Recognition of Pharmacological Bi-Heterocyclic Compounds by Using Terahertz Time Domain Spectroscopy and Chemometrics
Abstract
1. Introduction
1.1. Test Methods Used to Diagnose Drugs
1.2. Drug Detection Using Terahertz Spectroscopy Combined with Support Vector Machine (Svm)
2. Experimental Study
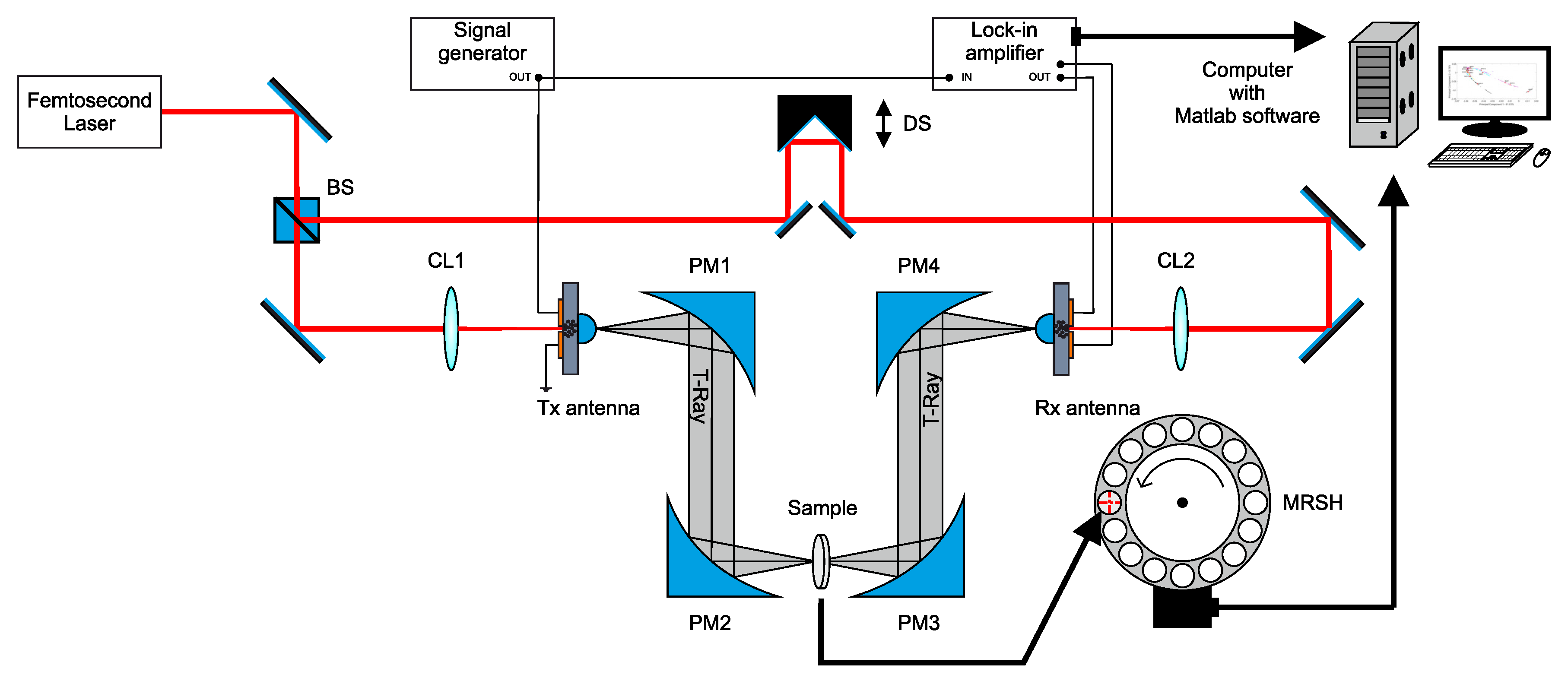
2.1. TDS-THz Spectrometer
2.2. Method of Manufacture of the Samples
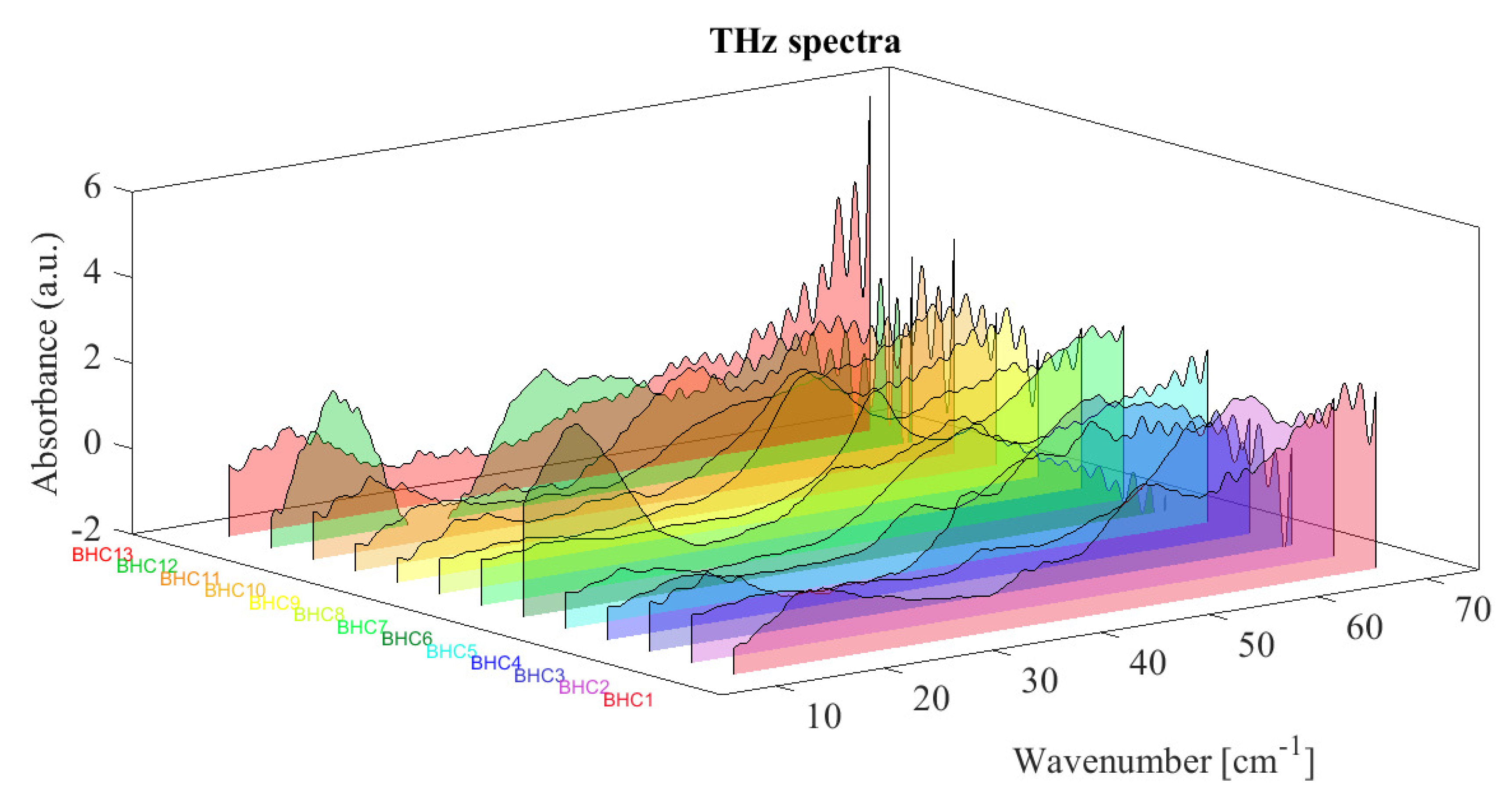
2.3. Chemical Structure of Bi-Heterocyclic Compounds
3. Statistical Analysis
3.1. PCA
3.2. KSVM
- d-degree polynomial:, where and ,
- Gaussian Radial Basis Functions (GRBF):, where
- two-layer perceptron:, where .
4. Classification Results
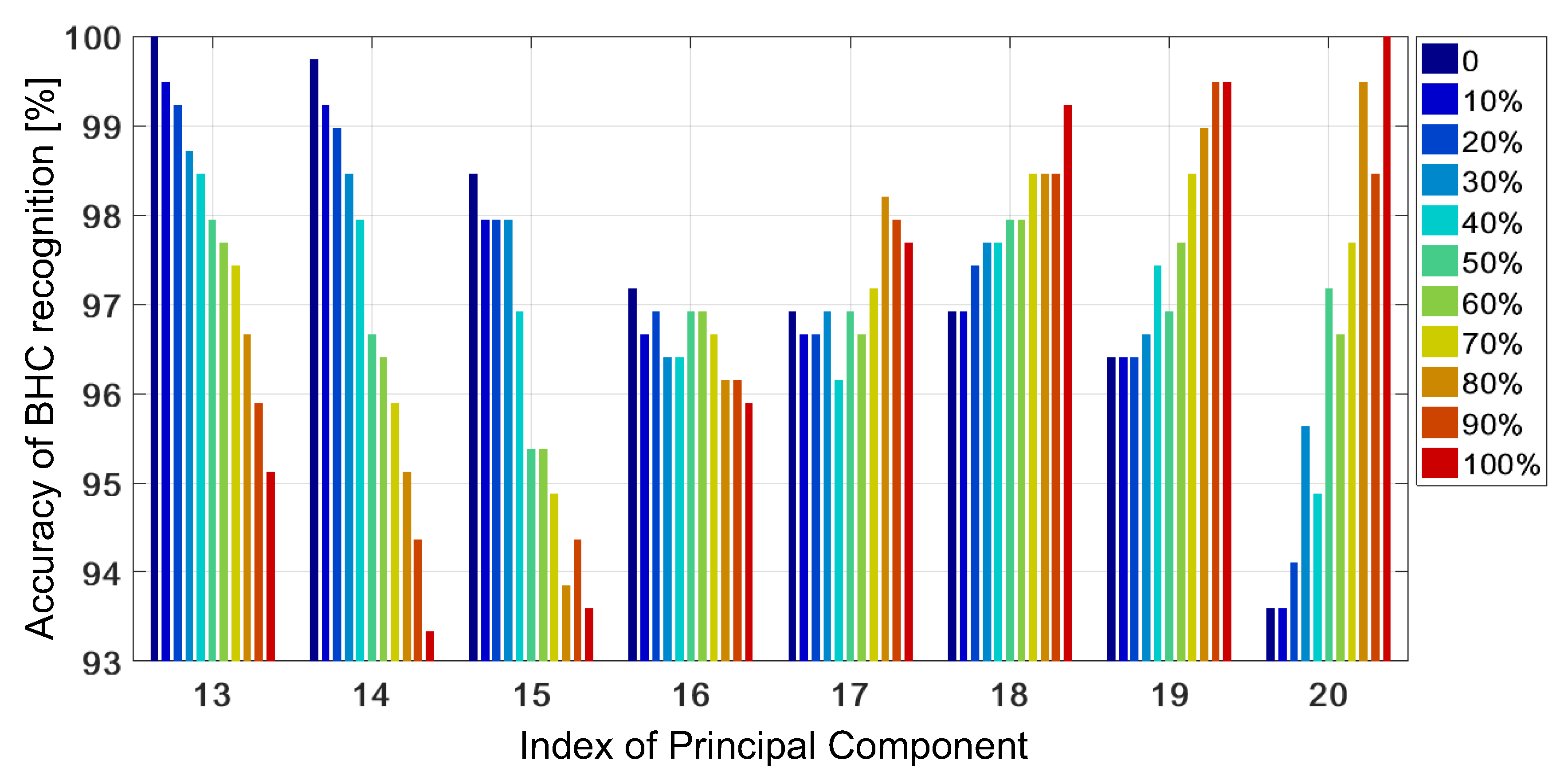
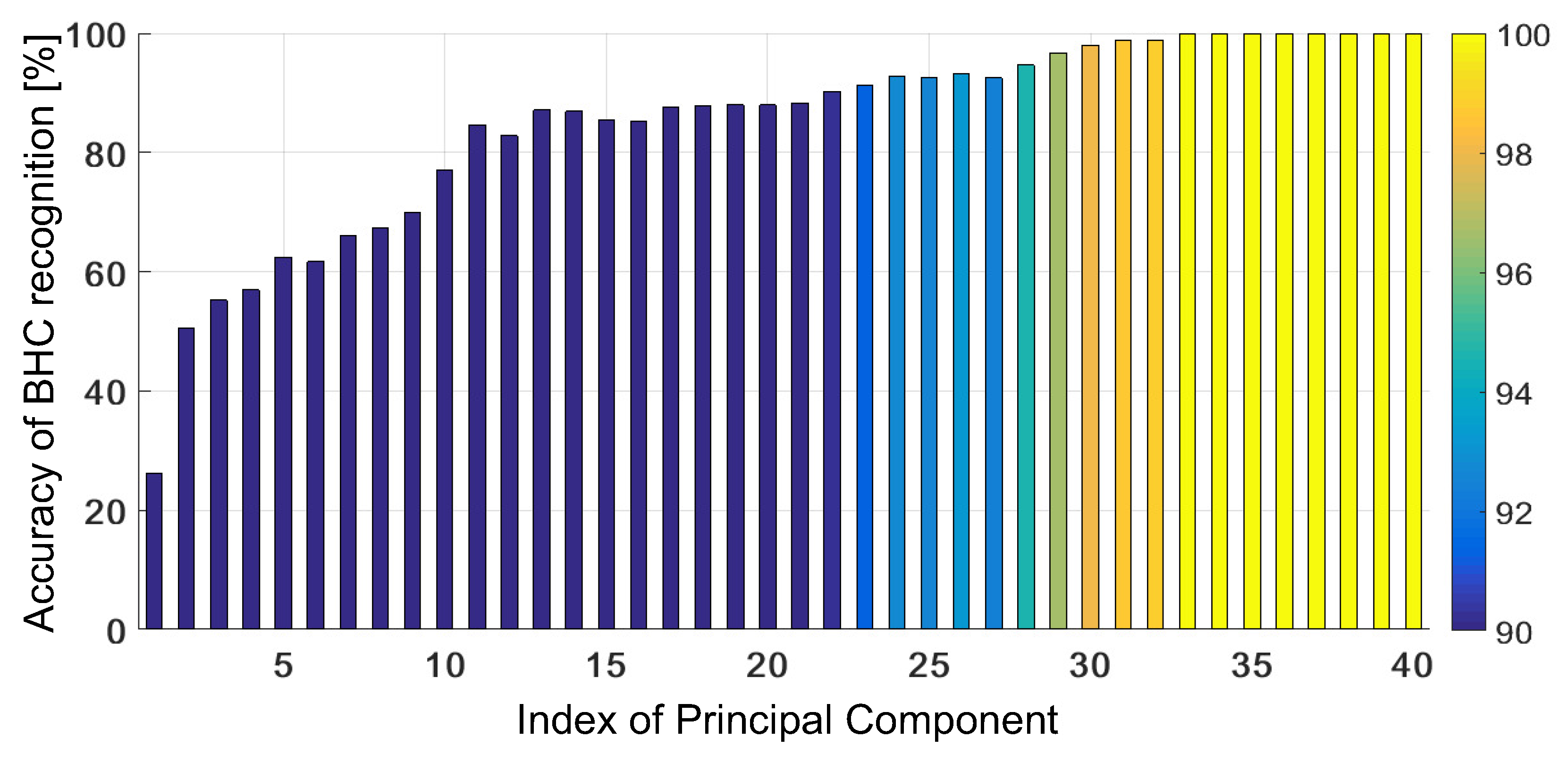
4.1. Untrained GRBF Kernel
4.2. Trained GRBF Kernel
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eicher, T.; Hauptmann, S.; Speicher, A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry at a Glance; John Wiley & Sons: West Sussex, UK, 2012. [Google Scholar]
- Yanagimoto, K.; Lee, K.G.; Ochi, H.; Shibamoto, T. Antioxidative activity of heterocyclic compounds found in coffee volatiles produced by Maillard reaction. J. Agric. Food Chem. 2002, 50, 5480–5484. [Google Scholar] [CrossRef] [PubMed]
- Agranat, I. Pharmaceutical Substances: Syntheses, Patents and Applications of the Most Relevant AIPs. Synthesis 2009, 2009, 2116. [Google Scholar] [CrossRef][Green Version]
- Lamberth, C.; Dinges, J. Bioactive Heterocyclic Compound Classes: Pharmaceuticals; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Kujawski, J.; Janusz, A.; Kuźma, W.; Ożarowski, M.; Kujawski, R. Biologicznie ważne substancje zawierające pierścień pirolu–działanie i występowanie. Farm Pol. 2010, 66, 117–125. [Google Scholar]
- Selvam, T.P.; James, C.R.; Dniandev, P.V.; Valzita, S.K. A mini review of pyrimidine and fused pyrimidine marketed drugs. Res. Pharm. 2012, 2, 4. [Google Scholar]
- Selvam, T.P.; Kumar, P.V. Quinazoline marketed drugs. Res. Pharm. 2011, 1, 1–21. [Google Scholar]
- Majumdar, K.C.; Chattopadhyay, S.K. Heterocycles in Natural Product Synthesis; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Alvarez-Builla, J.; Vaquero, J.J.; Barluenga, J. Modern Heterocyclic Chemistry, 4 Volume Set; Wiley-VCH: Weinheim, Germany, 2011; Volume 2. [Google Scholar]
- Paudel, A.; Raijada, D.; Rantanen, J. Raman spectroscopy in pharmaceutical product design. Adv. Drug Deliv. Rev. 2015, 89, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Nyadong, L.; Yang, F.; Fernandez, F.M.; Brown, C.D.; Newton, P.N.; Kazarian, S.G. Assessment of hand-held Raman instrumentation for in situ screening for potentially counterfeit artesunate antimalarial tablets by FT-Raman spectroscopy and direct ionization mass spectrometry. Anal. Chim. Acta 2008, 623, 178–186. [Google Scholar] [CrossRef]
- Jamrógiewicz, M. Application of the near-infrared spectroscopy in the pharmaceutical technology. J. Pharm. Biomed. Anal. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Igne, B.; Ciurczak, E.W. Pharmaceutical and Medical Applications of Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- De Beer, T.; Burggraeve, A.; Fonteyne, M.; Saerens, L.; Remon, J.P.; Vervaet, C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int. J. Pharm. 2011, 417, 32–47. [Google Scholar] [CrossRef]
- Bressolle, F.; Audran, M.; Pham, T.N.; Vallon, J.J. Cyclodextrins and enantiomeric separations of drugs by liquid chromatography and capillary electrophoresis: Basic principles and new developments. J. Chromatogr. Biomed. Sci. Appl. 1996, 687, 303–336. [Google Scholar] [CrossRef]
- Phadnis, N.V.; Cavatur, R.K.; Suryanarayanan, R. Identification of drugs in pharmaceutical dosage forms by X-ray powder diffractometry. J. Pharm. Biomed. Anal. 1997, 15, 929–943. [Google Scholar] [CrossRef]
- Rösner, P.; Junge, T.; Westphal, F.; Fritschi, G. Mass Spectra of Designer Drugs 2015; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Hummel, D.; Löffler, D.; Fink, G.; Ternes, T.A. Simultaneous determination of psychoactive drugs and their metabolites in aqueous matrices by liquid chromatography mass spectrometry. Environ. Sci. Technol. 2006, 40, 7321–7328. [Google Scholar] [CrossRef] [PubMed]
- Barras, J.; Althoefer, K.; Rowe, M.; Poplett, I.; Smith, J. The emerging field of medicines authentication by nuclear quadrupole resonance spectroscopy. Appl. Magn. Reson. 2012, 43, 511–529. [Google Scholar] [CrossRef]
- Barras, J.; Murnane, D.; Althoefer, K.; Assi, S.; Rowe, M.D.; Poplett, I.J.; Kyriakidou, G.; Smith, J.A. Nitrogen-14 nuclear quadrupole resonance spectroscopy: A promising analytical methodology for medicines authentication and counterfeit antimalarial analysis. Anal. Chem. 2013, 85, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Buffler, A. Contraband detection with fast neutrons. Radiat. Phys. Chem. 2004, 71, 853–861. [Google Scholar] [CrossRef]
- Buffler, A.; Tickner, J. Detecting contraband using neutrons: Challenges and future directions. Radiat. Meas. 2010, 45, 1186–1192. [Google Scholar] [CrossRef]
- AlNabooda, M.O.; Shubair, R.M.; Rishani, N.R.; Aldabbagh, G. Terahertz spectroscopy and imaging for the detection and identification of illicit drugs. In Proceedings of the Sensors Networks Smart And Emerging Technologies (Senset), Beirut, Lebanon, 12–14 September 2017; pp. 1–4. [Google Scholar]
- Puc, U.; Abina, A.; Rutar, M.; Zidanšek, A.; Jeglič, A.; Valušis, G. Terahertz spectroscopic identification of explosive and drug simulants concealed by various hiding techniques. Appl. Opt. 2015, 54, 4495–4502. [Google Scholar] [CrossRef] [PubMed]
- Federici, J.F.; Schulkin, B.; Huang, F.; Gary, D.; Barat, R.; Oliveira, F.; Zimdars, D. THz imaging and sensing for security applications—Explosives, weapons and drugs. Semicond. Sci. Technol. 2005, 20, S266–S280. [Google Scholar] [CrossRef]
- Burnett, A.D.; Cunningham, J.E.; Davies, A.G.; Dean, P.; Linfield, E.H. Terahertz frequency spectroscopy and its potential for security applications. In Infrared and Raman Spectroscopy in Forensic Science; Wiley Online Library: West Sussex, UK, 2012; pp. 295–314. [Google Scholar]
- Kawase, K.; Ogawa, Y.; Watanabe, Y.; Inoue, H. Non-destructive terahertz imaging of illicit drugs using spectral fingerprints. Opt. Express 2003, 11, 2549–2554. [Google Scholar] [CrossRef]
- Kato, M.; Tripathi, S.R.; Murate, K.; Imayama, K.; Kawase, K. Non-destructive drug inspection in covering materials using a terahertz spectral imaging system with injection-seeded terahertz parametric generation and detection. Opt. Express 2016, 24, 6425–6432. [Google Scholar] [CrossRef]
- Shen, Y.C.; Taday, P.F. Development and application of terahertz pulsed imaging for nondestructive inspection of pharmaceutical tablet. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 407–415. [Google Scholar] [CrossRef]
- Ho, L.; Cuppok, Y.; Muschert, S.; Gordon, K.C.; Pepper, M.; Shen, Y.; Siepmann, F.; Siepmann, J.; Taday, P.F.; Rades, T. Effects of film coating thickness and drug layer uniformity on in vitro drug release from sustained-release coated pellets: A case study using terahertz pulsed imaging. Int. J. Pharm. 2009, 382, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Porter, S.C. An update on pharmaceutical film coating for drug delivery. Expert Opin. Drug Deliv. 2013, 10, 421–435. [Google Scholar] [CrossRef] [PubMed]
- May, R.K.; Evans, M.J.; Zhong, S.; Warr, I.; Gladden, L.F.; Shen, Y.; Zeitler, J.A. Terahertz in-line sensor for direct coating thickness measurement of individual tablets during film coating in real-time. J. Pharm. Sci. 2011, 100, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, J.A.; Shen, Y.C. Industrial applications of terahertz imaging. In Terahertz Spectroscopy and Imaging; Springer: Berlin/Heidelberg, Germany, 2013; pp. 451–489. [Google Scholar]
- Taday, P.F.; Bradley, I.; Arnone, D.; Pepper, M. Using terahertz pulse spectroscopy to study the crystalline structure of a drug: A case study of the polymorphs of ranitidine hydrochloride. J. Pharm. Sci. 2003, 92, 831–838. [Google Scholar] [CrossRef]
- Taday, P.F. Applications of terahertz spectroscopy to pharmaceutical sciences. Philos. Trans. R. Soc. Lond. Ser. Math. Phys. Eng. Sci. 2003, 362, 351–364. [Google Scholar] [CrossRef]
- Kojima, S.; Shibata, T.; Igawa, H.; Mori, T. Broadband terahertz time-domain spectroscopy: Crystalline and glassy drug materials. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Tsukuba, Japan, 2014; Volume 54, p. 012001. [Google Scholar]
- Du, S.; Li, H.; Xie, L.; Chen, L.; Peng, Y.; Zhu, Y.; Li, H.; Dong, P.; Wang, J. Vibrational frequencies of anti-diabetic drug studied by terahertz time-domain spectroscopy. Appl. Phys. Lett. 2012, 100, 143702. [Google Scholar] [CrossRef]
- Ueno, Y.; Ajito, K.; Kukutsu, N.; Tamechika, E. Quantitative analysis of amino acids in dietary supplements using terahertz time-domain spectroscopy. Anal. Sci. 2011, 27, 351. [Google Scholar] [CrossRef]
- Müller, J.; Brock, D.; Knop, K.; Zeitler, J.A.; Kleinebudde, P. Prediction of dissolution time and coating thickness of sustained release formulations using Raman spectroscopy and terahertz pulsed imaging. Eur. J. Pharm. Biopharm. 2012, 80, 690–697. [Google Scholar] [CrossRef]
- Shen, Y.C. Terahertz pulsed spectroscopy and imaging for pharmaceutical applications: A review. Int. J. Pharm. 2011, 417, 48–60. [Google Scholar] [CrossRef]
- Ergün, S.; Sönmez, S. Terahertz technology for military applications. J. Manag. Inf. Sci. 2015, 3, 13–16. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; He, Y.; Liu, K.; Fan, S.; Parrott, E.P.; Pickwell-MacPherson, E. Recent advances in terahertz technology for biomedical applications. Quant. Imaging Med. Surg. 2017, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Nagatsuma, T. Handbook of Terahertz Technologies: Devices and Applications; CRC Press: Roca Raton, FL, USA, 2015. [Google Scholar]
- Saeedkia, D. Handbook of Terahertz Technology For Imaging, Sensing And Communications; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Dexheimer, S.L. Terahertz Spectroscopy: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sterczewski, L.A.; Nowak, K.; Szlachetko, B.; Grzelczak, M.P.; Szczesniak-Siega, B.; Plinska, S.; Malinka, W.; Plinski, E.F. Chemometric evaluation of THz spectral similarity for the selection of early drug candidates. Sci. Rep. 2017, 7, 14583. [Google Scholar] [CrossRef] [PubMed]
- Byvatov, E.; Fechner, U.; Sadowski, J.; Schneider, G. Comparison of support vector machine and artificial neural network systems for drug/nondrug classification. J. Chem. Inf. Comput. Sci. 2003, 43, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Zararsiz, G.; Goksuluk, D. Drug/nondrug classification using support vector machines with various feature selection strategies. Comput. Methods Programs Biomed. 2014, 117, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Burbidge, R.; Trotter, M.; Buxton, B.; Holden, S. Drug design by machine learning: Support vector machines for pharmaceutical data analysis. Comput. Chem. 2001, 26, 5–14. [Google Scholar] [CrossRef]
- Louis, B.; Agrawal, V.K.; Khadikar, P.V. Prediction of intrinsic solubility of generic drugs using MLR, ANN and SVM analyses. Eur. J. Med. Chem. 2010, 45, 4018–4025. [Google Scholar] [CrossRef]
- Sorich, M.J.; Miners, J.O.; McKinnon, R.A.; Winkler, D.A.; Burden, F.R.; Smith, P.A. Comparison of linear and nonlinear classification algorithms for the prediction of drug and chemical metabolism by human UDP-glucuronosyltransferase isoforms. J. Chem. Inf. Comput. Sci. 2003, 43, 2019–2024. [Google Scholar] [CrossRef]
- Liu, M.; Wu, Y.; Chen, Y.; Sun, J.; Zhao, Z.; Chen, X.W.; Matheny, M.E.; Xu, H. Large-scale prediction of adverse drug reactions using chemical, biological, and phenotypic properties of drugs. J. Am. Med Inform. Assoc. 2012, 19, e28–e35. [Google Scholar] [CrossRef]
- Heikamp, K.; Bajorath, J. Support vector machines for drug discovery. Expert Opin. Drug Discov. 2014, 9, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Zhao, G.; Wang, W.; Li, F. Chinese traditional medicine recognition by support vector machine (SVM) terahertz spectrum. Guang Xue Guang Fen 2009, 29, 2346–2350. [Google Scholar]
- Nowak, M.; Nowak, K.; Grzelczak, M.; Szlachetko, B.; Sterczewski, L.; Plinski, E.; Swiatek, P.; Strzelecka, M.; Plinska, S.; Malinka, W. Machine learning applied to bi-heterocyclic drugs recognition. In Proceedings of the 2017 42nd International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Cancún, Mexico, 27 August–1 September 2017; pp. 1–2. [Google Scholar]
- Selzer, P.M. Comprehensive Analysis of Parasite Biology: From Metabolism to Drug Discovery; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Shibany, K.A.; Tötemeyer, S.; Pratt, S.L.; Paine, S.W. Equine hepatocytes: Isolation, cryopreservation, and applications to in vitro drug metabolism studies. Pharmacol. Res. Perspect. 2016, 4, e00268. [Google Scholar] [CrossRef] [PubMed]
- Platt, J. Sequential Minimal Optimization: A Fast Algorithm for Training Support Vector Machines. Technical Report MSR-TR-98-14, Microsoft Research. Available online: https://pdfs.semanticscholar.org/59ee/e096b49d66f39891eb88a6c84cc89acba12d.pdf (accessed on 28 June 2019).
- Zdunek, R.; Nowak, M.; Plinski, E. Statistical classification of soft solder alloys by laser-induced breakdown spectroscopy: Review of methods. J. Eur. Opt. Soc. Rapid Publ. 2016, 11, 16006. [Google Scholar] [CrossRef]
- Wojcik, M.R.; Zdunek, R.; Antonczak, A.J. Unsupervised verification of laser-induced breakdown spectroscopy dataset clustering. Spectrochim. Acta Part At. Spectrosc. 2016, 126, 84–92. [Google Scholar] [CrossRef]
- Hofmann, T.; Schölkopf, B.; Smola, A. Kernel Methods in Machine Learning. Ann. Stat. 2008, 36, 1171–1220. [Google Scholar] [CrossRef]
- Liu, W.; Principe, J.C.; Haykin, S. Kernel Adaptive Filtering: A Comprehensive Introduction, 1st ed.; Wiley Publishing: Hoboken, NJ, USA, 2010. [Google Scholar]
- Gendreau, M.; Potvin, J.Y. Handbook of Metaheuristics, 2nd ed.; Springer Publishing Company, Incorporated: New York, NY, USA, 2010. [Google Scholar]
- De Jong, K.A. Evolutionary Computation: A Unified Approach; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Lewis, R.M.; Torczon, V.; Trosset, M.W. Direct search methods: Then and now. J. Comput. Appl. Math. 2000, 124, 191–207. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]




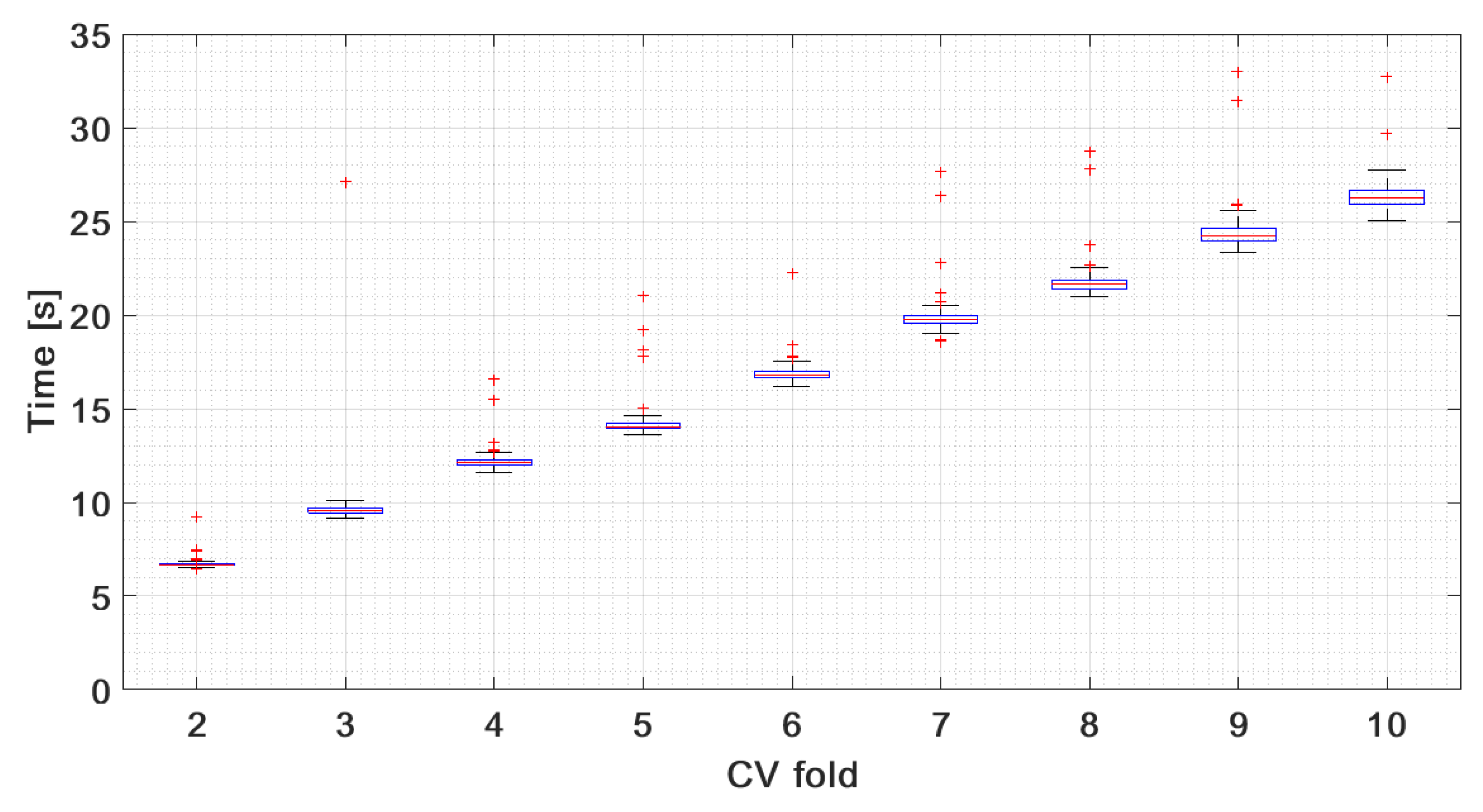

| No. initialization points: | 5 | 10 | 15 | 20 | 30 | 50 |
| Mean training time [s]: | 4.01 | 4.84 | 5.34 | 5.97 | 6.7 | 8.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, M.R.; Zdunek, R.; Pliński, E.; Świątek, P.; Strzelecka, M.; Malinka, W.; Plińska, S. Recognition of Pharmacological Bi-Heterocyclic Compounds by Using Terahertz Time Domain Spectroscopy and Chemometrics. Sensors 2019, 19, 3349. https://doi.org/10.3390/s19153349
Nowak MR, Zdunek R, Pliński E, Świątek P, Strzelecka M, Malinka W, Plińska S. Recognition of Pharmacological Bi-Heterocyclic Compounds by Using Terahertz Time Domain Spectroscopy and Chemometrics. Sensors. 2019; 19(15):3349. https://doi.org/10.3390/s19153349
Chicago/Turabian StyleNowak, Maciej Roman, Rafał Zdunek, Edward Pliński, Piotr Świątek, Małgorzata Strzelecka, Wiesław Malinka, and Stanisława Plińska. 2019. "Recognition of Pharmacological Bi-Heterocyclic Compounds by Using Terahertz Time Domain Spectroscopy and Chemometrics" Sensors 19, no. 15: 3349. https://doi.org/10.3390/s19153349
APA StyleNowak, M. R., Zdunek, R., Pliński, E., Świątek, P., Strzelecka, M., Malinka, W., & Plińska, S. (2019). Recognition of Pharmacological Bi-Heterocyclic Compounds by Using Terahertz Time Domain Spectroscopy and Chemometrics. Sensors, 19(15), 3349. https://doi.org/10.3390/s19153349






