3D-Printed Multilayer Sensor Structure for Electrical Capacitance Tomography
Abstract
:1. Introduction
2. Theoretical Fundamentals of 3D ECT Sensor Modeling
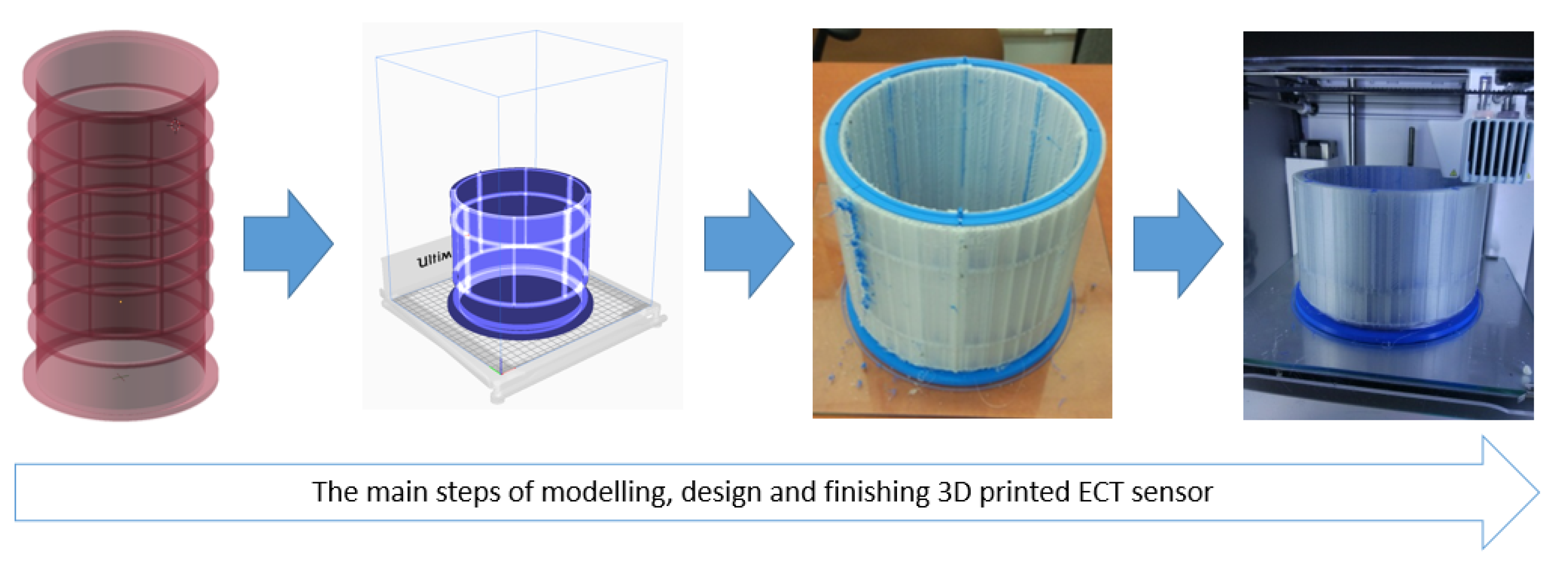
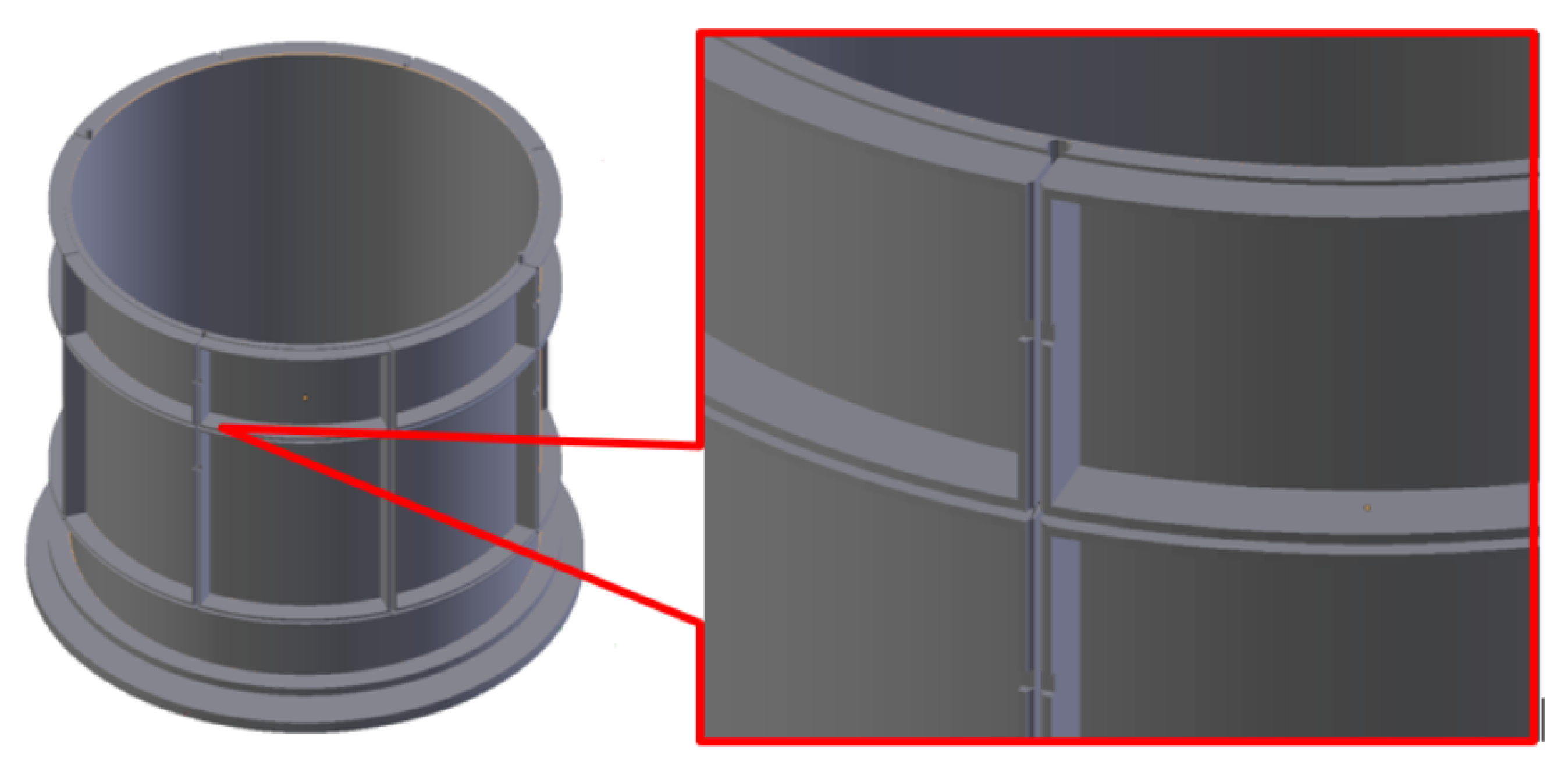
3. 3D Modeling & Printing of ECT Capacitance Sensors
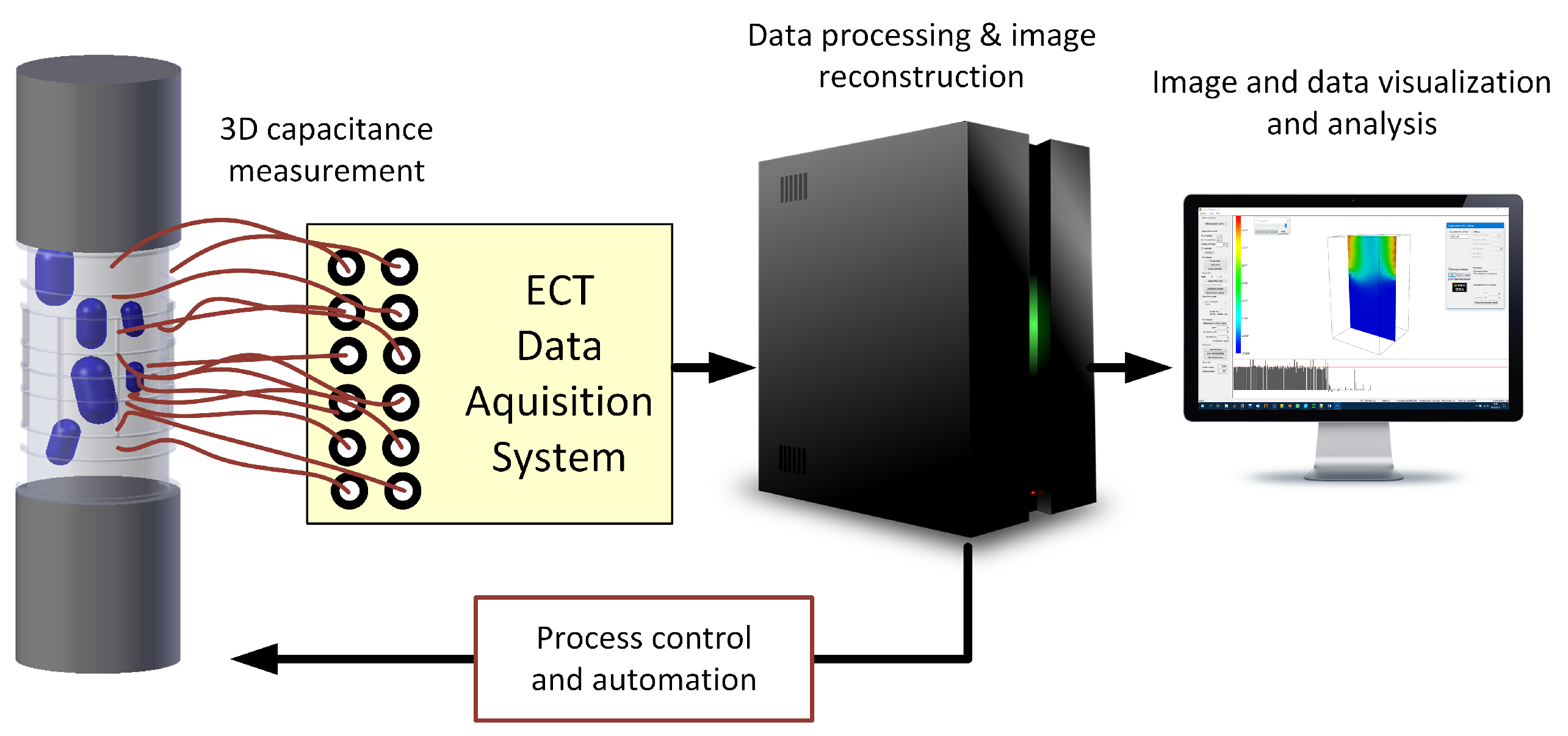
4. Experimental Setup
5. Results and Discussion
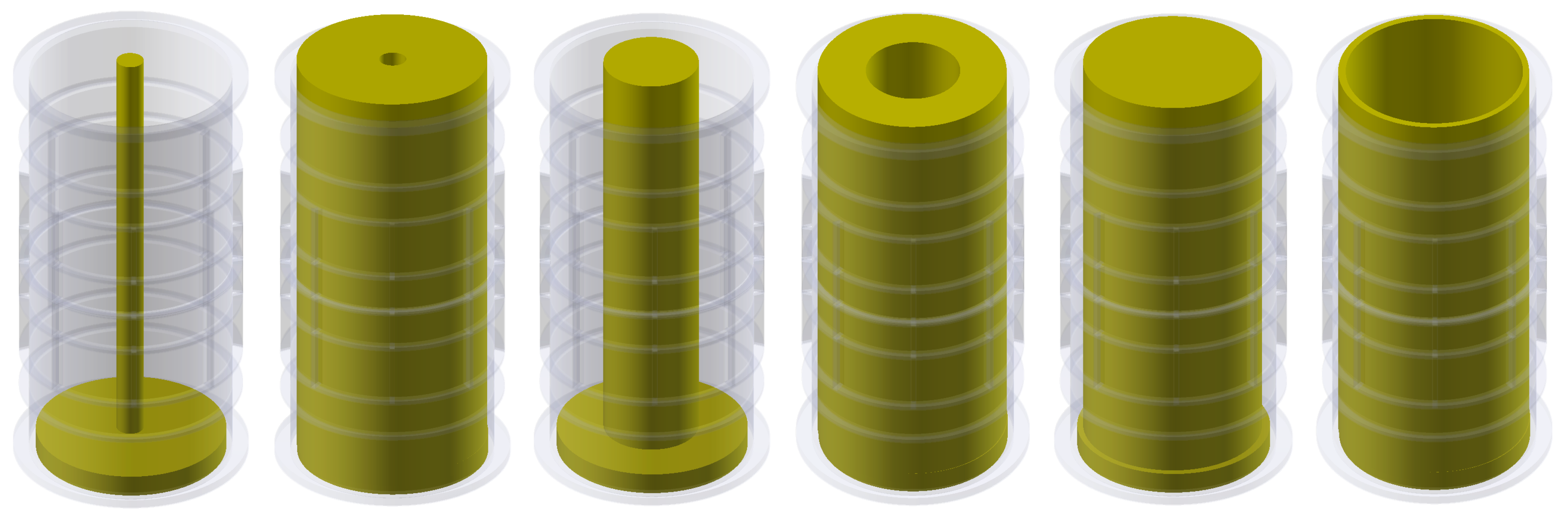
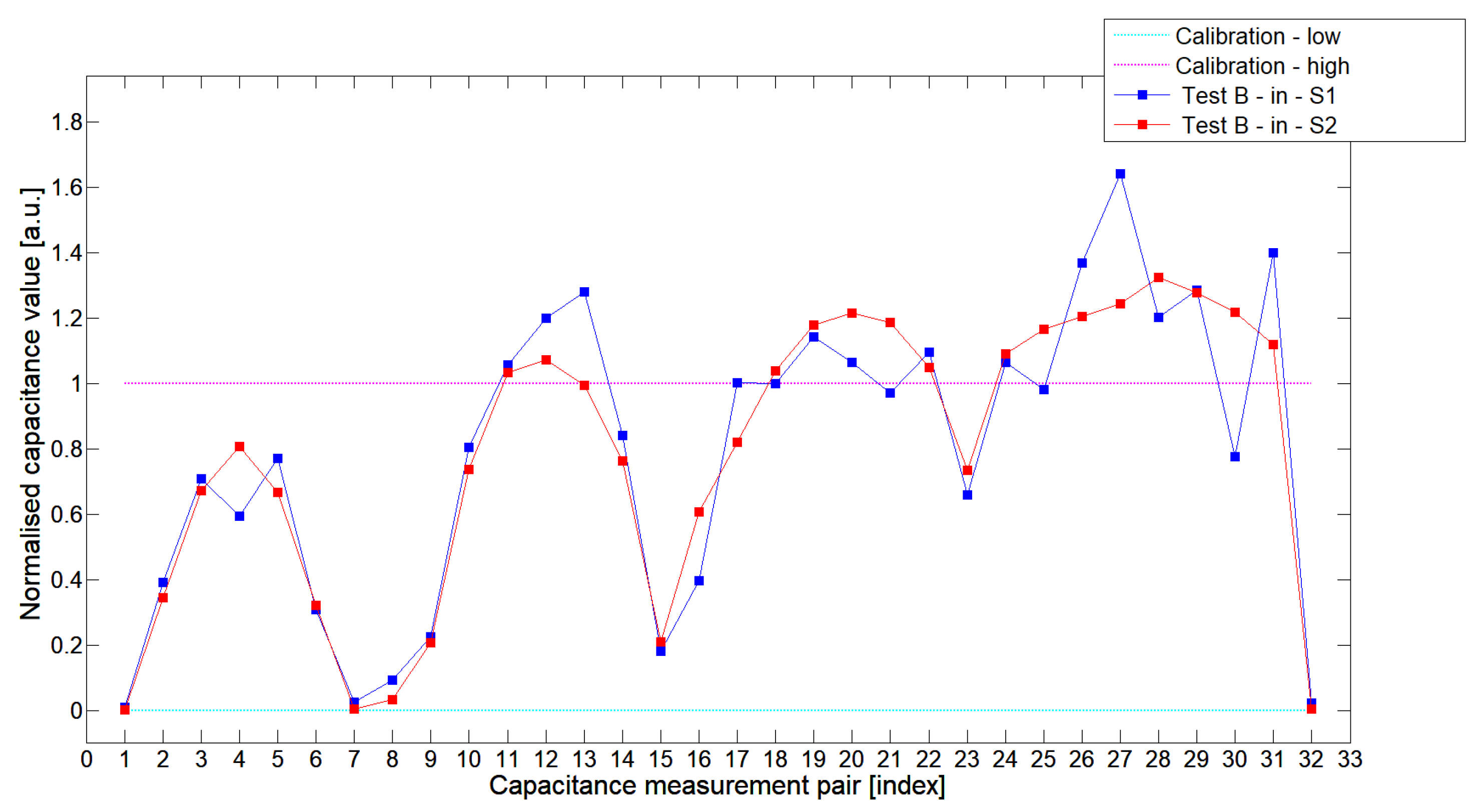
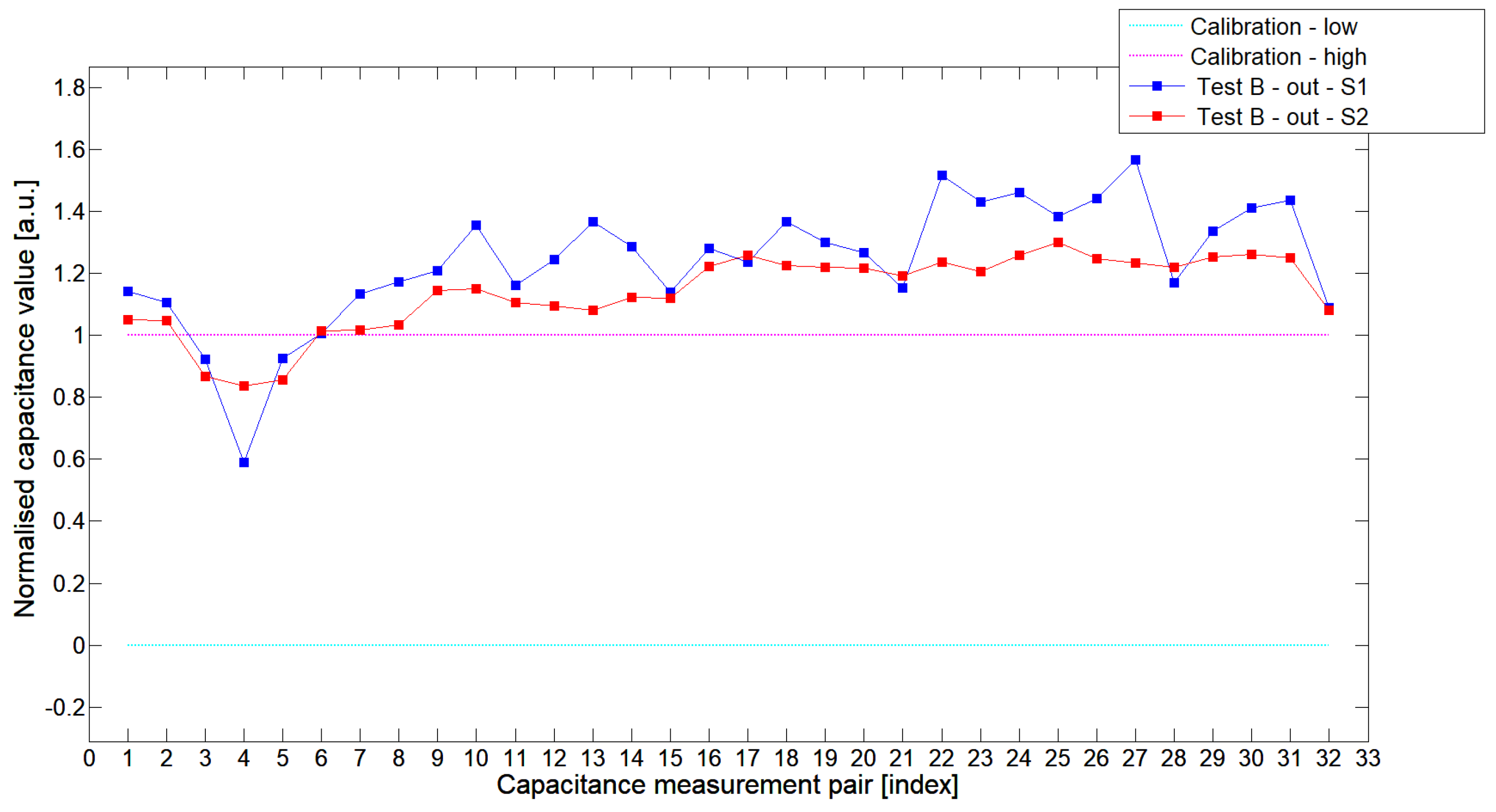
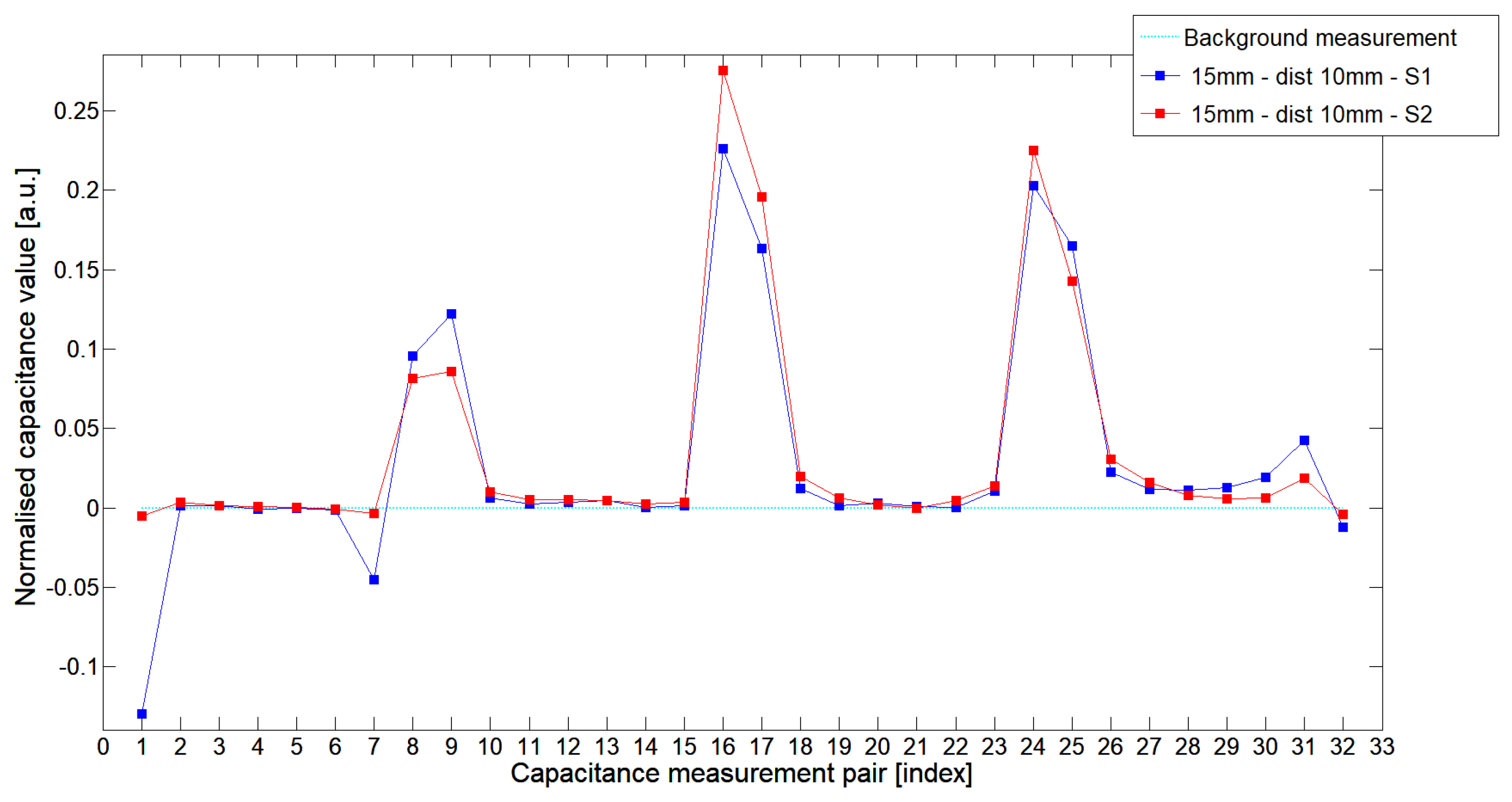
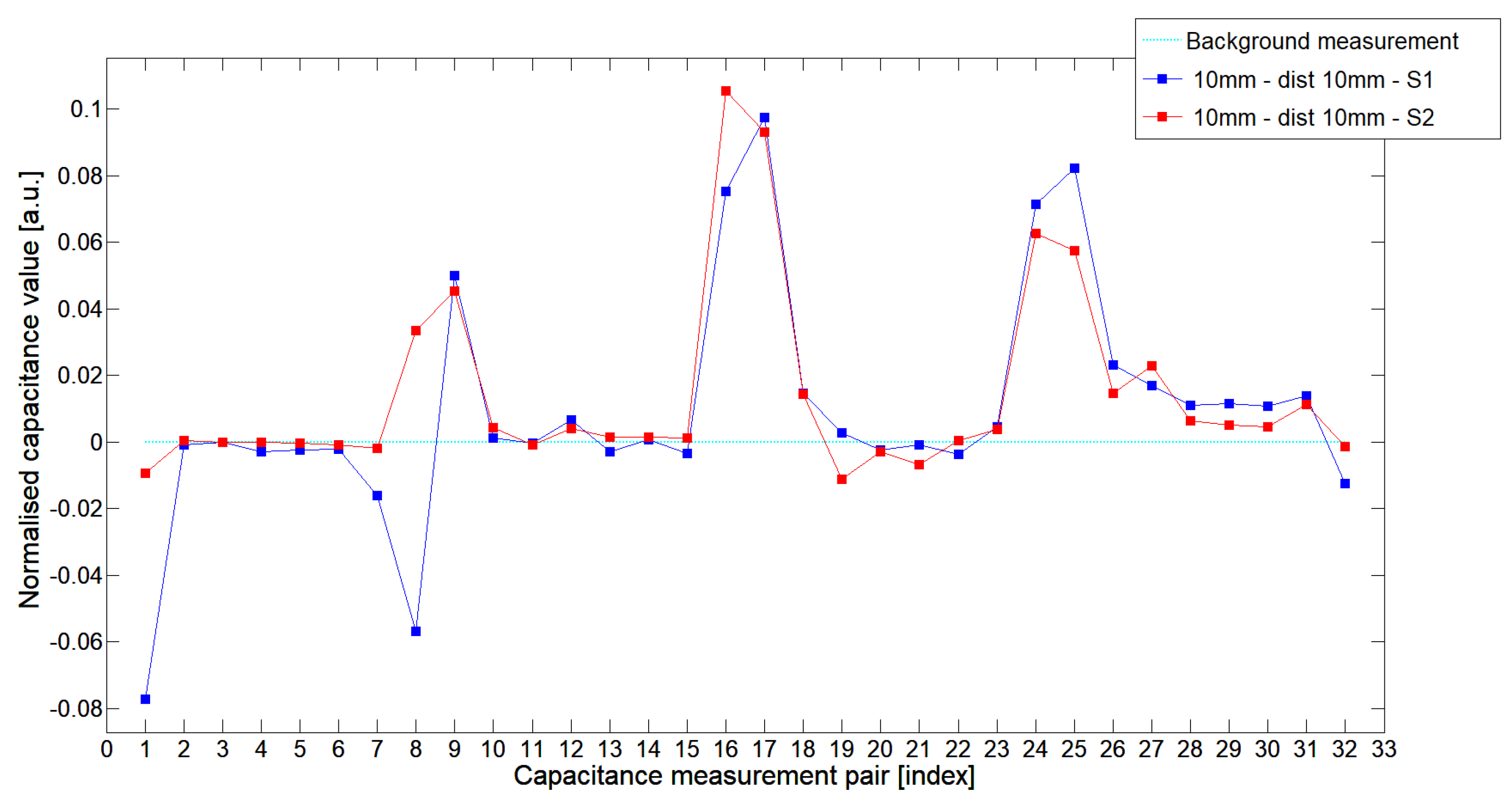
5.1. Low-Contrast Objects Investigation
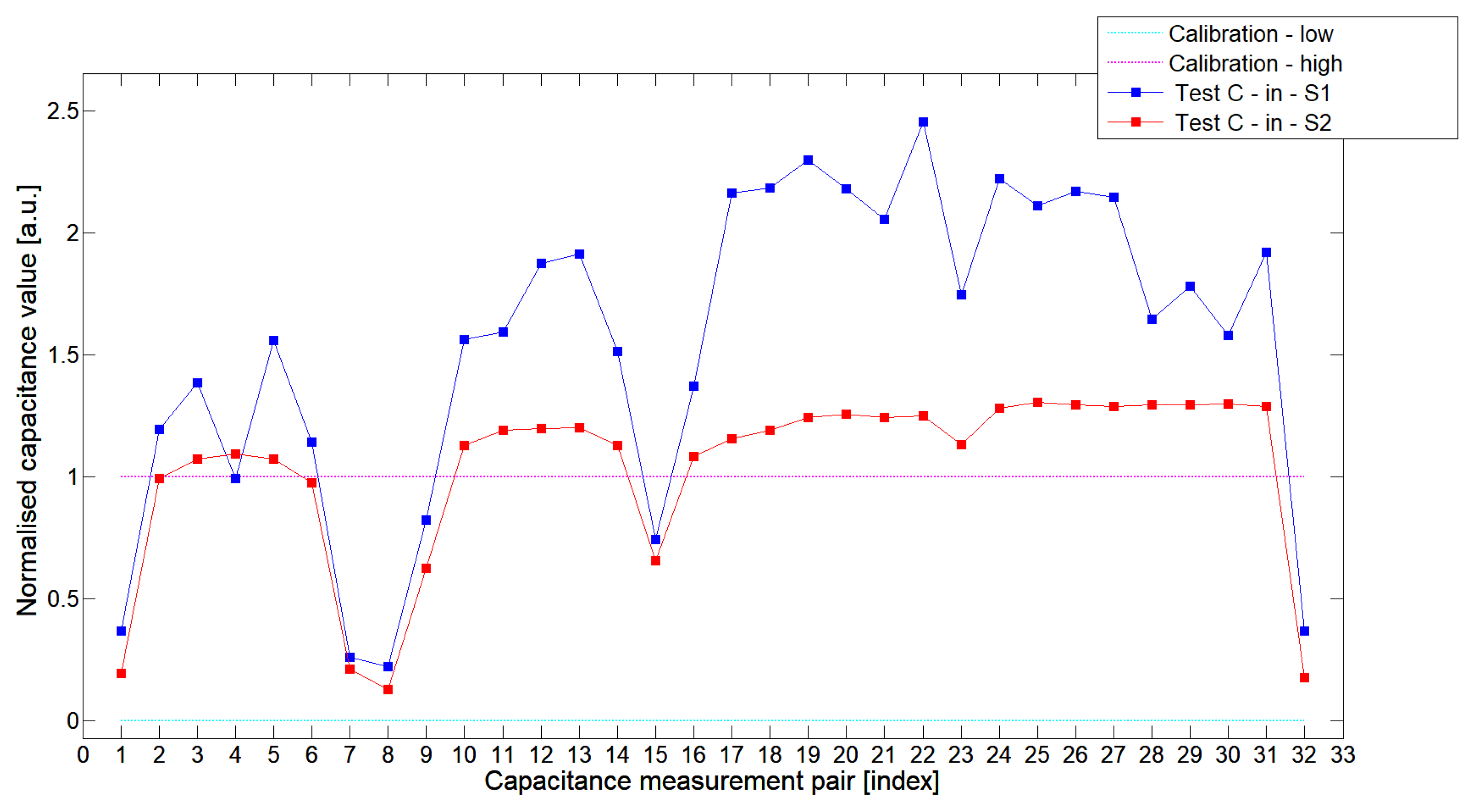
5.2. High-Contrast Objects Investigation
6. Conclusions and Directions for Further Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rybak, G.; Chaniecki, Z.; Grudzien, K.; Romanowski, A.; Sankowski, D. Non-invasive methods of industrial processes control. IAPGOS Inform. Control. Meas. Econ. Environ. Prot. 2004, 4, 41–45. [Google Scholar] [CrossRef]
- Che, H.Q.; Ye, J.M.; Yang, W.Q.; Wang, H.G. Measurement of the gas-solid flow in a wurster tube using 3D electrical capacitance tomography sensor. Lect. Notes Electr. Eng. 2019, 506, 367–383. [Google Scholar] [CrossRef]
- Grudzien, K.; Romanowski, A.; Sankowski, D.; Willams, R.A. Gravitational Granular Flow Dynamics Study Based on Tomographic Data Processing. Part. Sci. Technol. 2007, 26, 67–82. [Google Scholar] [CrossRef]
- Wajman, R.; Banasiak, R.; Mazurkiewicz, L.; Dyakowski, T.; Sankowski, D. Spatial imaging with 3D capacitance measurements. Meas. Sci. Technol. 2006, 17, 2113–2118. [Google Scholar] [CrossRef]
- Yang, W.Q.; Peng, L. Image reconstruction algorithms for electrical capacitance tomography. Meas. Sci. Technol. 2003, 14, R1–R13. [Google Scholar] [CrossRef]
- Marashdeh, Q.; Wang, F.; Fan, L.S.; Warsito, W. Velocity measurement of multi-phase flows based on electrical capacitance volume tomography. Proc. IEEE Sens. 2007, 1017–1019. [Google Scholar] [CrossRef]
- Plaskowski, A.; Beck, M.S.; Thorn, R.; Dyakowski, T. Imaging Industrial Flows—Applications of Electrical Process Tomography; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Rymarczyk, T. Using electrical impedance tomography to monitoring flood banks. Int. J. Appl. Electromagn. Mech. 2014, 45, 489–494. [Google Scholar] [CrossRef]
- Zeeshan, Z.; Zuccarelli, C.E.; Acero, D.O.; Marashdeh, Q.M.; Teixeira, F.L. Enhancing Resolution of Electrical Capacitive Sensors for Multiphase Flows by Fine-Stepped Electronic Scanning of Synthetic Electrodes. IEEE Trans. Instrum. Meas. 2019, 68, 462–473. [Google Scholar] [CrossRef]
- Banasiak, R.; Wajman, R.; Sankowski, D.; Soleimani, M. Three-dimensional nonlinear inversion of electrical capacitance tomography data using a complete sensor model. Insight Prog. Electromagn. Res. PIER 2010, 100, 219–234. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, S.; Dong, F. Focusing Sensor Design for Open Electrical Impedance Tomography Based on Shape Conformal Transformation. Sensors 2019, 19, 2060. [Google Scholar] [CrossRef]
- Wajman, R.; Fiderek, P.; Fidos, H.; Jaworski, T.; Nowakowski, J.; Sankowski, D.; Banasiak, R. Metrological evaluation of a 3D electrical capacitance tomography measurement system for two-phase flow fraction determination. Meas. Sci. Technol. 2013, 24. [Google Scholar] [CrossRef]
- Xie, W.Q.; Huang, S.M.; Lenn, C.P.; Stoll, A.L.; Beck, M.S. Experimental evaluation of capacitance tomographic flow imaging systems using physical models. IEE Proc. Circuits Devices Syst. 1994, 141, 357–368. [Google Scholar] [CrossRef]
- Romanowski, A.; Grudzien, K.; Aykroyd, R.G.; Williams, R.A. Advanced Statistical Analysis as a Novel Tool to Pneumatic Conveying Monitoring and Control Strategy Development. Part. Part. Syst. Charact. 2006, 23, 289–296. [Google Scholar] [CrossRef]
- Grudzien, K.; Romanowski, A.; Williams, R.A. Application of a Bayesian Approach to the Tomographic Analysis of Hopper Flow. Part. Part. Syst. Charact. 2017, 22, 246–253. [Google Scholar] [CrossRef]
- Grudzien, K. Visualization System for Large-Scale Silo Flow Monitoring Based on ECT Technique. IEEE Sens. J. 2017, 17, 8242–8250. [Google Scholar] [CrossRef]
- Romanowski, A. Big Data-Driven Contextual Processing Methods for Electrical Capacitance Tomography. IEEE Trans. Ind. Inform. 2019, 15, 1609–1618. [Google Scholar] [CrossRef]
- Rymarczyk, T.; Sikora, J. Applying industrial tomography to control and optimization flow systems. Open Phys. 2018, 16, 332–345. [Google Scholar] [CrossRef]
- Banasiak, R.; Wajman, R.; Soleimani, M. An efficient nodal Jacobian method for 3D electrical capacitance tomography image reconstruction. Insight Non-Destr. Test. Cond. Monit. 2009, 51, 36–38. [Google Scholar] [CrossRef]
- Kryszyn, J.; Smolik, W.T.; Radzik, B.; Olszewski, T.; Szabatin, R. Switchless charge-discharge circuit for electrical capacitance tomography. Meas. Sci. Technol. 2014, 25. [Google Scholar] [CrossRef]
- Mazurkiewicz, L.; Banasiak, R.; Wajman, R.; Dyakowski, T.; Sankowski, D. Towards 3D Capacitance Tomography. In Proceedings of the 4th World Congress Industrial Process Tomography, Aizu, Japan, 5–8 September 2005; pp. 546–551. [Google Scholar]
- Wajman, R.; Banasiak, R. Tunnel-based method of sensitivity matrix calculation for 3D-ECT imaging. Sens. Rev. 2014, 34, 273–283. [Google Scholar] [CrossRef]
- Ye, J.; Wang, H.; Yang, W. Characterization of a multi-plane electrical capacitance tomography sensor with different numbers of electrodes. Meas. Sci. Technol. 2016, 27. [Google Scholar] [CrossRef]
- Li, Y.; Holland, D.J. Optimizing the geometry of three-dimensional electrical capacitance tomography sensors. IEEE Sens. J. 2015, 15, 1567–1574. [Google Scholar] [CrossRef]
- Huang, A.; Cao, Z.; Sun, S.; Lu, F.; Xu, L. An agile electrical capacitance tomography system with improved frame rates. IEEE Sens. J. 2019, 19, 1416–1425. [Google Scholar] [CrossRef]
- Hu, X.; Yang, W. Planar capacitive sensors—Designs and applications. Sens. Rev. 2010, 30, 24–39. [Google Scholar] [CrossRef]
- Wu, M.; Ye, J.; Wang, H.; Yang, W. Evaluation of Excitation Strategy for a Large-Scale ECT Sensor with Internal-External Electrodes. IEEE Sens. J. 2017, 17, 8091–8098. [Google Scholar] [CrossRef]
- Xu, H.; Yang, H.; Wang, S. Effect of Axial Guard Electrodes on Sensing Field of Capacitance Tomographic Sensor. In Proceedings of the 1st World Congress on Industrial Process Tomography, Buxton, UK, 14–17 April 1999; pp. 348–352. [Google Scholar]
- Dichtl, C.; Sippel, P.; Krohns, S. Dielectric Properties of 3D Printed Polylactic Acid. Adv. Mater. Sci. Eng. Vol. 2017, 2017. [Google Scholar] [CrossRef]
- Chen, C.; Woźniak, P.W.; Romanowski, A.; Obaid, M.; Jaworski, T.; Kucharski, J.; Grudzien, K.; Zhao, S.; Fjeld, M. Using Crowdsourcing for Scientific Analysis of Industrial Tomographic Images. Part. Part. Syst. Charact. 2016, 7, 52:1–52:25. [Google Scholar] [CrossRef]
- Romanowski, A. Contextual processing of electrical capacitance tomography measurement data for temporal modeling of pneumatic conveying process. In Proceedings of the 2018 Federated Conference on Computer Science and Information Systems (FedCSIS), Annals of Computer Science and Information Systems, Poznan, Poland, 9–12 September 2018; IEEE: Piscataway, NJ, USA, 2018; Volume 15, pp. 283–286. [Google Scholar] [CrossRef]


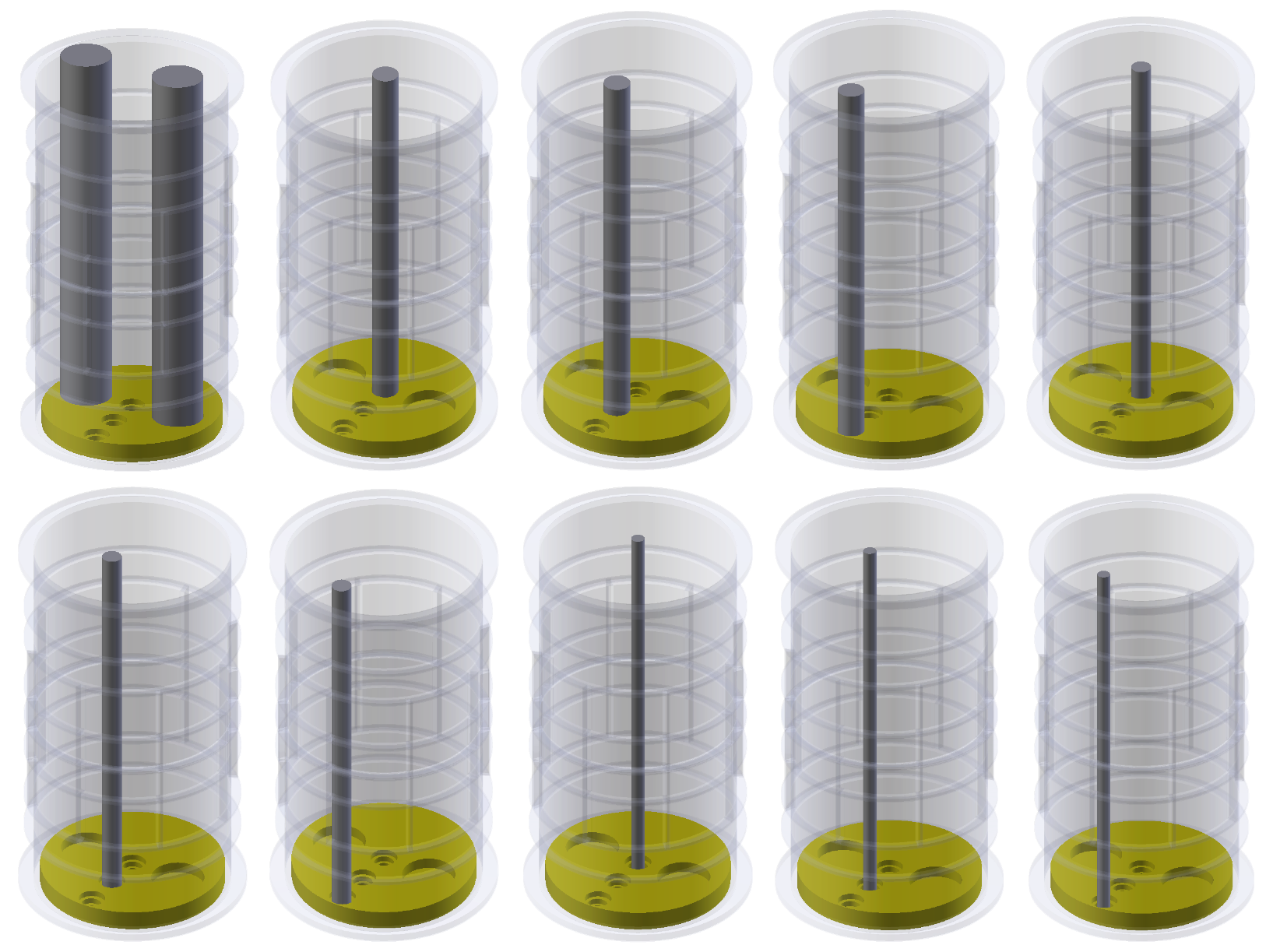


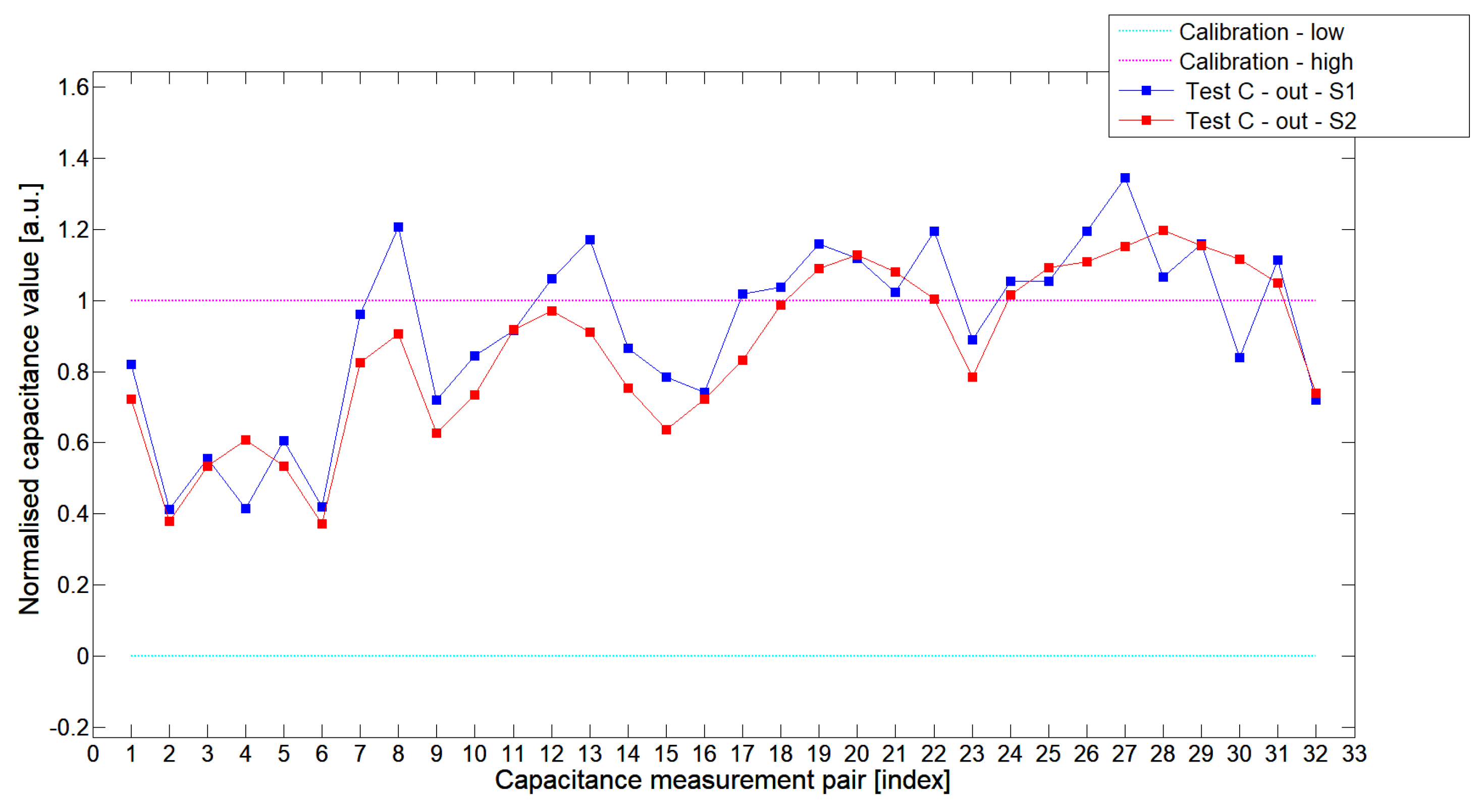

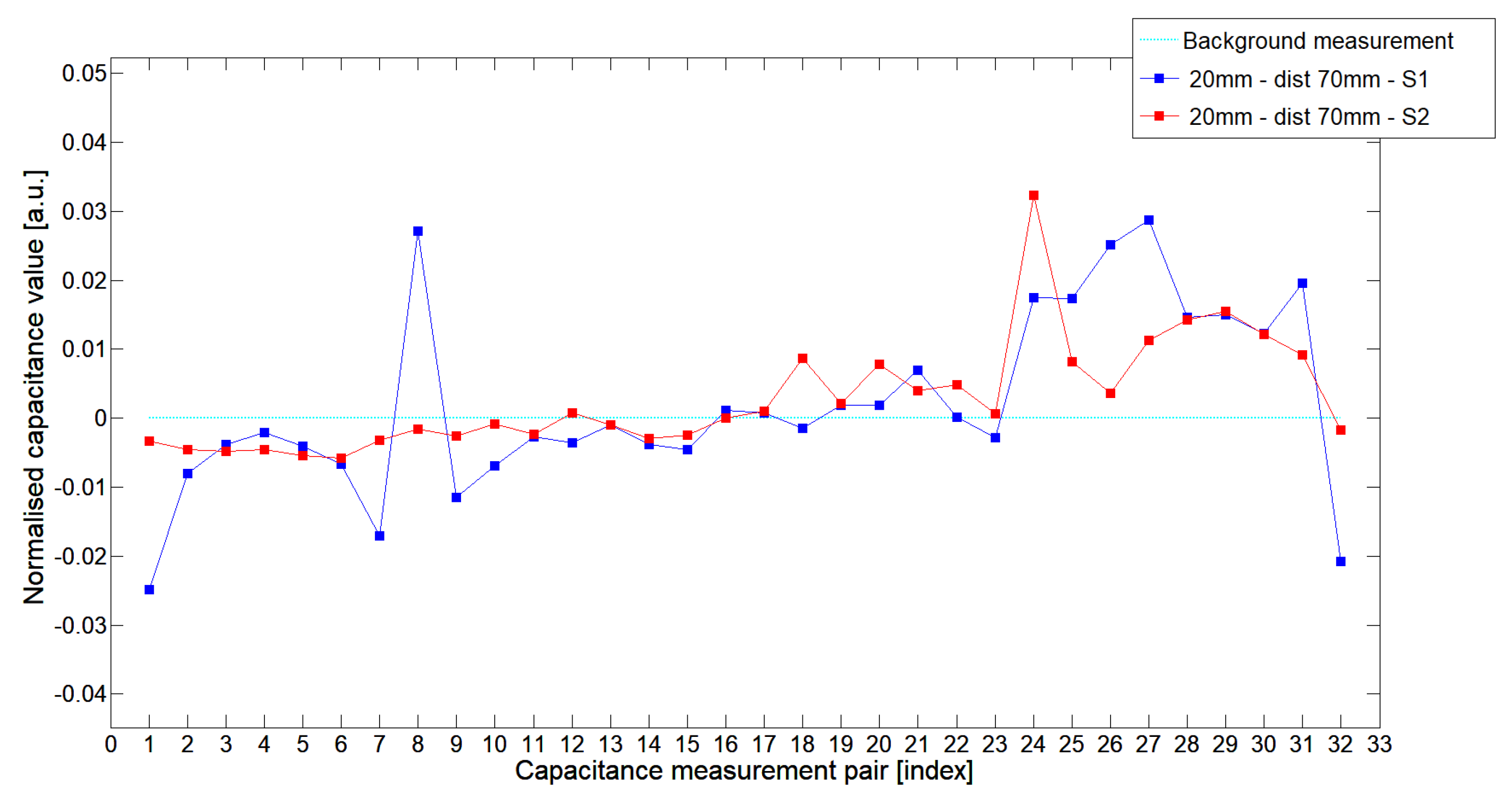


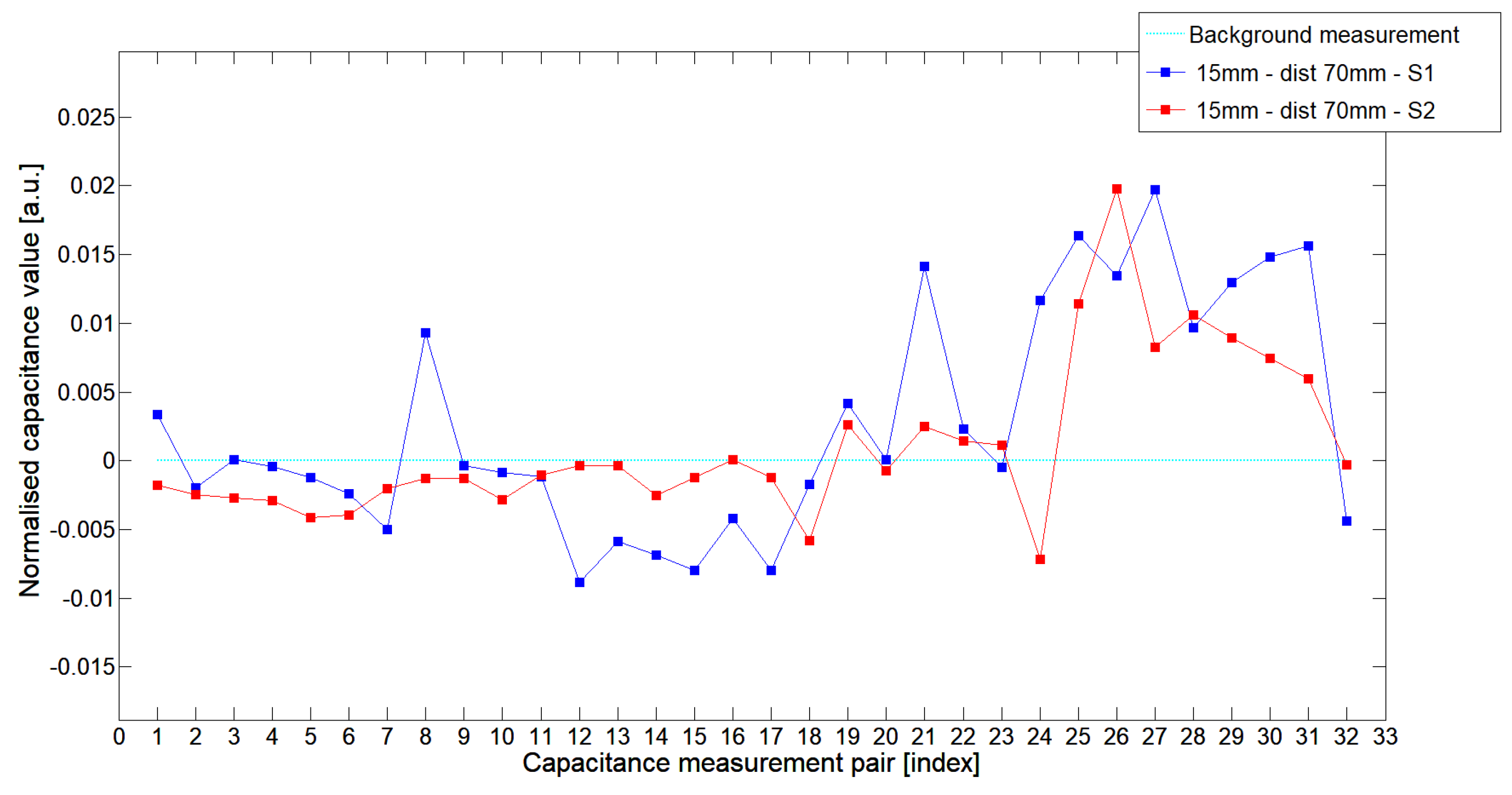
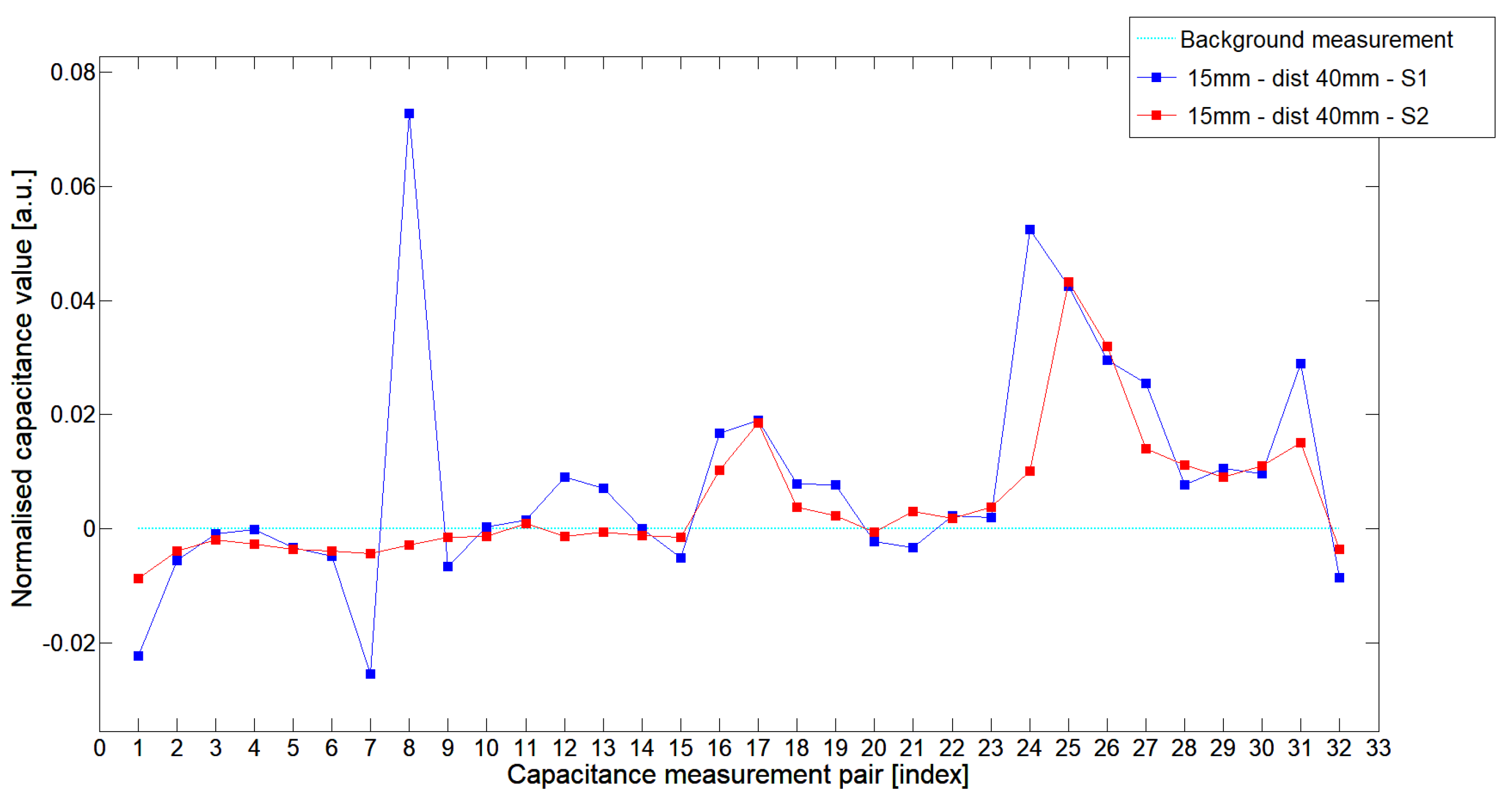


| Config | TestA | TestB | TestC |
|---|---|---|---|
| inside | Phantom Test | Phantom Test | Phantom Test |
| outside | Phantom Test | Phantom Test | Phantom Test |
| Test | Test | Test | Test | Test | Test | |
|---|---|---|---|---|---|---|
| S1-1-2 | 7.446 | 7.291 | 7.460 | 7.278 | 7.408 | 7.336 |
| S1-1-9 | 6.604 | 6.536 | 6.614 | 6.531 | 6.616 | 6.541 |
| S1-1-17 | 3.926 | 3.914 | 3.928 | 3.911 | 3.918 | 3.930 |
| S1-1-25 | 3.919 | 3.917 | 3.921 | 3.917 | 3.917 | 3.928 |
| S2-1-2 | 4.909 | 4.626 | 4.922 | 4.617 | 4.827 | 4.673 |
| S2-1-9 | 4.577 | 4.414 | 4.591 | 4.410 | 4.568 | 4.427 |
| S2-1-17 | 3.920 | 3.912 | 3.921 | 3.911 | 3.913 | 3.916 |
| S2-1-25 | 3.922 | 3.920 | 3.922 | 3.920 | 3.919 | 3.922 |
| std | 2 × 40 | 20 | 20 | 20 | 15 | 15 | 15 | 10 | 10 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.0883 | 0.0132 | 0.0310 | 0.1403 | 0.0083 | 0.0201 | 0.0728 | 0.0038 | 0.0143 | 0.0349 |
| S2 | 0.1296 | 0.0081 | 0.0223 | 0.1272 | 0.0057 | 0.0110 | 0.0717 | 0.0050 | 0.0097 | 0.0284 |
| % | −32% | 63% | 39% | 10% | 46% | 83% | 1.5% | −24% | 47% | 23% |
| mean | 2 × 40 | 20 | 20 | 20 | 15 | 15 | 15 | 10 | 10 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.0330 | 0.0020 | 0.0099 | 0.0332 | 0.0011 | 0.0083 | 0.0299 | −0.0024 | 0.0031 | 0.0097 |
| S2 | 0.0632 | 0.0028 | 0.0091 | 0.0683 | 0.0027 | 0.0046 | 0.0363 | 0.0011 | 0.0067 | 0.0143 |
| % | 48% | 29% | −8% | 51% | 45% | −80% | −18% | >100% | >100% | 68% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, A.; Banasiak, R.; Romanowski, A.; Sankowski, D. 3D-Printed Multilayer Sensor Structure for Electrical Capacitance Tomography. Sensors 2019, 19, 3416. https://doi.org/10.3390/s19153416
Kowalska A, Banasiak R, Romanowski A, Sankowski D. 3D-Printed Multilayer Sensor Structure for Electrical Capacitance Tomography. Sensors. 2019; 19(15):3416. https://doi.org/10.3390/s19153416
Chicago/Turabian StyleKowalska, Aleksandra, Robert Banasiak, Andrzej Romanowski, and Dominik Sankowski. 2019. "3D-Printed Multilayer Sensor Structure for Electrical Capacitance Tomography" Sensors 19, no. 15: 3416. https://doi.org/10.3390/s19153416
APA StyleKowalska, A., Banasiak, R., Romanowski, A., & Sankowski, D. (2019). 3D-Printed Multilayer Sensor Structure for Electrical Capacitance Tomography. Sensors, 19(15), 3416. https://doi.org/10.3390/s19153416





