Effects of AlN and BCN Thin Film Multilayer Design on the Reaction Time of Ni/Ni-20Cr Thin Film Thermocouples on Thermally Sprayed Al2O3
Abstract
:1. Introduction
- half-life period t50: time to reach 50% of the temperature jump
- ninety percent time t90: time to reach 90% of the temperature jump
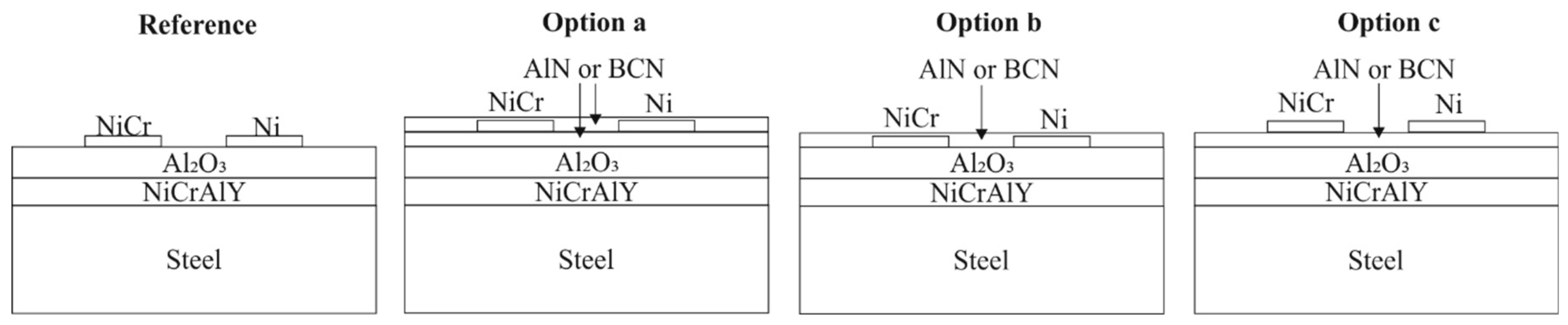
2. Materials and Methods
2.1. Deposition Process
2.2. Thin Film Characterization
3. Results and Discussion
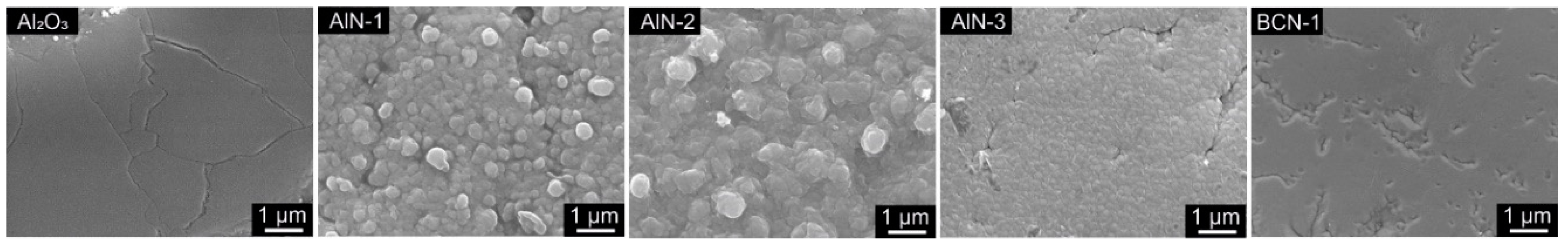
3.1. AlN/BCN Thin Films
3.1.1. Morphology and Physical Structure
3.1.2. Chemical Composition
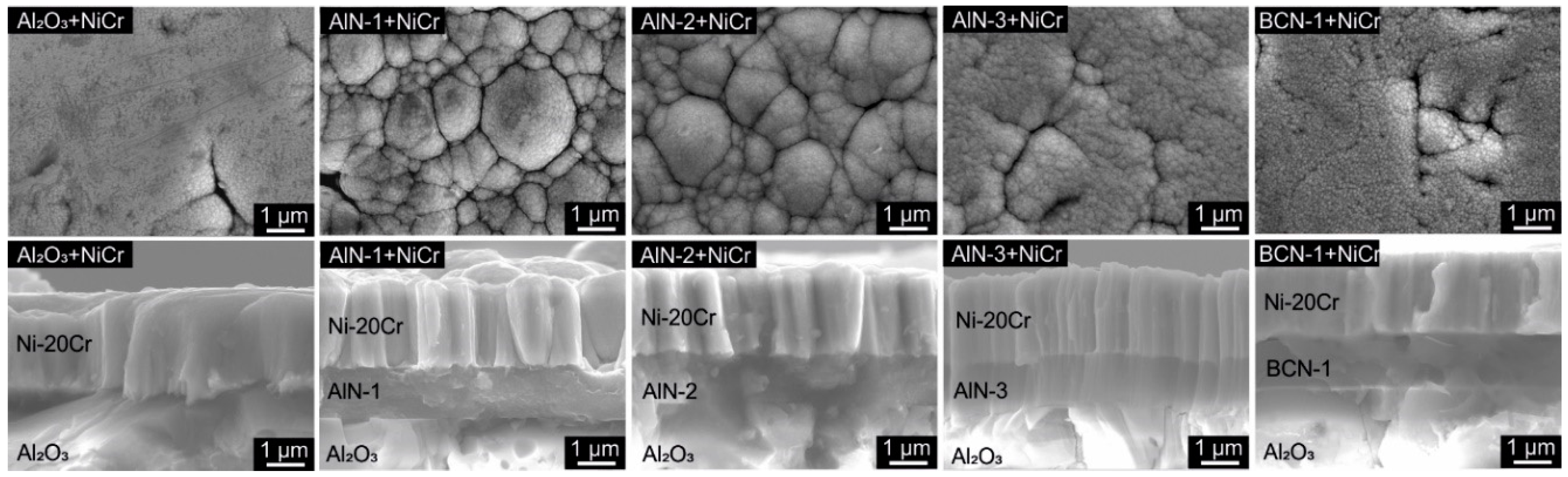
3.2. Ni/Ni-20Cr Thin Films
3.2.1. Morphology
3.2.2. Physical Structure
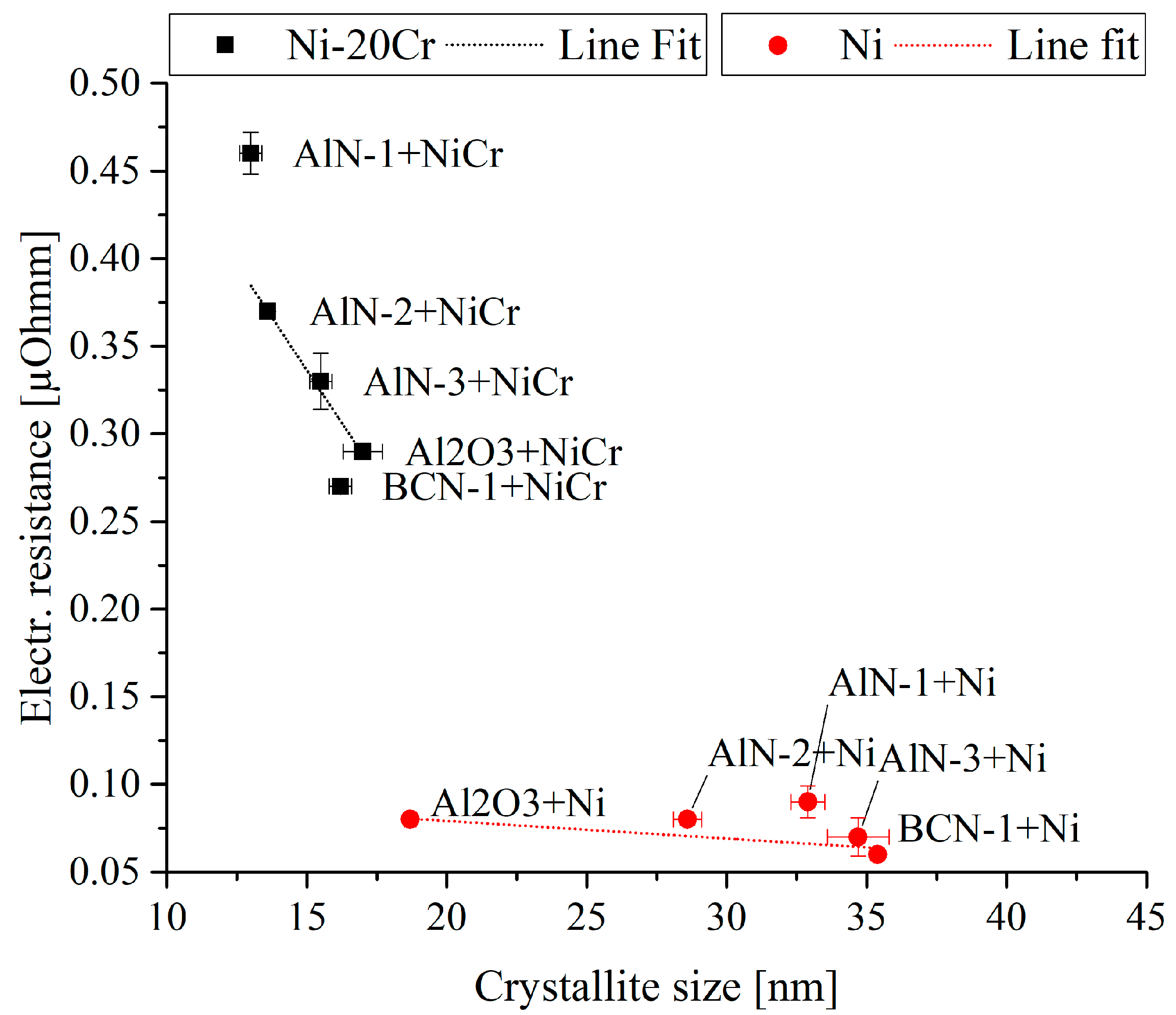
3.2.3. Electrical Properties
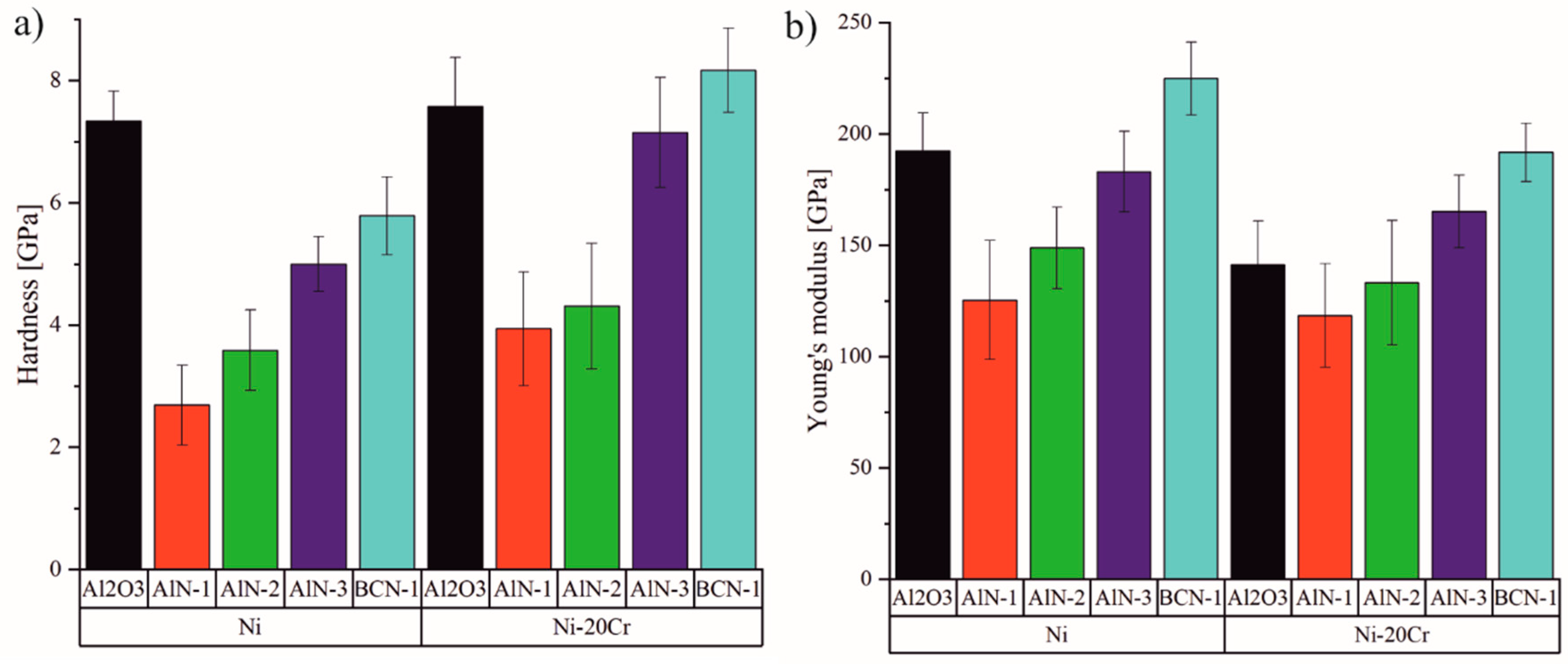
3.2.4. Mechanical Properties
3.3. Ni/Ni-20Cr Thermocouples
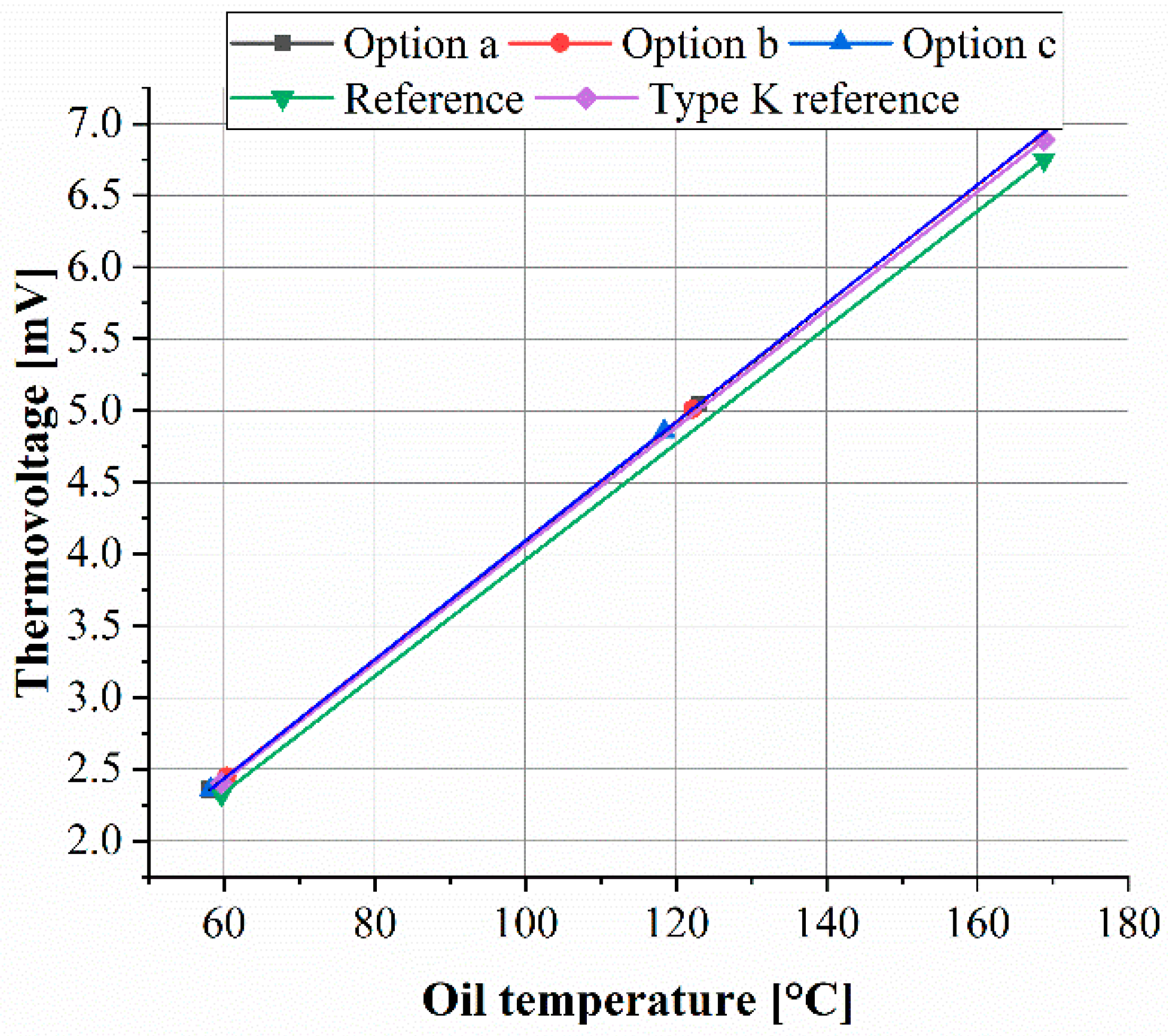
3.3.1. Calibration
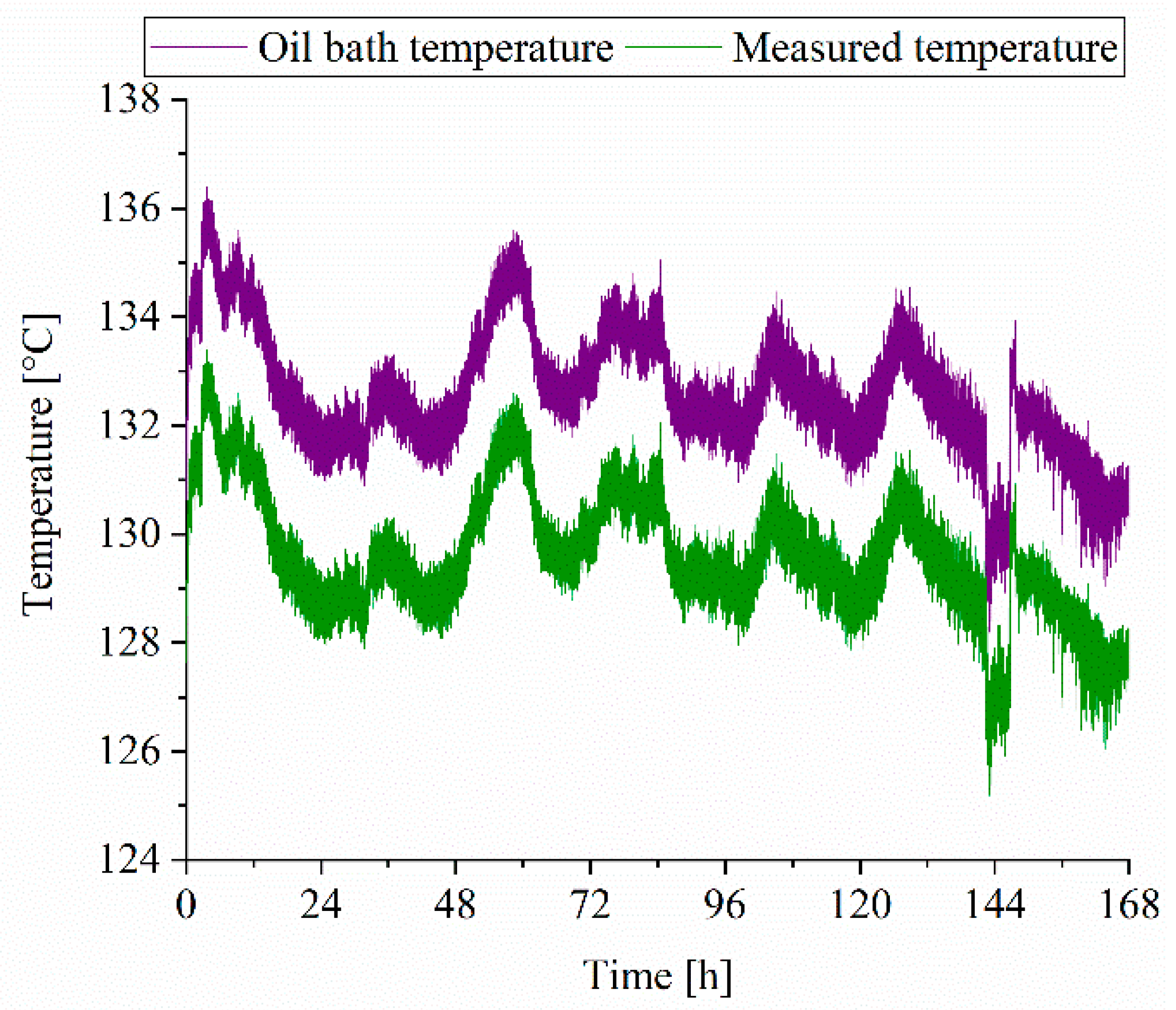
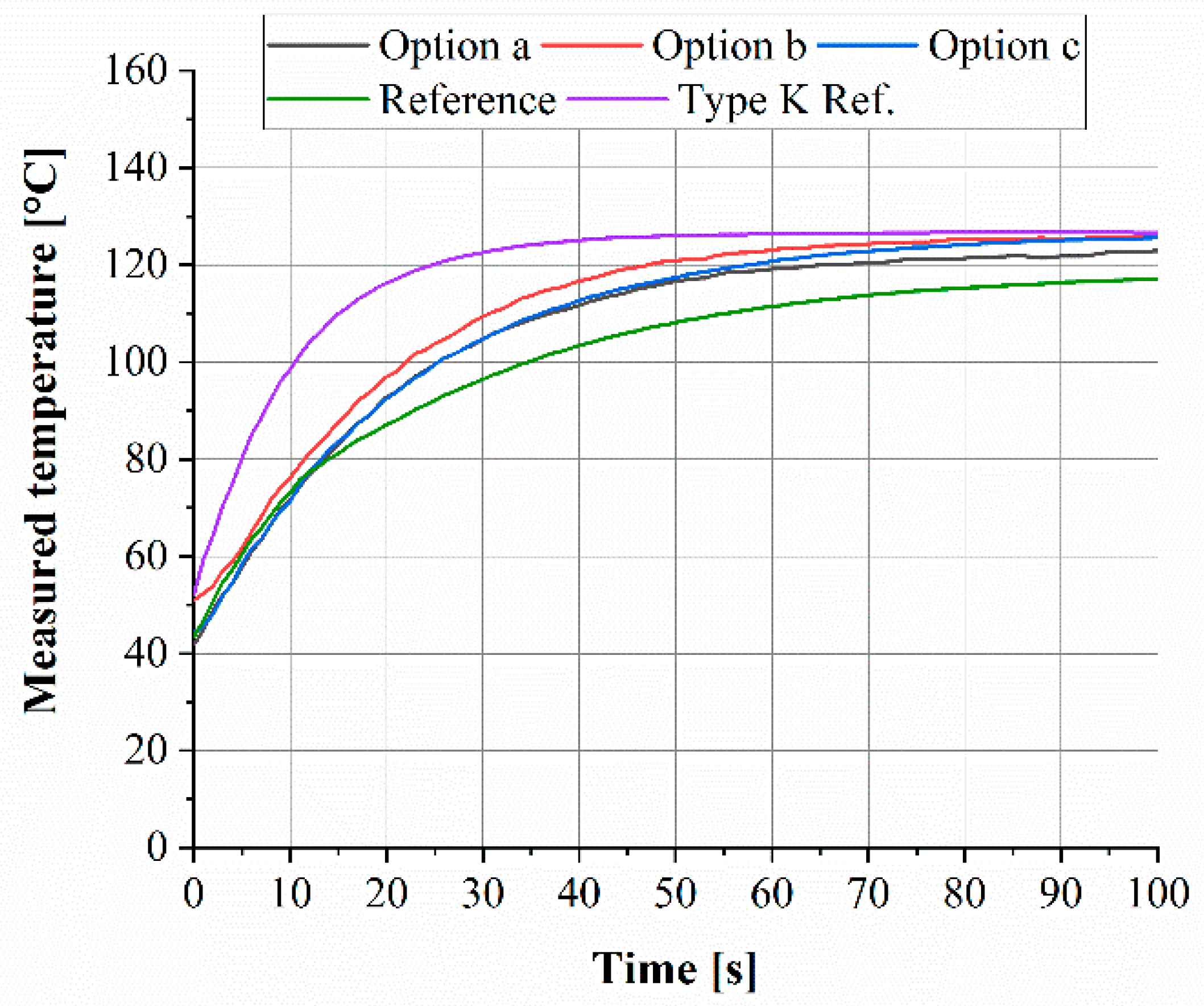
3.3.2. Reaction Time
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marr, M.A.; Wallace, J.S.; Chandra, S.; Pershin, L.; Mostaghimi, J. A fast response thermocouple for internal combustion engine surface temperature measurements. Exp. Therm. Fluid Sci. 2010, 34, 183–189. [Google Scholar] [CrossRef]
- Debey, D.; Bluhm, R.; Habets, N.; Kurz, H. Fabrication of planar thermocouples for real-time measurements of temperature profiles in polymer melts. Sens. Actuators A Phys. 1997, 58, 179–184. [Google Scholar] [CrossRef]
- Abeykoon, C.; Martin, P.J.; Kelly, A.L.; Brown, E.C. A review and evaluation of melt temperature sensors for polymer extrusion. Sens. Actuators A Phys. 2012, 182, 16–27. [Google Scholar] [CrossRef]
- Yust, M.; Kreider, K.G. Transparent thin film thermocouple. Thin Solid Films 1989, 176, 73–78. [Google Scholar] [CrossRef]
- Tougas, I.M.; Gregory, O.J. Thin film platinum–palladium thermocouples for gas turbine engine applications. Thin Solid Films 2013, 539, 345–349. [Google Scholar] [CrossRef]
- Bhatt, H.D.; Vedula, R.; Desu, S.B.; Fralick, G.C. Thin film TiC/TaC thermocouples. Thin Solid Films 1999, 342, 214–220. [Google Scholar] [CrossRef]
- Tian, X.; Kennedy, F.E.; Deacutis, J.J.; Henning, A.K. The Development and Use of Thin Film Thermocouples for Contact Temperature Measurement. Tribol. T 1992, 35, 491–499. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Zhao, W.; Zhang, W.; Liu, X.; Jiang, S. Fabrication and calibration of Pt–10%Rh/Pt thin film thermocouples. Measurement 2014, 48, 248–251. [Google Scholar] [CrossRef]
- Basti, A.; Obikawa, T.; Shinozuka, J. Tools with built-in thin film thermocouple sensors for monitoring cutting temperature. Int. J. Mach. Tool. Manuf. 2007, 47, 793–798. [Google Scholar] [CrossRef]
- Shinozuka, J.; Basti, A.; Obikawa, T. Development of Cutting Tool With Built-In Thin Film Thermocouples for Measuring High Temperature Fields in Metal Cutting Processes. J. Manuf. Sci. Eng. 2008, 130, 34501. [Google Scholar] [CrossRef]
- Danışman, M.; Cansever, N. Effect of Cr content on mechanical and electrical properties of Ni–Cr thin films. J. Alloys Compd. 2010, 493, 649–653. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, G.; Gao, G.; Xu, C.; Du, J.; Zou, D.; Chen, J. The resistance characteristics of the Ni-Cr thin films and their influence on the integrated circuits. In Proceedings of the 1998 5th International Conference on Solid-State and Integrated Circuit Technology, Beijing, China, 21–23 October 1998; Tu, K.N., Zhang, M., Eds.; IEEE Press: New York, NY, USA, 1998; pp. 123–126, ISBN 0-7803-4306-9. [Google Scholar]
- Tillmann, W.; Kokalj, D.; Stangier, D.; Schöppner, V.; Benis, H.B.; Malatyali, H. Influence of Cr-Content on the thermoelectric and mechanical properties of NiCr thin film thermocouples synthesized on thermally sprayed Al2O3. Thin Solid Films 2018. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, X.; Jiang, S.; Zhang, W.; Jiang, H. Microstructure Evolution and Thermoelectric Property of Pt-PtRh Thin Film Thermocouples. Crystals 2017, 7, 96. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Feng, C.; Zhou, H. The Influence of Size Effect on Sensitivity of Cu/CuNi Thin–film Thermocouple. Phys. Procedia 2011, 22, 95–100. [Google Scholar] [CrossRef]
- Tillmann, W.; Kokalj, D.; Stangier, D. Optimization of the deposition parameters of Ni-20Cr thin films on thermally sprayed Al2O3 for sensor application. Surf. Coat. Technol. 2018, 344, 223–232. [Google Scholar] [CrossRef]
- Lai, L.; Zeng, W.; Fu, X.; Sun, R.; Du, R. Optimization of sputtering parameters for Ni–Cr alloy deposition on copper foil as embedded thin film resistor. Surf. Coat. Technol. 2013, 218, 80–86. [Google Scholar] [CrossRef]
- Lai, L.; Fu, X.; Sun, R.; Du, R. Comparison of microstructure and electrical properties of NiCr alloy thin film deposited on different substrates. Surf. Coat. Technol. 2013, 235, 552–560. [Google Scholar] [CrossRef]
- Shinozuka, J.; Obikawa, T. Development of Cutting Tools with Built-In Thin Film Thermocouples. In Advances in Abrasive Technology VI; Pearce, T., Gao, Y., Eds.; Trans Tech Publ: Uetikon-Zürich, Switzerland, 2004; pp. 547–552. ISBN 978-0-87849-933-5. [Google Scholar]
- Biermann, D.; Kirschner, M.; Pantke, K.; Tillmann, W.; Herper, J. New coating systems for temperature monitoring in turning processes. Surf. Coat. Technol. 2013, 215, 376–380. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, N.H.; Choi, G.P.; Lee, W.S.; Seo, Y.J.; Park, J. Structural and surface properties of NiCr thin films prepared by DC magnetron sputtering under variation of annealing conditions. Microelectron. Eng. 2005, 82, 314–320. [Google Scholar] [CrossRef]
- Nowakowska-Langier, K.; Chodun, R.; Zdunek, K.; Minikayev, R.; Nietubyc, R. Structure of AlN films deposited by magnetron sputtering method. Mater. Sci. Pol. 2015, 33, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Kale, A.; Brusa, R.S.; Miotello, A. Structural and electrical properties of AlN films deposited using reactive RF magnetron sputtering for solar concentrator application. Appl. Surf. Sci. 2012, 258, 3450–3454. [Google Scholar] [CrossRef]
- Panda, P.; Ramaseshan, R.; Ravi, N.; Mangamma, G.; Jose, F.; Dash, S.; Suzuki, K.; Suematsu, H. Reduction of residual stress in AlN thin films synthesized by magnetron sputtering technique. Mater. Chem. Phys. 2017, 200, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Kim, S.H. Thermal conductivity of AlN thin films deposited by RF magnetron sputtering. Mater. Sci. Semicond. Process. 2012, 15, 6–10. [Google Scholar] [CrossRef]
- Arroyo, J.; Silvestre, S.; Coll, A.; Castañer, L. Thermal response of AlN and CNx films as coatings of thin film anemometers. Measurement 2013, 46, 1421–1426. [Google Scholar] [CrossRef]
- Duquenne, C.; Besland, M.P.; Tessier, P.Y.; Gautron, E.; Scudeller, Y.; Averty, D. Thermal conductivity of aluminium nitride thin films prepared by reactive magnetron sputtering. J. Phys. D Appl. Phys. 2011, 45, 15301. [Google Scholar] [CrossRef]
- Engelmark, F.; Fucntes, G.; Katardjiev, I.V.; Harsta, A.; Smith, U.; Berg, S. Synthesis of highly oriented piezoelectric AlN films by reactive sputter deposition. J. Vac. Sci. Technol. A 2000, 18, 1609–1612. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Mishra, S.C.; Mishra, P.; Limaye, P.K.; Singh, K. Mechanical and tribological properties of crystalline aluminum nitride coatings deposited on stainless steel by magnetron sputtering. J. Nucl. Mater. 2015, 466, 69–79. [Google Scholar] [CrossRef]
- Bogner, M.; Benstetter, G.; Fu, Y.Q. RETRACTED: Cross- and in-plane thermal conductivity of AlN thin films measured using differential 3-omega method. Surf. Coat. Technol. 2017, 320, 91–96. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Liu, C.; Fan, S.; Zhang, J.; Ding, X.; Golberg, D. Thermal conductivity of nanostructured boron nitride materials. J. Phys. Chem. B 2006, 110, 10354–10357. [Google Scholar] [CrossRef]
- Houska, J.; Steidl, P.; Vlcek, J.; Martan, J. Thermal, mechanical and electrical properties of hard B 4 C, BCN, ZrBC and ZrBCN ceramics. Ceram. Int. 2016, 42, 4361–4369. [Google Scholar] [CrossRef]
- Ong, C.W.; Zhao, X.A.; Chan, K.F.; Ng, Y.M.; Chan, P.W.; Choy, C.L.; Kwok, R.W.M. Effects of carbon incorporation on the structure and mechanical properties of cubic boron nitride films. Thin Solid Films 1997, 307, 152–155. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, J.; Jia, F.; Zhuang, C.; Bai, Y.; Jiang, X. Relationship between chemical compositions of magnetron sputtered B–C–N films and various experimental parameters. Vacuum 2012, 86, 1499–1504. [Google Scholar] [CrossRef]
- Bernhard, F. Technische Temperaturmessung. In Physikalische und Meßtechnische Grundlagen, Sensoren und Meßverfahren, Meßfehler und Kalibrierung; Springer: Berlin/Heidelberg, Germany, 2004; ISBN 9783642623448. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Librizzi, A. Entwicklung von Temperatursensitiven Dünnschichten und Untersuchungen zu deren Einsatz in Spritzgießwerkzeugen der Thermoplastverarbeitung, 1. Aufl; Shaker: Herzogenrath, Germany, 2015; ISBN 3844035257. [Google Scholar]
- Iriarte, G.F.; Rodríguez, J.G.; Calle, F. Synthesis of c-axis oriented AlN thin films on different substrates: A review. Mater. Res. Bull. 2010, 45, 1039–1045. [Google Scholar] [CrossRef]
- Sharma, N.; Ilango, S.; Dash, S.; Tyagi, A.K. X-ray photoelectron spectroscopy studies on AlN thin films grown by ion beam sputtering in reactive assistance of N+/N2+ ions: Substrate temperature induced compositional variations. Thin Solid Films 2017, 636, 626–633. [Google Scholar] [CrossRef]
- Petley, V.; Sathishkumar, S.; Thulasi Raman, K.H.; Rao, G.M.; Chandrasekhar, U. Microstructural and mechanical characteristics of Ni–Cr thin films. Mater. Res. Bull. 2015, 66, 59–64. [Google Scholar] [CrossRef]
- Vinayak, S.; Vyas, H.P.; Vankar, V.D. Microstructure and electrical characteristics of Ni–Cr thin films. Thin Solid Films 2007, 515, 7109–7116. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, Y.; Zhang, J.; Kuang, C.; Yue, M.; Zhou, S. Grain size-dependent electrical resistivity of bulk nanocrystalline Gd metals. Prog. Nat. Sci. Mater. Int. 2013, 23, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, N.W. Solid State Physics; Holt Rinehart and Winston: New York, NY, USA, 1976; ISBN 9780030839931. [Google Scholar]
- Wochnik, A.S.; Handloser, M.; Durach, D.; Hartschuh, A.; Scheu, C. Increasing crystallinity for improved electrical conductivity of TiO2 blocking layers. ACS Appl. Mater. Interfaces 2013, 5, 5696–5699. [Google Scholar] [CrossRef]
- Chou, C.C.; Hung, F.Y.; Lui, T.S. Variation of Microstructure and Electrical Conductivity of Amorphous AgInSbTe and SbTe Films during Crystallization. Mater. Trans. 2007, 48, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Absar, S.; Ruszkiewicz, B.J.; Skovron, J.D.; Mears, L.; Abke, T.; Zhao, X.; Choi, H. Temperature measurement in friction element welding process with micro thin film thermocouples. Procedia Manuf. 2018, 26, 485–494. [Google Scholar] [CrossRef]
- Chuang, N.C.; Lin, J.T.; Chen, H.R. TCR control of Ni–Cr resistive film deposited by DC magnetron sputtering. Vacuum 2015, 119, 200–203. [Google Scholar] [CrossRef]
- Lai, L.; Zeng, W.; Fu, X.; Sun, R.; Du, R. Annealing effect on the electrical properties and microstructure of embedded Ni–Cr thin film resistor. J. Alloys Compd. 2012, 538, 125–130. [Google Scholar] [CrossRef]
- Martan, J.; Beneš, P. Thermal properties of cutting tool coatings at high temperatures. Thermochim. Acta 2012, 539, 51–55. [Google Scholar] [CrossRef]
- Samani, M.K.; Ding, X.Z.; Amini, S.; Khosravian, N.; Cheong, J.Y.; Chen, G.; Tay, B.K. Thermal conductivity of titanium aluminum silicon nitride coatings deposited by lateral rotating cathode arc. Thin Solid Films 2013, 537, 108–112. [Google Scholar] [CrossRef]




| Coating | Bias MF [-V] | Target Power [W] | Heating Power [W] | Substrate Temperature [°C] | Chamber Pressure [mPa] | Gas Flow Rate Ratio |
|---|---|---|---|---|---|---|
| Ni | −150 | 2× 1875 DC (Ni) | 1200 | 260 ± 9 | 250 | Ar/Kr = 2.7 |
| Ni-20Cr | −150 | 2× 1875 DC (Ni), 1× 1035 DC (Cr) | 1200 | 265 ± 8 | 250 | Ar/Kr = 2.7 |
| AlN-1 | −150 | 2× 3180 MF (Al) | 5000 | 383 ± 4 | 330 | Ar/N2 = 6.0 |
| AlN-2 | −150 | 2× 3180 MF (Al) | 7000 | 475 ± 7 | 330 | Ar/N2 = 6.0 |
| AlN-3 | −150 | 2× 3180 MF (Al) | 3000 | 325 ± 8 | 330 | Ar/N2 = 5.1 |
| BCN-1 | −150 | 1× 3150 MF (B4C) | 7000 | 453 ± 3 | 330 | Ar/N2 = 26.0 |
| Composition | AlN-1 | AlN-2 | AlN-3 | BCN-1 |
|---|---|---|---|---|
| Al [at.-%] | 46.5 ± 0.5 | 48.2 ± 0.5 | 42.1 ± 0.3 | - |
| B [at.-%] | - | - | - | 42.2 ± 2.2 |
| C [at.-%] | - | - | - | 16.8 ± 0.8 |
| N [at.-%] | 53.5 ± 0.5 | 51.8 ± 0.5 | 57.9 ± 0.3 | 41.0 ± 2.5 |
| Coating | Option a | Option b | Option c |
|---|---|---|---|
| AlN-1 | y = 0.0414x − 0.0458 | y = 0.0414x − 0.0459 | y = 0.0414x − 0.0476 |
| AlN-2 | y = 0.0414x − 0.0455 | y = 0.0414x − 0.0461 | y = 0.0414x − 0.0476 |
| AlN-3 | y = 0.0414x − 0.0454 | y = 0.0414x − 0.0460 | y = 0.0414x − 0.0483 |
| BCN-1 | y = 0.0414x − 0.0464 | y = 0.0414x − 0.0468 | y = 0.0414x − 0.0484 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tillmann, W.; Kokalj, D.; Stangier, D.; Schöppner, V.; Malatyali, H. Effects of AlN and BCN Thin Film Multilayer Design on the Reaction Time of Ni/Ni-20Cr Thin Film Thermocouples on Thermally Sprayed Al2O3. Sensors 2019, 19, 3414. https://doi.org/10.3390/s19153414
Tillmann W, Kokalj D, Stangier D, Schöppner V, Malatyali H. Effects of AlN and BCN Thin Film Multilayer Design on the Reaction Time of Ni/Ni-20Cr Thin Film Thermocouples on Thermally Sprayed Al2O3. Sensors. 2019; 19(15):3414. https://doi.org/10.3390/s19153414
Chicago/Turabian StyleTillmann, Wolfgang, David Kokalj, Dominic Stangier, Volker Schöppner, and Hatice Malatyali. 2019. "Effects of AlN and BCN Thin Film Multilayer Design on the Reaction Time of Ni/Ni-20Cr Thin Film Thermocouples on Thermally Sprayed Al2O3" Sensors 19, no. 15: 3414. https://doi.org/10.3390/s19153414
APA StyleTillmann, W., Kokalj, D., Stangier, D., Schöppner, V., & Malatyali, H. (2019). Effects of AlN and BCN Thin Film Multilayer Design on the Reaction Time of Ni/Ni-20Cr Thin Film Thermocouples on Thermally Sprayed Al2O3. Sensors, 19(15), 3414. https://doi.org/10.3390/s19153414





