Microanalyser Prototype for On-Line Monitoring of Copper(II) Ion in Mining Industrial Processes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
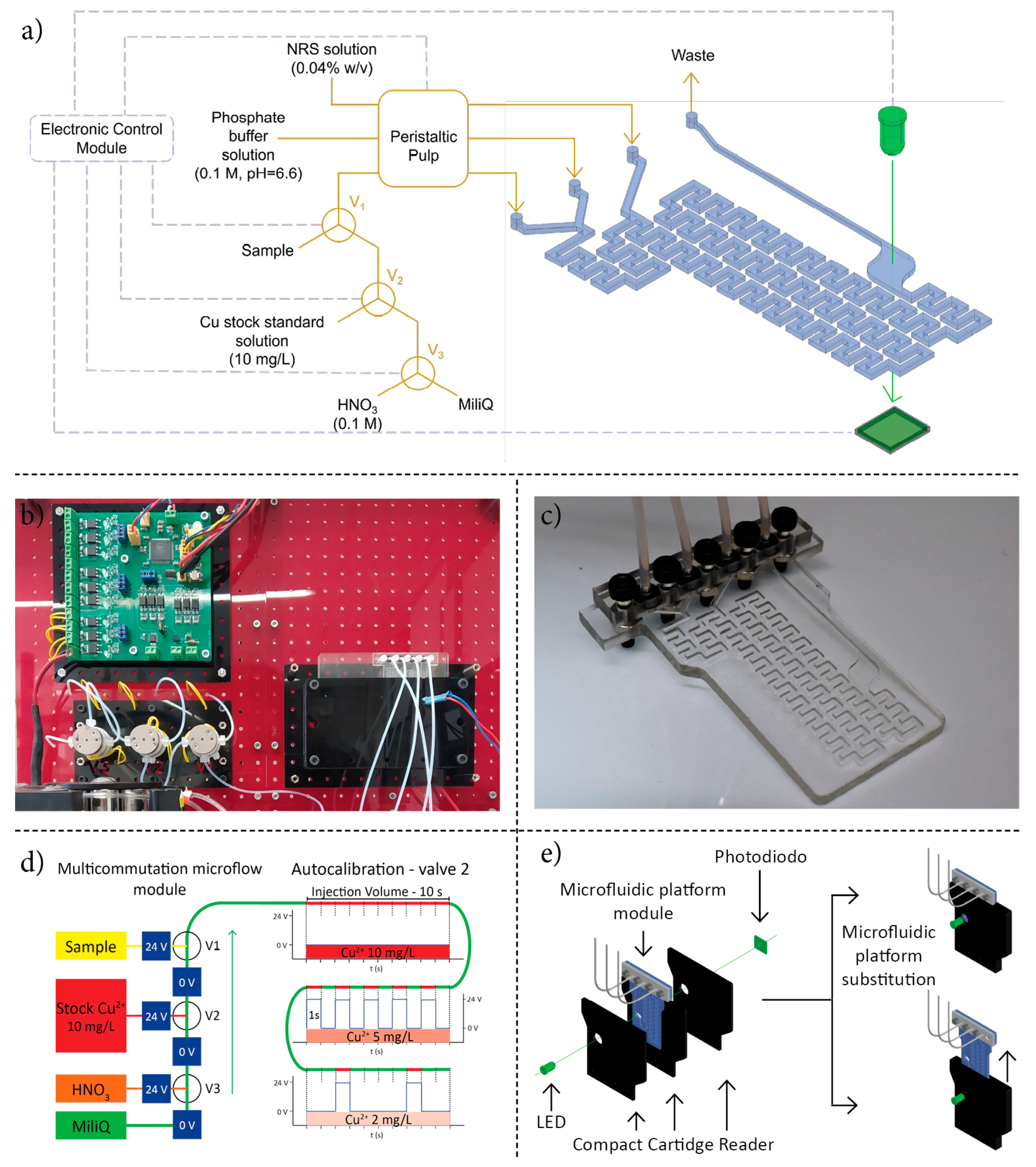
3.1. Microanalyzer Design and Fabrication
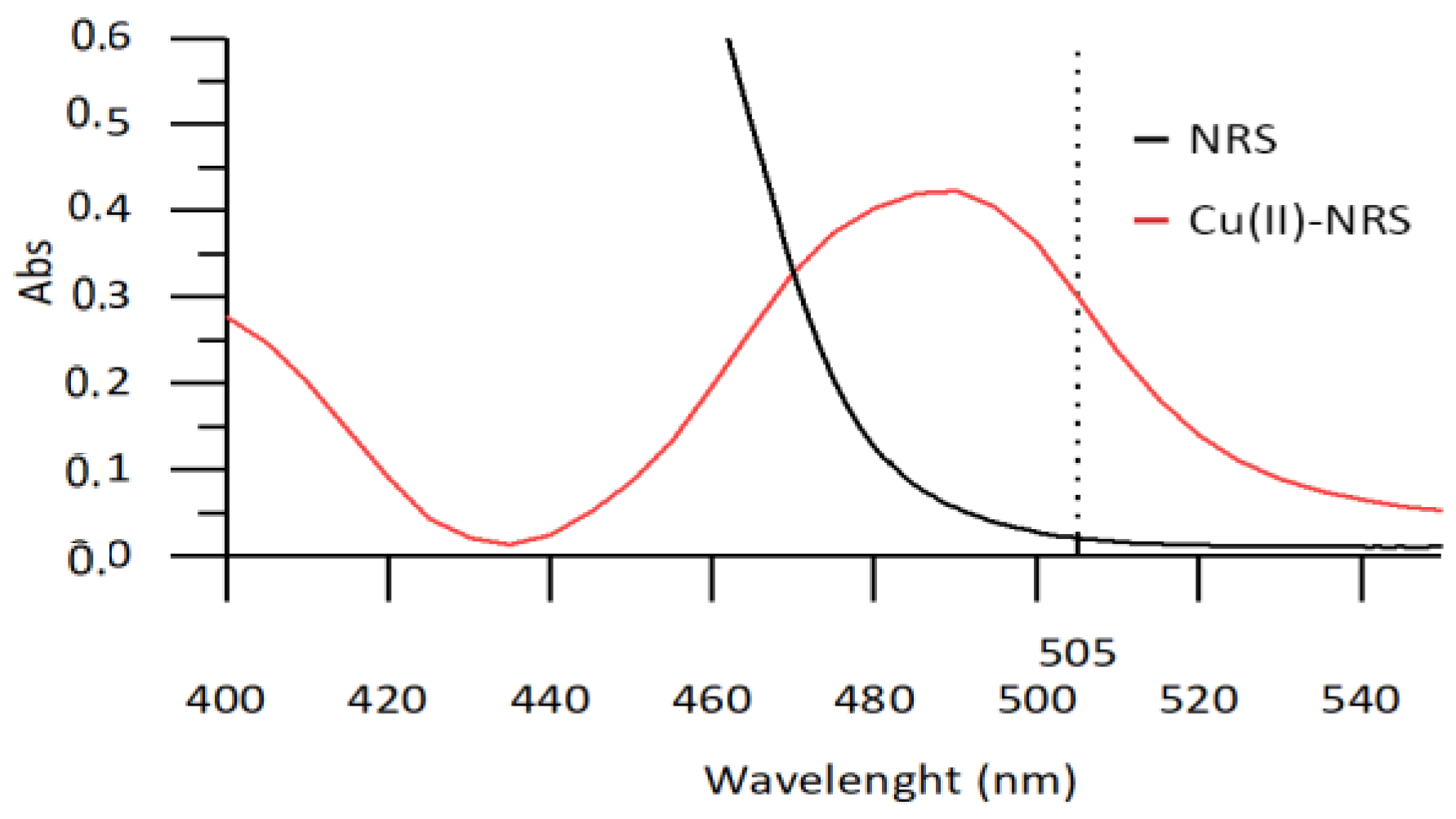
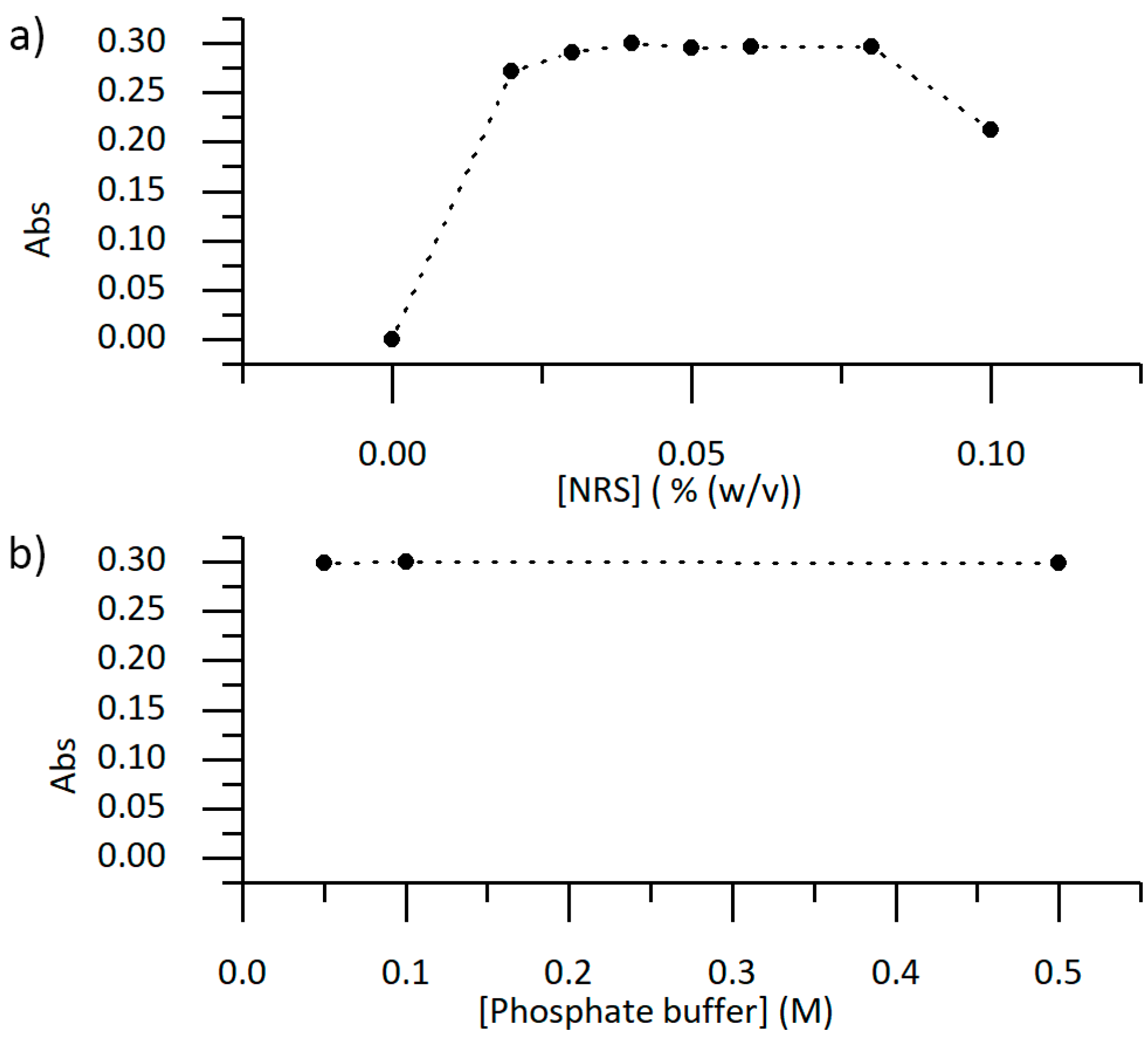
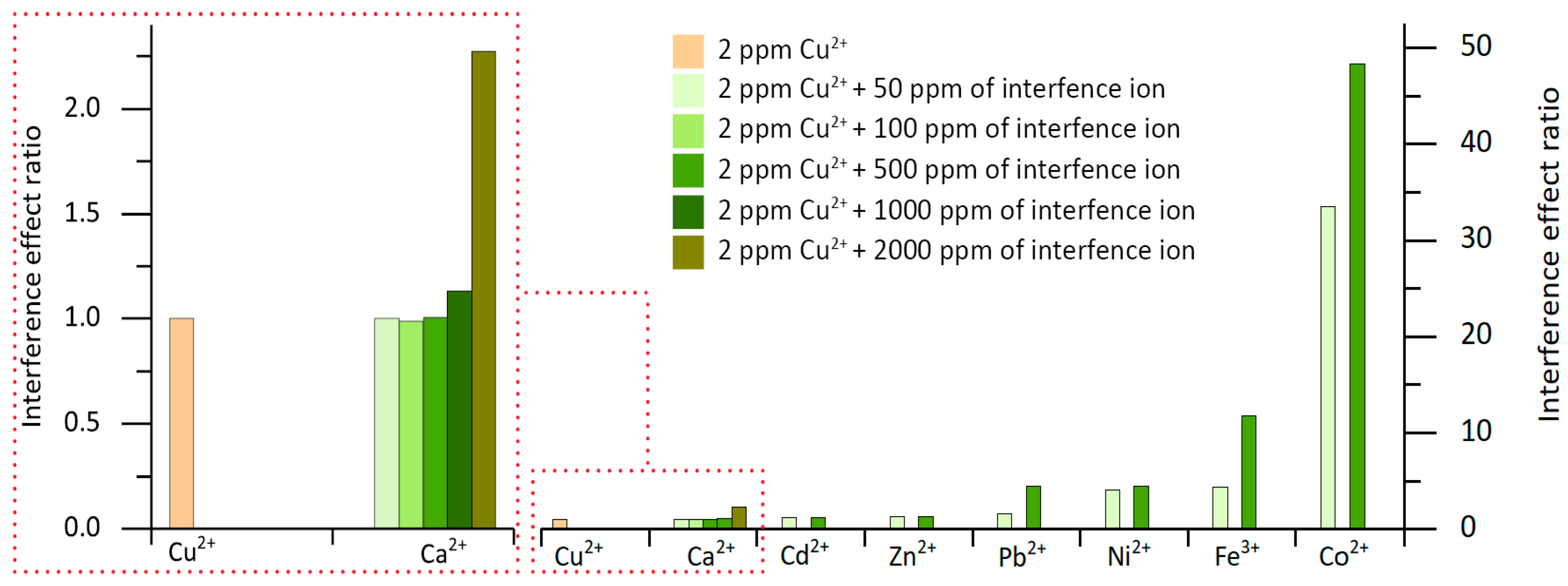
3.2. Chemical and Hydrodynamic Optimization of the Microanalyzer Prototype Variables
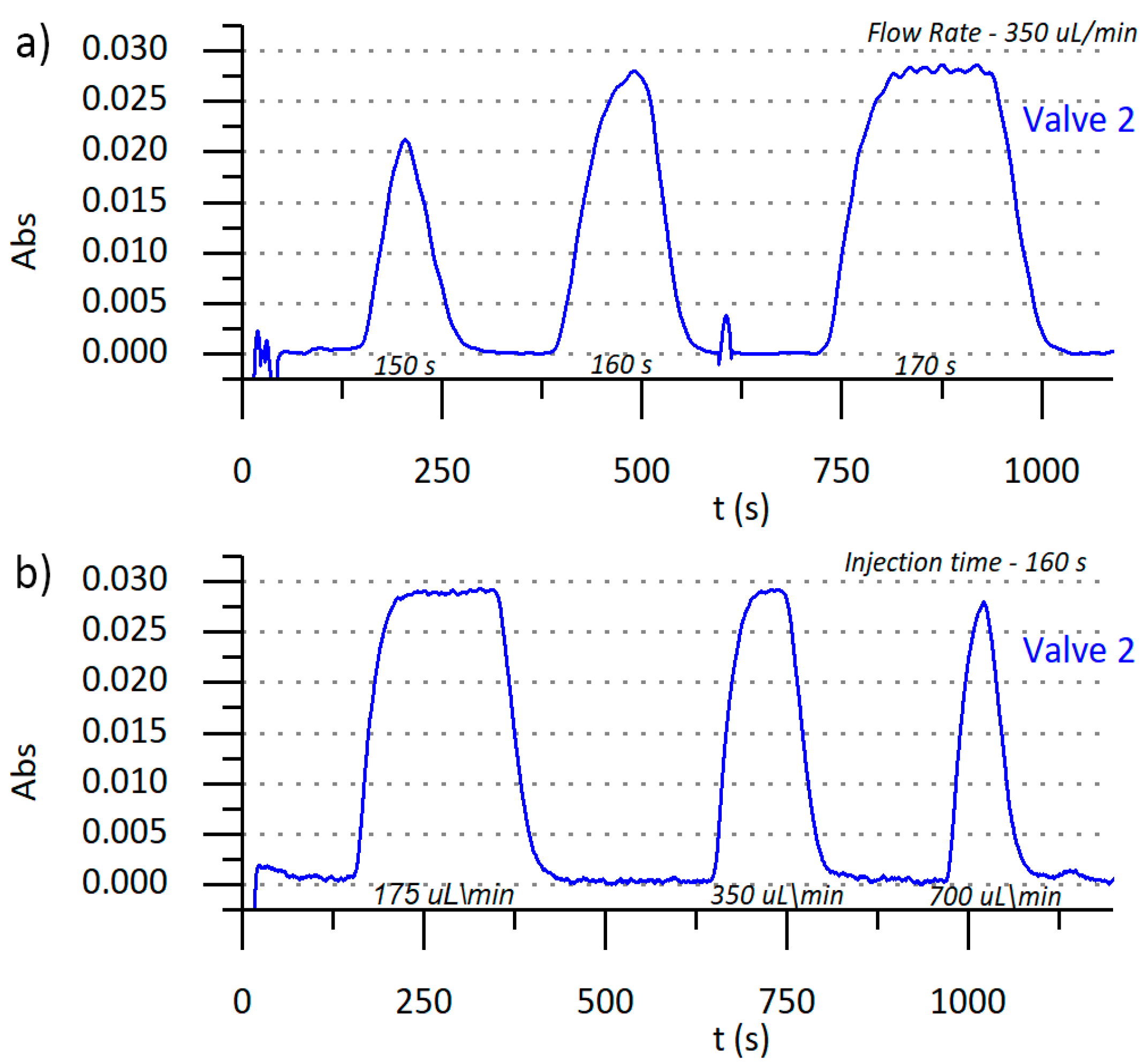
3.3. Microanalyzer Programable Sequence
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davenport, W.G.; King, M.; Schlesinger, M.; Biswas, A.K. Extractive Metalurgy of Cooper, 4th ed.; Pergamon Press: Oxford, UK, 2002; ISBN 0444502068. [Google Scholar]
- Ramos, L.; Ramos, J.J.; Brinkman, U.A.T. Miniaturization in sample treatment for environmental analysis. Anal. Bioanal. Chem. 2005, 381, 119–140. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Monochloramine in Drinking-Water. Background Document for Development of WHO Guide Lines for Drinking-Water Quality. Available online: http://www.who.int/water_sanitation_health/water-quality/guidelines/chemicals/chloramine-background.pdf (accessed on 1 August 2019).
- Widmer, H.M. Trends in industrial analytical chemistry. Trends Anal. Chem. 1983, 2, 1–3. [Google Scholar] [CrossRef]
- Van den Berg, A.; Bergveld, P. Labs-on-a-Chip: Origin, highlights and future perspectives on the occasion of the 10th microTAS conference. Lab Chip 2006, 6, 1266–1273. [Google Scholar] [PubMed]
- Manz, A.; Graber, N.; Widmers, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Patabadige, D.E.W.; Jia, S.; Sibbitts, J.; Sadeghi, J.; Sellens, K.; Culbertson, C.T. Micro Total Analysis Systems: Fundamental Advances and Applications. Anal. Chem. 2016, 88, 320–338. [Google Scholar] [CrossRef]
- Simonet, B.; Rios, A.; Escarpa, A. Miniaturization in Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Smith, M.J.; Manahan, S.E. Copper Determination in Water by Standard Addition Potentiometry. Anal. Chem. 1973, 45, 836–839. [Google Scholar] [CrossRef]
- Koplik, R.; Mestek, O.; Fingerová, H.; Suchánek, M. Validation protocol for the determination of copper in plant samples by isotope dilution inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1999, 14, 241–245. [Google Scholar] [CrossRef]
- Willard, H.H.; Kaufman, S. Colorimetric Determination of Cobalt Using Nitroso-R Salt. Anal. Chem. 1947, 19, 505. [Google Scholar] [CrossRef]
- Kruanetr, S.; Tengjaroenkul, U.; Liawruangrath, B.; Liawruangrath, S. A cleaner and simple spectrophotometric micro-fluidic procedure for copper determination using nitroso-R salt as chromogenic agent. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2008, 70, 1134–1140. [Google Scholar] [CrossRef]
- Pinto, J.J.; Moreno, C.; García-Vargas, M. A simple and very sensitive spectrophotometric method for the direct determination of copper ions. Anal. Bioanal. Chem. 2002, 373, 844–848. [Google Scholar] [CrossRef]
- Säbel, C.E.; Neureuther, J.M.; Siemann, S. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal. Biochem. 2010, 397, 218–226. [Google Scholar] [CrossRef]
- Phansi, P.; Henríquez, C.; Palacio, E.; Nacapricha, D.; Cerdà, V. An automated in-chip-catalytic-spectrophotometric method for determination of copper(ii) using a multisyringe flow injection analysis-multipumping flow system. Anal. Meth. 2014, 6, 8494–8504. [Google Scholar] [CrossRef]
- Wan Khalid, W.E.F.; Ahmad, M.; Heng, L.Y. Characterization of a simple optical chemical sensor for Cu(II) detection based on immobilization of lipophilized nitroso-r reagent in sol-gel matrix. Chiang Mai J. Sci. 2014, 41, 383–394. [Google Scholar]
- Leelasattarathkul, T.; Liawruangrath, S.; Rayanakorn, M.; Liawruangrath, B.; Oungpipat, W.; Youngvises, N. Greener analytical method for the determination of copper(II) in wastewater by micro flow system with optical sensor. Talanta 2007, 72, 126–131. [Google Scholar] [CrossRef]
- Purachat, B.; Liawruangrath, S.; Sooksamiti, P.; Rattanaphani, S.; Buddhasukh, D. Univariate and simplex optimization for the flow-injection spectrophotometric determination of copper using nitroso-R salt as a complexing agent. Anal. Sci. 2001, 17, 443–447. [Google Scholar] [CrossRef][Green Version]
- Lasi, H.; Fettke, P.; Kemper, H.-G.; Feld, T.; Hoffmann, M. Industry 4.0. Bus. Inf. Syst. Eng. 2014, 6, 239–242. [Google Scholar] [CrossRef]
- Dasgupta, P.K.; Eom, I.Y.; Morris, K.J.; Li, J. Light emitting diode-based detectors: Absorbance, fluorescence and spectroelectrochemical measurements in a planar flow-through cell. Anal. Chim. Acta 2003, 500, 337–364. [Google Scholar] [CrossRef]
- de Lima, K.M.G. A portable photometer based on LED for the determination of aromatic hydrocarbons in water. Microchem. J. 2012, 103, 62–67. [Google Scholar] [CrossRef]
- da Rocha, Z.M.; Martinez-Cisneros, C.S.; Seabra, A.C.; Valdés, F.; Gongora-Rubio, M.R.; Alonso-Chamarro, J. Compact and autonomous multiwavelength microanalyzer for in-line and in situ colorimetric determinations. Lab Chip 2012, 12, 109–117. [Google Scholar] [CrossRef]
- Pedro, S.G.; Puyol, M.; Izquierdo, D.; Salinas, I.; de la Fuente, J.M.; Alonso-Chamarro, J.A. Ceramic microreactor for the synthesis of water soluble CdS and CdS/ZnS nanocrystals with on-line optical characterization. Nanoscale 2012, 4, 1328–1335. [Google Scholar] [CrossRef]
- Calvo-López, A.; Ymbern, O.; Izquierdo, D.; Alonso-Chamarro, J. Low cost and compact analytical microsystem for carbon dioxide determination in production processes of wine and beer. Anal. Chim. Acta 2016, 931, 64–69. [Google Scholar] [CrossRef]
- Davies, J.L.; Andrews, G.S.; Miller, R.; Owen, H.G. Comparison of the stannous chloride and vanadate methods for estimation of serum inorganic phosphorus by use of the “SMA 12-60”. Clin. Chem. 1973, 19, 411–414. [Google Scholar]
- Ventura-Gayete, J.F.; Armenta, S.; Garrigues, S.; Morales-Rubio, Á.; De La Guardia, M. Multicommutation-NIR determination of Hexythiazox in pesticide formulations. Talanta 2006, 68, 1700–1706. [Google Scholar] [CrossRef]
- de Andrade, M.F.; de Assis, S.G.F.; Paim, A.P.S.; dos Reis, B.F. Multicommuted Flow Analysis Procedure for Total Polyphenols Determination in Wines Employing Chemiluminescence Detection. Food Anal. Meth. 2014, 7, 967–976. [Google Scholar] [CrossRef]
- de Souza, G.C.S.; Bezerra da Silva, P.A.; da S. Leotério, D.M.; Paim, A.P.S.; Lavorante, A.F. A multicommuted flow system for fast screening/sequential spectrophotometric determination of dichromate, salicylic acid, hydrogen peroxide and starch in milk samples. Food Control 2014, 46, 127–135. [Google Scholar] [CrossRef]
- Pessoa-Neto, O.D.; Dos Santos, V.B.; Vicentini, F.C.; Suarez, W.T.; Alonso-Chamarro, J.; Fatibello-Filho, O.; Faria, R.C. A low-cost automated flow analyzer based on low temperature co-fired ceramic and LED photometer for ascorbic acid determination. Cent. Eur. J. Chem. 2014, 12, 341–347. [Google Scholar] [CrossRef]
- Ymbern, O.; Sández, N.; Calvo-López, A.; Puyol, M.; Alonso-Chamarro, J. Gas diffusion as a new fluidic unit operation for centrifugal microfluidic platforms. Lab Chip 2014, 14, 1014–1022. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef]
- Sequeira, M.; Bowden, M.; Minogue, E.; Diamond, D. Towards autonomous environmental monitoring systems. Talanta 2002, 56, 355–363. [Google Scholar] [CrossRef]
- Estados Unidos Mexicanos.—Secretaría de Medio Ambiente, Recursos Naturales y Pesca. Norma Oficial Mexicana NOM-001-ECOL-1996. Available online: http://siga.jalisco.gob.mx/Assets/documentos/normatividad/nom001semarnat1996.htm (accessed on 1 August 2019).
- Ymbern, O.; Berenguel-Alonso, M.; Calvo-López, A.; Gómez-De Pedro, S.; Izquierdo, D.; Alonso-Chamarro, J. Versatile lock and key assembly for optical measurements with microfluidic platforms and cartridges. Anal. Chem. 2015, 87, 1503–1508. [Google Scholar] [CrossRef]
- Calvo López, A. Diseño, Construcción y Evaluación de Analizadores Miniaturizados para su Aplicación Aeroespacial, Medioambiental, Alimentaria, Biomédica e Industrial. Ph.D. Thesis, Universitat Autònoma de Barcelona, Catalonia, Spain, 2017. [Google Scholar]

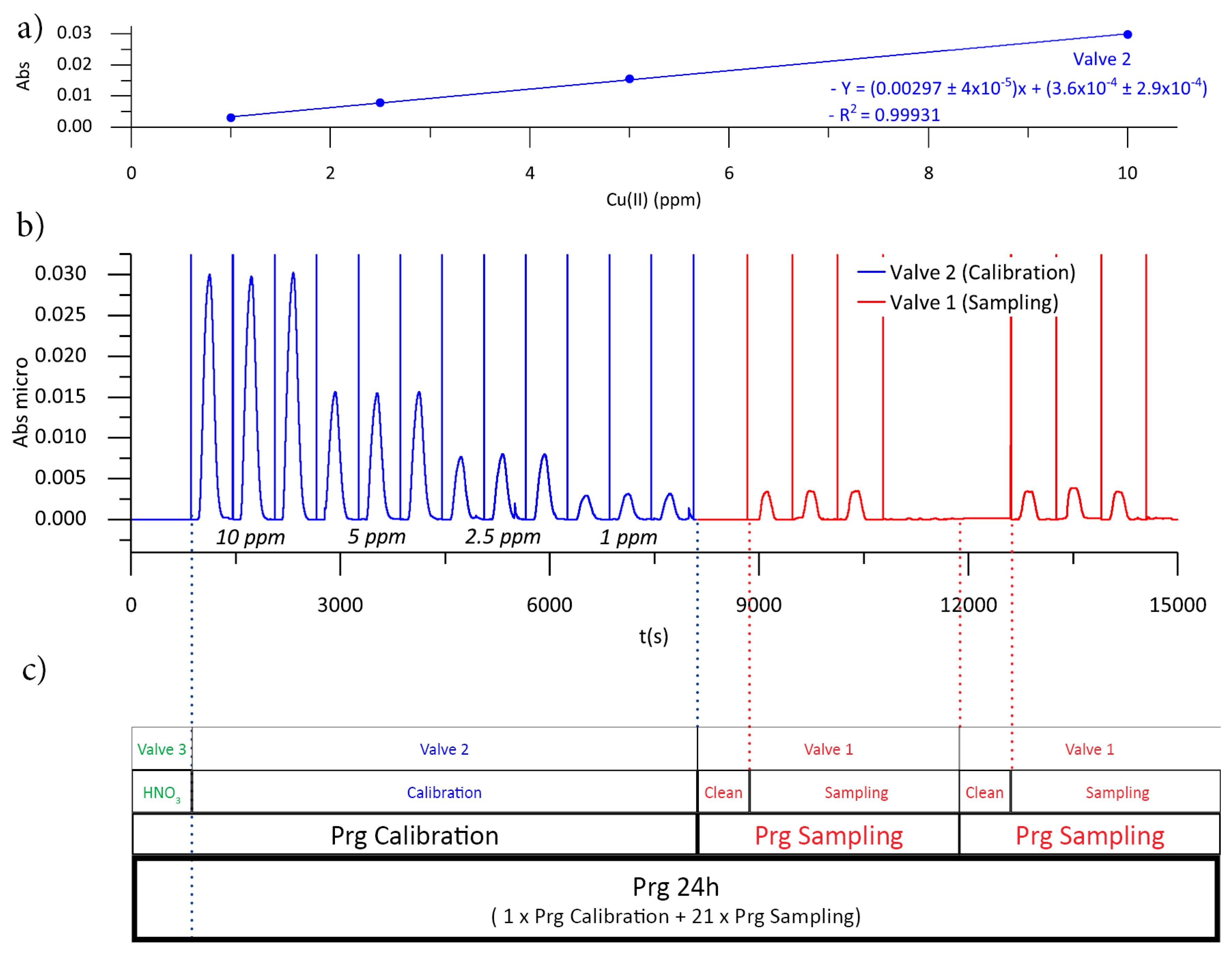
| Cu (mg/L) | Dilution (1/n) | Valve Position | ||
|---|---|---|---|---|
| Stock [Cu] = 10 mg/L | H2O (MiliQ) | Cycles | ||
| 10 | 1 | 160 s | 0 | ×1 |
| 5 | 1/2 | 1 s | 1 s | ×80 |
| 2.5 | 1/4 | 1 s | 3 s | ×40 |
| 1 | 1/10 | 1 s | 9 s | ×16 |
| 0.5 | 1/20 | 1 s | 19 s | ×8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara Amatón, K.V.; Couceiro, P.; Coto Fuentes, H.; Calvo-Lopez, A.; Sández, N.; Moreno Casillas, H.A.; Valdés Perezgasga, F.; Alonso-Chamarro, J. Microanalyser Prototype for On-Line Monitoring of Copper(II) Ion in Mining Industrial Processes. Sensors 2019, 19, 3382. https://doi.org/10.3390/s19153382
Guevara Amatón KV, Couceiro P, Coto Fuentes H, Calvo-Lopez A, Sández N, Moreno Casillas HA, Valdés Perezgasga F, Alonso-Chamarro J. Microanalyser Prototype for On-Line Monitoring of Copper(II) Ion in Mining Industrial Processes. Sensors. 2019; 19(15):3382. https://doi.org/10.3390/s19153382
Chicago/Turabian StyleGuevara Amatón, Karla Victoria, Pedro Couceiro, Hesner Coto Fuentes, Antonio Calvo-Lopez, Natàlia Sández, Héctor Aurelio Moreno Casillas, Francisco Valdés Perezgasga, and Julián Alonso-Chamarro. 2019. "Microanalyser Prototype for On-Line Monitoring of Copper(II) Ion in Mining Industrial Processes" Sensors 19, no. 15: 3382. https://doi.org/10.3390/s19153382
APA StyleGuevara Amatón, K. V., Couceiro, P., Coto Fuentes, H., Calvo-Lopez, A., Sández, N., Moreno Casillas, H. A., Valdés Perezgasga, F., & Alonso-Chamarro, J. (2019). Microanalyser Prototype for On-Line Monitoring of Copper(II) Ion in Mining Industrial Processes. Sensors, 19(15), 3382. https://doi.org/10.3390/s19153382




