Early, Non-Invasive Sensing of Sustained Hyperglycemia in Mice Using Millimeter-Wave Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Population
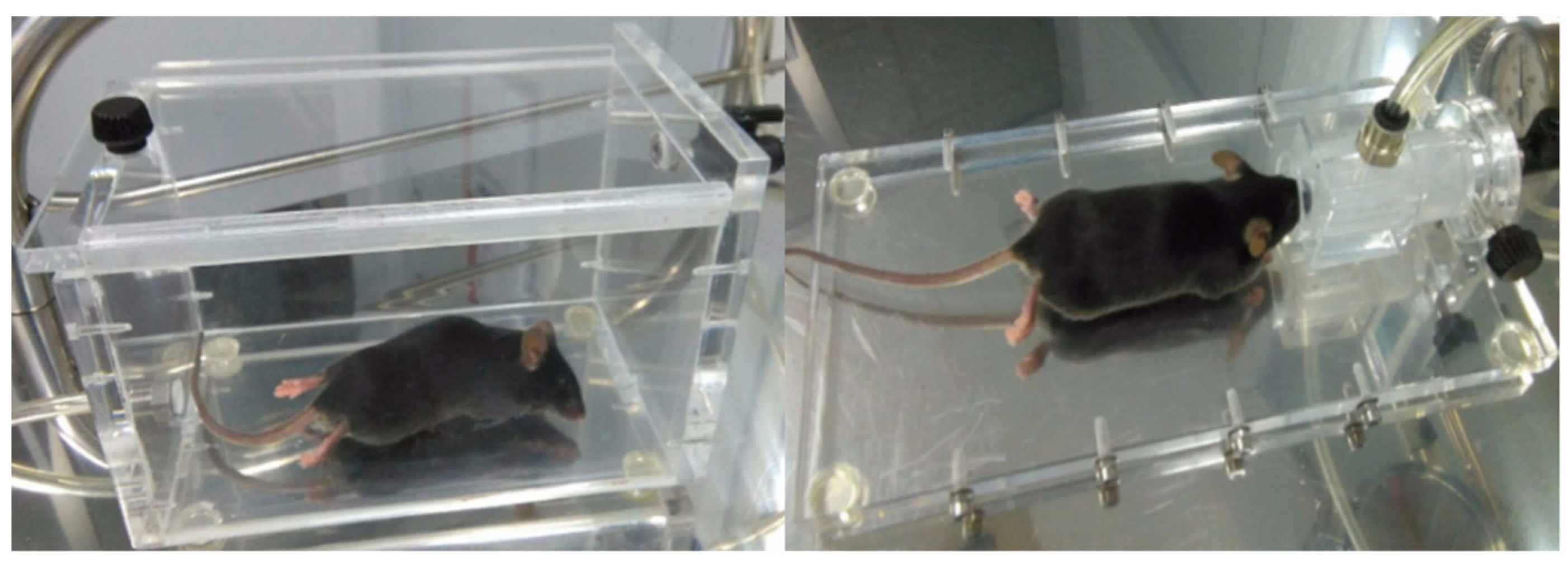
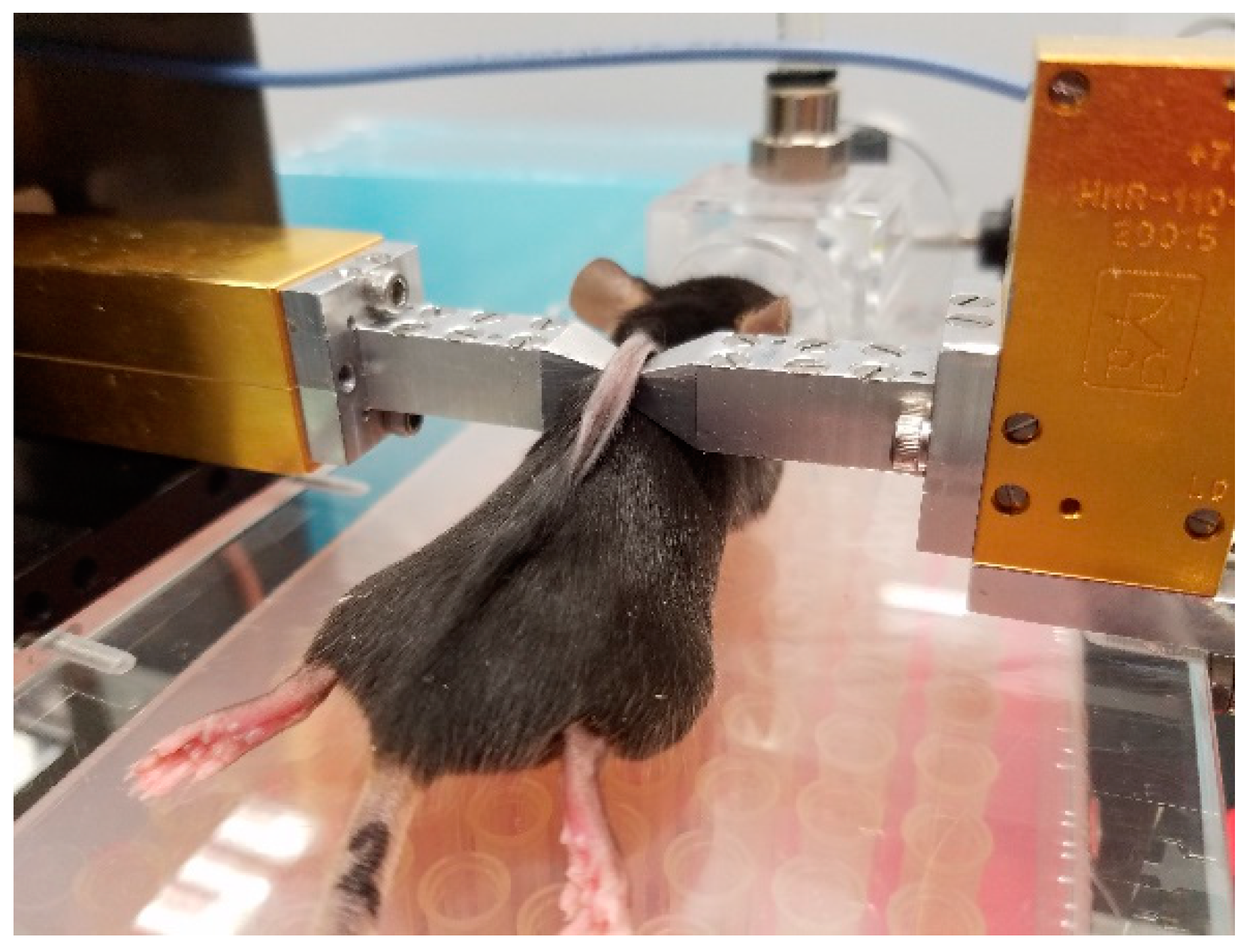
2.2. Experimental Protocol
2.3. Non-Invasive mm-Wave Spectroscopy System for Assessment of Glycemic States
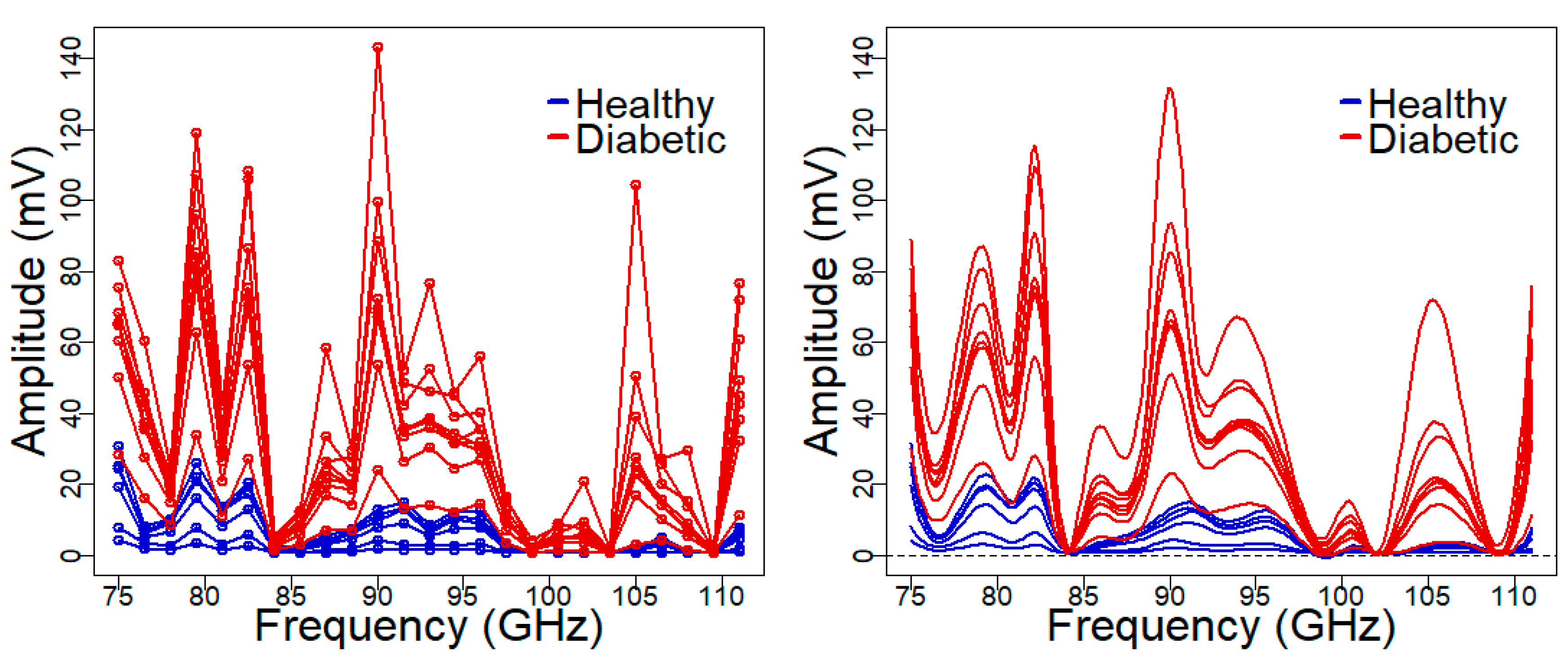
2.4. Functional Data Analysis for Spectral Data
3. Results
3.1. Blind (Predictive) Determination of Sustained Hyperglycemia in Animal Models
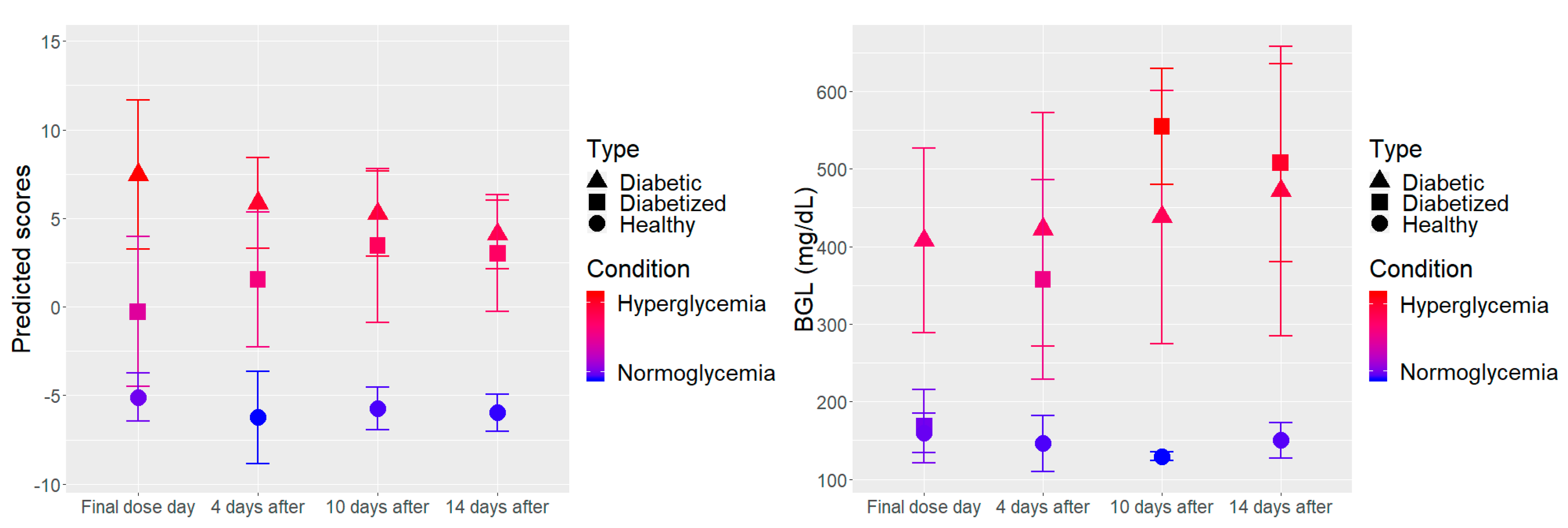
3.2. Temporal Evolution Study
4. Discussion
Data Availability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J. Minimally invasive and robotic surgery. JAMA 2001, 285, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jayaraman, S. Enhancing the Quality of Life Through Wearable Technology. IEEE Eng. Med. Biol. Mag. 2003, 22, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Ahn, D.; Drincic, A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res. Clin. Pract. 2017, 133, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Meetoo, D.; McGovern, P.; Safadi, R. An epidemiological overview of diabetes across the world. Br. J. Nurs. 2007, 16, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26, 3160–3167.
- Nathan, D.M. Long-Term complications of diabetes mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Milicevic, Z.; Raz, I.; Beattie, S.D.; Campaigne, B.N.; Sarwat, S.; Gromniak, E.; Kowalska, I.; Galic, E.; Tan, M.; Hanefeld, M. Natural history of cardiovascular disease in patients with diabetes: role of hyperglycemia. Diabetes Care 2008, 31, S155–S160. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.P.; Pavlovich, J.G.; Goldstein, D.; Little, R.; England, J.; Peterson, C.M. What is hemoglobin A1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin. Chem. 1998, 44, 1951–1958. [Google Scholar] [PubMed]
- Saudek, C.D.; Brick, J.C. The Clinical Use of Hemoglobin A1c. J. Diabetes Sci. Technol. 2009, 3, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.-C.; Lin, H.Y.-H.; Niu, S.-W.; Hwang, D.-Y.; Lee, J.-J.; Tsai, J.-C.; Hung, C.-C.; Hwang, S.-J.; Chen, H.-C. Glycated Hemoglobin and Outcomes in Patients with Advanced Diabetic Chronic Kidney Disease. Sci. Rep. 2016, 6, 20028. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013, 36, S11–S66. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes. Available online: https://www.who.int/diabetes/global-report/en/ (accessed on 28 July 2019).
- Bauer, A.; Hertzberg, O.; Küderle, A.; Strobel, D.; Pleitez, M.A.; Mäntele, W. IR-spectroscopy of skin invivo: Optimal skin sites and properties for non-invasive glucose measurement by photoacoustic and photothermal spectroscopy. J. Biophotonics 2017, 11, 1–12. [Google Scholar]
- Pleitez, M.; Von Lilienfeld-Toal, H.; Mäntele, W. Infrared spectroscopic analysis of human interstitial fluid in vitro and in vivo using FT-IR spectroscopy and pulsed quantum cascade lasers (QCL): Establishing a new approach to non invasive glucose measurement. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 85, 61–65. [Google Scholar] [CrossRef]
- Hertzberg, O.; Bauer, A.; Küderle, A.; Pleitez, M.A.; Mäntele, W. Depth-selective photothermal IR spectroscopy of skin: Potential application for non-invasive glucose measurement. Analyst 2017, 142, 495–502. [Google Scholar] [CrossRef]
- Vashist, S.K. Non-invasive glucose monitoring technology in diabetes management: A review. Anal. Chim. Acta 2012, 750, 16–27. [Google Scholar] [CrossRef]
- Nawaz, A.; Øhlckers, P.; Sælid, S.; Jacobsen, M.; Nadeem Akram, M. Review: Non-Invasive Continuous Blood Glucose Measurement Techniques. J. Bioinforma. Diabetes 2016, 1, 1–27. [Google Scholar] [CrossRef]
- Martín-Mateos, P.; Dornuf, F.; Duarte, B.; Hils, B.; Moreno-Oyervides, A.; Bonilla-Manrique, O.E.; Larcher, F.; Krozer, V.; Acedo, P. In-vivo, non-invasive detection of hyperglycemic states in animal models using mm-wave spectroscopy. Sci. Rep. 2016, 6, 34035. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, Y.; Someya, D. Non-invasive measurement of blood sugar level by millimeter waves. In Proceedings of the 2001 IEEE MTT-S International Microwave Sympsoium Digest (Cat. No.01CH37157), Phoenix, AZ, USA, 20–24 May 2001; pp. 171–174. [Google Scholar]
- Hancock, C.P.; Chaudhry, S. A non-invasive monitoring system. In Proceedings of the 2007 European Microwave Conference, Munich, Germany, 9–12 October 2007; pp. 313–316. [Google Scholar]
- Topsakal, E.; Karacolak, T.; Moreland, E.C. Glucose-dependent dielectric properties of blood plasma. In Proceedings of the 2011 30th URSI General Assembly and Scientific Symposium, Istanbul, Turkey, 13–20 August 2011; pp. 1–4. [Google Scholar]
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-Based Noninvasive Concentration Measurements for Biomedical Applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204. [Google Scholar] [CrossRef]
- Siegel, P.H.; Lee, Y.; Pikov, V. Millimeter-wave non-invasive monitoring of glucose in anesthetized rats. In Proceedings of the 39th International Conference on Infrared, Millimeter, and Terahertz waves (IRMMW-THz), Tucson, AZ, USA, 14–19 September 2014. [Google Scholar]
- Dornuf, F.; Martín-Mateos, P.; Duarte, B.; Hils, B.; Bonilla-Manrique, O.E.; Larcher, F.; Acedo, P.; Krozer, V. Classification of skin phenotypes caused by diabetes mellitus using complex scattering parameters in the millimeter-wave frequency range. Sci. Rep. 2017, 7, 5822. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.R.; Stevens, R.D.; Wenner, B.R.; Ilkayeva, O.; Muoio, D.M.; Newgard, C.B. Metabolomics applied to diabetes research: Moving from information to knowledge. Diabetes 2009, 58, 2429–2443. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, N. Metabolomics in diabetes research. J. Endocrinol. 2012, 215, 29–42. [Google Scholar] [CrossRef]
- Preet, A.; Karve, T.M.; Cheema, A.K. Metabolomics: Approaches and applications to diabetes research. J. Diabetes Metab. 2012, 6, 1–8. [Google Scholar] [CrossRef]
- Lutz, T.A.; Woods, S.C. Overview of animal models of obesity. Curr. Protoc. Pharmacol. 2012, 58, 5–61. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar]
- De Boor, C. A practical guide to splines. In Applied Mathematical Sciences; Marsden, J.E., Sirovich, L., Eds.; Springer: New York, NY, USA, 2001; ISBN 0387953663. [Google Scholar]
- Aguilera, A.M.; Aguilera-Morillo, M.C. Comparative study of different B-spline approaches for functional data. Math. Comput. Model. 2013, 58, 1568–1579. [Google Scholar] [CrossRef]
- Ramsay, J.O.; Silverman, B.W. Functional Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2005; ISBN 038740080X. [Google Scholar]
- Aguilera, A.M.; Aguilera-Morillo, M.C. Penalized PCA approaches for B-spline expansions of smooth functional data. Appl. Math. Comput. 2013, 219, 7805–7819. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. Available online: https://www.r-project.org (accessed on 1 May 2019).

| Sample | Mice strain | Condition | Variation | Expected Glucose Level (mg/dL) | Time with the Condition | Label | Quantity |
|---|---|---|---|---|---|---|---|
| Training sample | C57B16/J | Normoglycemia | - | 100 | 6 months | Healthy mice | 2 |
| BalbC | Normoglycemia | - | 100 | 6 months | Healthy mice | 2 | |
| NMRI-Foxn1nu/Foxn1nu | Normoglycemia | - | 100 | 1 month | Healthy mice | 4 | |
| Lepob/Lepob | Normoglycemia | Normoglycemic by leptin-pump | 100 | 25 days | Healthy mice | 2 | |
| NMRI-Foxn1nu/Foxn1nu | Hyperglycemia | Drug-induced diabetes | >300 | 12 days | Diabetized mice | 3 | |
| Lepob/Lepob | Hyperglycemia | - | >150 | 6 months | Obese mice | 5 | |
| Lepdb/Lepdb | Hyperglycemia | - | >250 | 6 months | Diabetic mice | 2 | |
| Test sample | C57B16/J | Normoglycemia | - | 100 | 9 weeks | Healthy mice | 6 |
| Lepdb/Lepdb | Hyperglycemia | - | >250 | 9 weeks | Diabetic mice | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Oyervides, A.; Martín-Mateos, P.; Aguilera-Morillo, M.C.; Ulisse, G.; Arriba, M.C.; Durban, M.; Del Rio, M.; Larcher, F.; Krozer, V.; Acedo, P. Early, Non-Invasive Sensing of Sustained Hyperglycemia in Mice Using Millimeter-Wave Spectroscopy. Sensors 2019, 19, 3347. https://doi.org/10.3390/s19153347
Moreno-Oyervides A, Martín-Mateos P, Aguilera-Morillo MC, Ulisse G, Arriba MC, Durban M, Del Rio M, Larcher F, Krozer V, Acedo P. Early, Non-Invasive Sensing of Sustained Hyperglycemia in Mice Using Millimeter-Wave Spectroscopy. Sensors. 2019; 19(15):3347. https://doi.org/10.3390/s19153347
Chicago/Turabian StyleMoreno-Oyervides, Aldo, Pedro Martín-Mateos, M. Carmen Aguilera-Morillo, Giacomo Ulisse, María C. Arriba, María Durban, Marcela Del Rio, Fernando Larcher, Viktor Krozer, and Pablo Acedo. 2019. "Early, Non-Invasive Sensing of Sustained Hyperglycemia in Mice Using Millimeter-Wave Spectroscopy" Sensors 19, no. 15: 3347. https://doi.org/10.3390/s19153347
APA StyleMoreno-Oyervides, A., Martín-Mateos, P., Aguilera-Morillo, M. C., Ulisse, G., Arriba, M. C., Durban, M., Del Rio, M., Larcher, F., Krozer, V., & Acedo, P. (2019). Early, Non-Invasive Sensing of Sustained Hyperglycemia in Mice Using Millimeter-Wave Spectroscopy. Sensors, 19(15), 3347. https://doi.org/10.3390/s19153347






