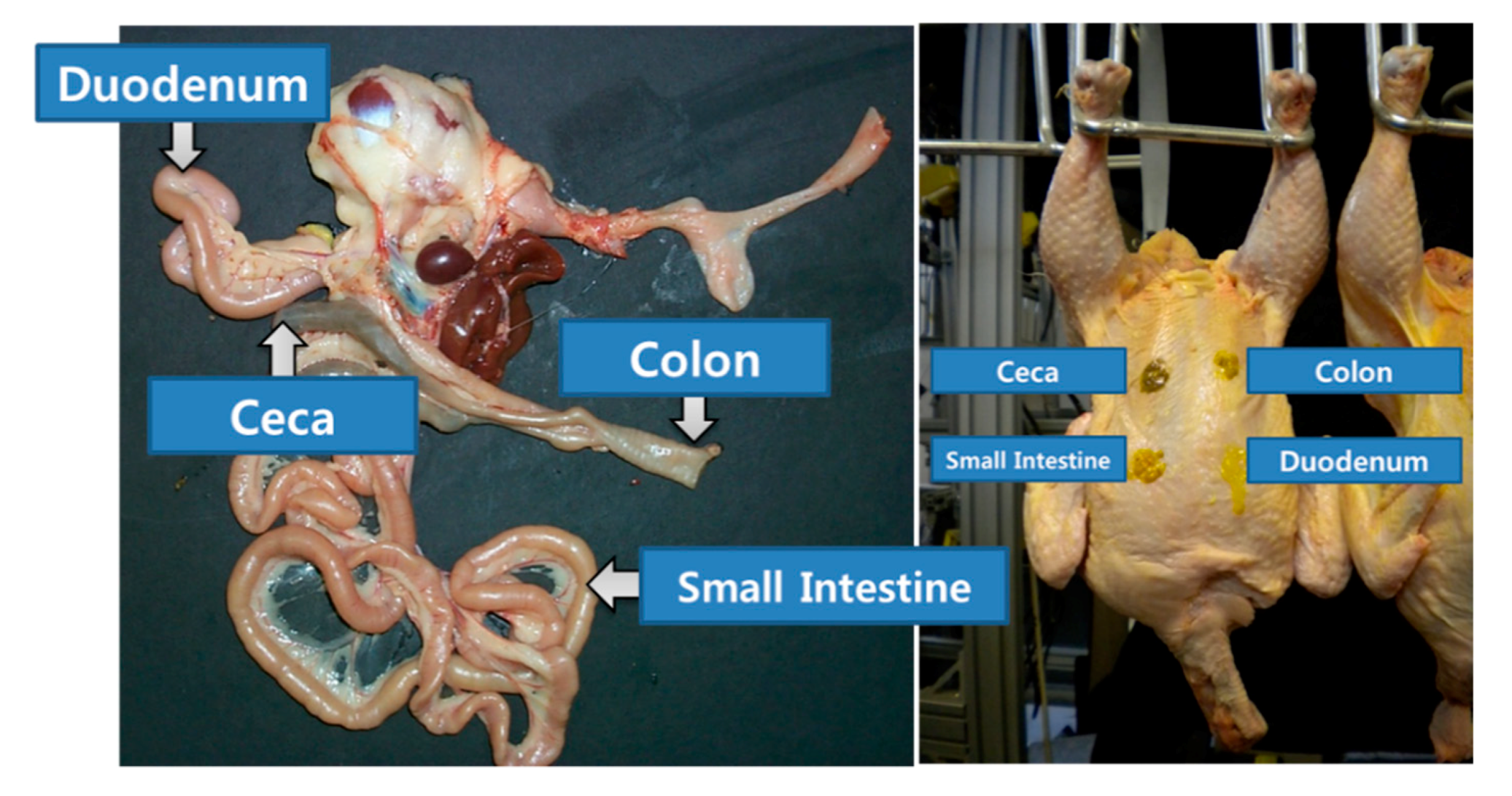
2.1. Fecal Matters from Chicken Organs
2.2. Data Acquisition and Image Correction
2.3. Analysis of Fluorescence Spectra
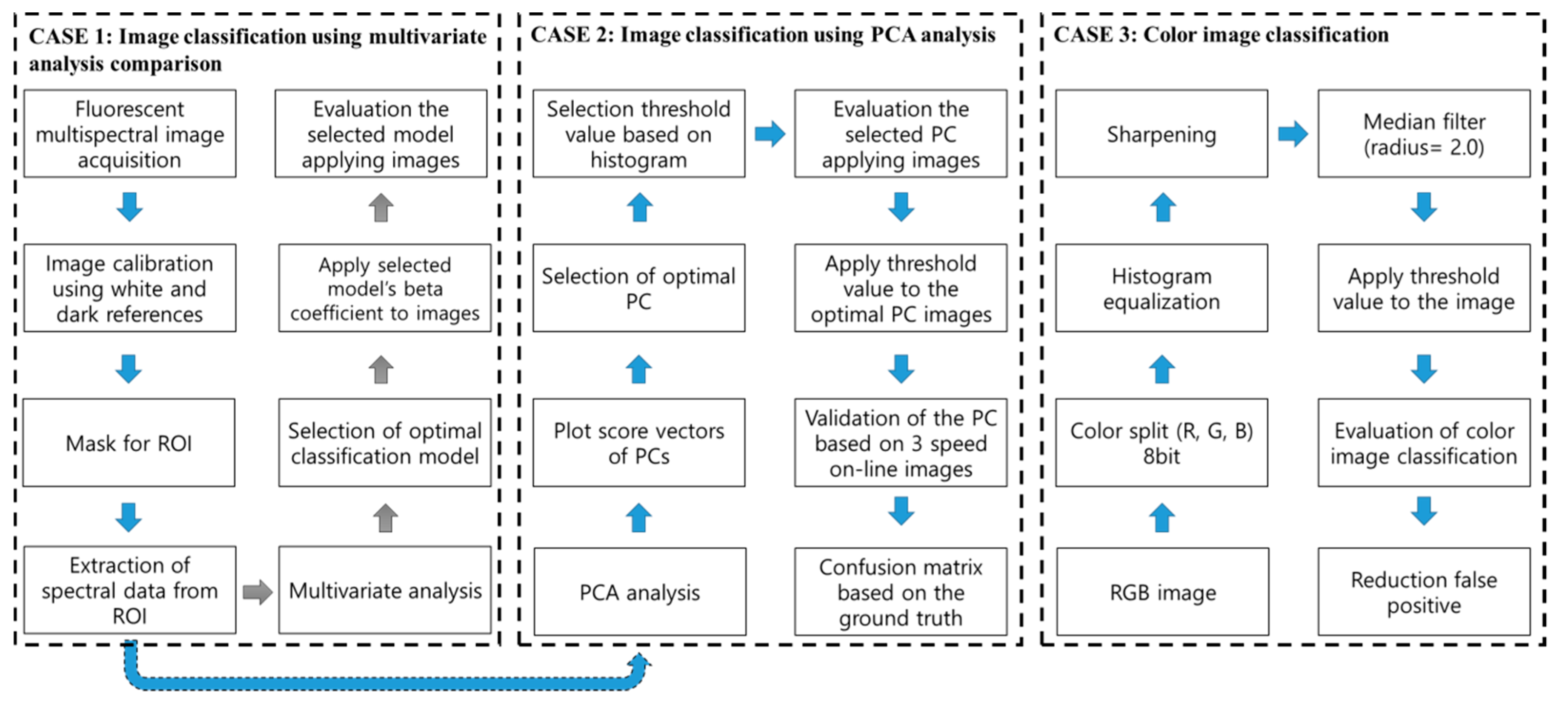
2.4. Analysis of Multispectral Fluorescence Image
3. Results and Discussion
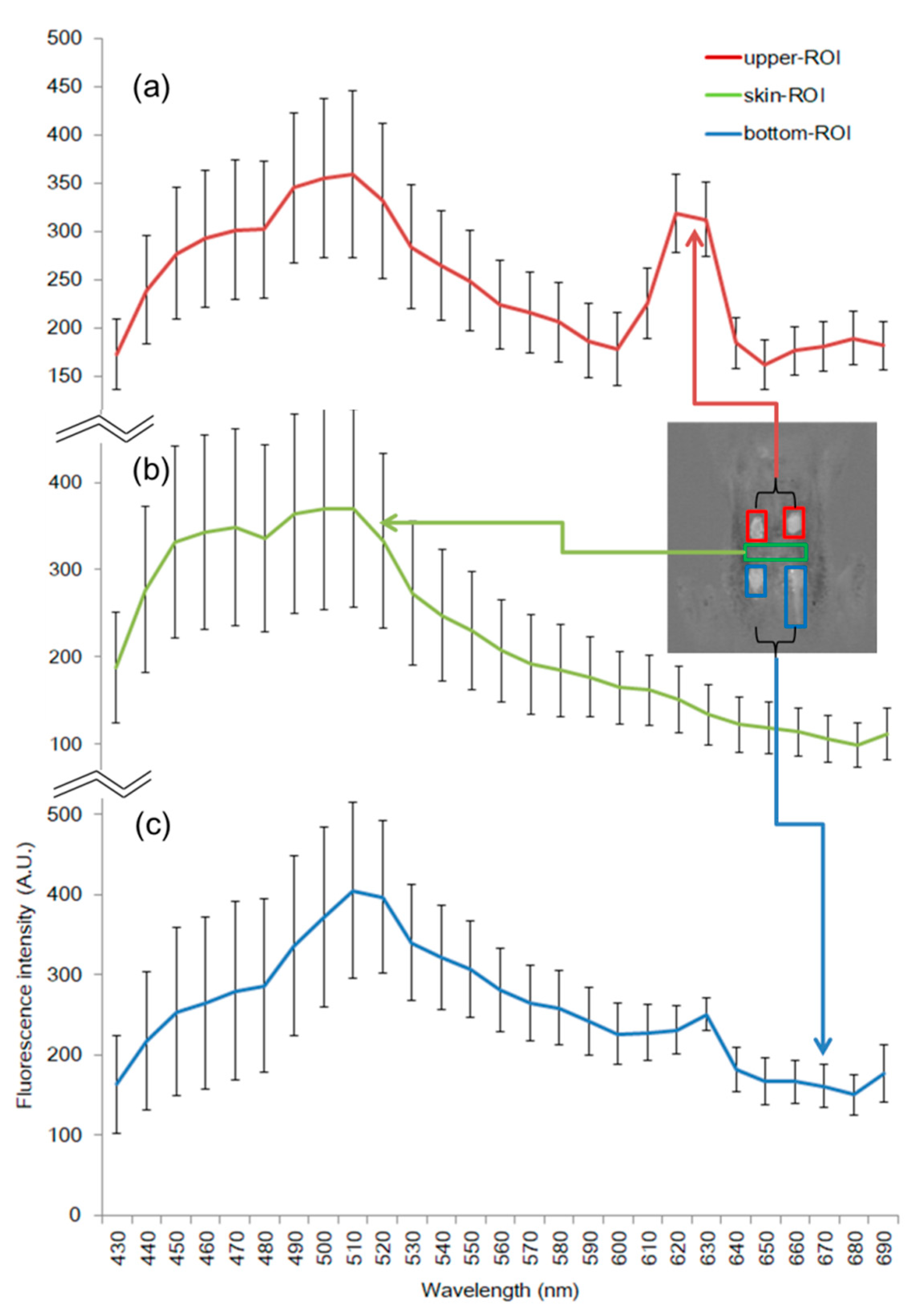
3.1. Spectral Characteristics
3.2. Spectral Data Analysis
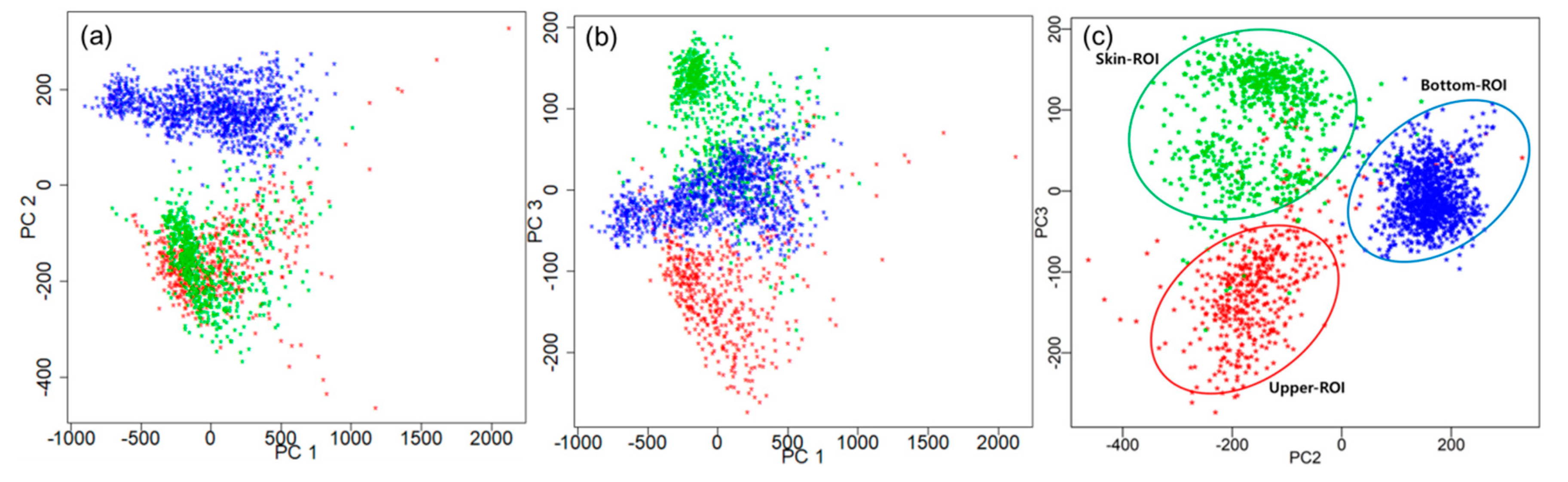
3.2.1. Spectral Analysis Using PCA
3.2.2. Spectral Classification Using PLS-DA
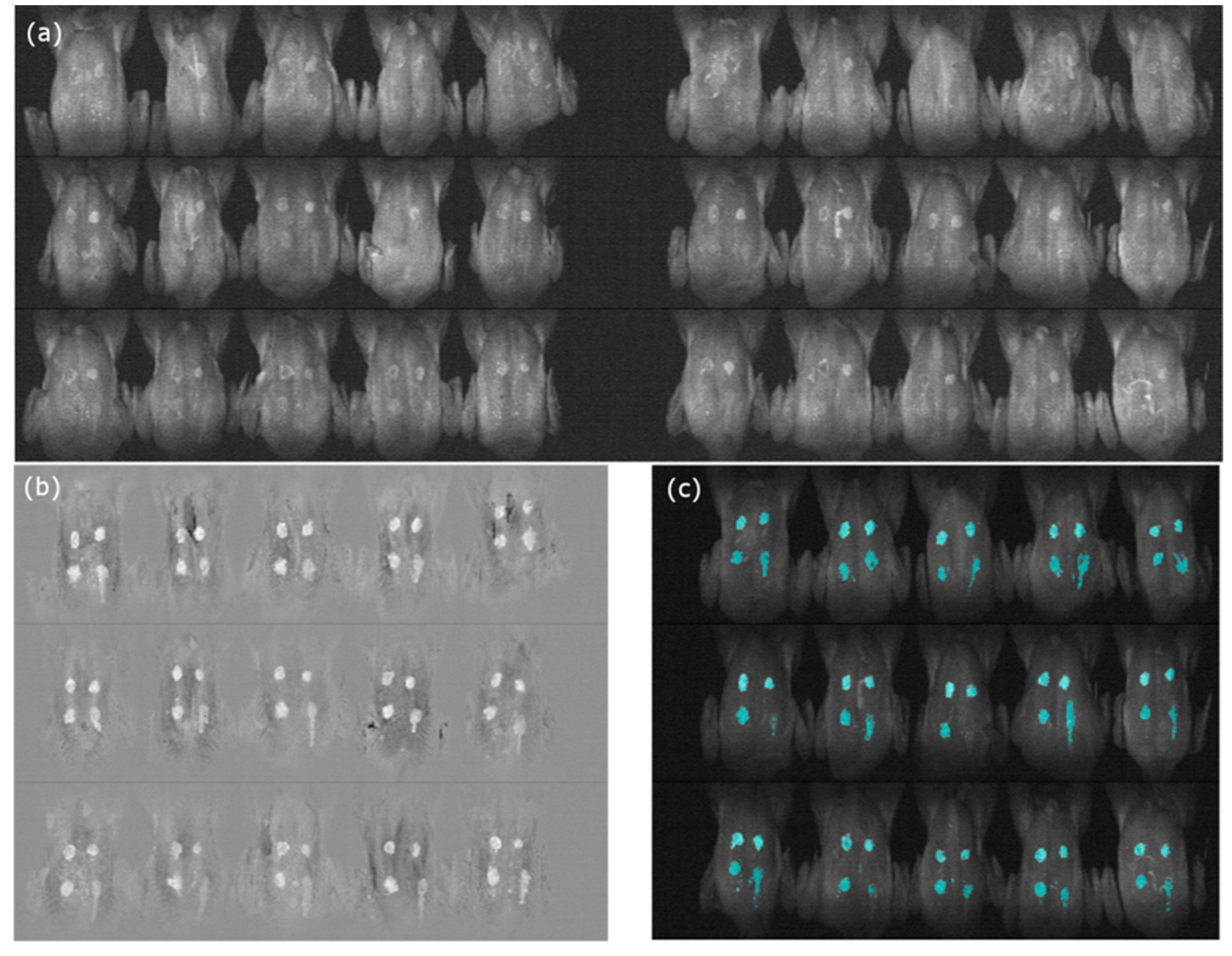
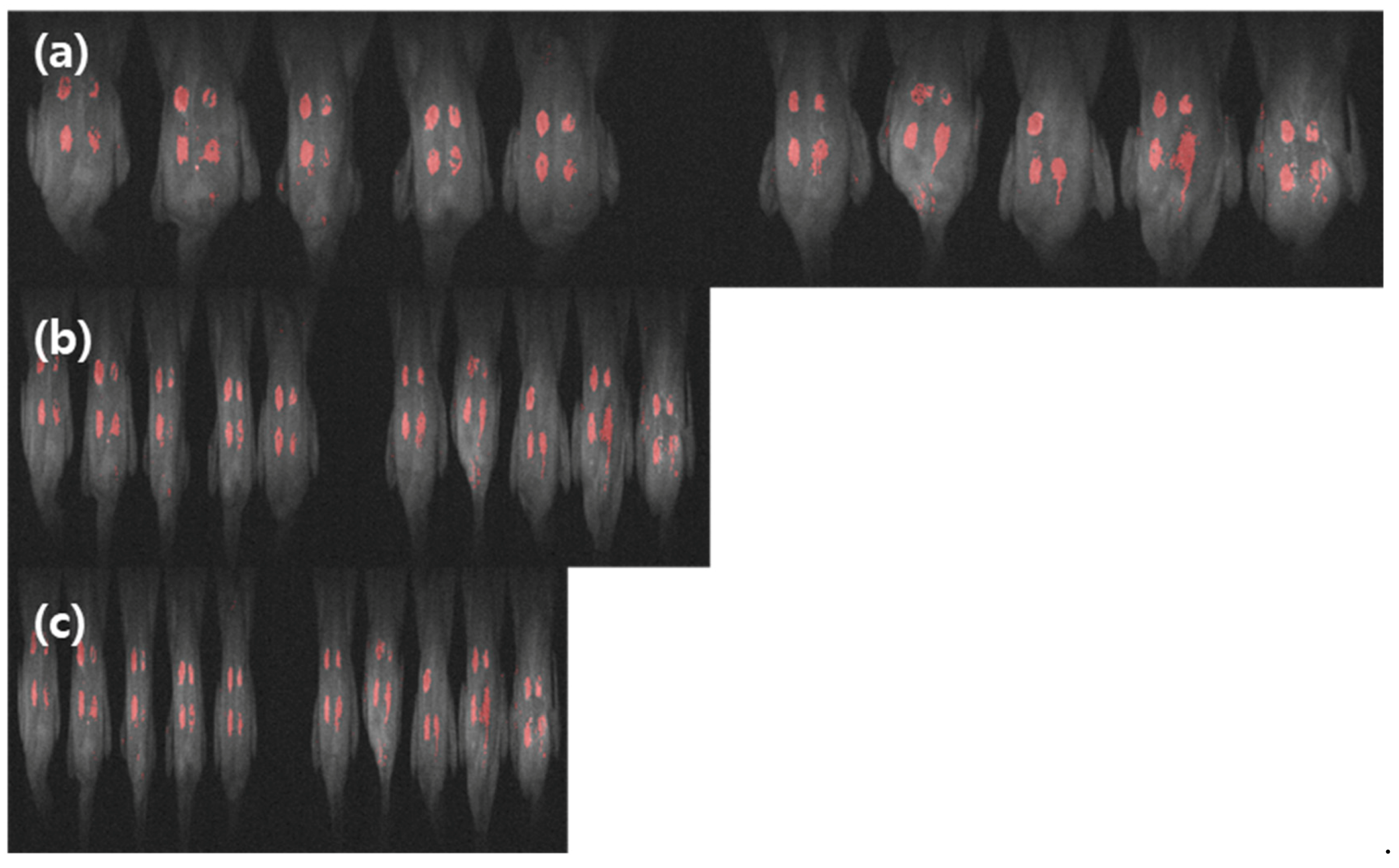
3.3. Image Data Analysis
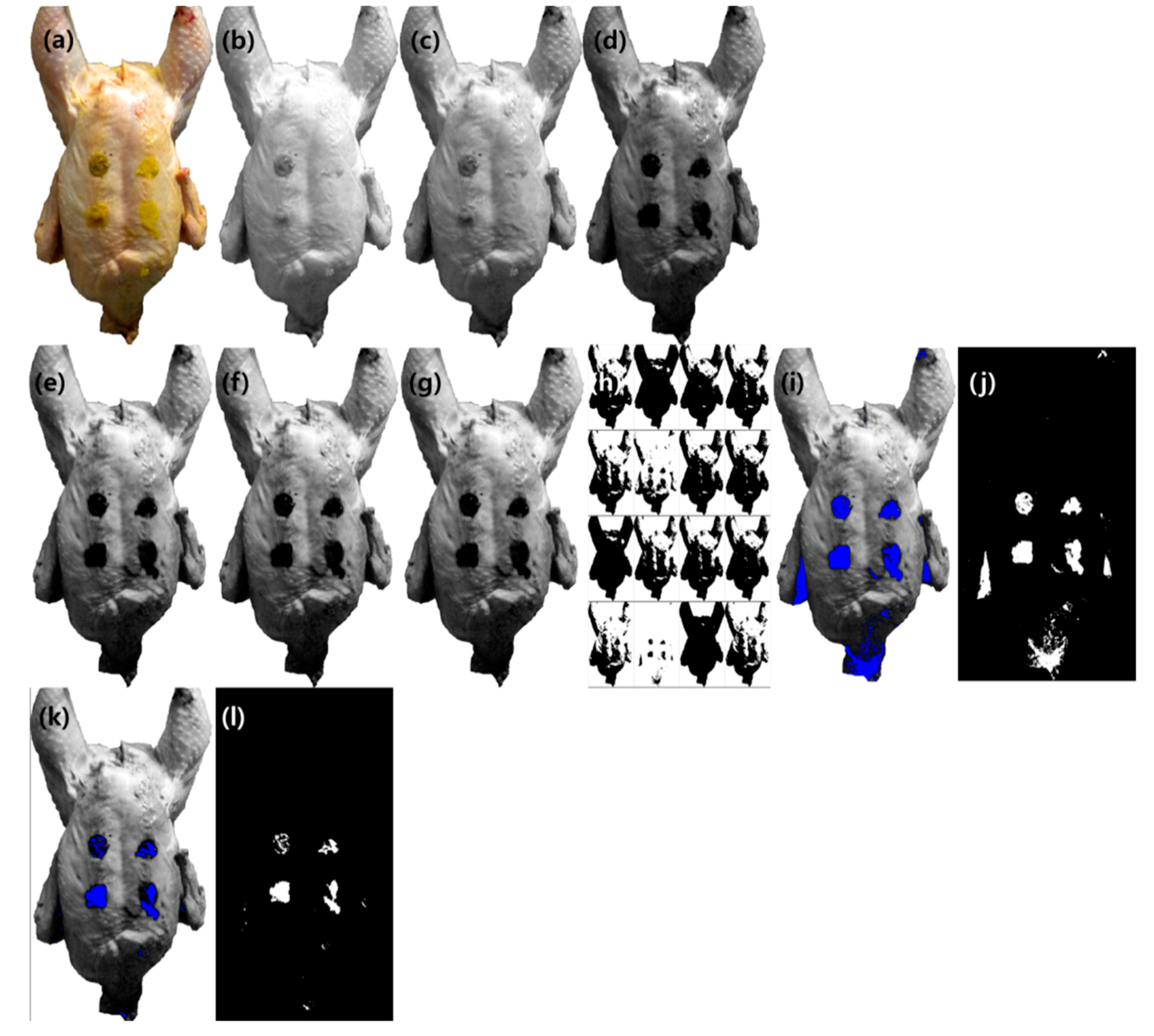
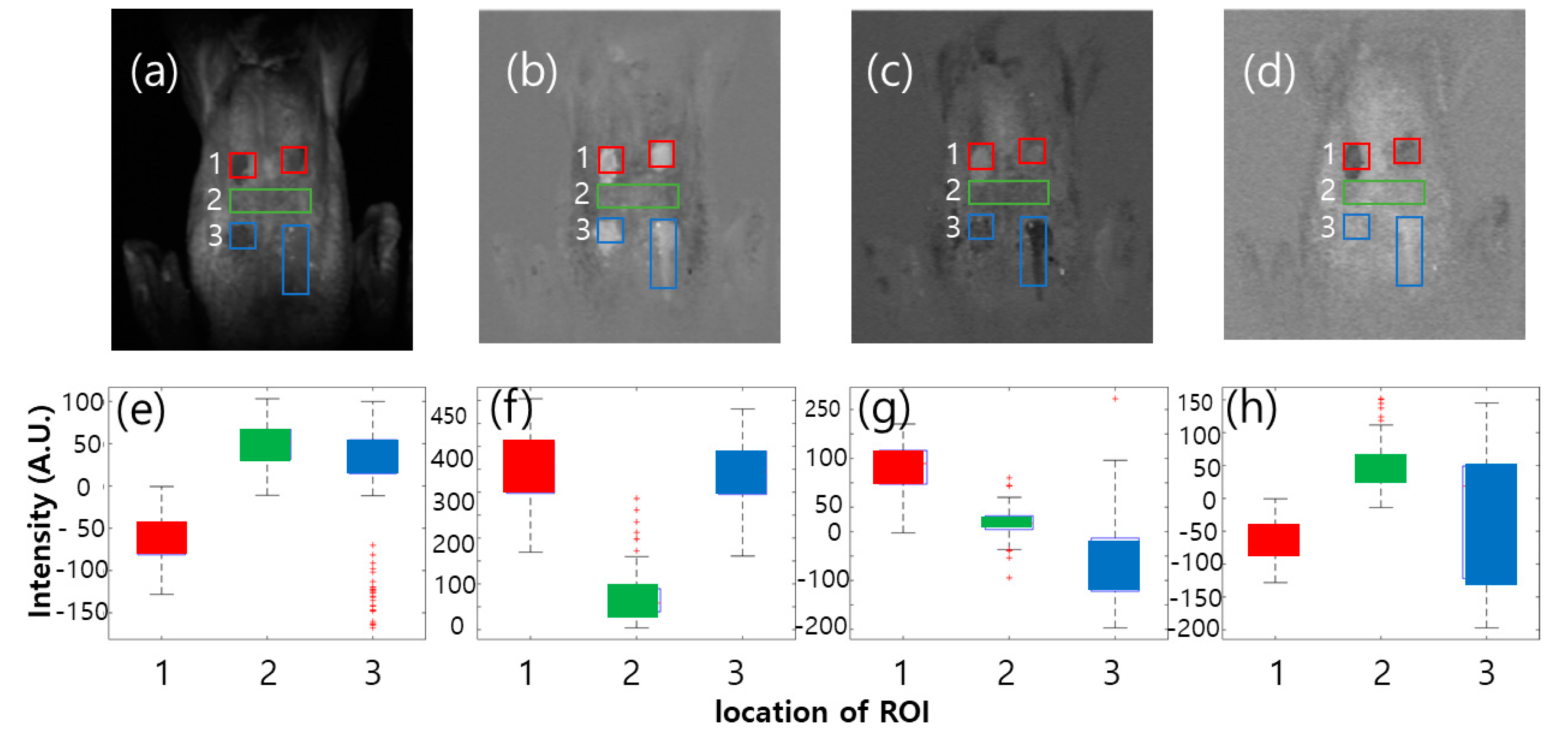
3.3.1. Color Image Classification
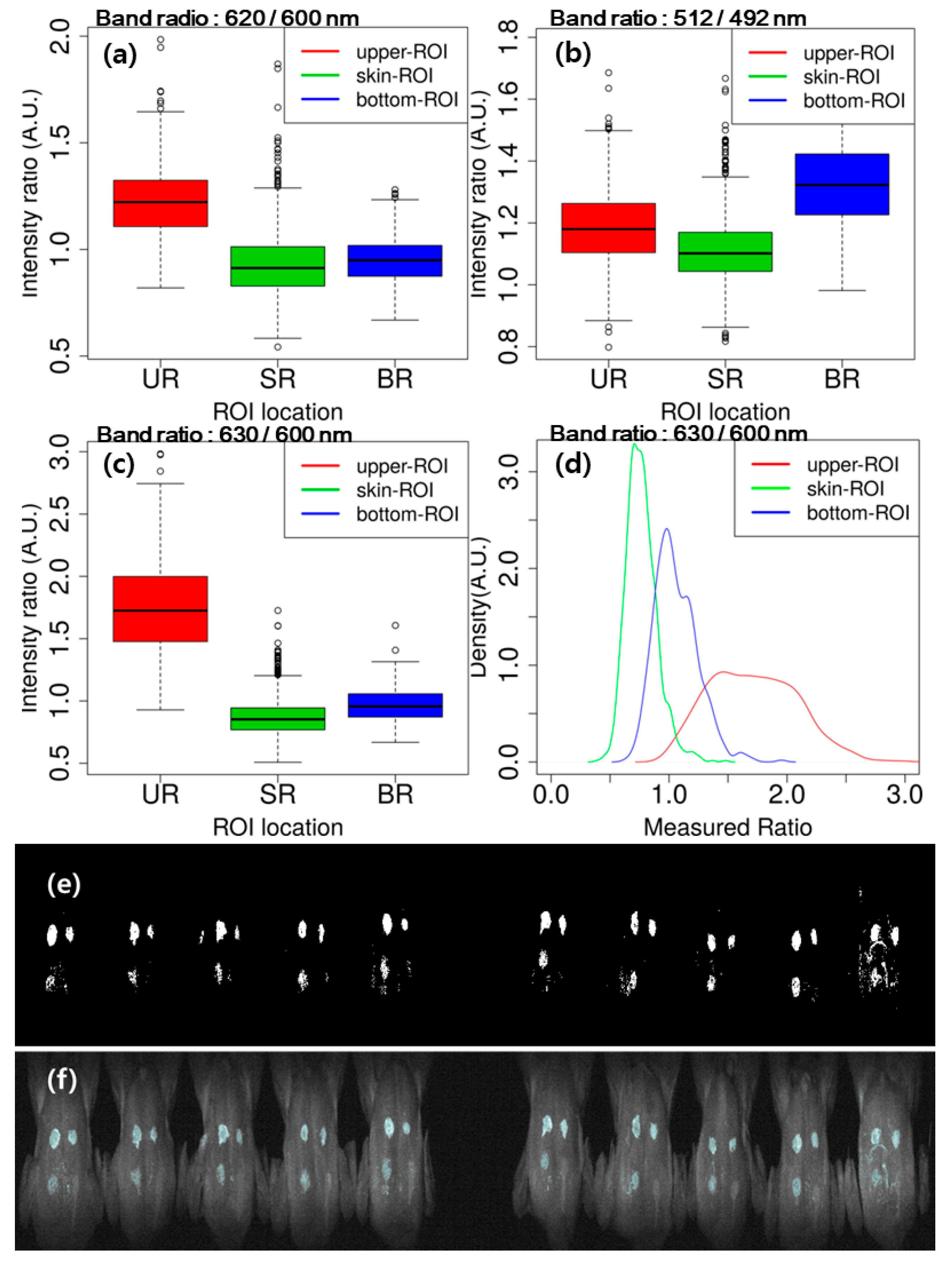
3.3.2. Image Classification Using Band Ratio
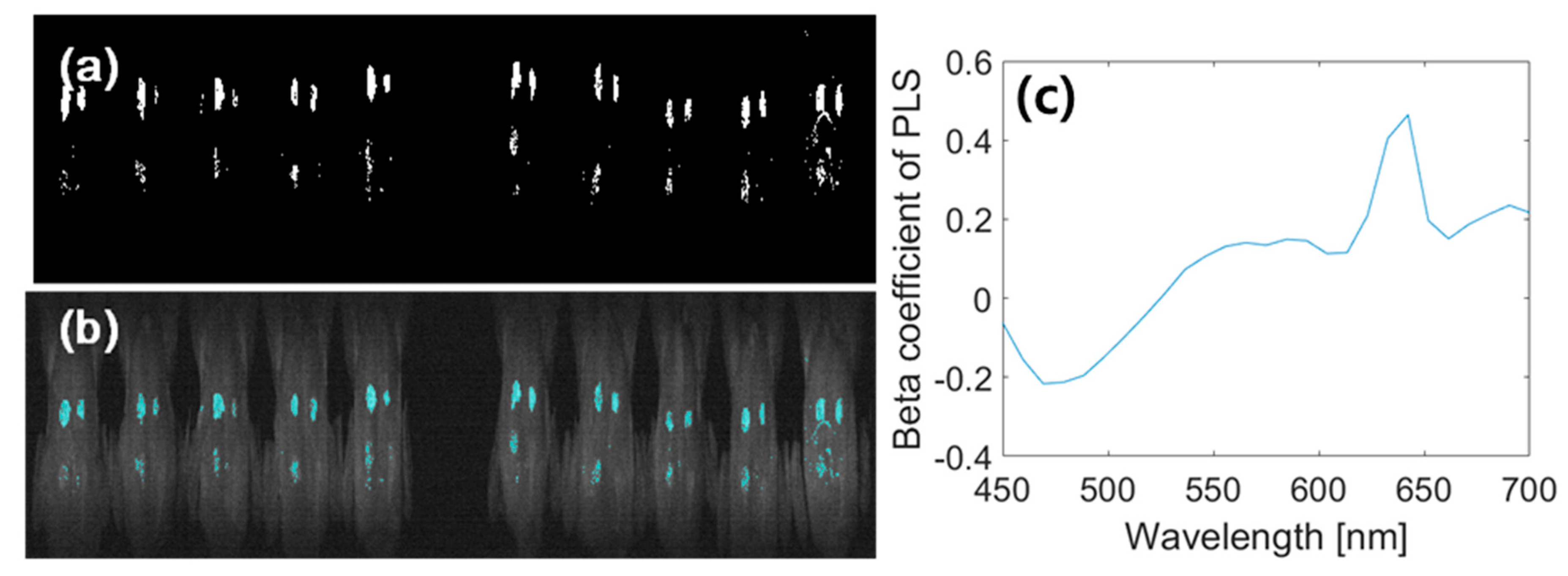
3.3.3. Image Classification Using PLS-DA
3.3.4. Image Classification Using PCA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vithu, P.; Moses, J.A. Machine vision system for food grain quality evaluation: A review. Trends Food Sci. Tech. 2016, 56, 13–20. [Google Scholar] [CrossRef]
- Mehta, S.S.; Burks, T.F. Vision-based control of robotic manipulator for citrus harvesting. Comput. Electron. Agr. 2014, 102, 146–158. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Leemans, D.; Theobald, V.J.; Fleming, H.R.; Gay, A.P. The development of chlorophyll-based markers in poultry diets to aid detection of fluorescent fecal contamination. Poult. Sci. 2013, 92, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Lefcourt, A.M.; Kim, M.S.; Chen, Y.-R. A transportable fluorescence imagining system for detecting fecal contaminants. Comput. Electron. Agr. 2005, 48, 63–74. [Google Scholar] [CrossRef]
- Brosnan, T.; Sun, D.-W. Improving quality inspection of food products by computer vision—A review. J. Food Eng. 2004, 611, 3–16. [Google Scholar] [CrossRef]
- Kim, M.S.; Lefcourt, A.M.; Chen, Y.R.; Tao, Y. Optimal fluorescence excitation and emission bands for detection of fecal contamination. J. Food Prot. 2003, 66, 1198–1207. [Google Scholar] [CrossRef]
- Mo, C.; Lim, J.; Kwon, S.W.; Lim, D.K.; Kim, M.S.; Kim, G.; Kang, J.; Kwon, K.D.; Cho, B.K. Hyperspectral Imaging and Partial Least Square Discriminant Analysis for Geographical Origin Discrimination of White Rice. J. Biosyst. Eng. 2017, 42, 293–300. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Leitenberger, M.; Longin, C.F.H.; Würschum, T.; Carle, R.; Schweiggert, R.M. Near-infrared reflectance spectroscopy for the rapid discrimination of kernels and flours of different wheat species. J. Food Compos. Anal. 2016, 51, 30–36. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Z.; Haughey, S.A.; Galvin-King, P.; Han, L.; Elliott, C.T. Classification the geographical origin of corn distillers dried grains with solubles by near infrared reflectance spectroscopy combined with chemometrics: A feasibility study. Food Chem. 2015, 189, 13–18. [Google Scholar] [CrossRef]
- Giunchi, A.; Berardinelli, A.; Ragni, L.; Fabbri, A.; Silaghi, F.A. Non-destructive freshness assessment of shell eggs using FT-NIR spectroscopy. J. Food Eng. 2008, 89, 142–148. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, A.Y.; Han, Y.H.; Park, J.M.; So, J.D.; Kim, G.S. Rancidity prediction of soybean oil by using near infrared spectroscopy techniques. J. Biosyst. Eng. 2018, 43, 219–228. [Google Scholar] [CrossRef]
- Vermeulen, P.; Fernández Pierna, J.A.; Abbas, O.; Dardenne, P.; Baeten, V. Origin identification of dried distillers grains with solubles using attenuated total reflection Fourier transform mid-infrared spectroscopy after in situ oil extraction. Food Chem. 2015, 189, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, S.H.; Choung, M.G. Nondestructive Determination of Lignans and Lignan Glycosides in Sesame Seeds by Near Infrared Reflectance Spectroscopy. J. Agric. Food Chem. 2006, 54, 4544–4550. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.R.; Yasmin, J.; Lee, W.H.; Mo, C.; Cho, B.K. Imaging Technologies for Nondestructive Measurement of Internal Properties of Agricultural Products: A Review. J. Biosyst. Eng. 2017, 42, 199–216. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.S.; Song, Y.; Oh, C.S.; Lim, H.S.; Lee, W.H.; Kang, J.S.; Cho, B.-K. Non-destructive evaluation of bacteria-infected watermelon seeds using visible/near-infrared multispectral imaging. J. Sci. Food Agric. 2017, 97, 1084–1092. [Google Scholar] [CrossRef]
- Khatiwada, B.P.; Subedi, P.P.; Hayes, C.; Carlos, L.C.C.; Walsh, K.B. Assessment of internal flesh browning in intact apple using visible-short wave near infrared spectroscopy. Postharvest Biol. Tec. 2016, 120, 103–111. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, K.; Noh, S.H. Study for nondestructive detection algorithm development of the internal browning and watercore of Fuji apple using VIS/NIR transmittance spectroscopy. Food Eng. Prog. 2007, 11, 38–44. [Google Scholar]
- Wang, J.; Nakano, K.; Ohashi, S. Nondestructive detection of internal insect infestation in jujubes using visible and near-infrared spectroscopy. Postharvest Biol. Tec. 2011, 59, 272–279. [Google Scholar] [CrossRef]
- Bowen, J.H.; Watkins, C.B. Fruit maturity, carbohydrate and mineral content relationships with watercore in ‘Fuji’ apples. Postharvest Biol. Tec. 1997, 11, 31–38. [Google Scholar] [CrossRef]
- Everard, C.D.; Kim, M.S.; Cho, H.; O’Donnell, C.P. Multispectral fluorescence imaging using violet LEDs as excitation sources for fecal matter contaminate identification on spinach leaves. J. Food Meas. Charact. 2016, 10, 56–63. [Google Scholar] [CrossRef]
- Everard, C.D.; Kim, M.S.; Lee, H. A comparison of multispectral reflectance and fluorescence imaging techniques for detection of contaminants on spinach leaves. J. Food Eng. 2014, 143, 139–145. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Non-invasive analytical technology for the detection of contamination, adulteration, and authenticity of meat, poultry, and fish: A review. Anal. Chim. Acta. 2015, 853, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Barbin, D.F.; ElMasry, G.; Sun, D.-W.; Allen, P. Non-destructive determination of chemical composition in intact and minced pork using near-infrared multispectral imaging. Food Chem. 2013, 138, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Kim, M.S.; Chao, K.; Lawrence, K.; Park, B.; Kim, K. Detection of fecal residues on poultry carcasses by laser-induced fluorescence imaging. J. Food Sci. 2009, 74, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Kim, M.S.; Chen, Y. Hyperspectral imaging technique for detection of poultry fecal residues on food processing equipments. In Proceedings of the SPIE Optical Sensors and Sensing Systems for Natural Resources and Food Safety and Quality, Boston, MA, USA, 8 November 2005; p. 59960L. [Google Scholar]
- Park, B.; Lawrence, K.C.; Windham, W.R.; Smith, D.P. Multispectral imaging system for fecal and ingesta detection on poultry carcasses. J. Food Process Eng. 2004, 27, 311–327. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S.; Kang, S.; Cho, B.K.; Jun, W. Detection of organic residues on poultry processing equipment surfaces by LED-induced fluorescence imaging. Applied engineering in agriculture 2011, 27, 153–161. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Stommel, J.R.; Kim, M.S.; Vinyard, B.T.; Esquerre, C. Hyperspectral fluorescence imaging for shelf life evaluation of fresh-cut Bell and Jalapeno Pepper. Sci. Hortic. 2019, 246, 749–758. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.S.; Lee, W.; Cho, B.-K. Determination of the total volatile basic nitrogen (TVB-N) content in pork meat using hyperspectral fluorescence imaging. Sens. Actuators B: Chem. 2018, 259, 532–539. [Google Scholar] [CrossRef]
- Ashby, K.D.; Wen, J.; Chowdhury, P.; Casey, T.A.; Rasmussen, M.A.; Petrich, J.W. Fluorescence of dietary porphyrins as a basis for real-time detection of fecal contamination on meat. J. Agric. Food Chem. 2003, 51, 3502–3507. [Google Scholar] [CrossRef]
- Naganathan, G.K.; Grimes, L.M.; Subbiah, J.; Calkins, C.R.; Samal, A.; Meyer, G.E. Visible/near-infrared multispectral imaging for beef tenderness prediction. Comput. Electron. Agric. 2008, 64, 225–233. [Google Scholar] [CrossRef]
- Wald, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemome. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Worley, B.; Halouska, S.; Powers, R. Utilities for quantifying separation in PCA/PLS-DA scores plots. Anal. Biochem. 2013, 433, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Kettaneh, N.; Berglund, A.; Wold, S. PCA and PLS with very large data sets. Comput. Stat. Data Anal. 2005, 48, 69–85. [Google Scholar] [CrossRef]
- Luo, J.; Ying, K.; Bai, J. Savitzky–Golay smoothing and differentiation filter for even number data. Signal Process. 2005, 85, 1429–1434. [Google Scholar] [CrossRef]
- Viera, A.J.; Garret, J.M. Understanding Interobserver Agreement: The Kappa Statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji - an Open Source platform for biological image analysis. Nat. Methods 2012, 9, 1–15. [Google Scholar] [CrossRef]
- Schenkman, K.A.; Marble, D.R.; Burns, D.H.; Feigl, E.O. Myoglobin oxygen dissociation by multiwavelength spectroscopy. J. Appl. Physiol. 1997, 82, 86–92. [Google Scholar] [CrossRef]
- Courrol, L.C.; Samad, R.E. Determination of chicken meat contamination by porphyrin fluorescence. J. Lumin. 2018, 199, 67–70. [Google Scholar] [CrossRef]
- Ishihara, R.; Katayama, Y.; Watanabe, T.; Yoshino, A.; Fukushima, T.; Sakatani, K. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol. Med.-Chir. 2007, 47, 53–57. [Google Scholar] [CrossRef]
- Cho, B.; Kim, M.S. Study on fluorescence excitation and emission bands for detection of fecal residue on poultry carcasses. J. Biosyst. Eng. 2008, 33, 136–141. [Google Scholar]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis – a marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Helland, I.S.; Naes, T.; Isaksson, T. Related versions of the multiplicative scatter correction method for preprocessing spectroscopic data. Chemome. Intell. Lab. Syst. 1995, 29, 233–241. [Google Scholar] [CrossRef]
- Duckworth, J. Mathematical data preprocessing. In Near-Infrared Spectroscopy in Agriculture; Roberts, C.A., Workman, J., Jr., Reeves, J.B., III, Eds.; American Society of Agronomy, Inc.: Madison, WI, USA; Crop Science Society of America, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 2004; pp. 115–132. ISBN 978-0-89118-236-8. [Google Scholar]
- Auto Threshold. A plugin for ImageJ Home Page. Available online: http://imagej.net/Auto_Threshold (accessed on 3 June 2019).
- Shanbhag, A.G. Utilization of information measure as a means of image thresholding. CVGIP: Graph Models Image Process. 1994, 56, 414–419. [Google Scholar] [CrossRef]
- Rendall, R.; Castillo, I.; Lu, B.; Colegrove, B.; Broadway, M.; Chiang, L.H.; Reis, M.S. Image-based manufacturing analytics: Improving the accuracy of an industrial pellet classification system using deep neural networks. Chemomet. Intellig. Lab. Sys. 2018, 180, 26–35. [Google Scholar] [CrossRef]
- Huang, L.K.; Wang, M.J. Image thresholding by minimizing the measure of fuzziness. Pattern Recognit. 1995, 28, 41–51. [Google Scholar] [CrossRef]


| Pre-Processing | Bottom-ROI | Upper-ROI | Skin-ROI | Accuracy | Kappa | |
|---|---|---|---|---|---|---|
| PLS-DA | X* | 92.4% | 97.8% | 99.8% | 97.6% | 0.96 |
| MSC | 81.0% | 86.7% | 99.7% | 90.1% | 0.84 | |
| D1 | 80.5% | 96.8% | 96.0% | 92.3% | 0.88 | |
| D2 | 79.7% | 98.2% | 96.3% | 92.8% | 0.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.; Lee, H.; Mo, C.; Kim, M.S.; Baek, I.; Lee, J.; Cho, B.-K. Multispectral Fluorescence Imaging Technique for On-Line Inspection of Fecal Residues on Poultry Carcasses. Sensors 2019, 19, 3483. https://doi.org/10.3390/s19163483
Seo Y, Lee H, Mo C, Kim MS, Baek I, Lee J, Cho B-K. Multispectral Fluorescence Imaging Technique for On-Line Inspection of Fecal Residues on Poultry Carcasses. Sensors. 2019; 19(16):3483. https://doi.org/10.3390/s19163483
Chicago/Turabian StyleSeo, Youngwook, Hoonsoo Lee, Changyeun Mo, Moon S. Kim, Insuck Baek, Jayoung Lee, and Byoung-Kwan Cho. 2019. "Multispectral Fluorescence Imaging Technique for On-Line Inspection of Fecal Residues on Poultry Carcasses" Sensors 19, no. 16: 3483. https://doi.org/10.3390/s19163483
APA StyleSeo, Y., Lee, H., Mo, C., Kim, M. S., Baek, I., Lee, J., & Cho, B.-K. (2019). Multispectral Fluorescence Imaging Technique for On-Line Inspection of Fecal Residues on Poultry Carcasses. Sensors, 19(16), 3483. https://doi.org/10.3390/s19163483






